The Effect of the Manner in Which Montane and Submontane Areas Are Utilized on the Quality of Leachate Water
Abstract
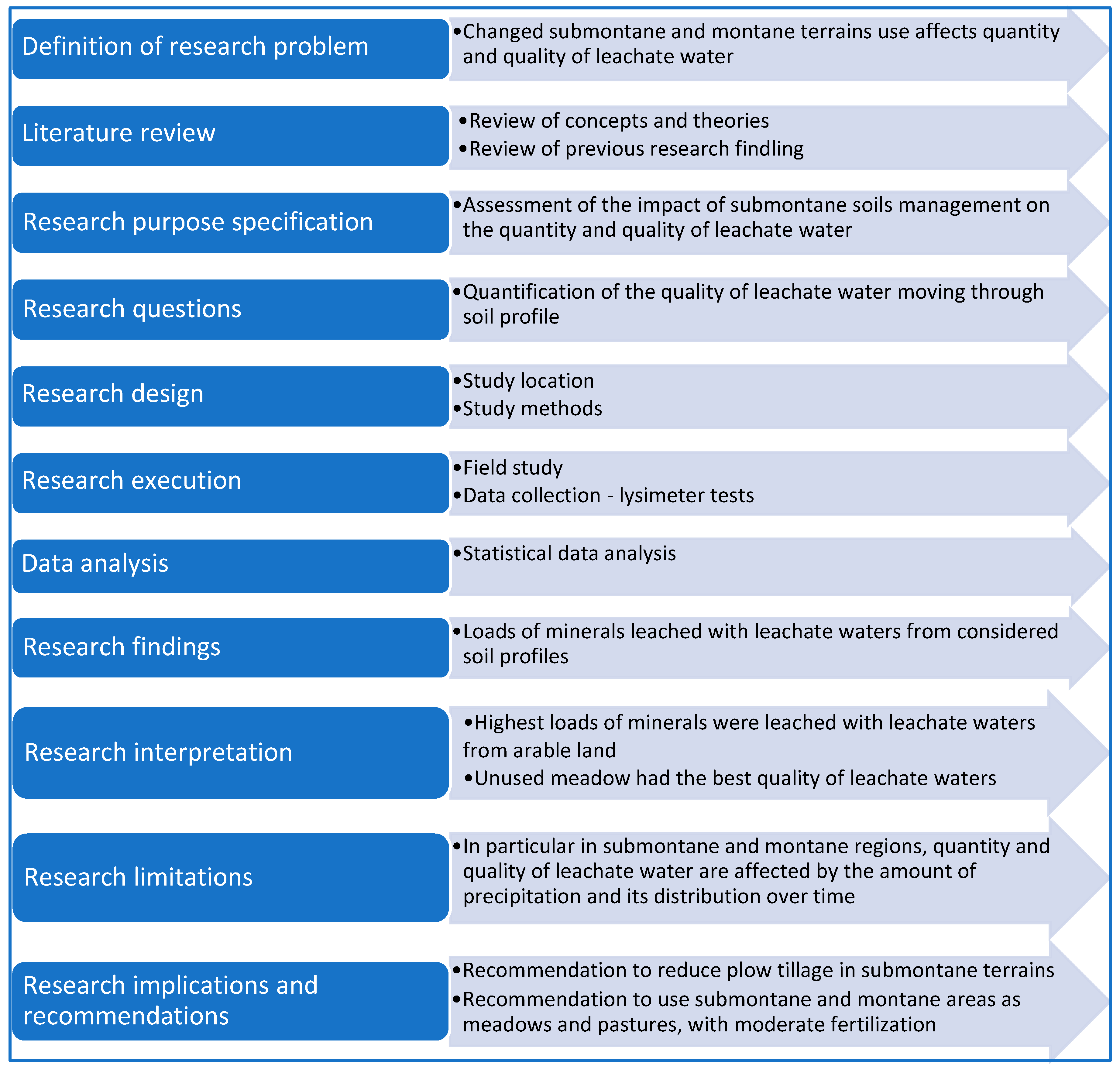
:1. Theoretical Framework
2. Materials and Methods
2.1. Location of the Study
2.2. Study Methods
- (A)
- non-fertilized meadow––control;
- (B)
- meadow fertilized with mineral fertilizers (P18K50N120);
- (C)
- meadow fertilized with 15 Mg·ha−1 liquid manure + supplementary mineral fertilization (P12N45);
- (D)
- meadow fertilized with 10 Mg·ha−1 manure + supplementary mineral fertilization (P4N51);
- (E)
- barren meadow (non-fertilized, non-mowed);
- (F)
- arable land receiving mineral fertilizers in the amount of P18K50N120 for the first two years and P18K50N60 in the third year of use.
- first (I)––between 1 April and 30 June 2015 (intensive growing season),
- second (II)––between 1 July and 31 October 2015 (slow growing season),
- third (III)––between 1 November and 31 March 2015 (non-growing season).
3. Results
3.1. Nitrogen
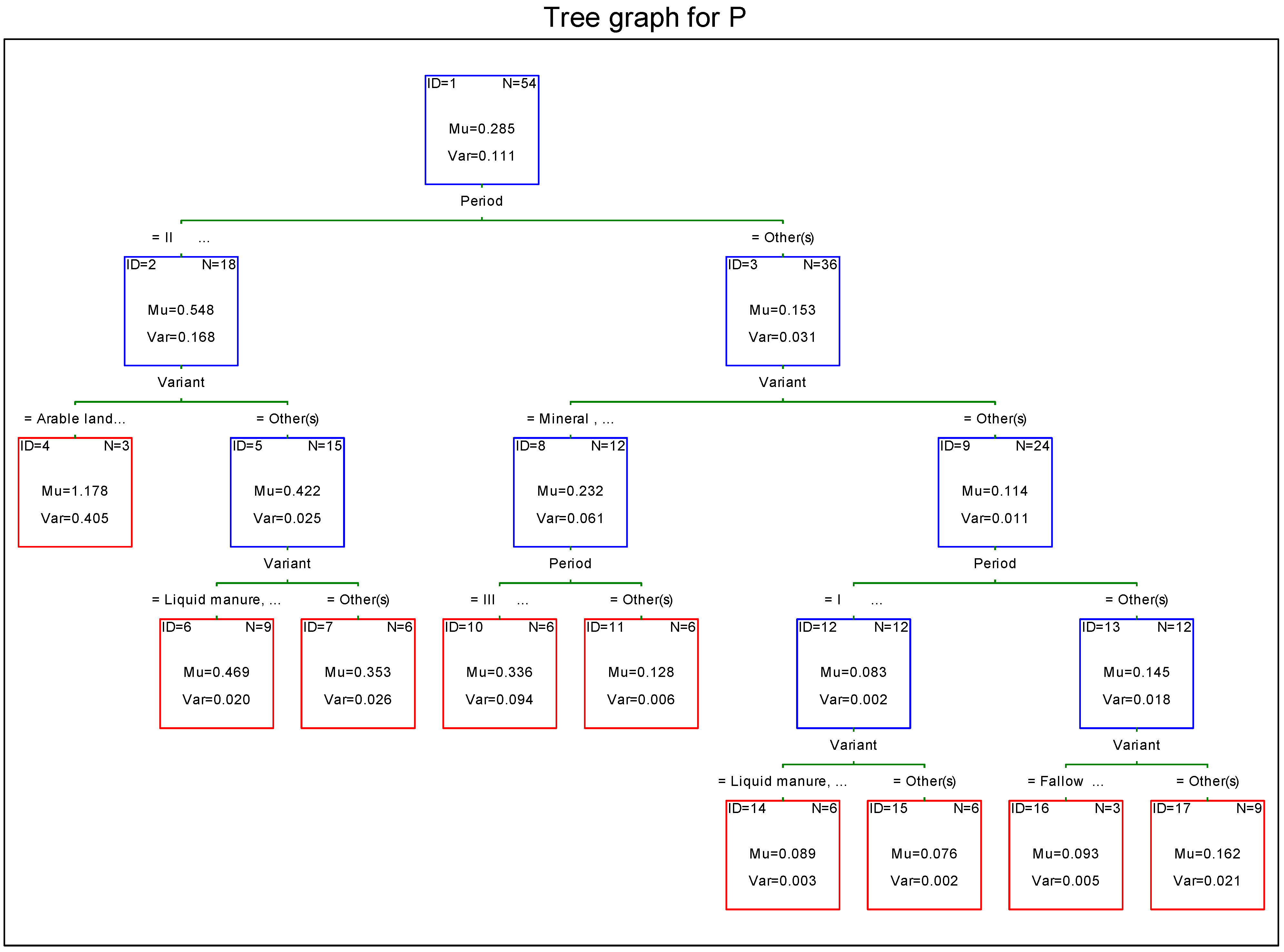
3.2. Phosphorus
3.3. Potassium
3.4. Calcium
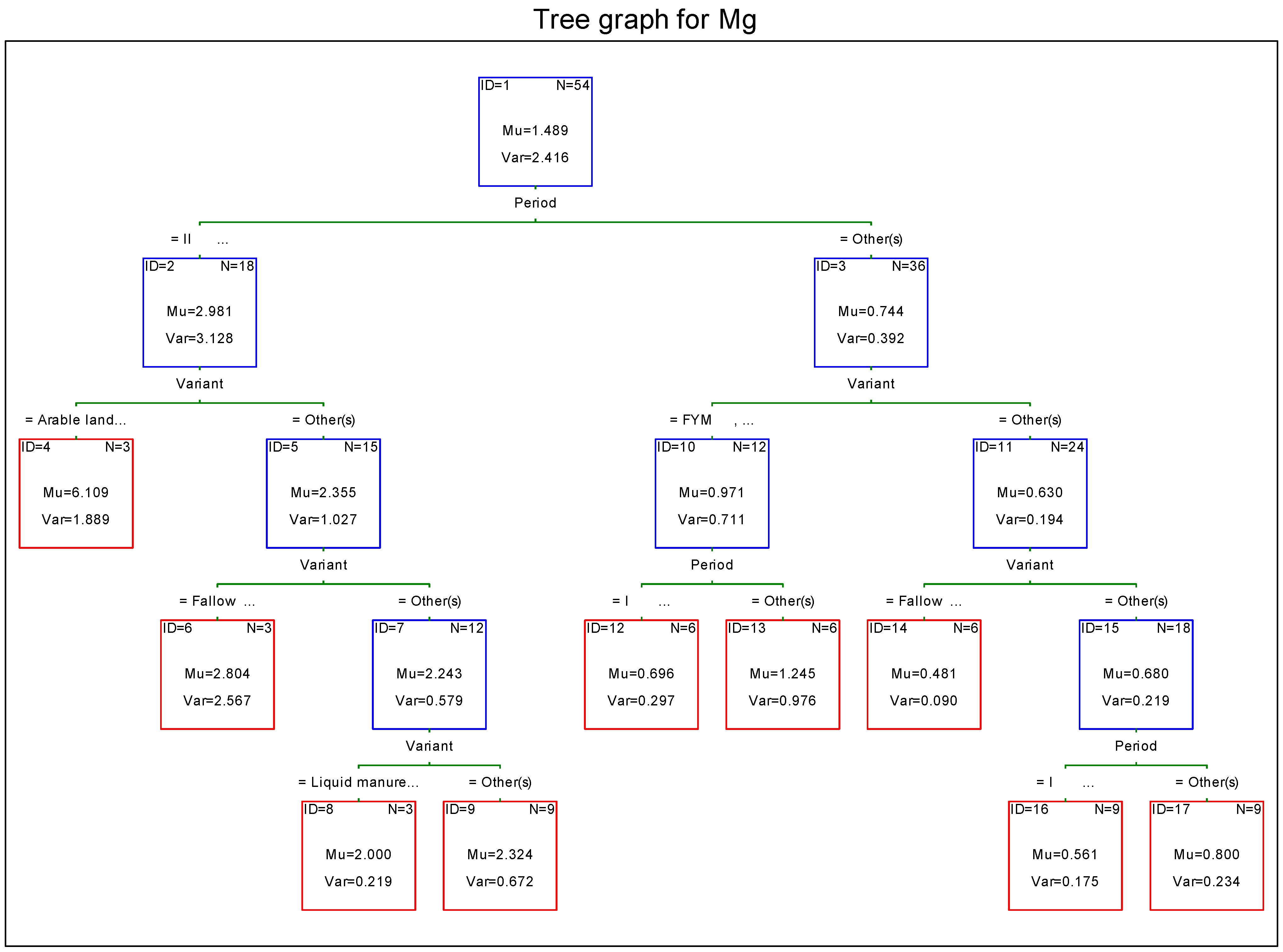
3.5. Magnesium
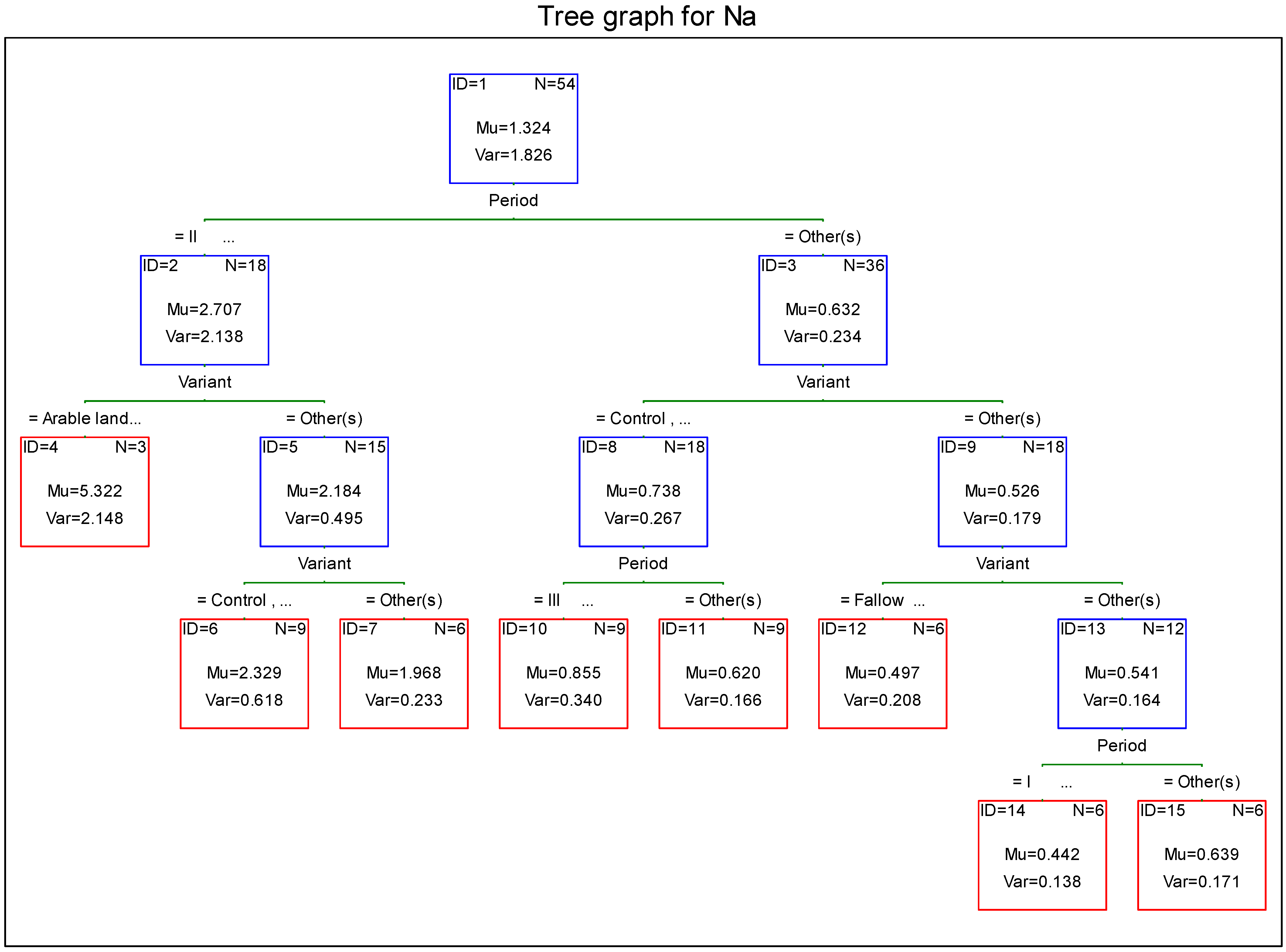
3.6. Sodium
4. Discussion
- the lowest concentration of macroelements in leachate water in the first period of the study (intensive growing);
- differences in concentration of potassium, phosphorus, and calcium in leachate water between objects receiving mineral and natural fertilizers;
- the highest load of minerals leached with leachate water from the soil in the second period of the study (slow growing season);
- considerable differences in the magnitude of load of macroelements leached from the soil between turf-covered objects (meadow objects) and arable soil.
5. Conclusions
- The highest annual load of nitrogen was leached from arable land. It was two- to three-fold higher than the loads leached from the other objects.
- Total annual load of leached phosphorus was generally highest in the first year, and in arable land in the third year. On average, annual loads of phosphorus leached from arable land were twice higher than the loads leached from meadow objects.
- The highest annual leaching of potassium was recorded in arable land, and the lowest in the control. The difference between these values was over two-fold.
- The highest annual load of calcium was leached from arable land. Its magnitude was 1.5 times higher than that of the load leached from the minerally fertilized object and over two times higher compared to the other objects.
- The lowest annual load of magnesium was leached from the object fertilized with liquid manure, and the highest from arable land. In the latter case, this load was over two-fold higher than the loads from the other meadow objects.
- The annual load of leached sodium in meadow objects was similar and ranged from 3.2 to 3.8 kg·ha−1, whereas in arable land it was twice as high.
- Concentration of macroelements in water depended on plant growth and microbiological activity in the soil. That concentration was positively correlated with the intensive plant growth and dynamics of organic substance decomposition in the soil.
- The load of minerals leached with leachate water from the soil was highest in the second period of the study. The magnitude of this load was affected by such factors as the amount of water moving through the soil and the intensity of organic substance decomposition in the soil.
- Grassy communities, compared to arable land, retained significantly more meteoric water and minerals, thereby protecting the soil against losing them.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A



References
- Koc, J.; Szyperek, U. Skuteczność barier biogeochemicznych w ograniczaniu spływu azotu w środowisku rolniczym. Ann. UMCS Sec. E 2004, 59, 93–100. [Google Scholar]
- Kornaś, M.; Grześkowiak, A. Wpływ użytkowania zlewni na kształtowanie jakości wody w zbiornikach wodnych zlewni rzeki Drawa. Woda Sr. Obsz. Wiej. 2011, 11, 125–137. [Google Scholar]
- Skorbiłowicz, M. Wpływ rodzaju zlewni na stężenie wybranych makroskładników w wodach Górnej Narwi. Woda Sr. Obsz. Wiej. 2004, 4, 117–123. [Google Scholar]
- Smoroń, S.; Twardy, S.; Kuźniar, A. Bilans azotu i fosforu w rolniczych obszarach karpackich o niekorzystnych warunkach gospodarowania. Woda Sr. Obsz. Wiej. 2010, 10, 225–236. [Google Scholar]
- Śmietanka, M. The influence of permanent grasslands on nitrate nitrogen loads in modelling approach. J. Water Land Dev. 2014, 21, 63–70. [Google Scholar] [CrossRef]
- Twardy, S.; Kopacz, M.; Kurnicki, R. Charakterystyka Zlewni Górnego Dunajca w Aspekcie Czynników Środowiskowych Determinujących Zrównoważony i Trwały Rozwój; Wyd. ITP: Kraków, Poland, 2015. [Google Scholar]
- Szpara, K. Agroturystyka w Karpatach Polskich. Prace Geogr. 2011, 125, 161–178. [Google Scholar]
- Kostuch, R. Przyrodnicze Podstawy Łąkowo-Pastwiskowej Gospodarki w Górach; PWRiL: Warszawa, Poland, 1976. [Google Scholar]
- Korzeniowski, L. Rola i znaczenie Lasów Województwa Nowosądeckiego dla Gospodarki Wodnej. Problemy Rolniczo-Leśne Województwa Nowosądeckiego; PTPN: Nowy Targ–Zakopane, Poland, 1982; pp. 205–212.
- Sapek, A. Procesy związane z wymywaniem azotu z gleb użytkowanych rolniczo. In Materiały Seminaryjne IMUZ; Wydawnictwo IMUZ: Falenty, Poland, 1990; Volume 26, pp. 17–29. [Google Scholar]
- Wójcikiewicz, M.; Barłowska, A.; Szpunar, E. Wybrane Problemy Zagrożenia i Ochrony Środowiska Rolniczego; Wydawnictwo Akademii Rolniczej: Kraków, Poland, 1990; pp. 89–96. [Google Scholar]
- Pawęska, K.; Bawiec, A.; Włodek, S.; Smaga, E. Wstępna analiza średniego zużycia wody w jednorodzinnych gospodarstwach domowych. Infrastrukt. Ekol. Teren. Wiej. 2013, 1, 170–178. [Google Scholar]
- Kasperczyk, M. Wpływ okrywy roślinnej gleby na ilość i jakość wód. In Materiały Konferencyjne. “Środowisko a zdrowie—2005”; Drukarnia Częstochowskie Zakłady Graficzne SA: Częstochowa, Poland, 2005; pp. 107–113. [Google Scholar]
- Koc, J.; Szymczyk, S.; Procyk, Z. Czynniki kształtujące wymycie azotu, fosforu i potasu z gleb uprawnych. Zesz. Probl. Post. Nauk Roln. 1999, 467, 119–125. [Google Scholar]
- Koc, J.; Szymczyk, S. Wpływ intensyfikacji rolnictwa na odpływ z gleb azotu mineralnego. Zesz. Probl. Post. Nauk Roln. 2003, 494, 175–181. [Google Scholar]
- Majewski, W. Powodzie a Gospodarka Wodna; Wyd. IMGW: Warszawa, Poland, 1997; pp. 379–384. [Google Scholar]
- Kondracki, J. Geografia Regionalna Polski; Wyd. Naukowe PWN: Warszawa, Poland, 2011. [Google Scholar]
- Wężyk, P.; de Kok, R. Automatic mapping of the dynamics of forest succession on abandoned parcels in south Poland. In Angewandte Geoinformatik; Strobl, J., Blaschke, T., Griesebner, G., Eds.; Wichmann Verlag: Heidelberg, Germany, 2005; pp. 774–779. [Google Scholar]
- Kozak, J. Forest cover changes and their drivers in the Polish Carpathian Mountains since 1800. In Reforesting Landscape: Linking pattern and Process; Nagendra, H., Southworth, J., Eds.; Springer: Dordrecht, The Netherlands; Berlin/Heidelberg, Germany; London, UK; New York, NY, USA, 2010; pp. 253–274. [Google Scholar]
- Duer, I.; Fotyma, M.; Madej, A. Kodeks Dobrej Praktyki Rolniczej; MRiRW, MŚ: Warszawa, Poland, 2004. [Google Scholar]
- Mioduszewski, W. Kształtowanie i wykorzystanie zasobów wodnych w krajobrazie rolniczym. In Woda w Krajobrazie Rolniczym; Woda−Środowisko−Obszary Wiejskie; Wydawnictwo IMUZ: Falenty, Poland, 2006; Volume 18, pp. 11–26. [Google Scholar]
- Kristensen, H.H.; Burgess, L.R.; Demmers, T.G.H.; Wathes, C.M. The preferences of laying hens for different concentrations of atmospheric ammonia. Appl. Anim. Behav. Sci. 2000, 68, 307–318. [Google Scholar] [CrossRef]
- Kangas, L.; Sanna, S. Regional nitrogen deposition model for integrated assessment of acidification and eutrophication. Atmos. Environ. 2001, 36, 1111–1122. [Google Scholar] [CrossRef]
- Kazutaka, K.; Hanajima, D.; Fukumoto, Y.; Suzuki, K.; Kawamoto, S.; Shima, J.; Haga, K. Isolation of thermophilic ammonium-tolerant bacterium and its application to reduce ammonia emission during composting of animal wastes. Biosci. Biotech. 2004, 68, 286–292. [Google Scholar]
- Bogdał, A.; Ostrowski, K. Wpływ rolniczego użytkowania zlewni podgórskiej i opadów atmosferycznych na jakość wód odpływających z jej obszaru. Woda Sr. Obsz. Wiej. 2007, 7, 59–69. [Google Scholar]
- Sapek, A. Rolnictwo polskie i ochrona jakości wody, zwłaszcza wody Bałtyku. Woda Sr. Obsz. Wiej. 2010, 10, 175–200. [Google Scholar]
- Twardy, S.; Kopacz, M. Ocena ilościowo-jakościowych zasobów wodnych zlewni pokrytych trwałą szatą roślinną. In Trwała Okrywa Roślinna Jako Podstawa Zrównoważonego Rozwoju Rolnictwa w Zlewniach Karpackich; Twardy, S., Ed.; Wyd. IM UZ: Falenty, Poland, 2001; pp. 147–158. [Google Scholar]
- Twardy, S.; Kopacz, M. Zrównoważony rozwój obszarów wiejskich. In Zrównoważona Konsumpcja Środowiska Przyrodniczego; Twardy, S., Ed.; Wyd. ITP: Falenty–Kraków, Poland, 2010; pp. 96–112. [Google Scholar]
- Traczyk, T. The role of plant subsystem in matter flow in the agricultural landscape. Pol. Ecol. Stud. 1985, 11, 445–466. [Google Scholar]
- Kasperczyk, M.; Szewczyk, W. Znaczenie żywienia pastwiskowego w gospodarstwie rolnym. Zesz. Nauk. AR w Krakowie 2000, 73, 117–123. [Google Scholar]
- Kuźniar, A.; Twardy, S. Ocena Potrzeb i Niedoborów Wodnych Użytków Zielonych w Polsce Południowej; PAN, Komitet Zagospodarowania Ziem Górskich: Kraków, Poland, 2001. [Google Scholar]
- Kuźniar, A.; Twardy, S. Warunki przyrodniczo-gospodarcze Karpat Polskich z uwzględnieniem niedoborów wodnych. In Niskonakładowa Produkcja Rolnicza z Wykorzystaniem Pasz z Użytków Zielonych w Karpatach Polskich; Jankowska-Huflejt, H., Zastawny, J., Eds.; Wydawnictwo IMUZ: Falenty, Poland, 2001; pp. 33–45. [Google Scholar]
- Kopeć, S. Rola użytków zielonych w ochronie wód. In Rola Użytków Zielonych i Zadrzewień w Ochronie Środowiska Rolniczego, Proceedings of the International Scientific and Technical Conference, October 21 1999, Kraków–Jaworki, Poland, Małopolski Ośrodek Badawczy; Wydawnictwo AR Kraków: Kraków, Poland, 1999; pp. 141–151. [Google Scholar]
- Jaguś, A. Zmiany jakościowe odpływów wód glebowych w warunkach recesji gospodarki nawozowej (na przykładzie górskich użytków zielonych). In Monitoring Środowiska Przyrodniczego 9; Kieleckie Towarzystwo Naukowe: Kielce, Poland, 2008; pp. 63–69. [Google Scholar]
- Jankowska-Huflejt, H.; Domański, J.P. Aktualne i możliwe kierunki wykorzystania trwałych użytków zielonych w Polsce. Woda Sr. Obsz. Wiej. 2009, 8, 31–49. [Google Scholar]
- Burzyńska, I. Ocena wpływu wybranych cech gleb łąkowych na przenikanie rozpuszczalnych form składników mineralnych do płytkich wód gruntowych. Woda Sr. Obsz. Wiej. 2007, 7, 95–106. [Google Scholar]
- Klima, K.; Wiśniowska-Kielian, B. Estimation of soil losses resulting from the surface run-off in the upland region depending on the type of land use. Adv. Agric. Sci. Probl. Issues 2007, 520, 821–827. [Google Scholar]
- Kopeć, M. Dynamika plonowania i jakości runi łąki górskiej w okresie 30 lat trwania doświadczenia nawozowego. Zesz. Nauk. AR, Ser. Rozprawy 2000, 267, 1–84. [Google Scholar]
- Jancovic, J.; Folkman, I. Wplyv hnojenia na floristicke złozenie travneho porastu a koncentraciu zivin v drenaznej vode. Polnohospodarstvo 1985, 31, 307–314. [Google Scholar]
- Jaguś, A.; Twardy, S. Wpływ Zróżnicowanego Użytkowania Łąki Górskiej na Plonowanie Runi i Cechy Jakościowe Odpływających Wód; Wydawnictwo IMUZ: Falenty–Kraków, Poland, 2006. [Google Scholar]
- Misztal, A. Produkcyjne Wykorzystanie Wody Oraz Odpływ Wgłębny w Zależności od Sposobu Użytkowania Gleby w Warunkach Górskich; Wydawnictwo IMUZ: Falenty–Kraków, Poland, 2001. [Google Scholar]
- Szajda, J.; Łabędzki, L. Wyznaczanie ewapotranspiracji rzeczywistej użytków zielonych na podstawie ewapotranspiracji maksymalnej i potencjału wody w glebie. Woda Sr. Obsz. Wiej. 2016, 16, 71–92. [Google Scholar]
- Jankowski, K.; Ciepiela, G.A.; Jodełka, J.; Kisielińska, G. Zmiany w składzie botanicznym runi łąkowej odłogowanej pod wpływem stosowania nawozów mineralnych i organicznych. Łąkarstwo Polsce 2005, 8, 255–262. [Google Scholar]
- Benson, V.; Potter, K.N.; Bogush, H.C.; Goss, D.; Wiliams, J.R. Nitrogen changing sensitivity to evaporation and soil water storage estimates in EPIC. J. Soil Water Cons. 1992, 47, 334–337. [Google Scholar]
- Filipek, J.; Kasperczyk, M. Wpływ liczby pokosów i poziomu nawożenia na skład botaniczny oraz produkcję masy roślinnej i białka ogólnego na łąkach górskich. Zesz. Nauk. AR Krakowie 1984, 182, 101–112. [Google Scholar]
- Jaszczyński, J. The groundwater quality against a background of human activities and impact of peatland area. Agron. Res. 2008, 6, 121–129. [Google Scholar]
- Koc, J.; Glińska-Lewczuk, K.; Solarski, K. Opady atmosferyczne jako medium chemicznej denudacji gleb. Zesz. Probl. Post. Nauk Roln. 2003, 493, 159–166. [Google Scholar]
- Paul, E.A.; Clark, F.E. Mikrobiologia i Biochemia Wód; Wyd. UMCS: Lublin, Poland, 2000. [Google Scholar]
- Sapek, B.; Kalińska, D. Mineralizacja organicznych związków azotu w glebie w świetle długoletnich doświadczeń łąkowych. Woda Sr. Obsz. Wiej. 2004, 4, 183–200. [Google Scholar]
- Sapek, A.; Sapek, B.; Chrzanowski, S.; Jaszczyński, J. Transfer of substances from soil solution in peat soil to ground- and surface water. Pol. J. Environ. Stud. 2006, 15, 367–374. [Google Scholar]
- Szajdak, L.; Szczepański, M.; Bogacz, A. Impact of secondary transformation of peat-moorsh soils on the decrease of nitrogen and carbon compounds in ground water. Agron. Res. 2007, 5, 189–200. [Google Scholar]
- Skorbiłowicz, M. Czynniki i Procesy Kształtujące Obieg Składników Mineralnych w Wodach Rzecznych Zlewni Górnej Narwi. Oficyna Wydaw; Politechniki Białostockiej: Bialystok, Poland, 2010. [Google Scholar]
- Terlikowski, J. Określanie ilości azotu mineralnego uwalnianego z gleby łąkowej (mady próchnicznej) na Żuławach Elbląskich. Woda Sr. Obsz. Wiej. 2013, 13, 149–159. [Google Scholar]
- Jaszczyński, J.; Urbaniak, M.; Nawalany, P. Wpływ stopnia zmurszenia gleb torfowych na wzbogacanie wody gruntowej w związki azotu, fosforu i RWO. Woda Sr. Obsz. Wiej. 2013, 13, 63–77. [Google Scholar]
- Kobuz, J. Rola mikroorganizmów w przemianach azotu w glebie. Zesz. Probl. Post. Nauk Roln. 1996, 440, 151–173. [Google Scholar]
- Krawczyk, W.; Walczak, J.; Paraponiak, P.; Dąbrowska-Wieczorek, M.; Herbut, E. Określenie stopnia uciążliwości nawozów organicznych dla środowiska glebowego i wodnego. Rocz. Nauk. Zoot. 2013, 40, 209–226. [Google Scholar]
- Pietrzak, S. Ocena potencjalnych strat azotu na podstawie bilansu w gospodarstwach rolnych o zróżnicowanym udziale użytków zielonych. Woda Sr. Obsz. Wiej. 2002, 2, 1–58. [Google Scholar]
- Simon, J.C.; Le Corre, L. Le bilan apparent de l’azote a l’échelle du l’exploitation agricole: Méthodologie, exemples de resultats. Fourrages 1992, 129, 79–94. [Google Scholar]
- Kacorzyk, P.; Kasperczyk, M.; Szewczyk, W. Wpływ rodzaju nawożenia na ilość wymywanych podstawowych składników nawozowych z gleby łąki górskiej. Fragm. Agron. 2016, 33, 48–54. [Google Scholar]
- Wasilewski, Z.; Sutkowska, E. Ocena wpływu użytkowania pastwiskowego i kośnego na plony oraz przenikanie związków azotu i potasu do wód gruntowych. Zesz. Nauk. Ar W Krakowie 368 2000, 73, 311–320. [Google Scholar]
- Michalczyk, Z. Rola obszarów wiejskich w tworzeniu i wykorzystaniu zasobów wodnych w Polsce. Woda Sr. Obsz. Wiej. 2004, 4, 13–24. [Google Scholar]
- Jones, J.B.; Case, V.W. Sampling, handling, and analyzing plant tissue samples. In Soil Testing and Plant Analysis; Westerman, R.L., Ed.; Soil Science Society of America: Madison, WI, USA, 1990; pp. 389–427. [Google Scholar]
- Kabała, C.; Karczewska, A. Metodyka Analiz Laboratoryjnych Gleb i Roślin. wyd. 8. 2019. Available online: http://www.up.wroc.pl/~kabala (accessed on 10 November 2019).
- Džeroski, S.; Lavrač, N. Relational Data Mining; Springer: Berlin, Germany, 2001. [Google Scholar]
- Jones, M.T. Artificial Intelligence: A Systems Approach; Jones and Bartlett Publishers: Sudbury, MA, USA, 2015. [Google Scholar]
- Navulur, K. Multispectral Image Analysis Using the Object-Oriented Paradigm; CRC Press: New York, NY, USA, 2006. [Google Scholar]
- Rao, C.R.; Wegman, E.J.; Solka, J.L. Data Mining and Data Visualization; Elsevier: North Holland, The Netherlands, 2005. [Google Scholar]
- Rokach, L.; Maimon, O. Data Mining with Decision Trees: Theory and Applications; World Scientific Publishing: Singapore, 2014. [Google Scholar]
- Wesołowski, P.; Durkowski, T. Stężenie składników mineralnych w wodach gruntowych na łąkach torfowych nawożonych gnojowicą i obornikiem. Woda Sr. Obsz. Wiej. 2004, 4, 139–145. [Google Scholar]
- Mazur, T.; Wojtas, A.; Mazur, Z. Wpływ nawożenia na zawartość jonu amonowego i azotanowego w roztworze glebowym. Zesz. Prob. Post. Nauk Roln. 1996, 440, 258–261. [Google Scholar]
- Koc, J.; Solarski, K.; Koc-Jurczyk, J. Wpływ warunków atmosferycznych na odpływ związków azotu ze zlewni drenarskiej. Inżynieria Ekol. 2007, 18, 223–225. [Google Scholar]
- Aarts, H.F.M.; Hack-ten Broeke, M.J.D.; Groot, W.J.M.; Dijkstra, J.P.D. Nitrogen budgets and nitrate leaching from an experimental system for sustainable dairy farming at ”De Marke“. In Proceedings of the 15th General Meeting of the European Grassland Federation, Wageningen, The Netherlands, 6–9 June 1994; pp. 377–381. [Google Scholar]
| Nutrient | Sheep Manure | Liquid Manure |
|---|---|---|
| (g kg−1 Fresh Matter) | ||
| N | 6.9 | 5.0 |
| P | 1.4 | 0.3 |
| K | 6.0 | 7.0 |
| Ca | 2.5 | 0.4 |
| Mg | 0.8 | 0.5 |
| Na | 0.6 | 3.4 |
| Variant | Period | Concentration of N (mg·dm−3) | SD | V% | Year of the Research | Mean | ||
|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | ||||||
| Load of N (kg·ha−1) | ||||||||
| Control | I | 0.74 | 0.42 | 56.97 | 0.39 | 0.27 | 0.02 | 0.22 |
| II | 2.05 | 0.72 | 34.90 | 1.61 | 1.69 | 2.12 | 1.81 | |
| III | 2.73 | 0.58 | 21.23 | 1.72 | 0.50 | 0.45 | 0.89 | |
| Σ | - | - | - | 3.72 | 2.46 | 2.59 | 2.92 | |
| Mineral P18K50N120 | I | 1.37 | 0.38 | 27.70 | 0.67 | 0.58 | 0.05 | 0.43 |
| II | 3.10 | 1.44 | 46.28 | 2.27 | 5.86 | 1.95 | 3.36 | |
| III | 5.36 | 1.77 | 32.92 | 2.68 | 1.79 | 0.66 | 1.71 | |
| Σ | - | - | - | 5.62 | 8.23 | 2.66 | 5.50 | |
| Liquid manure 15 Mg + P14N45 | I | 0.90 | 0.26 | 29.40 | 0.39 | 0.33 | 0.03 | 0.25 |
| II | 5.32 | 2.64 | 49.66 | 2.55 | 9.46 | 5.71 | 5.91 | |
| III | 3.47 | 1.02 | 29.46 | 2.62 | 1.05 | 0.34 | 1.33 | |
| Σ | - | - | - | 5.55 | 10.84 | 6.08 | 7.49 | |
| FYM (spring) 10 Mg + P4N51 | I | 0.81 | 0.24 | 29.89 | 0.33 | 0.39 | 0.02 | 0.25 |
| II | 2.48 | 0.64 | 25.70 | 2.25 | 4.52 | 2.74 | 3.17 | |
| III | 4.94 | 1.96 | 39.78 | 4.46 | 0.90 | 0.50 | 1.95 | |
| Σ | - | - | - | 7.05 | 5.81 | 3.26 | 5.37 | |
| Fallow | I | 0.34 | 0.14 | 40.00 | 0.20 | 0.19 | 0.01 | 0.13 |
| II | 2.05 | 0.76 | 37.15 | 2.24 | 1.61 | 2.38 | 2.08 | |
| III | 4.34 | 1.08 | 25.00 | 2.69 | 1.25 | 0.44 | 1.46 | |
| Σ | - | - | - | 5.14 | 3.04 | 2.83 | 3.67 | |
| Arable land P18K50N60-120 | I | 0.85 | 0.61 | 72.29 | 0.59 | 0.31 | 0.02 | 0.30 |
| II | 10.20 | 3.46 | 33.96 | 18.13 | 12.43 | 8.36 | 12.97 | |
| III | 5.67 | 1.50 | 26.49 | 5.27 | 1.52 | 0.67 | 2.49 | |
| Σ | - | - | - | 23.99 | 14.26 | 9.05 | 15.76 | |
| Variant | Period | Concentration of P (mg·dm−3) | SD | V% | Year of the Research | Mean | ||
|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | ||||||
| Load of P (kg·ha−1) | ||||||||
| Control | I | 0.36 | 0.11 | 30.50 | 0.10 | 0.12 | 0.02 | 0.08 |
| II | 0.36 | 0.23 | 63.09 | 0.09 | 0.49 | 0.38 | 0.32 | |
| III | 0.45 | 0.00 | 0.25 | 0.28 | 0.11 | 0.06 | 0.15 | |
| Σ | - | - | - | 0.47 | 0.72 | 0.47 | 0.55 | |
| Mineral P18K50N120 | I | 0.46 | 0.07 | 15.36 | 0.15 | 0.26 | 0.02 | 0.14 |
| II | 0.41 | 0.27 | 67.58 | 0.31 | 0.25 | 0.59 | 0.38 | |
| III | 0.65 | 0.43 | 66.04 | 0.59 | 0.22 | 0.02 | 0.28 | |
| Σ | - | - | - | 1.05 | 0.72 | 0.64 | 0.80 | |
| Liquid manure 15 Mg + P14N45 | I | 0.33 | 0.05 | 15.91 | 0.13 | 0.13 | 0.01 | 0.09 |
| II | 0.43 | 0.26 | 60.75 | 0.77 | 0.24 | 0.36 | 0.46 | |
| III | 0.33 | 0.32 | 96.43 | 0.47 | 0.03 | 0.02 | 0.18 | |
| Σ | - | - | - | 1.37 | 0.40 | 0.40 | 0.72 | |
| FYM (spring) 10 Mg + P4N51 | I | 0.32 | 0.07 | 22.73 | 0.13 | 0.12 | 0.01 | 0.09 |
| II | 0.34 | 0.04 | 12.76 | 0.46 | 0.44 | 0.38 | 0.43 | |
| III | 0.47 | 0.30 | 64.54 | 0.29 | 0.18 | 0.02 | 0.16 | |
| Σ | - | - | - | 0.88 | 0.74 | 0.41 | 0.68 | |
| Fallow | I | 0.27 | 0.17 | 61.25 | 0.05 | 0.15 | 0.02 | 0.07 |
| II | 0.51 | 0.13 | 24.65 | 0.54 | 0.49 | 0.54 | 0.52 | |
| III | 0.26 | 0.06 | 21.31 | 0.20 | 0.05 | 0.03 | 0.09 | |
| Σ | - | - | - | 0.79 | 0.68 | 0.60 | 0.69 | |
| Arable land P18K50N60-120 | I | 0.58 | 0.41 | 70.88 | 0.17 | 0.12 | 0.05 | 0.12 |
| II | 1.00 | 0.82 | 81.96 | 0.38 | 1.21 | 1.94 | 1.18 | |
| III | 0.81 | 0.55 | 68.16 | 0.88 | 0.29 | 0.03 | 0.40 | |
| Σ | - | - | - | 1.43 | 1.62 | 2.02 | 1.69 | |
| Variant | Period | Concentration of K (mg·dm−3) | SD | V% | Year of the Research | Mean | ||
|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | ||||||
| Load of K (kg·ha−1) | ||||||||
| Control | I | 1.85 | 0.42 | 22.57 | 0.75 | 0.76 | 0.06 | 0.52 |
| II | 1.39 | 0.09 | 6.47 | 1.37 | 1.53 | 0.96 | 1.29 | |
| III | 1.12 | 0.08 | 6.79 | 0.71 | 0.25 | 0.16 | 0.37 | |
| Σ | - | - | - | 2.82 | 2.54 | 1.18 | 2.18 | |
| Mineral P18K50N120 | I | 2.24 | 0.43 | 19.03 | 0.70 | 1.01 | 0.13 | 0.61 |
| II | 2.27 | 1.15 | 50.52 | 2.18 | 1.50 | 2.90 | 2.19 | |
| III | 2.17 | 0.23 | 10.68 | 1.42 | 0.58 | 0.28 | 0.76 | |
| Σ | - | - | - | 4.30 | 3.09 | 3.30 | 3.56 | |
| Liquid manure 15 Mg + P14N45 | I | 1.11 | 0.71 | 63.79 | 0.18 | 0.36 | 0.08 | 0.21 |
| II | 2.13 | 0.64 | 29.95 | 2.86 | 1.84 | 2.13 | 2.28 | |
| III | 1.14 | 0.26 | 23.24 | 0.76 | 0.22 | 0.20 | 0.39 | |
| Σ | - | - | - | 3.80 | 2.41 | 2.41 | 2.87 | |
| FYM (spring) 10 Mg + P4N51 | I | 1.07 | 0.50 | 47.01 | 0.20 | 0.45 | 0.07 | 0.24 |
| II | 1.96 | 0.87 | 44.17 | 3.56 | 1.61 | 2.07 | 2.41 | |
| III | 2.84 | 1.47 | 51.78 | 2.38 | 0.81 | 0.16 | 1.12 | |
| Σ | - | - | - | 6.14 | 2.87 | 2.30 | 3.77 | |
| Fallow | I | 1.97 | 0.66 | 33.44 | 0.52 | 1.32 | 0.13 | 0.66 |
| II | 2.46 | 0.40 | 16.13 | 2.14 | 3.45 | 2.26 | 2.62 | |
| III | 1.30 | 0.60 | 46.17 | 0.69 | 0.19 | 0.27 | 0.38 | |
| Σ | - | - | - | 3.34 | 4.97 | 2.65 | 3.65 | |
| Arable land P18K50N60-120 | I | 2.71 | 1.84 | 67.76 | 1.84 | 0.88 | 0.08 | 0.93 |
| II | 2.35 | 0.16 | 6.82 | 2.99 | 3.82 | 2.24 | 3.02 | |
| III | 1.76 | 0.90 | 51.14 | 1.29 | 0.72 | 0.14 | 0.72 | |
| Σ | - | - | - | 6.12 | 5.42 | 2.46 | 4.67 | |
| Variant | Period | Concentration of Ca (mg·dm−3) | SD | V% | Year of the Research | Mean | ||
|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | ||||||
| Load of Ca (kg·ha−1) | ||||||||
| Control | I | 17.17 | 2.70 | 15.74 | 4.59 | 7.68 | 0.81 | 4.36 |
| II | 16.65 | 5.06 | 30.39 | 11.17 | 17.26 | 16.28 | 14.90 | |
| III | 17.74 | 8.51 | 47.98 | 5.87 | 4.12 | 3.62 | 4.54 | |
| Σ | - | - | - | 21.63 | 29.06 | 20.71 | 23.80 | |
| Mineral P18K50N120 | I | 19.09 | 2.68 | 14.07 | 5.96 | 9.54 | 1.02 | 5.51 |
| II | 22.98 | 5.39 | 23.46 | 20.85 | 24.19 | 24.18 | 23.08 | |
| III | 23.94 | 5.88 | 24.56 | 12.55 | 5.36 | 4.34 | 7.42 | |
| Σ | - | - | - | 39.37 | 39.09 | 29.55 | 36.00 | |
| Liquid manure 15 Mg + P14N45 | I | 10.48 | 1.58 | 15.08 | 3.79 | 3.63 | 0.45 | 2.62 |
| II | 12.77 | 0.90 | 7.02 | 15.28 | 16.03 | 10.87 | 14.06 | |
| III | 11.30 | 1.77 | 15.63 | 7.58 | 3.25 | 1.39 | 4.08 | |
| Σ | - | - | - | 26.64 | 22.92 | 12.71 | 20.76 | |
| FYM (spring) 10 Mg + P4N51 | I | 12.76 | 2.58 | 20.26 | 3.48 | 5.36 | 0.67 | 3.17 |
| II | 14.58 | 3.42 | 23.47 | 14.56 | 21.44 | 18.55 | 18.18 | |
| III | 19.77 | 6.11 | 30.91 | 15.12 | 5.08 | 1.73 | 7.31 | |
| Σ | - | - | - | 33.16 | 31.88 | 20.96 | 28.67 | |
| Fallow | I | 12.41 | 1.33 | 10.75 | 4.66 | 7.16 | 0.74 | 4.19 |
| II | 15.27 | 2.73 | 17.90 | 16.32 | 22.84 | 10.69 | 16.62 | |
| III | 16.51 | 3.93 | 23.80 | 7.44 | 4.42 | 2.47 | 4.78 | |
| Σ | - | - | - | 28.42 | 34.41 | 13.91 | 25.58 | |
| Arable land P18K50N60-120 | I | 27.14 | 16.28 | 60.00 | 16.89 | 5.89 | 1.24 | 8.01 |
| II | 31.66 | 8.18 | 25.85 | 52.48 | 40.58 | 27.62 | 40.23 | |
| III | 18.63 | 5.58 | 29.93 | 13.63 | 3.54 | 3.86 | 7.01 | |
| Σ | - | - | - | 83.00 | 50.01 | 32.72 | 55.24 | |
| Variant | Period | Concentration of Mg (mg·dm−3) | SD | V% | Year of the Research | Mean | ||
|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | ||||||
| Load of Mg (kg·ha−1) | ||||||||
| Control | I | 1.87 | 0.60 | 32.29 | 0.42 | 1.06 | 0.08 | 0.52 |
| II | 2.51 | 0.36 | 14.36 | 2.31 | 3.15 | 1.58 | 2.35 | |
| III | 2.60 | 0.43 | 16.52 | 1.33 | 0.66 | 0.40 | 0.80 | |
| Σ | - | - | - | 4.06 | 4.86 | 2.06 | 3.66 | |
| Mineral P18K50N120 | I | 2.09 | 0.33 | 15.76 | 0.67 | 1.18 | 0.10 | 0.65 |
| II | 2.23 | 1.08 | 48.48 | 3.39 | 1.37 | 1.92 | 2.23 | |
| III | 2.50 | 0.75 | 30.21 | 1.63 | 0.79 | 0.25 | 0.89 | |
| Σ | - | - | - | 5.69 | 3.33 | 2.27 | 3.76 | |
| Liquid manure 15 Mg + P14N45 | I | 1.82 | 0.56 | 30.82 | 0.47 | 1.03 | 0.06 | 0.52 |
| II | 1.80 | 0.18 | 9.80 | 1.87 | 2.63 | 1.50 | 2.00 | |
| III | 1.96 | 0.61 | 31.32 | 1.31 | 0.64 | 0.20 | 0.72 | |
| Σ | - | - | - | 3.66 | 4.29 | 1.76 | 3.24 | |
| FYM (spring) 10 Mg + P4N51 | I | 1.76 | 0.69 | 39.47 | 0.39 | 0.69 | 0.11 | 0.40 |
| II | 1.85 | 0.59 | 31.78 | 1.62 | 3.71 | 1.87 | 2.40 | |
| III | 3.90 | 1.00 | 25.50 | 3.13 | 0.80 | 0.43 | 1.45 | |
| Σ | - | - | - | 5.14 | 5.20 | 2.41 | 4.25 | |
| Fallow | I | 1.54 | 0.20 | 13.13 | 0.56 | 0.93 | 0.09 | 0.53 |
| II | 2.46 | 1.36 | 55.39 | 2.63 | 4.85 | 0.94 | 2.80 | |
| III | 1.48 | 0.61 | 41.29 | 0.78 | 0.23 | 0.29 | 0.43 | |
| Σ | - | - | - | 3.96 | 6.02 | 1.32 | 3.77 | |
| Arable land P18K50N60-120 | I | 3.38 | 0.79 | 23.50 | 1.57 | 1.25 | 0.17 | 1.00 |
| II | 4.81 | 1.02 | 21.32 | 7.65 | 6.37 | 4.31 | 6.11 | |
| III | 2.69 | 0.06 | 2.21 | 1.96 | 0.71 | 0.44 | 1.04 | |
| Σ | - | - | - | 11.18 | 8.33 | 4.92 | 8.15 | |
| Variant | Period | Concentration of Na (mg·dm−3) | SD | V% | Year of the Research | Mean | ||
|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | ||||||
| Load of Na (kg·ha−1) | ||||||||
| Control | I | 2.69 | 0.51 | 18.91 | 0.84 | 0.95 | 0.14 | 0.64 |
| II | 2.73 | 0.85 | 31.05 | 1.83 | 2.81 | 2.69 | 2.44 | |
| III | 2.27 | 0.57 | 25.11 | 1.44 | 0.40 | 0.39 | 0.74 | |
| Σ | - | - | - | 4.10 | 4.15 | 3.22 | 3.82 | |
| Mineral P18K50N120 | I | 1.69 | 0.66 | 39.07 | 0.35 | 0.94 | 0.10 | 0.47 |
| II | 1.98 | 0.54 | 27.17 | 2.50 | 1.70 | 1.78 | 1.99 | |
| III | 1.86 | 0.34 | 18.28 | 1.22 | 0.37 | 0.31 | 0.63 | |
| Σ | - | - | - | 4.06 | 3.01 | 2.20 | 3.09 | |
| Liquid manure 15 Mg + P14N45 | I | 1.50 | 0.78 | 52.28 | 0.24 | 0.96 | 0.06 | 0.42 |
| II | 1.74 | 0.34 | 19.50 | 1.58 | 2.76 | 1.48 | 1.94 | |
| III | 1.76 | 0.59 | 33.30 | 1.18 | 0.58 | 0.17 | 0.65 | |
| Σ | - | - | - | 3.01 | 4.31 | 1.72 | 3.01 | |
| FYM (spring) 10 Mg + P4N51 | I | 2.24 | 0.93 | 41.35 | 1.09 | 0.89 | 0.06 | 0.68 |
| II | 1.78 | 0.57 | 32.15 | 1.70 | 3.68 | 1.62 | 2.33 | |
| III | 2.18 | 0.93 | 42.53 | 1.34 | 0.72 | 0.17 | 0.74 | |
| Σ | - | - | - | 4.13 | 5.29 | 1.85 | 3.75 | |
| Fallow | I | 1.74 | 0.94 | 54.27 | 0.33 | 1.48 | 0.10 | 0.64 |
| II | 2.01 | 0.59 | 29.58 | 2.14 | 3.30 | 1.20 | 2.21 | |
| III | 1.37 | 0.67 | 49.32 | 0.53 | 0.26 | 0.29 | 0.36 | |
| Σ | - | - | - | 3.00 | 5.03 | 1.59 | 3.21 | |
| Arable land P18K50N60-120 | I | 2.12 | 1.12 | 52.60 | 0.37 | 1.10 | 0.16 | 0.54 |
| II | 4.12 | 0.62 | 15.01 | 4.76 | 7.33 | 3.87 | 5.32 | |
| III | 2.78 | 0.21 | 7.42 | 2.03 | 0.81 | 0.41 | 1.08 | |
| Σ | - | - | - | 7.16 | 9.24 | 4.44 | 6.95 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kacorzyk, P.; Strojny, J. The Effect of the Manner in Which Montane and Submontane Areas Are Utilized on the Quality of Leachate Water. Sustainability 2021, 13, 6299. https://doi.org/10.3390/su13116299
Kacorzyk P, Strojny J. The Effect of the Manner in Which Montane and Submontane Areas Are Utilized on the Quality of Leachate Water. Sustainability. 2021; 13(11):6299. https://doi.org/10.3390/su13116299
Chicago/Turabian StyleKacorzyk, Piotr, and Jacek Strojny. 2021. "The Effect of the Manner in Which Montane and Submontane Areas Are Utilized on the Quality of Leachate Water" Sustainability 13, no. 11: 6299. https://doi.org/10.3390/su13116299
APA StyleKacorzyk, P., & Strojny, J. (2021). The Effect of the Manner in Which Montane and Submontane Areas Are Utilized on the Quality of Leachate Water. Sustainability, 13(11), 6299. https://doi.org/10.3390/su13116299







