Worldwide Research Analysis on Natural Zeolites as Environmental Remediation Materials
Abstract
1. Introduction
- A high degree of hydration;
- A high degree of crystallinity;
- Low density and the large volume of free spaces (dehydrated);
- Shape selectivity;
- Ion exchange characteristics;
- Catalytic characteristics;
- Sorption of molecules and ions;
- Electric conductivity.
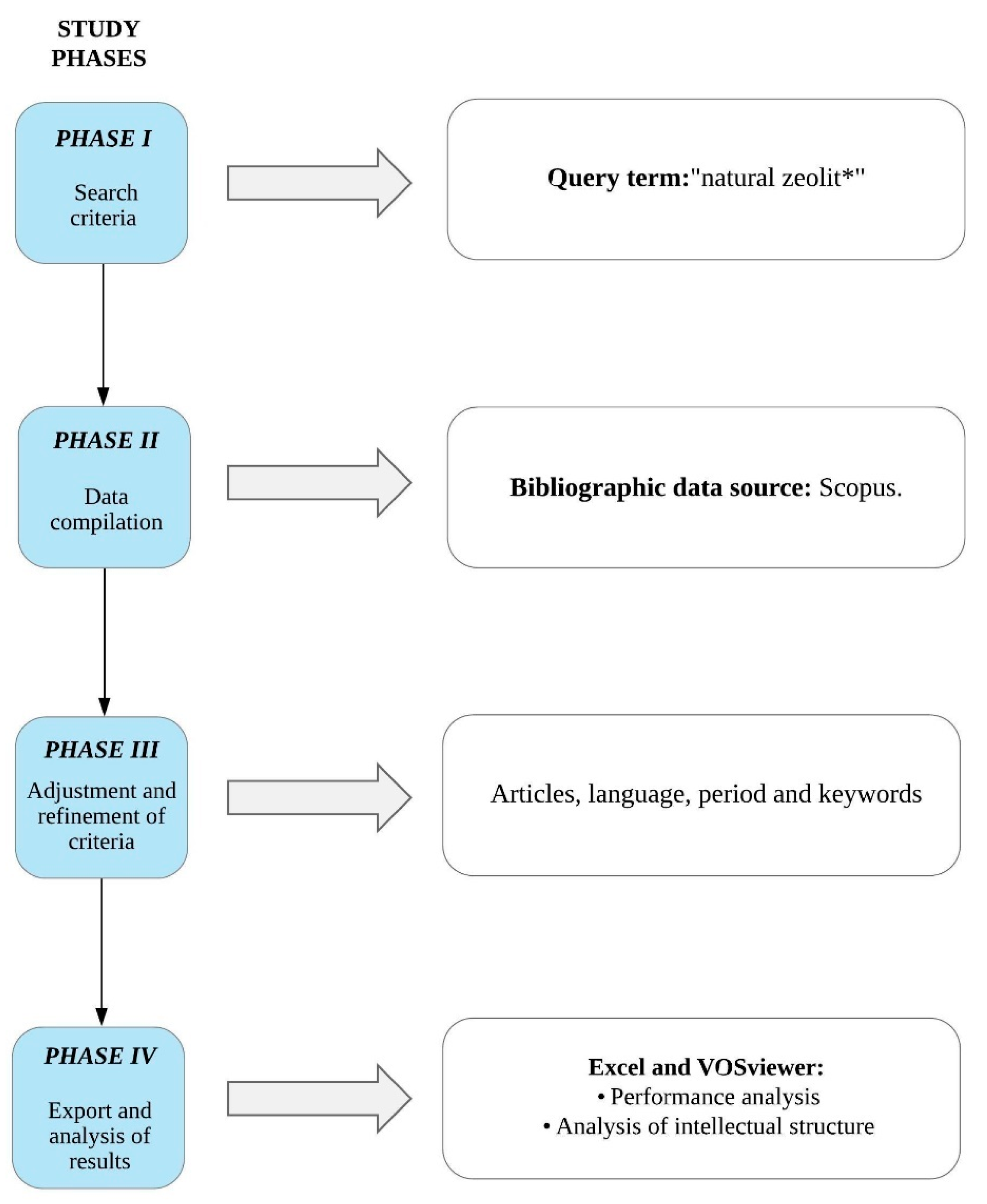
2. Materials and Methods
2.1. Defining the Appropriate Search Terms
2.2. Initial Search Results
2.3. Refinement of Search Results
2.4. Data Extraction and Analysis
- (i)
- (ii)
- VOSviewer—a tool that allows the construction, exploration, and visualization of two-dimensional bibliometric networks, exposing a specific field’s intellectual structure [52]. Moreover, it is handy since it allows the visualization of bibliometric maps in great detail through graphical representations of straightforward interpretations [64,65]. Science maps allow observing relationships between various variables that allow information on this structure obtained at a micro-level (co-occurrence of author keywords), meso-level (co-citation of cited authors), and macro level (co-citation of cited journals) [66,67]. VOSviewer is a friendly platform used to carry out these analyses; its creators provided documentation (manual and research articles) that allowed us to understand its functionality and use [52,64,68]. This program is used to study various scientific disciplines related to geology, for example, geotourism [69], fracking [70], and geological risks [71], as well as other areas of knowledge management [72], occupational health [73], education [74], etc.
3. Results
3.1. Performance Analysis
3.1.1. Scientific Production Analysis
3.1.2. Country Contribution
3.1.3. Journal’s Performance
3.1.4. Author Contribution
3.1.5. Frequently Cited Documents
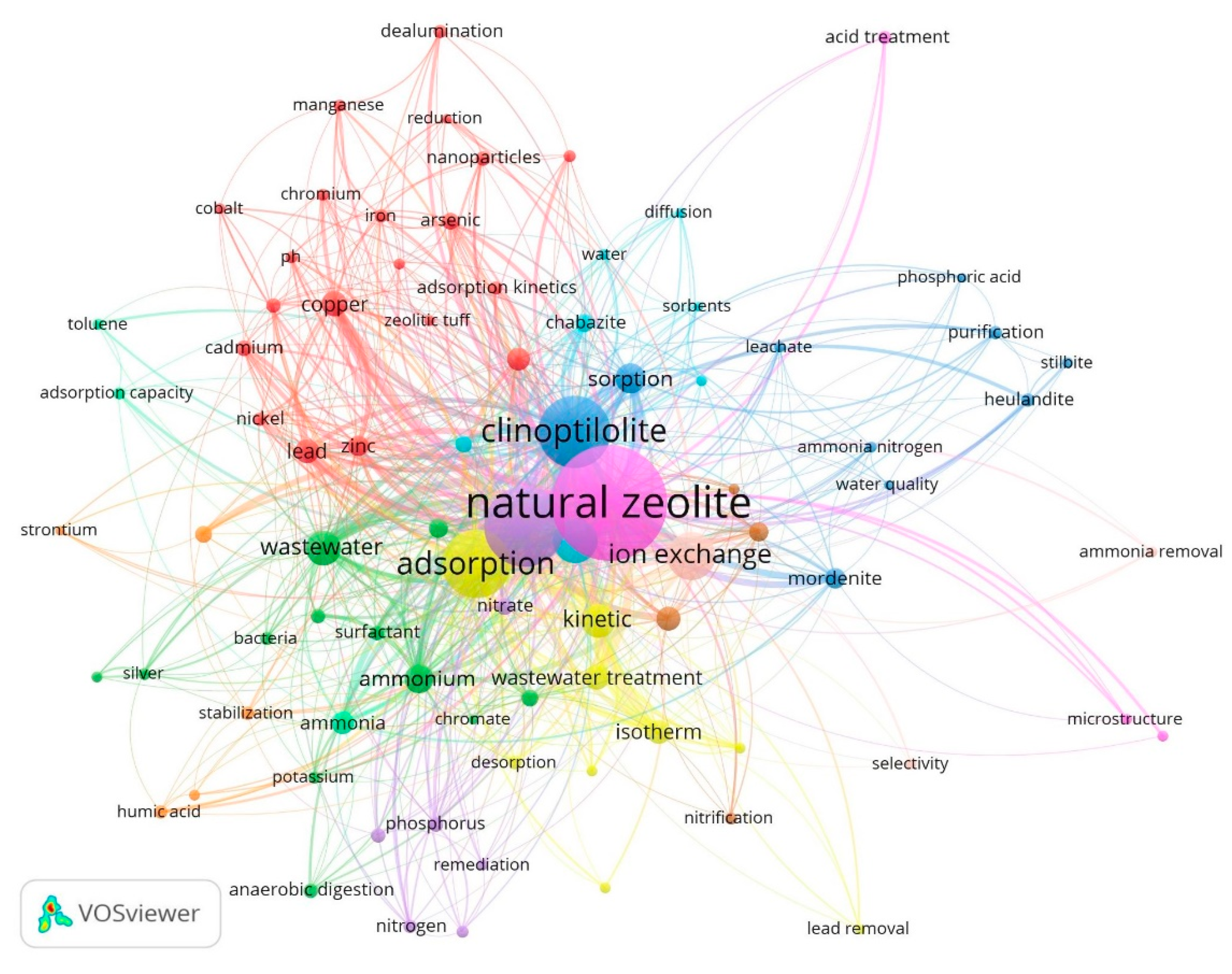
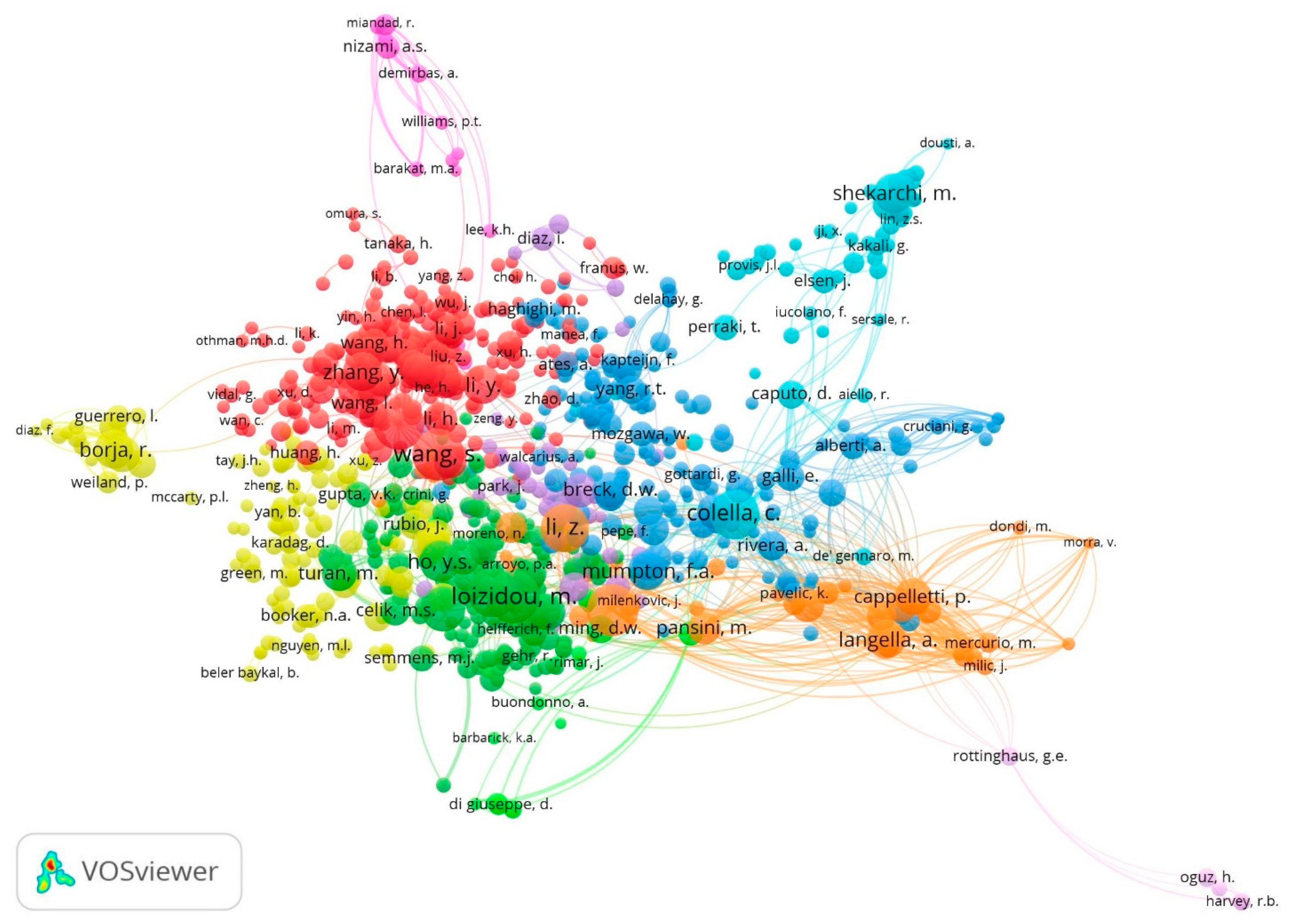
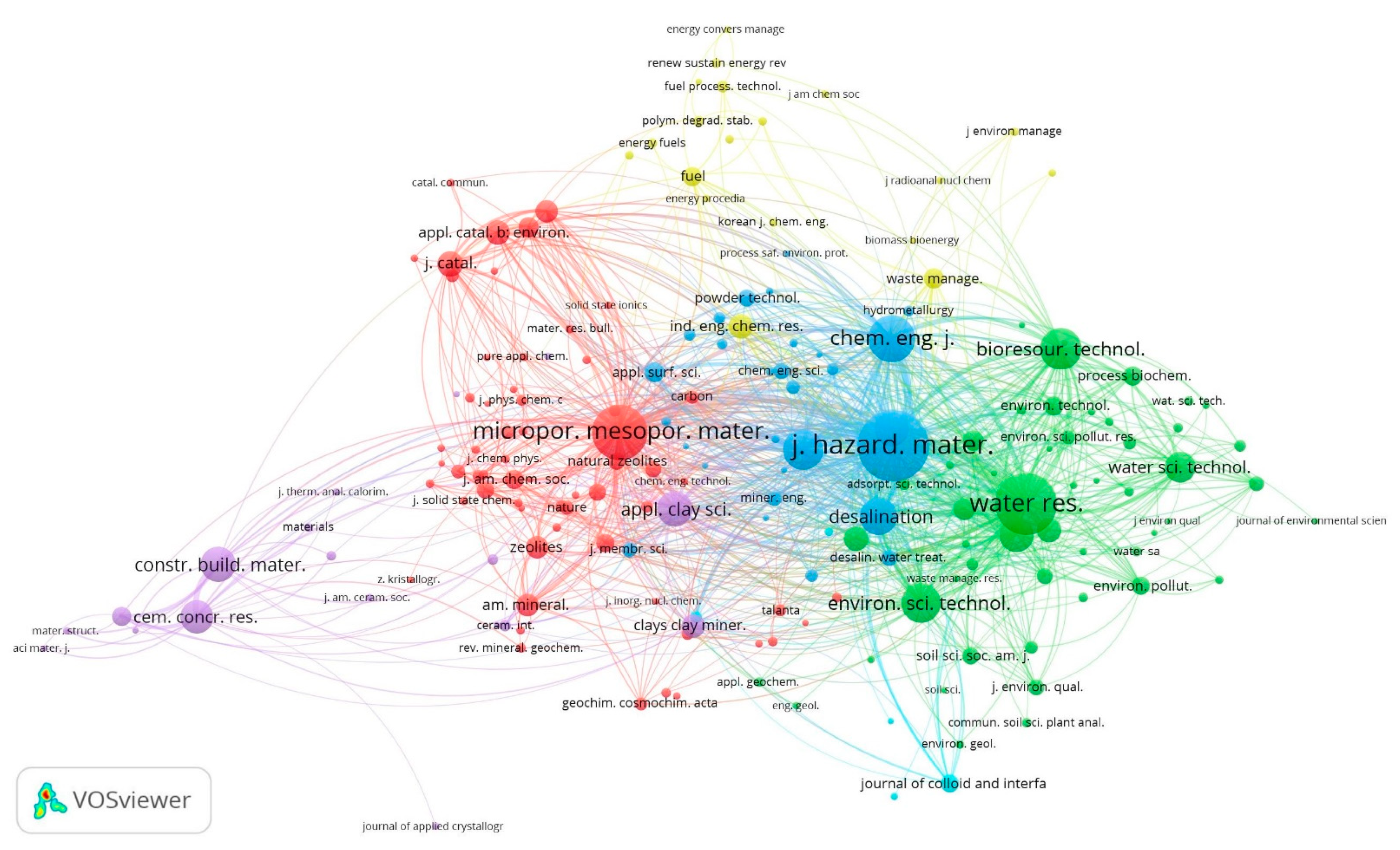
3.2. Intellectual Structure Analysis
3.2.1. Author’s Keyword Co-Occurrence Network
3.2.2. Cited Authors Co-Citation Network
3.2.3. Journal Co-Citation Network
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AC | Adsorption Capacity |
| ACI | Annual Citation Index |
| AT | Number of Articles |
| AMD | Acid Mine Drainage |
| AW | Acid Water |
| CSV | Comma-Separated Values |
| DW | Drinking Water |
| HI | H-Index |
| HM | Heavy Metals |
| HOCs | Hydrophobic Organic Pollutants |
| IE | Ion Exchange |
| MOCZ | Manganese oxide-coated zeolite |
| NOC | Non-ionic Organic Pollutants |
| NZ | Natural Zeolites |
| PAHs | Polycyclic Aromatic Hydrocarbons |
| PBDEs | Polybrominated Diphenyl Ethers |
| PTE | Potentially toxic elements |
| R | Rank |
| SD | Soil Degradation |
| SJR | SCImago Journal Rank |
| TC | Total number of citations |
| WW | WasteWater |
References
- Cruz-Guzman, M. La Contaminación de Suelos y Aguas: Su Prevención con Nuevas Sustancias Naturales; Universidad d Sevilla: Sevilla, Spain, 2007. [Google Scholar]
- Inglezakis, V.J.; Loizidou, M.D.; Grigoropoulou, H.P. Ion exchange of Pb2+, Cu2+, Fe3+, and Cr3+ on natural clinoptilolite: Selectivity determination and influence of acidity on metal uptake. J. Colloid Interface Sci. 2003, 261, 49–54. [Google Scholar] [CrossRef]
- Reyes, Y.C.; Vergara, I.; Torres, O.E.; Díaz, M.; González, E.E. Heavy metals contamination: Implications for health and food safety. Ing. Investig. Desarro. 2016, 16, 66–77. Available online: https://dialnet.unirioja.es/servlet/articulo?codigo=6096110 (accessed on 2 November 2020).
- ZHuang, Z.; Pan, X.-D.; Wu, P.-G.; Han, J.-L.; Chen, Q. Heavy metals in vegetables and the health risk to population in Zhejiang, China. Food Control. 2014, 36, 248–252. [Google Scholar] [CrossRef]
- SLin, H.; Juang, R.S. Heavy metal removal from water by sorption using surfactant-modified montmorillonite. J. Hazard. Mater. 2002, 92, 315–326. [Google Scholar] [CrossRef]
- Mumpton, F.A. La roca magica: Uses of natural zeolites in agriculture and industry. Proc. Natl. Acad. Sci. USA 1999, 96, 3463–3470. [Google Scholar] [CrossRef] [PubMed]
- Morante, F. Las Zeolitas de la Costa De Ecuador (Guayaquil): Geología, Caracterización y Aplicaciones. Ph.D. Thesis, E.T.S.I. Minas (UPM), Madrid, Spain, 2004. [Google Scholar]
- Calvo, B.; Canoira, L.; Morante, F.; Martínez-Bedia, J.M.; Vinagre, C.; García-González, J.-E.; Elsen, J.; Alcántara, R. Continuous elimination of Pb2+, Cu2+, Zn2+, H+ and NH4+ from acidic waters by ionic exchange on natural zeolites. J. Hazard. Mater. 2009, 166, 619–627. [Google Scholar] [CrossRef]
- An, W.; Zhou, X.; Liu, X.; Chai, P.; Kuznicki, T.; Kuznicki, S. Natural zeolite clinoptilolite-phosphate composite Membranes for water desalination by pervaporation. J. Membr. Sci. 2014, 470, 431–438. [Google Scholar] [CrossRef]
- Rege, S.U.; Yang, R.T.; Qian, K.; Buzanowski, M.A. Air-prepurification by pressure swing adsorption using single/layered beds. Chem. Eng. Sci. 2001, 56, 2745–2759. [Google Scholar] [CrossRef]
- An, W.; Swenson, P.; Wu, L.; Waller, T.; Ku, A.; Kuznicki, S.M. Selective separation of hydrogen from C1/C2 hydrocarbons and CO2 through dense natural zeolite membranes. J. Membr. Sci. 2011, 369, 414–419. [Google Scholar] [CrossRef]
- Parham, W. Natural zeolites: Some potential agricultural applications for developing countries. Nat. Resour. Forum 1989, 13, 107–115. [Google Scholar] [CrossRef]
- Costafreda, J.; Sanchez, M.; Velazquez, C.; Govea, P.; Tobon, I.; Gutierrez, A.; Vasquez, B.; Andres, L.; Alfonso, D.; Luis, J.; et al. Las Zeolitas Naturales de Iberoamérica; Fundación Gómez Pardo: Madrid, Spain, 2018. [Google Scholar]
- Moscou, L. Chapter 1 The Zeolite Scene. Stud. Surf. Sci. Catal. 1991, 58, 1–12. [Google Scholar] [CrossRef]
- Breck, D.W. Zeolite Molecular Sieves: Structure, Chemistry and Use; John Wiley and Sons: Hoboken, NJ, USA, 1973. [Google Scholar]
- Waisberg, M.; Joseph, P.; Hale, B.; Beyersmann, D. Molecular and cellular mechanisms of cadmium carcinogenesis. Toxicology 2003, 192, 95–117. [Google Scholar] [CrossRef]
- Franco, L.F.L.; Muñoz, P.T.L.; Garcia, F.G.M. Los riesgos de los metales pesados en la salud humana y animal. Biotecnol. Sect. Agropecu. Agroind. 2016, 14, 145. [Google Scholar] [CrossRef]
- Kothandaraman, V. Industrial Water Pollution Control. JAWRA J. Am. Water Resour. Assoc. 1969, 5, 73–74. [Google Scholar] [CrossRef]
- Guo, X.; Zeng, L.; Jin, X. Advanced regeneration and fixed-bed study of ammonium and potassium removal from anaerobic digested wastewater by natural zeolite. J. Environ. Sci. 2013, 25, 954–961. [Google Scholar] [CrossRef]
- Hailu, Y.; Tilahun, E.; Brhane, A.; Resky, H.; Sahu, O. Ion exchanges process for calcium, magnesium and total hardness from ground water with natural zeolite. Groundw. Sustain. Dev. 2019, 8, 457–467. [Google Scholar] [CrossRef]
- Blanchard, G.; Maunaye, M.; Martin, G. Removal of heavy metals from waters by means of natural zeolites. Water Res. 1984, 18, 1501–1507. [Google Scholar] [CrossRef]
- Kiurski, J.; Adamović, S.; Oros, I.; Krstić, J.; Miloradov, M.V. The removal efficiency of heavy metals from spent printing developer. Carpathian J. Earth Environ. Sci. 2012, 7, 5–16. [Google Scholar]
- Dwairi, R.A.A.; Al-Rawajfeh, A.E. Removal of cobalt and nickel from wastewater by using jordan low-cost zeolite and bentonite. J. Univ. Chem. Technol. Metall. 2012, 47, 69–76. [Google Scholar]
- Galletti, C.; Dosa, M.; Russo, N.; Fino, D. Zn2+ and Cd2+ removal from wastewater using clinoptilolite as adsorbent. Environ. Sci. Pollut. Res. 2020. [Google Scholar] [CrossRef]
- Díaz, I. Environmental uses of zeolites in Ethiopia. Catal. Today 2017, 285, 29–38. [Google Scholar] [CrossRef]
- Mustelier, J.L.C. Geología, caracterización y aplicaciones de las rocas zeolíticas del complejo volcánico de Cabo de Gata (Almería). Ph.D. Thesis, E.T.S.I. Minas, Madrid, Spain, 2008. [Google Scholar]
- Colella, C. Environmental Applications of Natural Zeolitic Materials Based on Their Ion Exchange Properties. In Natural Microporous Materials in Environmental Technology; Springer: Dordrecht, The Netherlands, 1999; pp. 207–224. [Google Scholar]
- Mier, M.V.; Callejas, R.L.; Gehr, R.; Cisneros, B.E.J.; Alvarez, P. Heavy metal removal with mexican clinoptilolite. Water Res. 2001, 35, 373–378. [Google Scholar] [CrossRef]
- Shavandi, M.; Haddadian, Z.; Ismail, M.; Abdullah, N.; Abidin, Z. Removal of Fe(III), Mn(II) and Zn(II) from palm oil mill effluent (POME) by natural zeolite. J. Taiwan Inst. Chem. Eng. 2012, 43, 750–759. [Google Scholar] [CrossRef]
- RMalekian, R.; Abedi-Koupai, J.; Eslamian, S.S.; Mousavi, S.F.; Abbaspour, K.C.; Afyuni, M. Ion-exchange process for ammonium removal and release using natural Iranian zeolite. Appl. Clay Sci. 2011, 51, 323–329. [Google Scholar] [CrossRef]
- Fu, H.; Li, Y.; Yu, Z.; Shen, J.; Li, J.; Zhang, M.; Ding, T.; Xu, L.; Lee, S.S. Ammonium removal using a calcined natural zeolite modified with sodium nitrate. J. Hazard. Mater. 2020, 393, 122481. [Google Scholar] [CrossRef] [PubMed]
- Tomečková, V.; Lichardusová, L.; Komanicky, V.; Pelegrinová, D. The Spectrofluorometric Monitoring of Water Purification by Natural Alternatives. Spectrosc. Lett. 2012, 45, 447–451. [Google Scholar] [CrossRef]
- Echevarría, M.S.; Déniz, R.D.T.; Castellanos, E.M.; A Sherbakov, G.; Moya, J.J.R. Uses of natural zeolite in the removal of Pb2+ from contaminated water. Eclética Química J. 1997, 22, 15–22. [Google Scholar] [CrossRef]
- Swenson, P.; Tanchuk, B.; Bastida, E.; An, W.; Kuznicki, S.M. Water desalination and de-oiling with natural zeolite membranes—Potential application for purification of SAGD process water. Desalination 2012, 286, 442–446. [Google Scholar] [CrossRef]
- Meshko, V.D.; Markovska, L.T.; Minceva, M.B.; Cibulic, V.V. Modelling of a fixed bed adsorption column for waste water treatment. J. Serb. Chem. Soc. 1998, 63, 891–901. [Google Scholar]
- Pucarevic, M.; Stojic, N.; Kuzmanovski, I. Removal of pesticides from water using zeolites. Kuwait J. Sci. 2017, 44, 99–105. [Google Scholar]
- Markovic, R.; Gardic, V.; Obradovic, L.; Djordjievski, S.; Stevanović, Z.; Stevanovic, J.; Gvozdenovic, M. The Application of a Natural Zeolite for Acid Mine Drainage Purification. Mater. Trans. 2015, 56, 2053–2057. [Google Scholar] [CrossRef]
- Baykara, H.; Martinez, M.C.; Rey, D.V.; Urbina, D.S.; Paredes, C.; Rigail-Cedeño, A.; Aviles, M.O. Preparation and Determination of Antimicrobial Property of Cation-Exchanged Ecuadorian Natural Zeolite to be Used as Filler for Polyethylene and Polypropylene Matrices. J. Polym. Environ. 2017, 26, 2566–2578. [Google Scholar] [CrossRef]
- Chen, Y.; Yu, W.; Jin, L.; Wang, Q.; Yin, J.; Lin, J.; Li, J.-Y. Stabilization of hydrophobic organic contaminants in sediments by natural zeolites: Bioavailability-based assessment of efficacy using equilibrium passive sampling. J. Soils Sediments 2019, 19, 3898–3907. [Google Scholar] [CrossRef]
- Ghiaci, M.; Abbaspur, A.; Kia, R.; Seyedeyn-Azad, F. Equilibrium isotherm studies for the sorption of benzene, toluene, and phenol onto organo-zeolites and as-synthesized MCM-41. Sep. Purif. Technol. 2004, 40, 217–229. [Google Scholar] [CrossRef]
- Cappelletti, P.; Colella, A.; Langella, A.; Mercurio, M.; Catalanotti, L.; Monetti, V.; de Gennaro, B. Use of surface modified natural zeolite (SMNZ) in pharmaceutical preparations Part 1. Mineralogical and technological characterization of some industrial zeolite-rich rocks. Microporous Mesoporous Mater. 2017, 250, 232–244. [Google Scholar] [CrossRef]
- Faghihian, H.; Marageh, M.G.; Kazemian, H. The use of clinoptilolite and its sodium form for removal of radioactive cesium, and strontium from nuclear wastewater and Pb2+, Ni2+, Cd2+, Ba2+ from municipal wastewater. Appl. Radiat. Isot. 1999, 50, 655–660. [Google Scholar] [CrossRef]
- Osmanlioglu, A.E. Treatment of radioactive liquid waste by sorption on natural zeolite in Turkey. J. Hazard. Mater. 2006, 137, 332–335. [Google Scholar] [CrossRef]
- Chelishchev, N.F. Use of natural zeolites at Chernobyl. Nat. Zeolites 1995, 93, 525–532. Available online: https://ci.nii.ac.jp/naid/20001587662 (accessed on 5 November 2020).
- Malczyk, P. Mercury inactivation in contaminated soil using natural zeolites of the clinoptilolite type. Fresenius Environ. Bull. 2009, 18, 1090–1093. [Google Scholar]
- Taheri-Sodejani, H.; Ghobadinia, M.; Tabatabaei, S.-H.; Kazemian, H. Using natural zeolite for contamination reduction of agricultural soil irrigated with treated urban wastewater. Desalination Water Treat. 2014, 54, 2723–2730. [Google Scholar] [CrossRef]
- Tranfield, D.; Denyer, D.; Smart, P. Towards a Methodology for Developing Evidence-Informed Management Knowledge by Means of Systematic Review. Br. J. Manag. 2003, 14, 207–222. [Google Scholar] [CrossRef]
- Fahimnia, B.; Sarkis, J.; Davarzani, H. Green supply chain management: A review and bibliometric analysis. Int. J. Prod. Econ. 2015, 162, 101–114. [Google Scholar] [CrossRef]
- Carrión-Mero, P.; Montalván-Burbano, N.; Paz-Salas, N.; Morante-Carballo, F. Volcanic Geomorphology: A Review of Worldwide Research. Geosciences 2020, 10, 347. [Google Scholar] [CrossRef]
- Zupic, I.; Čater, T. Bibliometric methods in management and organization. Organ. Res. Methods 2014, 18, 429–472. [Google Scholar] [CrossRef]
- Herrera-Franco, G.; Montalván-Burbano, N.; Carrión-Mero, P.; Jaya-Montalvo, M.; Gurumendi-Noriega, M. Worldwide Research on Geoparks through Bibliometric Analysis. Sustainability 2021, 13, 1175. [Google Scholar] [CrossRef]
- Van Eck, N.J.; Waltman, L. Software survey: VOSviewer, a computer program for bibliometric mapping. Scientometrics 2010, 84, 523–538. [Google Scholar] [CrossRef]
- Ogawa, T.; Iyoki, K.; Fukushima, T.; Kajikawa, Y. Landscape of Research Areas for Zeolites and Metal-Organic Frameworks Using Computational Classification Based on Citation Networks. Materials 2017, 10, 1428. [Google Scholar] [CrossRef]
- Reháková, M.; Sopková, A.; Casciola, M.; Failla, S. A study of the silver form of a natural zeolitic material of the clinoptilolite type. J. Incl. Phenom. Mol. Recognit. Chem. 1994, 20, 233–240. [Google Scholar] [CrossRef]
- Syamsiah, S.; Hadi, I.S. Adsorption cycles and effect of microbial population on phenol removal using natural zeolit. Sep. Purif. Technol. 2004, 34, 125–133. [Google Scholar] [CrossRef]
- Andres, A. Measuring Academic Research. Meas. Acad. Res. 2009. [Google Scholar] [CrossRef]
- Pico-Saltos, R.; Garz, J. Research Trends in Career Success: A Bibliometric Review. Sustainability 2021, 13, 4625. [Google Scholar] [CrossRef]
- Baas, J.; Schotten, M.; Plume, A.; Côté, G.; Karimi, R. Scopus as a curated, high-quality bibliometric data source for academic research in quantitative science studies. Quant. Sci. Stud. 2020, 1, 377–386. [Google Scholar] [CrossRef]
- Del Río-Rama, M.; Maldonado-Erazo, C.; Álvarez-García, J.; Durán-Sánchez, A. Cultural and Natural Resources in Tourism Island: Bibliometric Mapping. Sustainability 2020, 12, 724. [Google Scholar] [CrossRef]
- Ruban, D.A.; Ponedelnik, A.A.; Yashalova, N.N. Megaclasts: Term Use and Relevant Biases. Geosciences 2018, 9, 14. [Google Scholar] [CrossRef]
- Meseguer-Sánchez, V.; Abad-Segura, E.; Belmonte-Ureña, L.J.; Molina-Moreno, V. Examining the Research Evolution on the Socio-Economic and Environmental Dimensions on University Social Responsibility. Int. J. Environ. Res. Public Health 2020, 17, 4729. [Google Scholar] [CrossRef] [PubMed]
- Najmi, A.; Rashidi, T.H.; Abbasi, A.; Waller, T. Reviewing the transport domain: An evolutionary bibliometrics and network analysis. Scientometrics 2017, 110, 843–865. [Google Scholar] [CrossRef]
- Aznar-Sánchez, J.A.; Velasco-Muñoz, J.F.; Belmonte-Ureña, L.J.; Manzano-Agugliaro, F. The worldwide research trends on water ecosystem services. Ecol. Indic. 2019, 99, 310–323. [Google Scholar] [CrossRef]
- Van Eck, N.J.; Waltman, L. Visualizing Bibliometric Networks. In Measuring Scholarly Impact; Springer International Publishing: Cham, Switzerland, 2014; pp. 285–320. [Google Scholar]
- Van Eck, N.J.; Waltman, L.; Noyons, E.C.M.; Buter, R.K. Automatic term identification for bibliometric mapping. Scientometrics 2010, 82, 581–596. [Google Scholar] [CrossRef]
- Chandra, Y. Mapping the evolution of entrepreneurship as a field of research (1990–2013): A scientometric analysis. PLoS ONE 2018, 13, e0190228. [Google Scholar] [CrossRef]
- Herrera-Franco, G.; Montalván-Burbano, N.; Carrión-Mero, P.; Bravo-Montero, L. Worldwide Research on Socio-Hydrology: A Bibliometric Analysis. Water 2021, 13, 1283. [Google Scholar] [CrossRef]
- Van Eck, N.J.; Waltman, L. Citation-based clustering of publications using CitNetExplorer and VOSviewer. Scientometrics 2017, 111, 1053–1070. [Google Scholar] [CrossRef] [PubMed]
- Herrera-Franco, G.; Montalván-Burbano, N.; Carrión-Mero, P.; Apolo-Masache, B.; Jaya-Montalvo, M. Research Trends in Geotourism: A Bibliometric Analysis Using the Scopus Database. Geosciences 2020, 10, 379. [Google Scholar] [CrossRef]
- Li, J.; Jovanovic, A.; Klimek, P.; Guo, X. Bibliometric analysis of fracking scientific literature. Scientometrics 2015, 105, 1273–1284. [Google Scholar] [CrossRef]
- Briones-Bitar, J.; Carrión-Mero, P.; Montalván-Burbano, N.; Morante-Carballo, F. Rockfall Research: A Bibliometric Analysis and Future Trends. Geosciences 2020, 10, 403. [Google Scholar] [CrossRef]
- Montalván-Burbano, N.; Pérez-Valls, M.; Plaza-Úbeda, J. Analysis of scientific production on organizational innovation. Cogent Bus. Manag. 2020, 7, 1745043. [Google Scholar] [CrossRef]
- Zhu, B.; Fan, H.; Xie, B.; Su, R.; Zhou, C.; He, J. Mapping the Scientific Research on Healthcare Workers’ Occupational Health: A Bibliometric and Social Network Analysis. Int. J. Environ. Res. Public Health 2020, 17, 2625. [Google Scholar] [CrossRef]
- Durán-Sánchez, A.; del Río-Rama, M.d.; Álvarez-García, J.; García-Vélez, D.F. Mapping of scientific coverage on education for Entrepreneurship in Higher Education. J. Enterprising Communities 2019, 13, 84–104. [Google Scholar] [CrossRef]
- Vdovina, E.D.; Radyuk, R.I.; Sultanov, A.S.; Aripov, E.A. Use of natural zeolites of uzbekistan for the purification of low activity sewage. II. Sorption of radioactive cobalt. Sov. Radiochem. 1977, 19, 155–157. [Google Scholar]
- Sims, R.C.; Little, L.W. Enhanced Nitrification by Addition of Clinoptilolite to Tertiary Activated Sludge Units. Environ. Lett. 1973, 4, 27–34. [Google Scholar] [CrossRef]
- Semmens, M.; Martin, W. The influence of pretreatment on the capacity and selectivity of clinoptilolite for metal ions. Water Res. 1988, 22, 537–542. [Google Scholar] [CrossRef]
- Ćurković, L.; Cerjan-Stefanović, Š.; Filipan, T. Metal ion exchange by natural and modified zeolites. Water Res. 1997, 31, 1379–1382. [Google Scholar] [CrossRef]
- Saltalı, K.; Sarı, A.; Aydın, M. Removal of ammonium ion from aqueous solution by natural Turkish (Yıldızeli) zeolite for environmental quality. J. Hazard. Mater. 2007, 141, 258–263. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Ariyanto, E. Competitive adsorption of malachite green and Pb ions on natural zeolite. J. Colloid Interface Sci. 2007, 314, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Llanes-Monter, M.M.; Olguín, M.T.; Solache-Ríos, M.J. Lead sorption by a Mexican, clinoptilolite-rich tuff. Environ. Sci. Pollut. Res. 2007, 14, 397–403. [Google Scholar] [CrossRef]
- Motsi, T.; Rowson, N.; Simmons, M. Adsorption of heavy metals from acid mine drainage by natural zeolite. Int. J. Miner. Process. 2009, 92, 42–48. [Google Scholar] [CrossRef]
- Li, H.; Shi, W.-Y.; Shao, H.-B.; Shao, M.-A. The remediation of the lead-polluted garden soil by natural zeolite. J. Hazard. Mater. 2009, 169, 1106–1111. [Google Scholar] [CrossRef] [PubMed]
- Zou, W.; Zhao, L.; Han, R. Removal of Uranium (VI) by Fixed Bed Ion-exchange Column Using Natural Zeolite Coated with Manganese Oxide. Chin. J. Chem. Eng. 2009, 17, 585–593. [Google Scholar] [CrossRef]
- Yousef, R.I.; El-Eswed, B.; Al-Muhtaseb, A.H. Adsorption characteristics of natural zeolites as solid adsorbents for phenol removal from aqueous solutions: Kinetics, mechanism, and thermodynamics studies. Chem. Eng. J. 2011, 171, 1143–1149. [Google Scholar] [CrossRef]
- Mansouri, N.; Rikhtegar, N.; Panahi, H.A.; Atabi, F.; Shahraki, B.K. Porosity, characterization and structural properties of natural zeolite—Clinoptilolite—As a sorbent. Environ. Prot. Eng. 2013, 39, 139–152. [Google Scholar] [CrossRef]
- Erdem, E.; Karapinar, N.; Donat, R. The removal of heavy metal cations by natural zeolites. J. Colloid Interface Sci. 2004, 280, 309–314. [Google Scholar] [CrossRef]
- Meshko, V.; Markovska, L.; Mincheva, M.; Rodrigues, A. Adsorption of basic dyes on granular acivated carbon and natural zeolite. Water Res. 2001, 35, 3357–3366. [Google Scholar] [CrossRef]
- MBissen, M.; Frimmel, F.H. Arsenic—A Review. Part II: Oxidation of Arsenic and its Removal in Water Treatment. Acta Hydrochim. Hydrobiol. 2003, 31, 97–107. [Google Scholar] [CrossRef]
- Perić, J.; Trgo, M.; Medvidović, N.V. Removal of zinc, copper and lead by natural zeolite—A comparison of adsorption isotherms. Water Res. 2004, 38, 1893–1899. [Google Scholar] [CrossRef]
- Álvarez-Ayuso, E. Purification of metal electroplating waste waters using zeolites. Water Res. 2003, 37, 4855–4862. [Google Scholar] [CrossRef]
- Han, R.; Zhang, J.; Han, P.; Wang, Y.; Zhao, Z.; Tang, M. Study of equilibrium, kinetic and thermodynamic parameters about methylene blue adsorption onto natural zeolite. Chem. Eng. J. 2009, 145, 496–504. [Google Scholar] [CrossRef]
- Montalván-Burbano, N.; Velastegui-Montoya, A.; Gurumendi-Noriega, M.; Morante-Carballo, F.; Adami, M. Worldwide Research on Land Use and Land Cover in the Amazon Region. Sustainability 2021, 13, 6039. [Google Scholar] [CrossRef]
- Szollosi-Mota, A.; Prodan, M.; Ghicioi, E.; Nalboc, I.; Moldovan, C. Heavy metals removal from mining drainage acid water by use of natural zeolites. Environ. Eng. Manag. J. 2017, 16, 1383–1388. [Google Scholar] [CrossRef]
- Varvara, S.; Popa, M.; Bostan, R.; Damian, G. Preliminary considerations on the adsorption of heavy metals from acidic mine drainage using natural zeolite. J. Environ. Prot. Ecol. 2013, 14, 1506–1514. [Google Scholar]
- Buenaño, X.; Canoira, L.; Sánchez, D.M.; Costafreda, J. Zeolitic tuffs for acid mine drainage (AMD) treatment in Ecuador: Breakthrough curves for Mn2+, Cd2+, Cr3+, Zn2+, and Al3+. Environ. Sci. Pollut. Res. 2017, 24, 6794–6806. [Google Scholar] [CrossRef] [PubMed]
- Gaikwad, R.W.; Misal, S.A.; Dhirendra; Gupta, D.V. Removal of metal from acid mine drainage (AMD) by using natural zeolite of Nizarneshwar Hills of Western India. Arab. J. Geosci. 2009, 4, 85–89. [Google Scholar] [CrossRef]
- Nasrabadi, M.; Omid, M.H.; Mazdeh, A.M.; Shahriari, T. Cadmium adsorption by natural zeolite in a circular flume. Int. J. Environ. Technol. Manag. 2018, 21, 174. [Google Scholar] [CrossRef]
- Ivanova, E.P.; Kostova, M.A.; Koumanova, B.K. Kinetics of water and alcohol vapors adsorption on natural zeolite. Asia-Pacific J. Chem. Eng. 2009, 5, 869–881. [Google Scholar] [CrossRef]
- Smical, I. Sorption kinetics of pbspi 2+, cuspi 2+ and znspi 2+ ions by natural zeolites from maramures county (northern romania): Potential application in wastewater reuse. AACL Bioflux 2011, 4, 481–489. [Google Scholar]
- Vrînceanu, N.; Motelică, D.; Dumitru, M.; Calciu, I.; Tănase, V.; Preda, M. Assessment of using bentonite, dolomite, natural zeolite and manure for the immobilization of heavy metals in a contaminated soil: The Copșa Mică case study (Romania). Catena 2019, 176, 336–342. [Google Scholar] [CrossRef]
- Contin, M.; Miho, L.; Pellegrini, E.; Gjoka, F.; Shkurta, E. Effects of natural zeolites on ryegrass growth and bioavailability of Cd, Ni, Pb, and Zn in an Albanian contaminated soil. J. Soils Sediments 2019, 19, 4052–4062. [Google Scholar] [CrossRef]
- Janoš, P.; Vávrová, J.; Herzogová, L.; Pilařová, V. Effects of inorganic and organic amendments on the mobility (leachability) of heavy metals in contaminated soil: A sequential extraction study. Geoderma 2010, 159, 335–341. [Google Scholar] [CrossRef]
- Doula, M.K.; Elaiopoulos, K.; Kavvadias, V.A.; Mavraganis, V. Use of clinoptilolite to improve and protect soil quality from the disposal of olive oil mills wastes. J. Hazard. Mater. 2012, 207-208, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Asl, M.K.; Hasani, A.H.; Naserkhaki, E. Evaluation of nitrate removal from water using activated carbon and clinoptilolite by adsorption method. Biosci. Biotechnol. Res. Asia 2016, 13, 1045–1054. [Google Scholar] [CrossRef]
- Polloni-Silva, J.; Valdehita, A.; Fracácio, R.; Navas, J.M. Remediation efficiency of three treatments on water polluted with endocrine disruptors: Assessment by means of in vitro techniques. Chemosphere 2017, 173, 267–274. [Google Scholar] [CrossRef]
- Nagaiah, E.; Sonkamble, S.; Mondal, N.C.; Ahmed, S. Natural zeolites enhance groundwater quality: Evidences from Deccan basalts in India. Environ. Earth Sci. 2017, 76, 536. [Google Scholar] [CrossRef]
- Margeta, K.; Stefanović, Š.C.; Kaučič, V.; Logar, N.Z. The potential of clinoptilolite-rich tuffs from Croatia and Serbia for the reduction of toxic concentrations of cations and anions in aqueous solutions. Appl. Clay Sci. 2015, 116-117, 111–119. [Google Scholar] [CrossRef]
- Liu, X.; Tian, R.; Ding, W.; He, Y.; Li, H. Adsorption selectivity of heavy metals by Na-clinoptilolite in aqueous solutions. Adsorption 2019, 25, 747–755. [Google Scholar] [CrossRef]
- Pepe, F.; de Gennaro, B.; Aprea, P.; Caputo, D. Natural zeolites for heavy metals removal from aqueous solutions: Modeling of the fixed bed Ba2+/Na+ ion-exchange process using a mixed phillipsite/chabazite-rich tuff. Chem. Eng. J. 2013, 219, 37–42. [Google Scholar] [CrossRef]
- Seliman, A.F.; Borai, E.H. Utilization of natural chabazite and mordenite as a reactive barrier for immobilization of hazardous heavy metals. Environ. Sci. Pollut. Res. 2011, 18, 1098–1107. [Google Scholar] [CrossRef]
- Torosyan, G.; Matevosyan, G.; Harutyunyan, S. Use of Natural Materials for Wastewater Treatment & Improvement of Water Quality. In Advanced Water Supply and Wastewater Treatment: A Road to Safer Society and Environment; NATO Science for Peace and Security Series C: Environmental Security; Hlavinek, P., Winkler, I., Marsalek, J., Mahrikova, I., Eds.; Springer: Dordrecht, The Netherlands, 2011; Volume 106, pp. 171–180. [Google Scholar]
- Filatova, E.G.; Pomazkina, O.I.; Pozhidaev, Y.N. Development of the zeolite-sorption process for electroplating wastewater treatment. J. Water Chem. Technol. 2014, 36, 303–308. [Google Scholar] [CrossRef]
- Ellersdorfer, M. The ion-exchanger–loop-stripping process: Ammonium recovery from sludge liquor using NaCl-treated clinoptilolite and simultaneous air stripping. Water Sci. Technol. 2018, 77, 695–705. [Google Scholar] [CrossRef]
- Yousefi, A.; Matavos-Aramyan, S. Mix Design Optimization of Silica Fume-Based Pervious Concrete for Removal of Heavy Metals from Wastewaters. Silicon 2018, 10, 1737–1744. [Google Scholar] [CrossRef]
- Salem, A.; Sene, R.A. Removal of lead from solution by combination of natural zeolite–kaolin–bentonite as a new low-cost adsorbent. Chem. Eng. J. 2011, 174, 619–628. [Google Scholar] [CrossRef]
- Kragović, M.; Daković, A.; Marković, M.; Petković, A.; Milojković, J.; Zildžović, S. Removal of lead and cadmium ions from aqueous solutions by using natural and modified zeolite. Adv. Sci. Lett. 2017, 23, 5862–5865. [Google Scholar] [CrossRef]
- Moussavi, G.; Talebi, S.; Farrokhi, M.; Sabouti, R.M. The investigation of mechanism, kinetic and isotherm of ammonia and humic acid co-adsorption onto natural zeolite. Chem. Eng. J. 2011, 171, 1159–1169. [Google Scholar] [CrossRef]
- Zhou, L.; Boyd, C.E. Total ammonia nitrogen removal from aqueous solutions by the natural zeolite, mordenite: A laboratory test and experimental study. Aquaculture 2014, 432, 252–257. [Google Scholar] [CrossRef]
- Eva, C. Ammonia removal from water with natural zeolite clinoptilolite using the coagulation/flocculation vs. filtration techniques. Res. J. Chem. Environ. 2015, 19, 8–13. [Google Scholar]
- Mažeikien, A.; Valentukevičien, M.; Jankauskas, J. Laboratory study of ammonium ion removal by using zeolite (Clinoptilolite) to treat drinking water. J. Environ. Eng. Landsc. Manag. 2010, 18, 54–61. [Google Scholar] [CrossRef]
- Torma, S.; Vilček, J.; Adamišin, P.; Huttmanová, E.; Hronec, O. Influence of natural zeolite on nitrogen dynamics in soil. Turk. J. Agric. For. 2014, 38, 739–744. [Google Scholar] [CrossRef]
- Ferretti, G.; Keiblinger, K.M.; Faccini, B.; Di Giuseppe, D.; Mentler, A.; Zechmeister-Boltenstern, S.; Coltorti, M. Effects of Different Chabazite Zeolite Amendments to Sorption of Nitrification Inhibitor 3,4-Dimethylpyrazole Phosphate (DMPP) in Soil. J. Soil Sci. Plant Nutr. 2020, 20, 973–978. [Google Scholar] [CrossRef]
- Ferretti, G.; Keiblinger, K.M.; Zimmermann, M.; Di Giuseppe, D.; Faccini, B.; Colombani, N.; Mentler, A.; Zechmeister-Boltenstern, S.; Coltorti, M.; Mastrocicco, M. High resolution short-term investigation of soil CO2, N2O, NOx and NH3 emissions after different chabazite zeolite amendments. Appl. Soil Ecol. 2017, 119, 138–144. [Google Scholar] [CrossRef]
- Tian, J.; Guan, J.; Gao, H.; Wen, Y.; Ren, Z. The adsorption and mass-transfer process of cationic red X-GRL dye on natural zeolite. Water Sci. Technol. 2016, 73, 2119–2131. [Google Scholar] [CrossRef]
- Zolfaghari, M.; Magdouli, S.; Tanabene, R.; Komtchou, S.P.; Martial, R.; Saffar, T. Pragmatic strategy for the removal of ammonia from gold mine effluents using a combination of electro-coagulation and zeolite cation exchange processes: A staged approach. J. Water Process. Eng. 2020, 37, 101512. [Google Scholar] [CrossRef]
- Khan, G.F.; Wood, J. Information technology management domain: Emerging themes and keyword analysis. Scientometrics 2015, 105, 959–972. [Google Scholar] [CrossRef]
- Baier-Fuentes, H.; Merigó, J.M.; Amorós, J.E.; Gaviria-Marín, M. International entrepreneurship: A bibliometric overview. Int. Entrep. Manag. J. 2019, 15, 385–429. [Google Scholar] [CrossRef]
- Dong, D.; Chen, M.-L. Publication trends and co-citation mapping of translation studies between 2000 and 2015. Scientometrics 2015, 105, 1111–1128. [Google Scholar] [CrossRef]
- Jorgensen, T.; Weatherley, L. Ammonia removal from wastewater by ion exchange in the presence of organic contaminants. Water Res. 2003, 37, 1723–1728. [Google Scholar] [CrossRef]
- Sheppard, R.A. Characterization of Zeolitic Materials in Agricultural Research; Westview Press: Boulder, CO, USA, 1984; pp. 81–90. [Google Scholar]

| Rank | Country | Documents | Citations Count |
|---|---|---|---|
| 1 | Turkey | 195 | 6940 |
| 2 | China | 161 | 5618 |
| 3 | Iran | 155 | 3866 |
| 4 | United States | 90 | 3970 |
| 5 | Italy | 81 | 1733 |
| 6 | Australia | 62 | 3056 |
| 7 | Indonesia | 62 | 384 |
| 8 | Mexico | 62 | 1621 |
| 9 | Greece | 60 | 2162 |
| 10 | Spain | 60 | 1751 |
| Rank | Journal | AT 1 | % 2 | HI 3 | SJR 4 | Quartile |
|---|---|---|---|---|---|---|
| 1 | Microporous and Mesoporous Materials | 69 | 4.36 | 151 | 1 | Q1 |
| 2 | Journal of Hazardous Materials | 65 | 4.11 | 260 | 2.01 | Q1 |
| 3 | Construction and Building Materials | 38 | 2.40 | 147 | 1.49 | Q1 |
| 4 | Desalination and Water Treatment | 32 | 2.02 | 51 | 0.33 | Q2 |
| 5 | Chemical Engineering Journal | 27 | 1.71 | 198 | 2.32 | Q1 |
| 6 | Water Research | 26 | 1.64 | 285 | 2.93 | Q1 |
| 7 | Water Science and Technology | 24 | 1.52 | 131 | 0.47 | Q2 |
| 8 | Desalination | 24 | 1.52 | 169 | 1.81 | Q1 |
| 9 | Bioresource Technology | 22 | 1.39 | 273 | 2.43 | Q1 |
| 10 | Journal of Environmental Science and Health—Part A Toxic/Hazardous Substances and Environmental Engineering | 22 | 1.39 | 67 | 0.48 | Q2 |
| R 1 | Authors | Country | Institute/University | Citations Count | Publications | HI 2 |
|---|---|---|---|---|---|---|
| 1 | Karapinar N. | Turkey | Pamukkale Üniversitesi | 1172 | 2 | 6 |
| 2 | Wang S. | Australia | The University of Adelaide | 1151 | 12 | 111 |
| 3 | Erdem E. | Turkey | Pamukkale Üniversitesi | 1061 | 1 | 11 |
| 4 | Donat R. | Turkey | Pamukkale Üniversitesi | 1061 | 1 | 12 |
| 5 | Maunaye M. | France | ENSCR Ecole Nationale Supérieure de Chimie de Rennes | 883 | 1 | 10 |
| 6 | Martin G. | France | ENSCR Ecole Nationale Supérieure de Chimie de Rennes | 883 | 1 | 17 |
| 7 | Blanchard G. | France | ENSCR Ecole Nationale Supérieure de Chimie de Rennes | 883 | 1 | 1 |
| 8 | Shekarchi M. | Iran | Iranian Ministry of Health and Medical Education | 814 | 15 | 13 |
| 9 | Lin J. | Taiwan | National Chiao Tung University Taiwan | 758 | 10 | 35 |
| 10 | Trgo M. | Croatia | Sveučilište u Splitu | 703 | 13 | 10 |
| Rank | Article Title | Authors | Type of Document | TC 1 | Year | ACI 2 |
|---|---|---|---|---|---|---|
| 1 | The removal of heavy metal cations by natural zeolites | [87] | Article | 1061 | 2004 | 62.4 |
| 2 | Removal of heavy metals from waters by means of natural zeolites | [21] | Article | 883 | 1984 | 23.9 |
| 3 | La roca magica: Uses of natural zeolites in agriculture and industry | [6] | Review | 477 | 1999 | 21.7 |
| 4 | Adsorption of basic dyes on granular activated carbon and natural zeolite | [88] | Article | 467 | 2001 | 23.4 |
| 5 | Arsenic—A review. Part II: Oxidation of arsenic and its removal in water treatment | [89] | Review | 398 | 2003 | 22.1 |
| 6 | Removal of zinc, copper and lead by natural zeolite—A comparison of adsorption isotherms | [90] | Article | 394 | 2004 | 23.2 |
| 7 | Purification of metal electroplating waste waters using zeolites | [91] | Article | 370 | 2003 | 20.6 |
| 8 | Adsorption characteristics of natural zeolites as solid adsorbents for phenol removal from aqueous solutions: Kinetics, mechanism, and thermodynamics studies | [85] | Article | 337 | 2011 | 33.7 |
| 9 | Study of equilibrium, kinetic and thermodynamic parameters about methylene blue adsorption onto natural zeolite | [92] | Article | 319 | 2009 | 26.6 |
| 10 | Adsorption of heavy metals from acid mine drainage by natural zeolite | [82] | Article | 316 | 2009 | 26.3 |
| Keyword | Occurrences | 1973–1989 | 1990–1999 | 2000–2009 | 2010–2020 |
|---|---|---|---|---|---|
| natural zeolite | 667 | - | 39 | 159 | 469 |
| zeolite | 329 | 1 | 11 | 84 | 233 |
| clinoptilolite | 259 | 3 | 10 | 97 | 149 |
| adsorption | 237 | - | 2 | 52 | 183 |
| ion exchange | 119 | 3 | 9 | 53 | 54 |
| heavy metal | 99 | - | 3 | 31 | 65 |
| wastewater | 61 | 1 | 4 | 18 | 38 |
| kinetic | 60 | - | - | 15 | 45 |
| sorption | 48 | - | 2 | 15 | 31 |
| ammonium | 43 | - | 2 | 10 | 31 |
| copper | 34 | - | 1 | 14 | 19 |
| wastewater treatment | 32 | - | 1 | 8 | 23 |
| isotherm | 31 | - | - | 7 | 24 |
| water treatment | 31 | - | 1 | 11 | 19 |
| lead | 30 | - | 3 | 12 | 15 |
| adsorption isotherm | 28 | - | 1 | 4 | 23 |
| ammonia | 26 | 1 | 2 | 10 | 13 |
| mordenite | 22 | 1 | 4 | 6 | 11 |
| zinc | 22 | - | - | 11 | 11 |
| ammonium removal | 20 | 1 | 2 | 4 | 13 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morante-Carballo, F.; Montalván-Burbano, N.; Carrión-Mero, P.; Jácome-Francis, K. Worldwide Research Analysis on Natural Zeolites as Environmental Remediation Materials. Sustainability 2021, 13, 6378. https://doi.org/10.3390/su13116378
Morante-Carballo F, Montalván-Burbano N, Carrión-Mero P, Jácome-Francis K. Worldwide Research Analysis on Natural Zeolites as Environmental Remediation Materials. Sustainability. 2021; 13(11):6378. https://doi.org/10.3390/su13116378
Chicago/Turabian StyleMorante-Carballo, Fernando, Néstor Montalván-Burbano, Paúl Carrión-Mero, and Kelly Jácome-Francis. 2021. "Worldwide Research Analysis on Natural Zeolites as Environmental Remediation Materials" Sustainability 13, no. 11: 6378. https://doi.org/10.3390/su13116378
APA StyleMorante-Carballo, F., Montalván-Burbano, N., Carrión-Mero, P., & Jácome-Francis, K. (2021). Worldwide Research Analysis on Natural Zeolites as Environmental Remediation Materials. Sustainability, 13(11), 6378. https://doi.org/10.3390/su13116378








