Rapid Assessment of Anthocyanins Content of Onion Waste through Visible-Near-Short-Wave and Mid-Infrared Spectroscopy Combined with Machine Learning Techniques
Abstract
1. Introduction
2. Materials and Methods
2.1. Onion Samples (OS)
2.2. Chemical Characterization of the OS Extract
2.2.1. Chemicals
2.2.2. Preparation of OS Extract
2.2.3. Determination of Total Phenolic Content (TPC)
2.2.4. Determination of Total Monomeric Anthocyanin Content (TAC)
2.3. Spectroscopic Characterization of the Dry OS
2.3.1. VNIR-SWIR Analysis
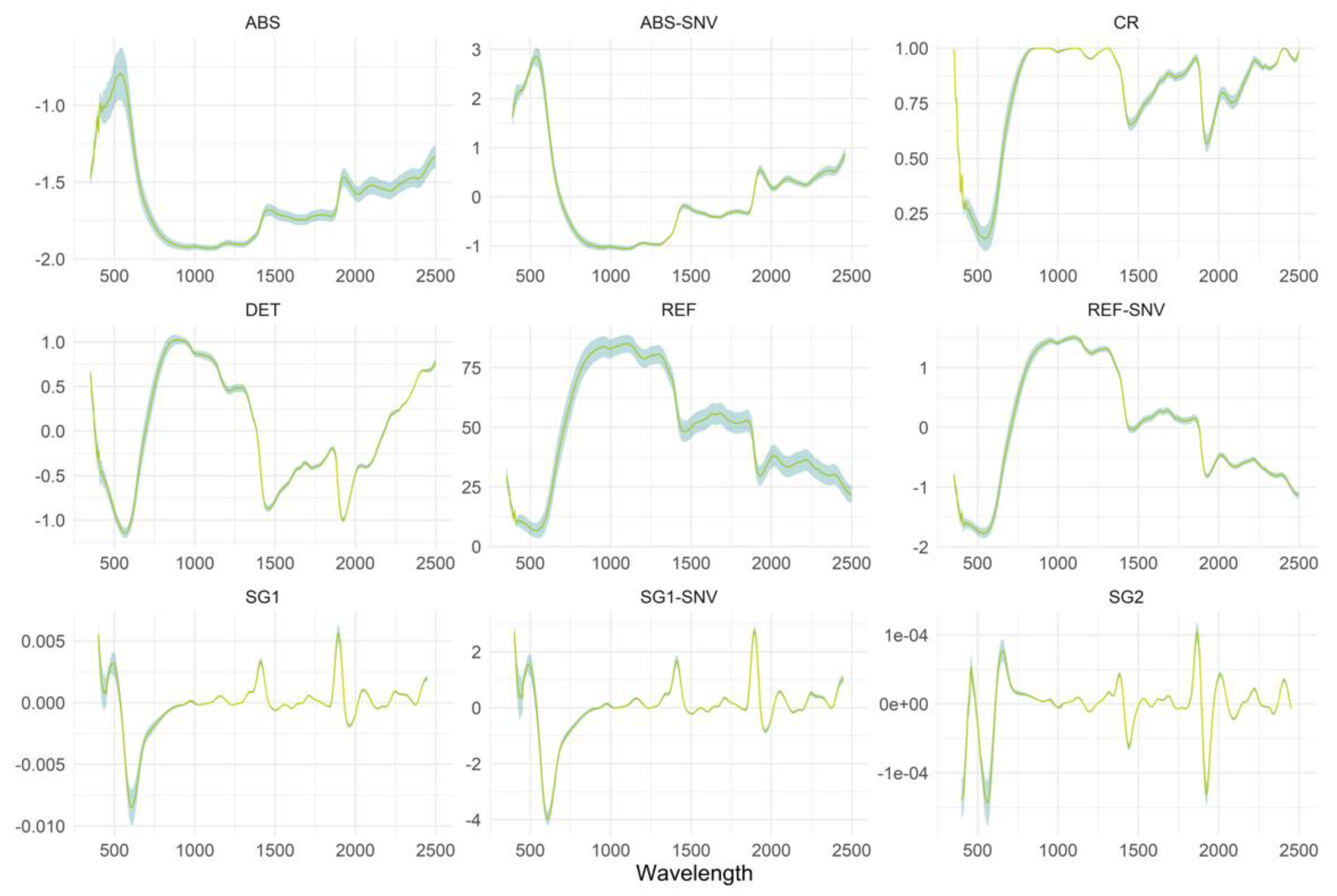
Spectral Preprocessing Techniques
Machine Learning Modeling
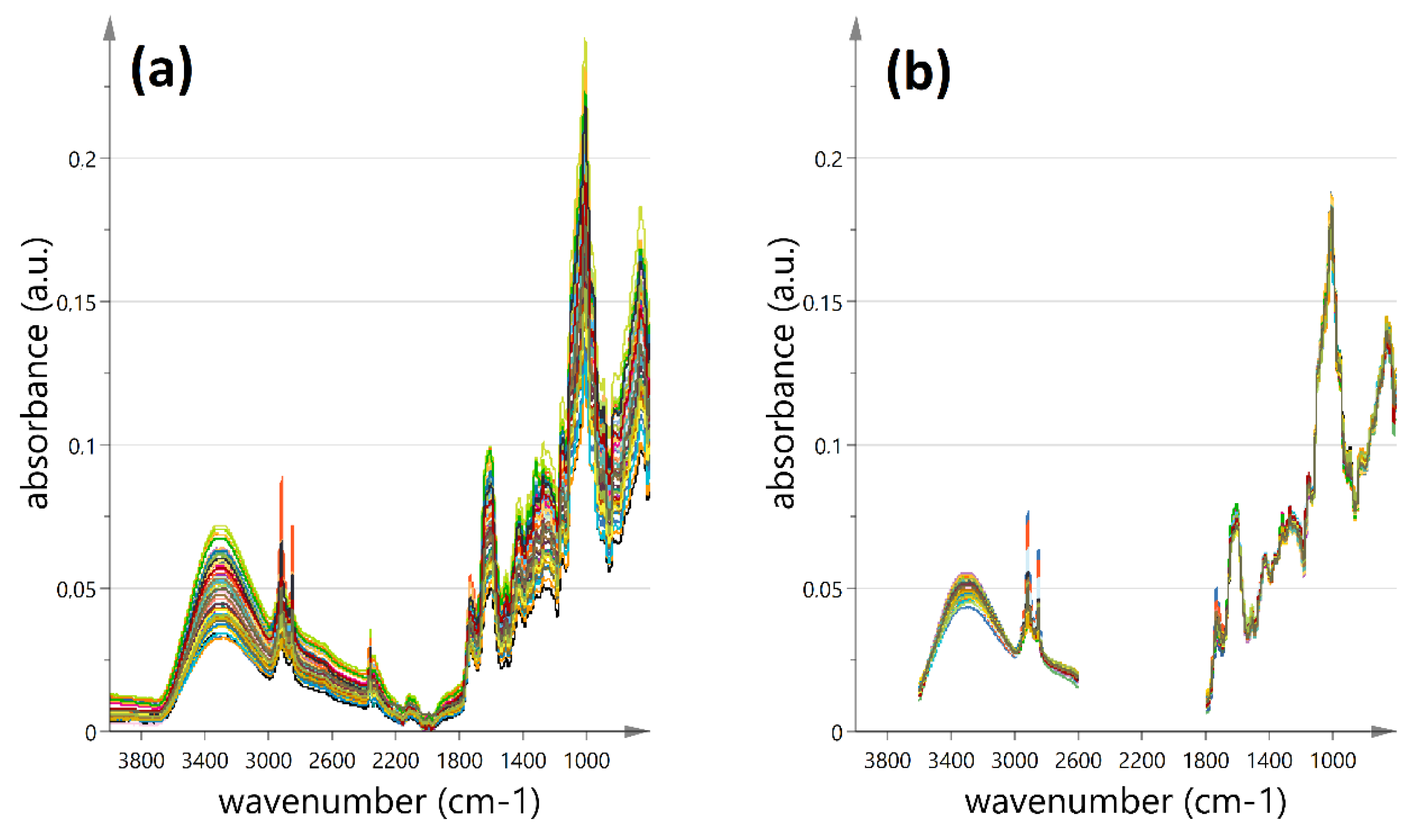
2.3.2. ATR-FT-MIR Analysis
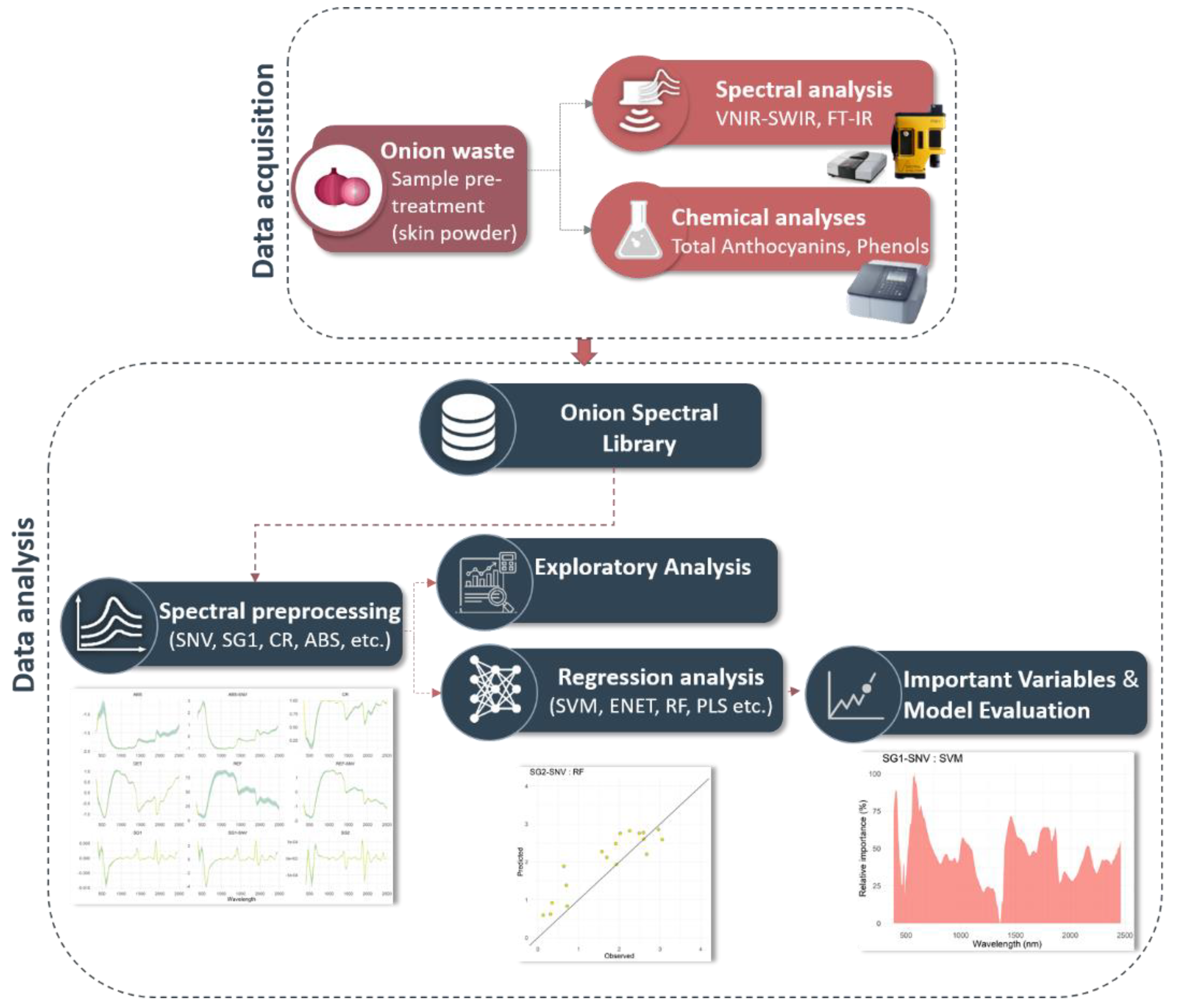
2.4. Implementation
3. Results and Discussion
3.1. Chemical Analyses Data
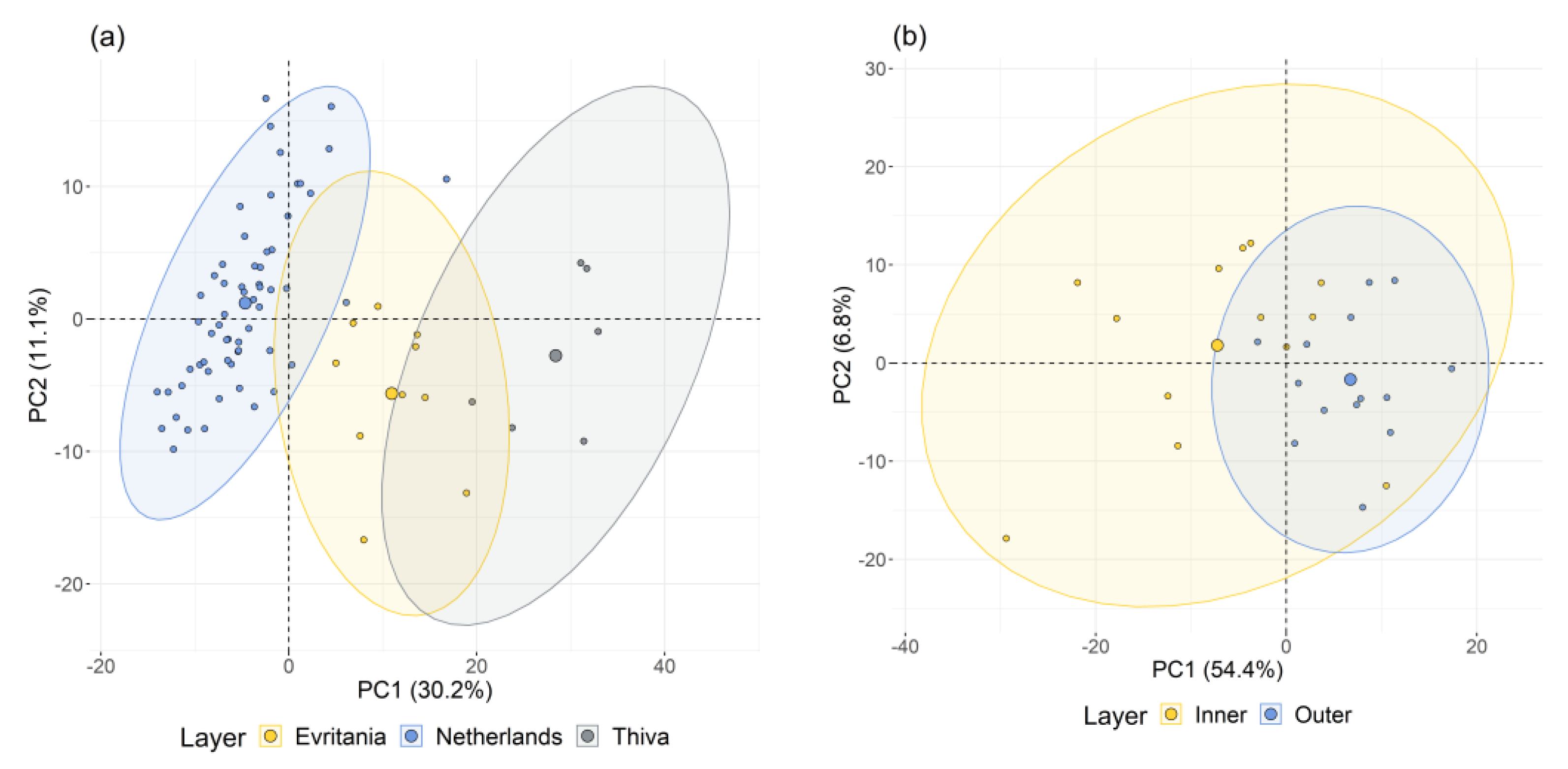
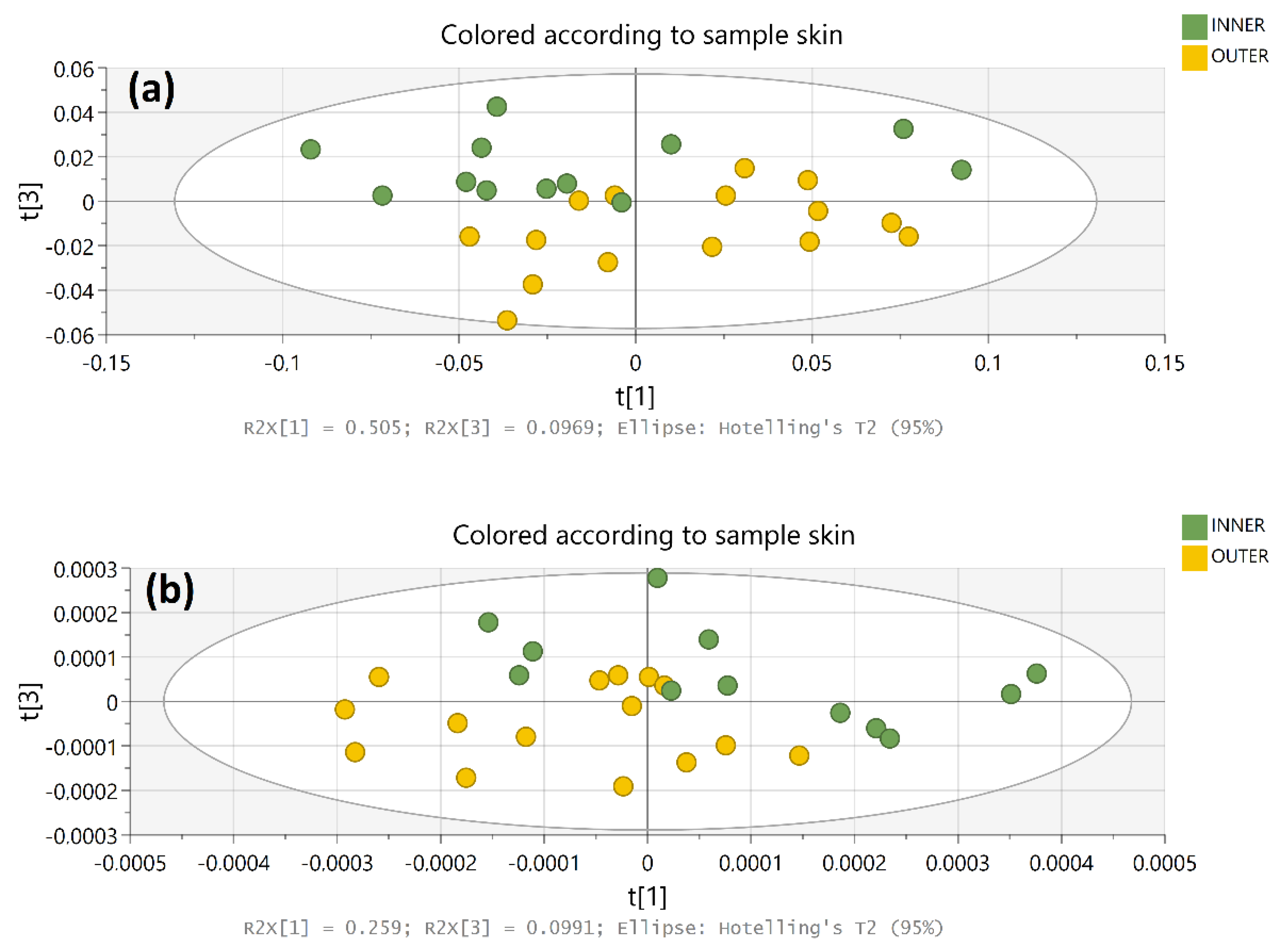
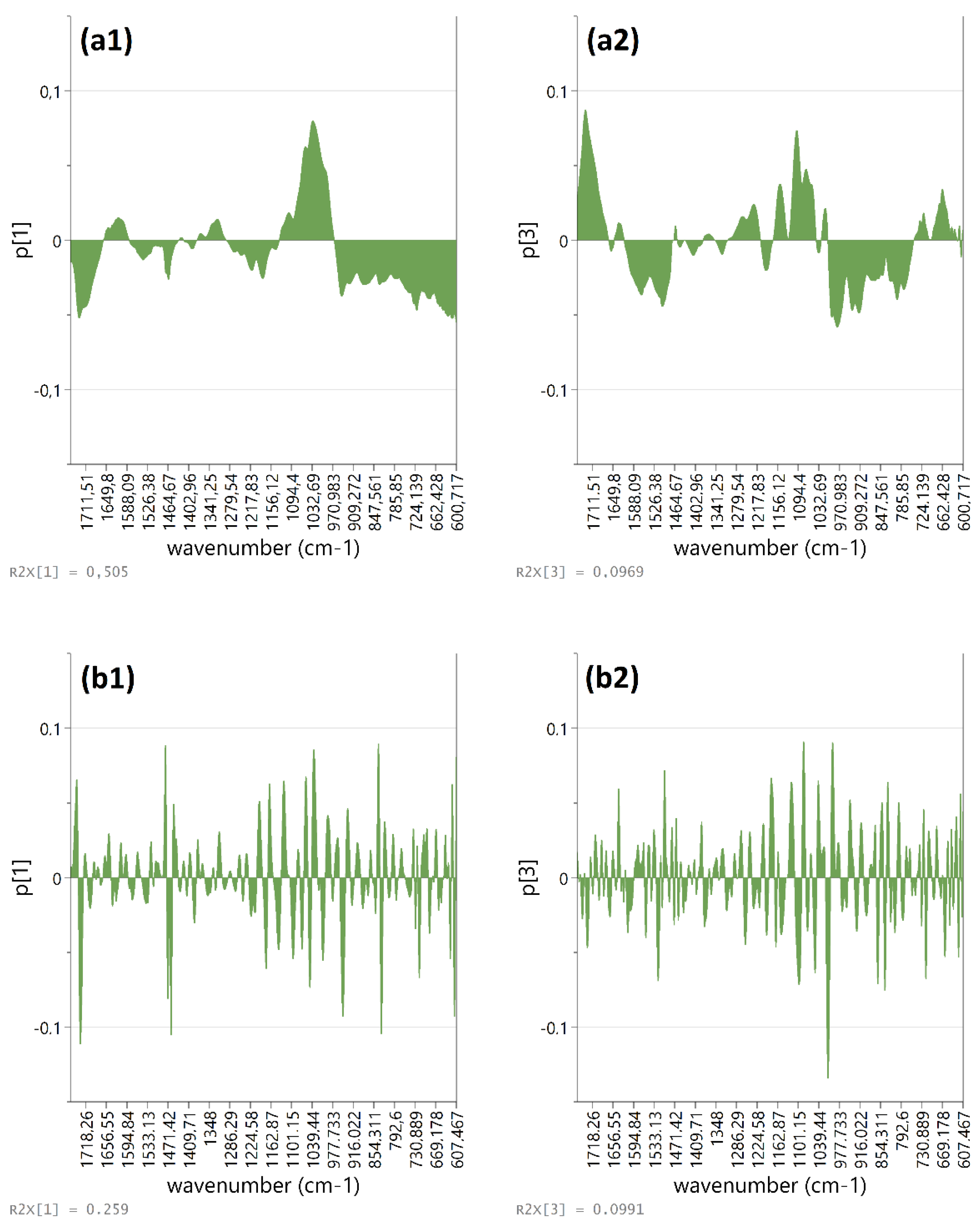
3.2. VNIR-SWIR Exploratory Approach
3.3. Monomeric Anthocyanins Prediction Based on the VNIR-SWIR Spectral Datasets
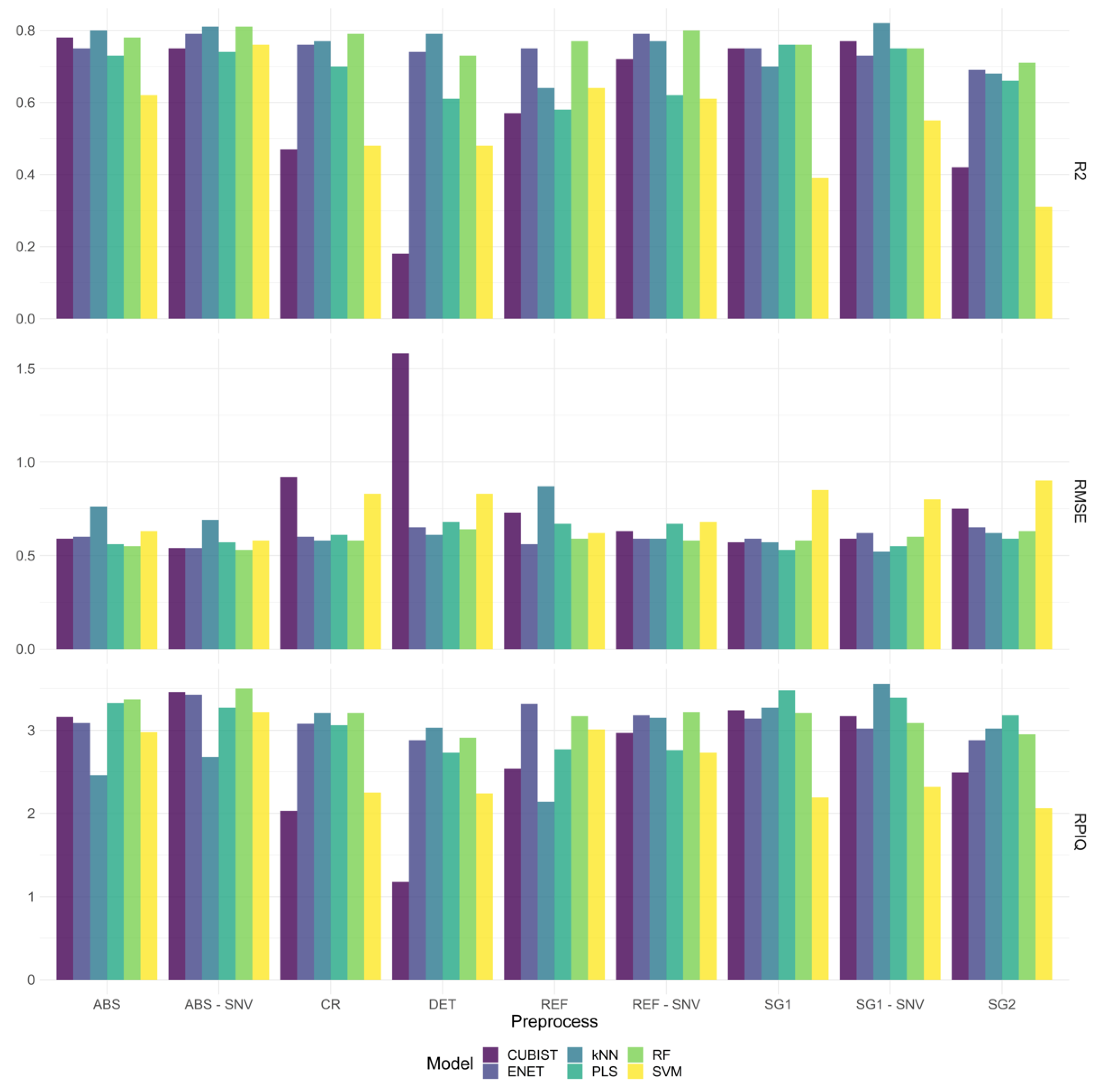
3.3.1. Performance of the ML Models
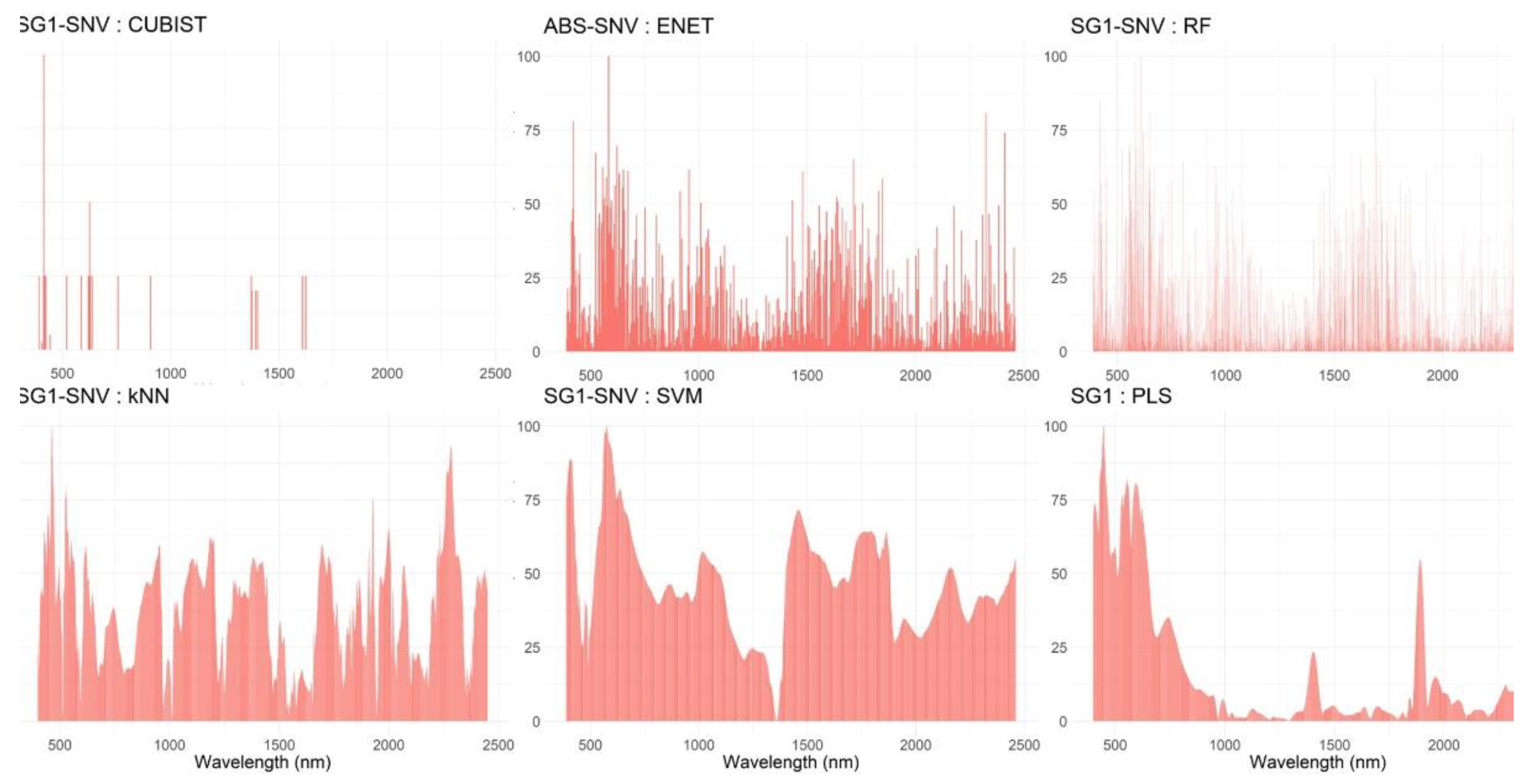
3.3.2. Exploring Shorter Diagnostic Regions in the VNIR-SWIR
3.4. Identification of Phenolic-Group Diagnostic Bands in the MIR
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Pre-Treatment Methods | PLS | RF | Cubist | ENET | k-NN | SVM | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| LVs | mtry | ntree | C | n | s | λ2 | k | C | sigma | |
| ABS | 3 | 9 | 200 | 50 | 0 | 0.75 | 0.010 | 8 | 1 | 0.001 |
| ABS-SNV | 2 | 4 | 250 | 10 | 0 | 0.75 | 0.010 | 12 | 1 | 0.001 |
| CR | 2 | 19 | 150 | 100 | 9 | 0.75 | 0.005 | 6 | 1 | 0.001 |
| DET | 2 | 3 | 150 | 100 | 0 | 0.50 | 0.005 | 8 | 1 | 0.001 |
| REF | 2 | 20 | 150 | 1 | 0 | 0.75 | 0.010 | 13 | 1 | 0.001 |
| REF-SNV | 3 | 17 | 200 | 10 | 0 | 0.50 | 0.010 | 5 | 1 | 0.001 |
| SG1 | 2 | 13 | 250 | 10 | 5 | 0.50 | 0.010 | 6 | 1 | 0.001 |
| SG1-SNV | 2 | 2 | 150 | 50 | 0 | 0.50 | 0.010 | 3 | 1 | 0.001 |
| SG2 | 2 | 20 | 150 | 1 | 5 | 0.50 | 0.010 | 3 | 1 | 0.001 |
Appendix B
| Pre-Processing Techniques | PLS | RF | CUBIST | ENET | K-NN | SVM | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| R2 | RMSE | RPIQ | R2 | RMSE | RPIQ | R2 | RMSE | RPIQ | R2 | RMSE | RPIQ | R2 | RMSE | RPIQ | R2 | RMSE | RPIQ | |
| ABS | 0.57 | 0.67 | 2.76 | 0.76 | 0.58 | 3.17 | 0.57 | 0.73 | 2.54 | 0.75 | 0.56 | 3.32 | 0.64 | 0.87 | 2.13 | 0.63 | 0.61 | 3.01 |
| ABS-SNV | 0.73 | 0.55 | 3.32 | 0.78 | 0.55 | 3.37 | 0.78 | 0.58 | 3.15 | 0.74 | 0.60 | 3.09 | 0.79 | 0.75 | 2.46 | 0.62 | 0.62 | 2.97 |
| CR | 0.76 | 0.53 | 3.48 | 0.76 | 0.57 | 3.21 | 0.74 | 0.57 | 3.24 | 0.75 | 0.59 | 3.14 | 0.69 | 0.56 | 3.27 | 0.38 | 0.84 | 2.19 |
| DET | 0.66 | 0.58 | 3.17 | 0.70 | 0.63 | 2.94 | 0.42 | 0.74 | 2.49 | 0.69 | 0.64 | 2.87 | 0.65 | 0.61 | 3.03 | 0.31 | 0.90 | 2.05 |
| REF | 0.69 | 0.60 | 3.06 | 0.78 | 0.57 | 3.20 | 0.46 | 0.91 | 2.02 | 0.75 | 0.60 | 3.07 | 0.77 | 0.57 | 3.20 | 0.48 | 0.82 | 2.25 |
| REF-SNV | 0.62 | 0.67 | 2.76 | 0.80 | 0.57 | 3.22 | 0.72 | 0.62 | 2.97 | 0.79 | 0.58 | 3.17 | 0.76 | 0.59 | 3.14 | 0.60 | 0.68 | 2.72 |
| SG1 | 0.60 | 0.68 | 2.72 | 0.73 | 0.63 | 2.91 | 0.18 | 1.58 | 1.17 | 0.74 | 0.64 | 2.87 | 0.79 | 0.61 | 3.02 | 0.48 | 0.83 | 2.23 |
| SG1-SNV | 0.74 | 0.54 | 3.39 | 0.75 | 0.60 | 3.08 | 0.77 | 0.58 | 3.16 | 0.73 | 0.61 | 3.02 | 0.81 | 0.52 | 3.56 | 0.54 | 0.80 | 2.31 |
| SG2 | 0.74 | 0.56 | 3.27 | 0.81 | 0.53 | 3.49 | 0.74 | 0.53 | 3.46 | 0.79 | 0.54 | 3.43 | 0.81 | 0.69 | 2.68 | 0.76 | 0.57 | 3.22 |
References
- Sharma, K.; Mahato, N.; Nile, S.H.; Lee, E.T.; Lee, Y.R. Economical and environmentally-friendly approaches for usage of onion (Allium cepa L.) waste. Food Funct. 2016, 7, 3354–3369. [Google Scholar] [CrossRef]
- FAO Production/Yield Quantities of Onions, Dry in Europe. Available online: http://www.fao.org/faostat/en/#data/QC/visualize (accessed on 9 June 2021).
- Benítez, V.; Mollá, E.; Martín-Cabrejas, M.A.; Aguilera, Y.; López-Andréu, F.J.; Cools, K.; Terry, L.A.; Esteban, R.M. Characterization of Industrial Onion Wastes (Allium cepa L.): Dietary Fibre and Bioactive Compounds. Plant Foods Hum. Nutr. 2011, 66, 48–57. [Google Scholar] [CrossRef]
- Celano, R.; Docimo, T.; Piccinelli, A.L.; Gazzerro, P.; Tucci, M.; Di Sanzo, R.; Carabetta, S.; Campone, L.; Russo, M.; Rastrelli, L.; et al. Onion Peel: Turning a Food Waste into a Resource. Antioxidants 2021, 10, 304. [Google Scholar] [CrossRef]
- Albishi, T.; John, J.A.; Al-Khalifa, A.S.; Shahidi, F. Antioxidative phenolic constituents of skins of onion varieties and their activities. J. Funct. Foods 2013, 5, 1191–1203. [Google Scholar] [CrossRef]
- Rhodes, M.J.C.; Price, K.R. Analytical problems in the study of flavonoid compounds in onions. Food Chem. 1996, 57, 113–117. [Google Scholar] [CrossRef]
- Mourtzinos, I.; Prodromidis, P.; Grigorakis, S.; Makris, D.P.; Biliaderis, C.G.; Moschakis, T. Natural food colorants derived from onion wastes: Application in a yoghurt product. Electrophoresis 2018, 39, 1975–1983. [Google Scholar] [CrossRef]
- Santiago, B.; Arias Calvo, A.; Gullón, B.; Feijoo, G.; Moreira, M.T.; González-García, S. Production of flavonol quercetin and fructooligosaccharides from onion (Allium cepa L.) waste: An environmental life cycle approach. Chem. Eng. J. 2020, 392, 123772. [Google Scholar] [CrossRef]
- Kimoto-Nira, H.; Ohashi, Y.; Amamiya, M.; Moriya, N.; Ohmori, H.; Sekiyama, Y. Fermentation of onion (Allium cepa L.) peel by lactic acid bacteria for production of functional food. J. Food Meas. Charact. 2020, 14, 142–149. [Google Scholar] [CrossRef]
- Patra, J.K.; Kwon, Y.; Baek, K.H. Green biosynthesis of gold nanoparticles by onion peel extract: Synthesis, characterization and biological activities. Adv. Powder Technol. 2016, 27, 2204–2213. [Google Scholar] [CrossRef]
- Imbert, E. Food waste valorization options: Opportunities from the bioeconomy. Open Agric. 2017, 2, 195–204. [Google Scholar] [CrossRef]
- Mustafa, F.; Andreescu, S. Chemical and biological sensors for food-quality monitoring and smart packaging. Foods 2018, 7, 168. [Google Scholar] [CrossRef] [PubMed]
- Tsakanikas, P.; Karnavas, A.; Panagou, E.Z.; Nychas, G.J. A machine learning workflow for raw food spectroscopic classification in a future industry. Sci. Rep. 2020, 10, 11212. [Google Scholar] [CrossRef]
- Lu, X.; Wang, J.; Al-Qadiri, H.M.; Ross, C.F.; Powers, J.R.; Tang, J.; Rasco, B.A. Determination of total phenolic content and antioxidant capacity of onion (Allium cepa) and shallot (Allium oschaninii) using infrared spectroscopy. Food Chem. 2011, 129, 637–644. [Google Scholar] [CrossRef] [PubMed]
- Vincke, D.; Baeten, V.; Sinnaeve, G.; Dardenne, P.; Pierna, J.A.F. Determination of Outer Skin in Dry Onions by Hyperspectral Imaging Spectroscopy and Chemometrics. NIR News 2014, 25, 9–12. [Google Scholar] [CrossRef]
- Wang, W.; Li, C.; Gitaitis, R.D. Optical properties of healthy and diseased onion tissues in the visible and near-infrared spectral region. Trans. ASABE 2014, 57, 1771–1782. [Google Scholar] [CrossRef]
- Lee, J.; Durst, R.W.; Wrolstad, R.E. Determination of total monomeric anthocyanin pigment content of fruit juices, beverages, natural colorants, and wines by the pH differential method: Collaborative study. J. AOAC Int. 2005, 88, 1269–1278. [Google Scholar] [CrossRef]
- Rinnan, Å.; van den Berg, F.; Engelsen, S.B. Review of the most common pre-processing techniques for near-infrared spectra. TrAC Trends Anal. Chem. 2009, 28, 1201–1222. [Google Scholar] [CrossRef]
- Minasny, B.; McBratney, A.B. A conditioned Latin hypercube method for sampling in the presence of ancillary information. Comput. Geosci. 2006, 32, 1378–1388. [Google Scholar] [CrossRef]
- Geladi, P.; Kowalski, B.R. Partial least-squares regression: A tutorial. Anal. Chim. Acta 1986. [Google Scholar] [CrossRef]
- Svetnik, V.; Liaw, A.; Tong, C.; Christopher Culberson, J.; Sheridan, R.P.; Feuston, B.P. Random Forest: A Classification and Regression Tool for Compound Classification and QSAR Modeling. J. Chem. Inf. Comput. Sci. 2003. [Google Scholar] [CrossRef]
- Quinlan, J.R. Learning with continuous classes. In Proceedings of the Australian Joint Conference on Artificial Intelligence, Hobart, Australia, 16–18 November 1992; pp. 343–348. [Google Scholar]
- Drucker, H.; Surges, C.J.C.; Kaufman, L.; Smola, A.; Vapnik, V. Support vector regression machines. In Proceedings of the Advances in Neural Information Processing Systems; MIT Press: Cambridge, MA, USA, 1997; pp. 155–161. [Google Scholar]
- Engel, J.; Gerretzen, J.; Szymańska, E.; Jansen, J.J.; Downey, G.; Blanchet, L.; Buydens, L.M.C. Breaking with trends in pre-processing? TrAC Trends Anal. Chem. 2013, 50, 96–106. [Google Scholar] [CrossRef]
- Ordoudi, S.A.; de los Mozos Pascual, M.; Tsimidou, M.Z. On the quality control of traded saffron by means of transmission Fourier-transform mid-infrared (FT-MIR) spectroscopy and chemometrics. Food Chem. 2014, 150, 414–421. [Google Scholar] [CrossRef] [PubMed]
- R Development Core Team. R: A Language and Environment for Statistical Computing; R Found. Stat. Comput.: Vienna, Austria, 2013. [Google Scholar]
- Kuhn, M. Building Predictive Models in R Using the caret Package. J. Stat. Softw. Artic. 2008, 28, 1–26. [Google Scholar] [CrossRef]
- Kiassos, E.; Mylonaki, S.; Makris, D.P.; Kefalas, P. Implementation of response surface methodology to optimise extraction of onion (Allium cepa) solid waste phenolics. Innov. Food Sci. Emerg. Technol. 2009. [Google Scholar] [CrossRef]
- Dykes, L.; Hoffmann, L.; Portillo-Rodriguez, O.; Rooney, W.L.; Rooney, L.W. Prediction of total phenols, condensed tannins, and 3-deoxyanthocyanidins in sorghum grain using near-infrared (NIR) spectroscopy. J. Cereal Sci. 2014, 60, 138–142. [Google Scholar] [CrossRef]
- Caramês, E.T.S.; Alamar, P.D.; Poppi, R.J.; Pallone, J.A.L. Rapid Assessment of Total Phenolic and Anthocyanin Contents in Grape Juice Using Infrared Spectroscopy and Multivariate Calibration. Food Anal. Methods 2017, 10, 1609–1615. [Google Scholar] [CrossRef]
- Lohumi, S.; Lee, S.; Lee, W.H.; Kim, M.S.; Mo, C.; Bae, H.; Cho, B.K. Detection of starch adulteration in onion powder by FT-NIR and FT-IR spectroscopy. J. Agric. Food Chem. 2014, 62, 9246–9251. [Google Scholar] [CrossRef]
- Tang, Y.; Jones, E.; Minasny, B. Evaluating low-cost portable near infrared sensors for rapid analysis of soils from South Eastern Australia. Geoderma Reg. 2020, 20, e00240. [Google Scholar] [CrossRef]
- Tsakiridis, N.L.; Keramaris, K.D.; Theocharis, J.B.; Zalidis, G.C. Simultaneous prediction of soil properties from VNIR-SWIR spectra using a localized multi-channel 1-D convolutional neural network. Geoderma 2020, 367, 114208. [Google Scholar] [CrossRef]
- McCann, M.C.; Hammouri, M.; Wilson, R.; Belton, P.; Roberts, K. Fourier transform infrared microspectroscopy is a new way to look at plant cell walls. Plant Physiol. 1992, 100, 1940–1947. [Google Scholar] [CrossRef] [PubMed]
- Sun, D.-W. Infrared Spectroscopy for Food Quality Analysis and Control, 1st ed.; Sun, D.-W., Ed.; Elsevier Inc.: New York, NY, USA, 2009; ISBN 9780123741363. [Google Scholar]
- Socrates, G. Infrared and Raman Characteristic Group Frequencies, 3rd ed.; Socrates, G., Ed.; John Wiley and Sons, Ltd.: Chichester, UK, 2004; Volume 35, ISBN 978-0-470-09307-8. [Google Scholar]
- Lu, X.; Ross, C.F.; Powers, J.R.; Rasco, B.A. Determination of quercetins in onion (Allium cepa) using infrared spectroscopy. J. Agric. Food Chem. 2011, 59, 6376–6382. [Google Scholar] [CrossRef] [PubMed]
- Schulz, H.; Baranska, M. Identification and quantification of valuable plant substances by IR and Raman spectroscopy. Vib. Spectrosc. 2007, 43, 13–25. [Google Scholar] [CrossRef]
- Abbas, O.; Compère, G.; Larondelle, Y.; Pompeu, D.; Rogez, H.; Baeten, V. Phenolic compound explorer: A mid-infrared spectroscopy database. Vib. Spectrosc. 2017, 92, 111–118. [Google Scholar] [CrossRef]
- Khiari, Z.; Makris, D.P. Stability and transformation of major flavonols in onion (Allium cepa) solid wastes. J. Food Sci. Technol. 2012, 49, 489–494. [Google Scholar] [CrossRef]
- Tram, N.L.; Hazama, C.; Shimoyamada, M.; Ando, H.; Kato, K.; Yamauchi, R. Antioxidative compounds from the outer scales of onion. J. Agric. Food Chem. 2005, 53, 8183–8189. [Google Scholar] [CrossRef]

| Parameter | Min | Max | Q1 | Q2 | Q3 | Mean |
|---|---|---|---|---|---|---|
| TPC (mg GAE/g) | 13 | 79 | 30 | 43 | 52 | 43 |
| TAC (mg Cyanidin/g) | 0.13 | 3.82 | 1.49 | 2.08 | 2.71 | 2.01 |
| Spectral Range | R2 | RMSE | RPIQ |
|---|---|---|---|
| Full spectrum | 0.82 | 0.52 | 3.56 |
| VNIR (400–1000 nm) | 0.70 | 0.66 | 2.80 |
| SWIR (1350–2500 nm) | 0.55 | 0.75 | 2.49 |
| Sample Code | MIR Peaks | MIR Valleys | Functional Group Vibrations | Possible Identity |
|---|---|---|---|---|
| 0th Order | 2nd Order | |||
| entry 1 | 3299.7 | v(O-H), v(N-H) | carbohydrates, water, proteins | |
| entry 2 | 2923.6 | 2917–2918 | vas(C-H) | -CH3 and –CH2- alkanes |
| 2850.3 | 2848–2850 | vas(C-H) | CH3 or CH3 – Ar | |
| 1734.7 | 1734–1736 | v (Ar-C = O) | aryl carboxylic acid monomers, e.g., hydroxybenzoic acids | |
| 1717–1718 | v(Ar-C = O) | aryl ketones, aldehydes | ||
| 1637–8 | 1636–1642 | v(Ar-C = O), δ(H-OH) | aryl carboxylic acids and flavonoids, polygalacturonic acid peptides (amide I), water | |
| 1600–2 | v(C = C aromatic), vas(COO-) | flavonoids, polygalacturonic acid | ||
| 1561–1562 | flavonoids, nucleic acid ring base | |||
| 1521 | v(C = C aromatic) | aryl carboxylic acids and flavonoids | ||
| 1509 | 1503–1507 | δ(C-H aromatic) | flavonoids | |
| 1489 | δ(C-H aromatic) | flavonoids, e.g., quercetin and cyanidin glucosides | ||
| 1465 | 1471, 1464 | δ(C-H aromatic) | aryl carboxylic acids and flavonoids | |
| 1423.2 | 1440, 1420 | δ(C-H aromatic) & vs(COO-) | aryl carboxylic acids, polygalacturonic acid esters | |
| 1385 | δ(O-H aromatic) | |||
| 1368 | 1363–1371 | δ(O-H aromatic) | flavonoids, e.g., catechin, polysaccharides | |
| 1334 | v(C-O), δ(C-H aromatic) | phenol | ||
| 1321 | 1316–1319 | δsym (-CH3 ), v(C- H) β(O-H) | alkanes, alkenes, phenol or tertiary alcohol | |
| 1271 | ||||
| 1200, 1230 | v(Ar C-C-O), | phenols, carbohydrates | ||
| 1152.3 | 1159–1166 | v(C-O) v(C-CO-C) | C–O–C glycosidic linkages of oligosaccharides aliphatic ketones | |
| 1101–1103 | v (C-O), v (C-C), ring | carbohydrates | ||
| 1096 | v(C-C) | carbohydrates | ||
| 1072 | v(C-OH) | oligosaccharides | ||
| 1051 | 1049–1050 | |||
| 1008.6 | v(C-O) | C–O–C glycosidic linkages of oligosaccharides, polysaccharides | ||
| 987–988 | 985–986 | ω( = C-H), δ( = C-H) | polysaccharides, e.g., cellulose, aryl carboxylic acids, flavanols, | |
| 972–973 | ω( = C-H), δ( = C-H) | polysaccharides, e.g., pectin, aryl carboxylic acids | ||
| 954 | 951–952 | carbohydrates, aryl carboxylic acids | ||
| 892 | 891–894 | ω( = C-H) | substituted aromatic ring | |
| 863–865 | ω( = C-H) | substituted aromatic ring | ||
| 831–833 | 832–833 | ω( = C-H) | substituted aromatic ring | |
| 756–758 | γ(C-H) | hydroxybenzoic acids | ||
| 717–718 | γ(C-H) | flavonoids | ||
| 706–710 | γ(C-H) | flavonoids, e.g., quercetin glycosides, epicatechin | ||
| 665.3 | ||||
| 641–649 | ||||
| 627–632 | flavonoids, e.g., anthocyanin diglycosides |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tziolas, N.; Ordoudi, S.A.; Tavlaridis, A.; Karyotis, K.; Zalidis, G.; Mourtzinos, I. Rapid Assessment of Anthocyanins Content of Onion Waste through Visible-Near-Short-Wave and Mid-Infrared Spectroscopy Combined with Machine Learning Techniques. Sustainability 2021, 13, 6588. https://doi.org/10.3390/su13126588
Tziolas N, Ordoudi SA, Tavlaridis A, Karyotis K, Zalidis G, Mourtzinos I. Rapid Assessment of Anthocyanins Content of Onion Waste through Visible-Near-Short-Wave and Mid-Infrared Spectroscopy Combined with Machine Learning Techniques. Sustainability. 2021; 13(12):6588. https://doi.org/10.3390/su13126588
Chicago/Turabian StyleTziolas, Nikolaos, Stella A. Ordoudi, Apostolos Tavlaridis, Konstantinos Karyotis, George Zalidis, and Ioannis Mourtzinos. 2021. "Rapid Assessment of Anthocyanins Content of Onion Waste through Visible-Near-Short-Wave and Mid-Infrared Spectroscopy Combined with Machine Learning Techniques" Sustainability 13, no. 12: 6588. https://doi.org/10.3390/su13126588
APA StyleTziolas, N., Ordoudi, S. A., Tavlaridis, A., Karyotis, K., Zalidis, G., & Mourtzinos, I. (2021). Rapid Assessment of Anthocyanins Content of Onion Waste through Visible-Near-Short-Wave and Mid-Infrared Spectroscopy Combined with Machine Learning Techniques. Sustainability, 13(12), 6588. https://doi.org/10.3390/su13126588








