Soil Nutrient and Vegetation Diversity Patterns of Alpine Wetlands on the Qinghai-Tibetan Plateau
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Site
2.2. Plant Species Diversity Index Measurements
2.3. Soil and Plant Sampling and Analysis
2.4. Data Analysis
3. Results
3.1. Plant Composition
3.2. Spatial Patterns of Vegetation Biomass and Species Diversity
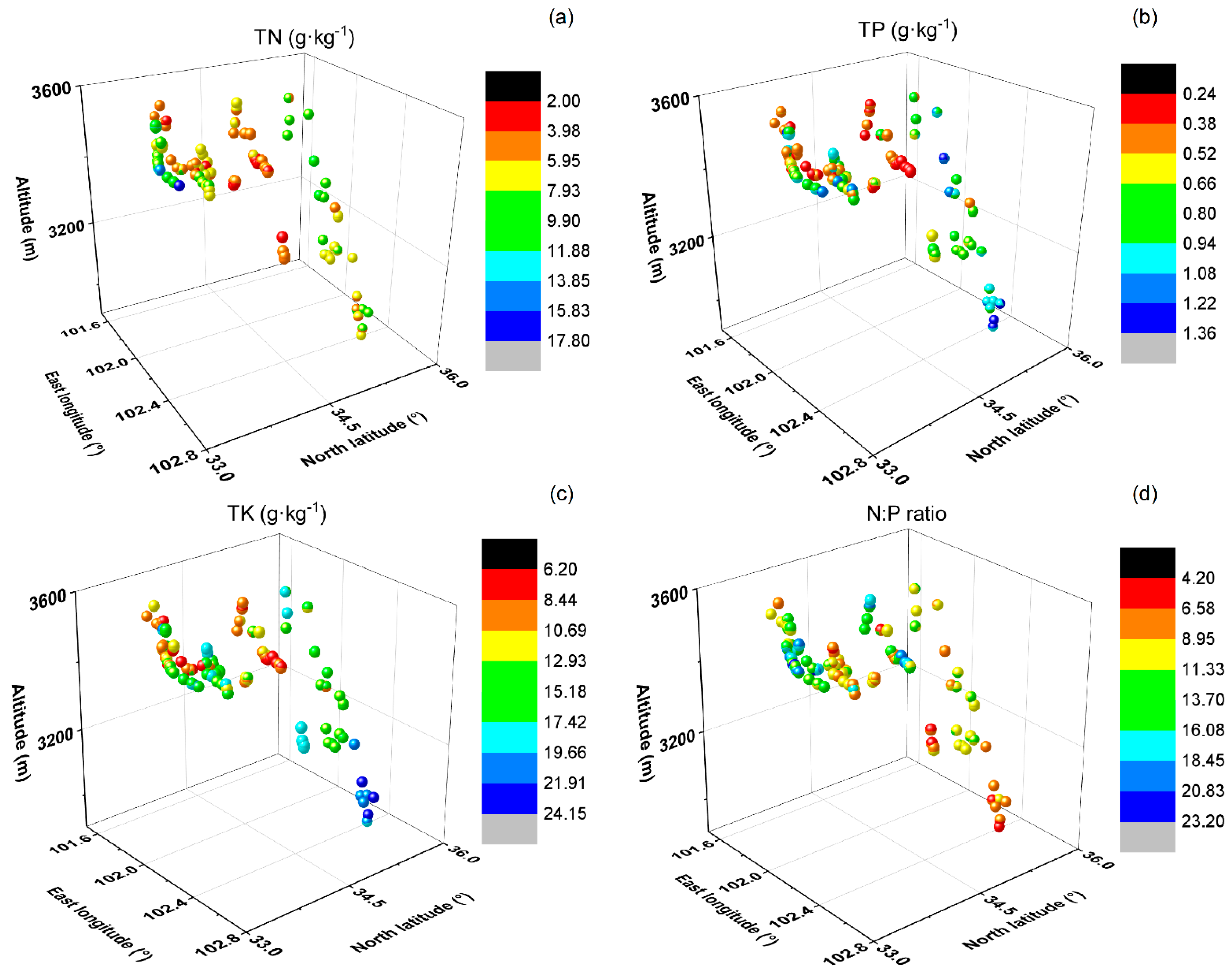
3.3. Nutrient Concentrations and N:P Ratios in Surface Soils
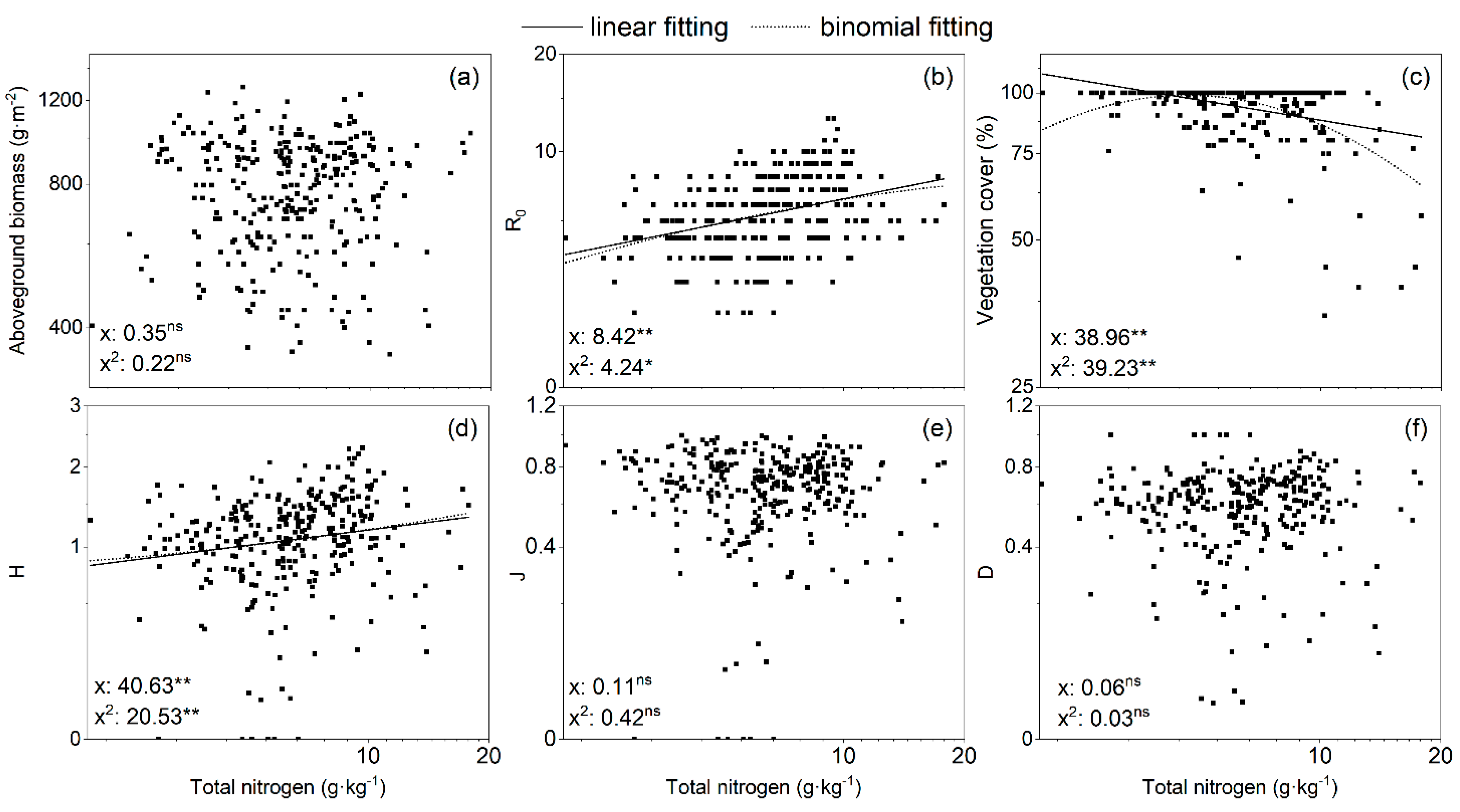
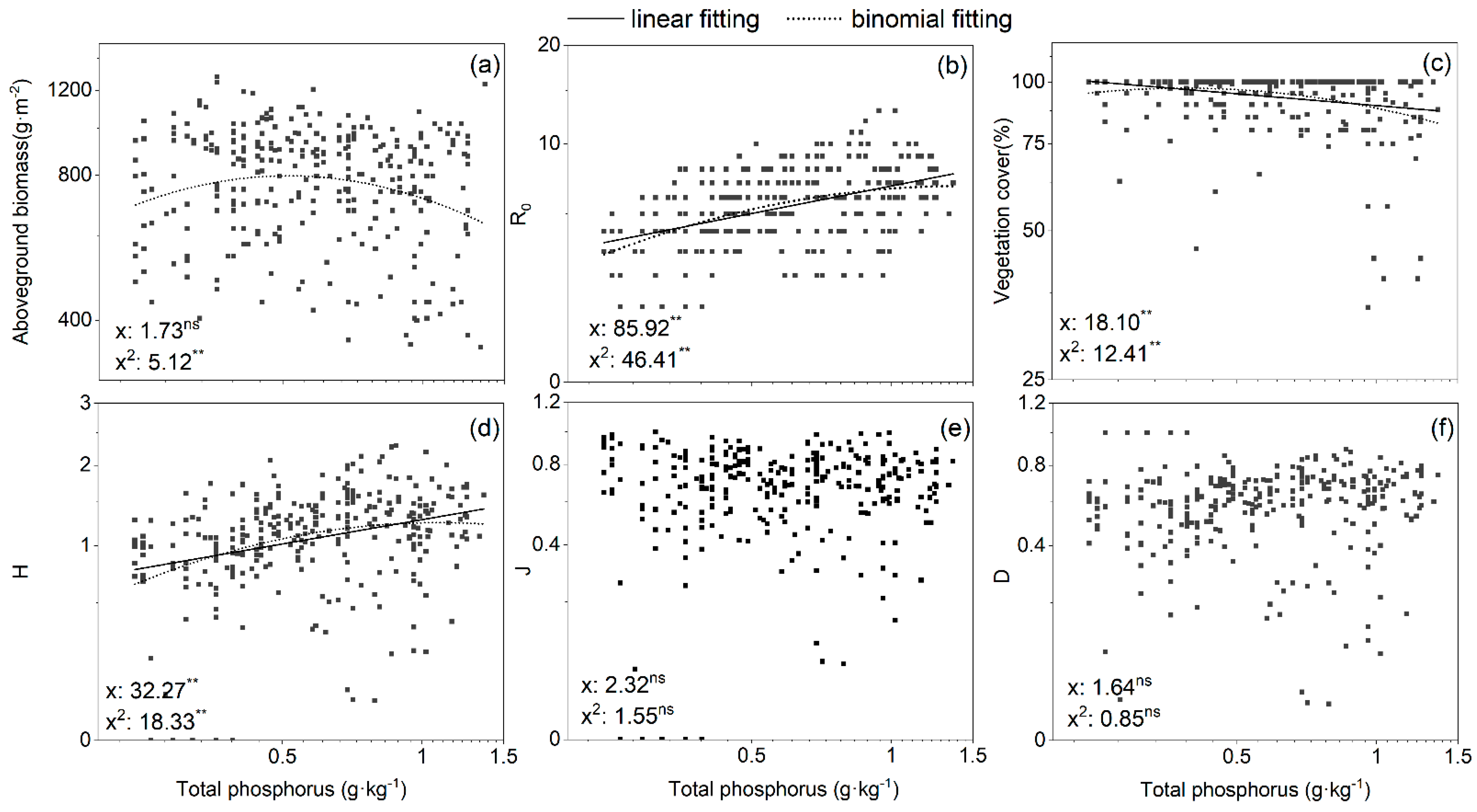
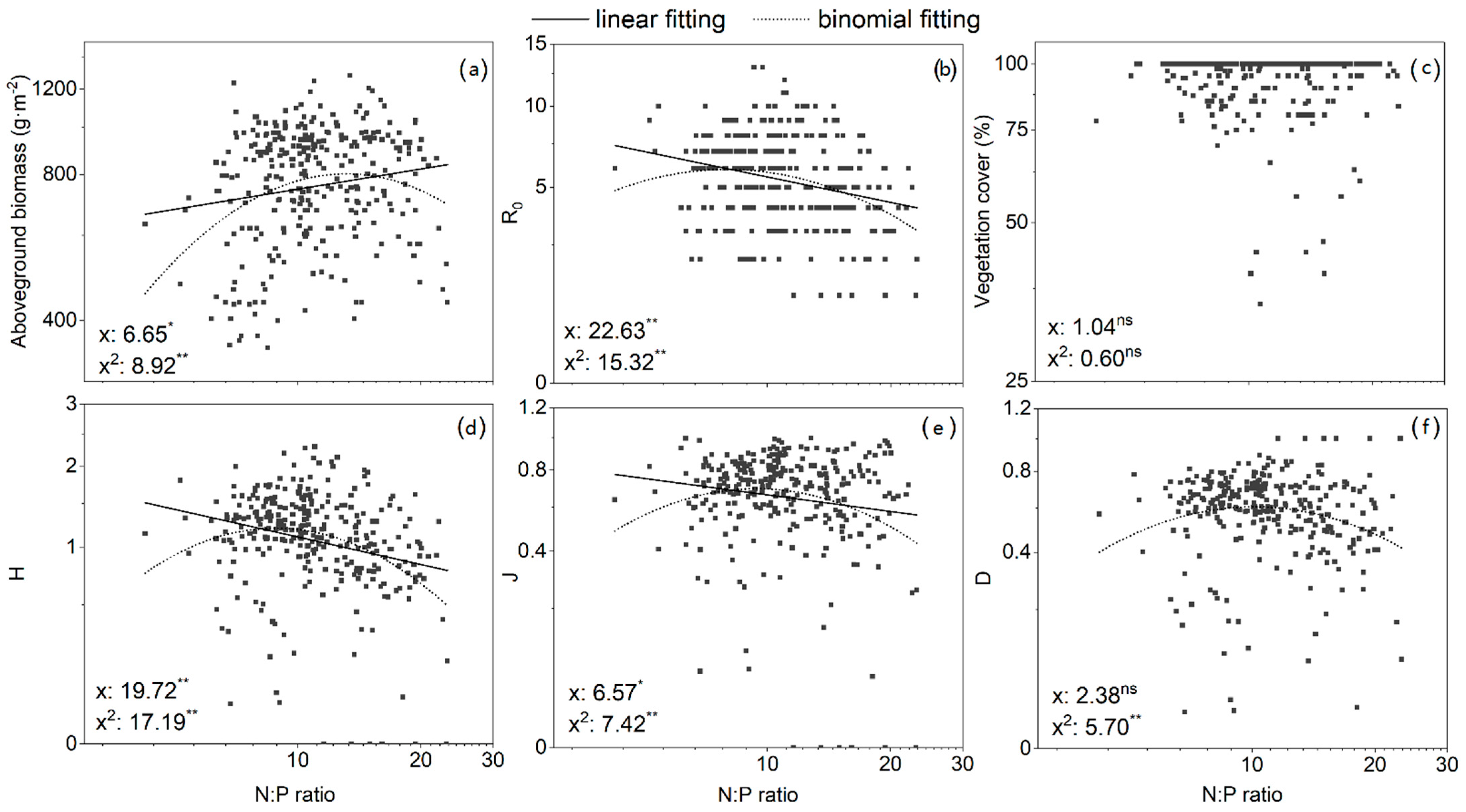
3.4. Relationships between Vegetation Biomass, Cover, Species Diversity, and Nutrient Availability
3.5. Relationships between Vegetation Biomass, Cover, Species Diversity, and Soil Attributes
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, C.; Guo, Q.; Tan, D.; Shi, Z.; Ma, C. Haloxylon ammodendron community patterns in different habitats along southeastern edge of Zhunger basin. Ying Yong Sheng Tai Xue Bao/J. Appl. Ecol. 2005, 16, 1224–1229. [Google Scholar]
- Xiao, H.; Chen, J.; Zhang, Z. Influence of Deposition Temperature on the Structure of Si3N4 Thin Film Prepared by MWECR-PECVD. Plasma Sci. Technol. 2004, 6, 2485–2488. [Google Scholar] [CrossRef]
- Trindade, C.R.T.; Landeiro, V.L.; Schneck, F. Macrophyte functional groups elucidate the relative role of environmental and spatial factors on species richness and assemblage structure. Hydrobiologia 2018, 823, 217–230. [Google Scholar] [CrossRef]
- Dong, S.-K.; Sha, W.; Su, X.-K.; Zhang, Y.; Li, S.; Gao, X.; Liu, S.-L.; Shi, J.-B.; Liu, Q.-R.; Hao, Y. The impacts of geographic, soil and climatic factors on plant diversity, biomass and their relationships of the alpine dry ecosystems: Cases from the Aerjin Mountain Nature Reserve, China. Ecol. Eng. 2019, 127, 170–177. [Google Scholar] [CrossRef]
- De Deyn, G.B.; Raaijmakers, C.E.; Van Der Putten, W.H. Plant community development is affected by nutrients and soil biota. J. Ecol. 2004, 92, 824–834. [Google Scholar] [CrossRef]
- Perroni-Ventura, Y.; Montaña, C.; García-Oliva, F. Relationship between soil nutrient availability and plant species richness in a tropical semi-arid environment. J. Veg. Sci. 2006, 17, 719–728. [Google Scholar] [CrossRef]
- Bedford, B.L.; Walbridge, M.R.; Aldous, A. Patterns in Nutrient Availability and Plant Diversity of Temperate North American Wetlands. Ecology 1999, 80, 2151–2169. [Google Scholar] [CrossRef]
- Wheeler, B.D.; Shaw, S.C. Above-Ground Crop Mass and Species Richness of the Principal Types of Herbaceous Rich-Fen Vegetation of Lowland England and Wales. J. Ecol. 1991, 79, 285. [Google Scholar] [CrossRef]
- Zhang, D.; Qi, Q.; Tong, S.; Wang, X.; An, Y.; Zhang, M.; Lu, X. Soil Degradation Effects on Plant Diversity and Nutrient in Tussock Meadow Wetlands. J. Soil Sci. Plant Nutr. 2019, 19, 535–544. [Google Scholar] [CrossRef]
- Mylliemngap, W.; Barik, S.K. Plant diversity, net primary productivity and soil nutrient contents of a humid subtropical grassland remained low even after 50 years of post-disturbance recovery from coal mining. Environ. Monit. Assess. 2019, 191, 697. [Google Scholar] [CrossRef] [PubMed]
- Seabloom, E.W.; Adler, P.B.; Alberti, J.; Biederman, L.; Buckley, Y.M.; Cadotte, M.W.; Collins, S.L.; Dee, L.; Fay, P.A.; Firn, J.; et al. Increasing effects of chronic nutrient enrichment on plant diversity loss and ecosystem productivity over time. Ecology 2021, 102. [Google Scholar] [CrossRef]
- Janssens, F.; Peeters, A.; Tallowin, J.; Bakker, J.; Bekker, R.; Fillat, F.; Oomes, M. Relationship between soil chemical factors and grassland diversity. Plant Soil 1998, 202, 69–78. [Google Scholar] [CrossRef]
- Venterink, H.O.; Wassen, M.; Belgers, J.D.M.; Verhoeven, J.T.A. Control of environmental variables on species density in fens and meadows: Importance of direct effects and effects through community biomass. J. Ecol. 2001, 89, 1033–1040. [Google Scholar] [CrossRef]
- Duranel, A.; Acreman, M.C.; Stratford, C.J.; Thompson, J.R.; Mould, D.J. Assessing the hydrological suitability of floodplains for species-rich meadow restoration: A case study of the Thames floodplain, UK. Hydrol. Earth Syst. Sci. 2007, 11, 170–179. [Google Scholar] [CrossRef]
- Tiessen, H.; Cuevas, E.; Chacon, P. The role of soil organic matter in sustaining soil fertility. Nat. Cell Biol. 1994, 371, 783–785. [Google Scholar] [CrossRef]
- Fornara, D.A.; Tilman, D. Plant functional composition influences rates of soil carbon and nitrogen accumulation. J. Ecol. 2008, 96, 314–322. [Google Scholar] [CrossRef]
- Lange, M.; Eisenhauer, N.; Sierra, C.A.; Bessler, H.; Engels, C.; Griffiths, R.I.; Mellado-Vázquez, P.G.; Malik, A.A.; Roy, J.; Scheu, S.; et al. Plant diversity increases soil microbial activity and soil carbon storage. Nat. Commun. 2015, 6, 6707. [Google Scholar] [CrossRef] [PubMed]
- Fischer, C.; Tischer, J.; Roscher, C.; Eisenhauer, N.; Ravenek, J.; Gleixner, G.; Attinger, S.; Jensen, B.; de Kroon, H.; Mommer, L.; et al. Plant species diversity affects infiltration capacity in an experimental grassland through changes in soil properties. Plant Soil 2015, 397, 1–16. [Google Scholar] [CrossRef]
- Bai, J.-H.; Lu, Q.-Q.; Wang, J.-J.; Zhao, Q.-Q.; Ouyang, H.; Deng, W.; Li, A.-N. Landscape pattern evolution processes of alpine wetlands and their driving factors in the Zoige Plateau of China. J. Mt. Sci. 2013, 10, 54–67. [Google Scholar] [CrossRef]
- Niu, S.W.; Ma, L.B.; Zeng, M.M. Effect of overgrazing on grassland desertification in Maqu County. Acta Ecol. Sin. 2008, 28, 145–153. [Google Scholar] [CrossRef]
- Zou, L.; Zhou, Z.; Yan, S.; Qin, Y. Response of Soil Nutrients to Different Land Utilization Types in Alpine Meadow in Maqu Chinese. J. Grassl. 2009, 31, 80–87. [Google Scholar]
- Brandt, J.S.; Haynes, M.A.; Kuemmerle, T.; Waller, D.M.; Radeloff, V.C. Regime shift on the roof of the world: Alpine meadows converting to shrublands in the southern Himalayas. Biol. Conserv. 2013, 158, 116–127. [Google Scholar] [CrossRef]
- Niu, K.; Choler, P.; de Bello, F.; Mirotchnick, N.; Du, G.; Sun, S. Fertilization decreases species diversity but increases functional diversity: A three-year experiment in a Tibetan alpine meadow. Agric. Ecosyst. Environ. 2014, 182, 106–112. [Google Scholar] [CrossRef]
- Mittelbach, G.G.; Steiner, C.F.; Scheiner, S.M.; Gross, K.L.; Reynolds, H.L.; Waide, R.B.; Willig, M.R.; Dodson, S.I.; Gough, L. What is the observed relationship between species richiness and productivity? Ecology 2001, 82, 2381–2396. [Google Scholar] [CrossRef]
- Dingaan, M.N.V.; Tsubo, M.; Walker, S.; Newby, T. Influence of grazing on plant diversityproductivity relationship in semi-arid grassland of South Africa. Appl. Ecol. Environ. Res. 2016, 14, 1–13. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, X.; Zhang, Q.; Luo, L.; Xue, B. The evaluation of famers’ climate change adaptation strategies in high-frigid ecological vulnerable region: A case of Gannan Plateau. Acta Ecol. Sin. 2017, 37, 2392–2402. [Google Scholar] [CrossRef][Green Version]
- Arrhenius, O. Species and Area. J. Ecol. 1921, 9, 95. [Google Scholar] [CrossRef]
- Shannon, C.E. A Mathematical Theory of Communication. Bell Syst. Tech. J. 1948, 27, 379–423. [Google Scholar] [CrossRef]
- Pielou, E.C. The measurement of diversity in different types of biological collections. J. Theor. Biol. 1966, 13, 131–144. [Google Scholar] [CrossRef]
- Simpson, E. Measurement of diversity. Nature 1949, 163, 688. [Google Scholar] [CrossRef]
- Skeen, J.N. An Extension of the Concept of Importance Value in Analyzing Forest Communities. Ecology 1973, 54, 655–656. [Google Scholar] [CrossRef]
- Nelson, D.W.; Sommer, L.E. Total carbon, organic carbon and organic matter. In Methods of Soil Analysis, Part 2; Page, A.L., Miller, R.H., Keeney, D.R., Eds.; ASA and SSSA: Madison, WI, USA, 1982; pp. 539–579. [Google Scholar]
- Page, A.L.; Miller, R.H.; Keeney, D.R. Methods of Soil Analysis. Part 2. Chemical and Microbiological Properties; Page, A.L., Ed.; American Society of Agronomy, Soil Science Society of America: Madison, WI, USA, 1982; p. 1159. [Google Scholar]
- Troumbis, A.Y. No observational evidence for diversity-enhancing productivity in Mediterranean shrublands? Oecologia 2001, 129, 622–623. [Google Scholar] [CrossRef]
- Mahdavi, P.; Bergmeier, E. Plant functional traits and diversity in sand dune ecosystems across different biogeographic regions. Acta Oecol. 2016, 74, 37–45. [Google Scholar] [CrossRef]
- Zhang, J.; Zhao, N.; Liu, C.; Yang, H.; Li, M.; Yu, G.; Wilcox, K.; Yu, Q.; He, N. C:N:P stoichiometry in China’s forests: From organs to ecosystems. Funct. Ecol. 2018, 32, 50–60. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, C.; Wang, M. Linkages of C:N:P stoichiometry between soil and leaf and their response to climatic factors along altitudinal gradients. J. Soils Sediments 2019, 19, 1820–1829. [Google Scholar] [CrossRef]
- Chen, S.Y.; Yu, H.; Feng, Q.S.; Liang, T.G. Spatial heterogeneity of soil N:P ratio on Gannan Plateau. Acta Agrestia Sin. 2013, 21, 34–40. [Google Scholar] [CrossRef]
- Bo, L.-Q. Feasible study on the integration system for the space monitoring of major earthquakes and volcanoes in terrestrial land. Chin. Geogr. Sci. 2002, 12, 350–353. [Google Scholar] [CrossRef]
- Gu, F.-X.; Zhang, Y.-D.; Chu, Y.; Shi, Q.-D.; Pan, X.-L. Primary analysis on groundwater, soil moisture and salinity in fukang oasis of southern Junggar basin. Chin. Geogr. Sci. 2002, 12, 333–338. [Google Scholar] [CrossRef]
- Tilman, D. Resource Competition and Community Structure. Monographs in Population Biology; Princeton University Press: Princeton, NJ, USA, 1982; Volume 17. [Google Scholar]
- Abanda, P.A.; Compton, J.S.; Hannigan, R.E. Soil nutrient content, above-ground biomass and litter in a semi-arid shrubland, South Africa. Geoderma 2011, 164, 128–137. [Google Scholar] [CrossRef]
- Yuan, F.; Wu, J.; Li, A.; Rowe, H.; Bai, Y.; Huang, J.; Han, X. Spatial patterns of soil nutrients, plant diversity, and aboveground biomass in the Inner Mongolia grassland: Before and after a biodiversity removal experiment. Landsc. Ecol. 2015, 30, 1737–1750. [Google Scholar] [CrossRef]
- Vitousek, P.M.; Howarth, R.W. Nitrogen limitation on land and in the sea: How can it occur? Biogeochemistry 1991, 13, 87–115. [Google Scholar] [CrossRef]
- Bridgham, S.D.; Pastor, J.; Janssens, J.A.; Chapin, C.; Malterer, T.J. Multiple limiting gradients in Peatlands: A call for a new paradigm. Wetlands 1996, 16, 45–65. [Google Scholar] [CrossRef]
- Verhoeven, J.; Koerselman, W.; Meuleman, A. Nitrogen- or phosphorus-limited growth in herbaceous, wet vegetation: Relations with atmospheric inputs and management regimes. Trends Ecol. Evol. 1996, 11, 494–497. [Google Scholar] [CrossRef]
- Bi, X.; Li, B.; Fu, Q.; Fan, Y.; Ma, L.; Yang, Z.; Nan, B.; Dai, X.; Zhang, X. Effects of grazing exclusion on the grassland ecosystems of mountain meadows and temperate typical steppe in a mountain-basin system in Central Asia’s arid regions, China. Sci. Total Environ. 2018, 630, 254–263. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.-L.; Liu, Z.-H.; Zhang, L.; Chen, J.-M.; Hu, T.-M. Long-term fencing improved soil properties and soil organic carbon storage in an alpine swamp meadow of western China. Plant Soil 2010, 332, 331–337. [Google Scholar] [CrossRef]
- Hu, Z.; Li, S.; Guo, Q.; Niu, S.; He, N.; Li, L.; Yu, G. A synthesis of the effect of grazing exclusion on carbon dynamics in grasslands in China. Glob. Chang. Biol. 2016, 22, 1385–1393. [Google Scholar] [CrossRef]
- Yuan, Z.-Q.; Jiang, X.-J. Vegetation and soil covariation, not grazing exclusion, control soil organic carbon and nitrogen in density fractions of alpine meadows in a Tibetan permafrost region. Catena 2021, 196, 104832. [Google Scholar] [CrossRef]
- Shi, X.-M.; Li, X.G.; Li, C.T.; Zhao, Y.; Shang, Z.H.; Ma, Q. Grazing exclusion decreases soil organic C storage at an alpine grassland of the Qinghai–Tibetan Plateau. Ecol. Eng. 2013, 57, 183–187. [Google Scholar] [CrossRef]
- Shang, W.; Wu, X.; Zhao, L.; Yue, G.; Zhao, Y.; Qiao, Y.; Li, Y. Seasonal variations in labile soil organic matter fractions in permafrost soils with different vegetation types in the central Qinghai–Tibet Plateau. Catena 2016, 137, 670–678. [Google Scholar] [CrossRef]
- Sitters, J.; Edwards, P.J.; Venterink, H.O. Increases of Soil C, N, and P Pools Along an Acacia Tree Density Gradient and Their Effects on Trees and Grasses. Ecosystems 2012, 16, 347–357. [Google Scholar] [CrossRef]
- Zhou, Y.; Boutton, T.W.; Ben Wu, X. Soil C:N:P stoichiometry responds to vegetation change from grassland to woodland. Biogeochemitry. 2018, 140, 341–357. [Google Scholar] [CrossRef]
- Liu, Y.L.J.; Luo, Y.; Li, D.-Z.; Gao, L. Evolution and maintenance mechanisms of plant diversity in the Qinghai-Tibet Plateau and adjacent regions: Retrospect and prospect. Biodivers. Sci. 2017, 25, 41–45. [Google Scholar] [CrossRef]
- Fayiah, M.; Dong, S.; Li, Y.; Xu, Y.; Gao, X.; Li, S.; Shen, H.; Xiao, J.; Yang, Y.; Wessell, K. The relationships between plant diversity, plant cover, plant biomass and soil fertility vary with grassland type on Qinghai-Tibetan Plateau. Agric. Ecosyst. Environ. 2019, 286, 106659. [Google Scholar] [CrossRef]
- Huang, C.; Peng, F.; You, Q.; Liao, J.; Duan, H.; Wang, T.; Xue, X. The Response of Plant and Soil Properties of Alpine Grassland to Long-Term Exclosure in the Northeastern Qinghai–Tibetan Plateau. Front. Environ. Sci. 2020, 8, 589104. [Google Scholar] [CrossRef]
- Shaver, G.R.; Melillo, J.M. Nutrient Budgets of Marsh Plants: Efficiency Concepts and Relation to Availability. Ecology 1984, 65, 1491–1510. [Google Scholar] [CrossRef]
- Koerselman, W.; Meuleman, A.F.M. The Vegetation N:P Ratio: A New Tool to Detect the Nature of Nutrient Limitation. J. Appl. Ecol. 1996, 33, 1441. [Google Scholar] [CrossRef]
- Pollock, M.M.; Naiman, R.J.; Hanley, T.A. Plant Species Richness in Riparian Wetlands—A Test of Biodiversity Theory. Ecology 1998, 79, 94. [Google Scholar] [CrossRef]
- Tilman, G.D. Plant Dominance Along an Experimental Nutrient Gradient. Ecology 1984, 65, 1445–1453. [Google Scholar] [CrossRef]
- Tilman, D. Secondary Succession and the Pattern of Plant Dominance Along Experimental Nitrogen Gradients. Ecol. Monogr. 1987, 57, 189–214. [Google Scholar] [CrossRef]
- Gough, L.; Osenberg, C.W.; Gross, K.L.; Collins, S.L. Fertilization effects on species density and primary productivity in herbaceous plant communities. Oikos 2000, 89, 428–439. [Google Scholar] [CrossRef]
- Gough, L.; Gross, K.L.; Cleland, E.; Clark, C.M.; Collins, S.L.; Fargione, J.E.; Pennings, S.C.; Suding, K. Incorporating clonal growth form clarifies the role of plant height in response to nitrogen addition. Oecologia 2012, 169, 1053–1062. [Google Scholar] [CrossRef]
- Laliberté, E.; Zemunik, G.; Turner, B.L. Environmental filtering explains variation in plant diversity along resource gradients. Science 2014, 345, 1602–1605. [Google Scholar] [CrossRef] [PubMed]
- Isermann, M. Soil pH and species diversity in coastal dunes. Plant Ecol. 2005, 178, 111–120. [Google Scholar] [CrossRef]
- Fu, B.; Liu, S.; Ma, K.; Zhu, Y. Relationships between soil characteristics, topography and plant diversity in a heterogeneous deciduous broad-leaved forest near Beijing, China. Plant Soil 2004, 261, 47–54. [Google Scholar] [CrossRef]
- Koceja, M.E.; Bledsoe, R.B.; Goodwillie, C.; Peralta, A.L. Nutrient enrichment increases soil bacterial diversity and decomposition rates of different litter types in a coastal plain wetland. bioRxiv 2019, 732883. [Google Scholar] [CrossRef]
- Dingaan, M.N.; Tsubo, M.; Walker, S.; Newby, T. Soil chemical properties and plant species diversity along a rainfall gradient in semi-arid grassland of South Africa. Plant Ecol. Evol. 2017, 150, 35–44. [Google Scholar] [CrossRef]
- Deng, L.; Wang, K.; Li, J.; Zhao, G.; Shangguan, Z. Effect of soil moisture and atmospheric humidity on both plant productivity and diversity of native grasslands across the Loess Plateau, China. Ecol. Eng. 2016, 94, 525–531. [Google Scholar] [CrossRef]
- Aerts, R. Interspecific competition in natural plant communities: Mechanisms, trade-offs and plant-soil feedbacks. J. Exp. Bot. 1999, 50, 29–37. [Google Scholar] [CrossRef]
- Aerts, R.; De Caluwe, H.; Beltman, B. Is the relation between nutrient supply and biodiversity co-determined by the type of nutrient limitation? Oikos 2003, 101, 489–498. [Google Scholar] [CrossRef]
- Bhandari, J.; Zhang, Y. Effect of altitude and soil properties on biomass and plant richness in the grasslands of Tibet, China, and Manang District, Nepal. Ecosphere 2019, 10, 02915. [Google Scholar] [CrossRef]
- Ji, C.-J.; Yang, Y.-H.; Han, W.-X.; He, Y.-F.; Smith, J.; Smith, P. Climatic and Edaphic Controls on Soil pH in Alpine Grasslands on the Tibetan Plateau, China: A Quantitative Analysis. Pedosphere 2014, 24, 39–44. [Google Scholar] [CrossRef]
- Davidson, E.A.; Janssens, I.A. Temperature sensitivity of soil carbon decomposition and feedbacks to climate change. Nat. Cell Biol. 2006, 440, 165–173. [Google Scholar] [CrossRef]
- Brady, N.C.; Weil, R.R. The nature and properties of soils. J. Range Manag. 2002, 5, 333. [Google Scholar]
- Robertson, G.; Groffman, P. Nitrogen Transformations. In Soil Microbiology, Ecology and Biochemistry; Elsevier: Amsterdam, The Netherlands, 2015; pp. 421–446. [Google Scholar]
- Güsewell, S.; Bailey, K.M.; Roem, W.J.; Bedford, B.L. Nutrient limitation and botanical diversity in wetlands: Can fertilisation raise species richness? Oikos 2005, 109, 71–80. [Google Scholar] [CrossRef]
- Neina, D. The Role of Soil pH in Plant Nutrition and Soil Remediation. Appl. Environ. Soil Sci. 2019, 2019, 1–9. [Google Scholar] [CrossRef]
- Taiz, L.; Zeiger, E. Plant Physiology; Sinauer Associates: Sunderland, UK, 1980. [Google Scholar]
- Laughlin, D.C.; Abella, S.R. Abiotic and biotic factors explain independent gradients of plant community composition in ponderosa pine forests. Ecol. Model. 2007, 205, 231–240. [Google Scholar] [CrossRef]
- Güsewell, S.; Koerselman, W.; Verhoeven, J.T.A. Biomass N:P Ratios as Indicators of Nutrient Limitation for Plant Populations in Wetlands. Ecol. Appl. 2003, 13, 372–384. [Google Scholar] [CrossRef]
- Tessier, J.T.; Raynal, D.J. Use of nitrogen to phosphorus ratios in plant tissue as an indicator of nutrient limitation and nitrogen saturation. J. Appl. Ecol. 2003, 40, 523–534. [Google Scholar] [CrossRef]
- Ågren, G.I. The C:N:P stoichiometry of autotrophs-theory and observations. Ecol. Lett. 2004, 7, 185–191. [Google Scholar] [CrossRef]
- Ladanai, S.; Ågren, G.I.; Olsson, B.A. Relationships between Tree and Soil Properties in Picea abies and Pinus sylvestris Forests in Sweden. Ecosystems 2010, 13, 302–316. [Google Scholar] [CrossRef]




| Variables | Aboveground Biomass | Vegetation Cover | R0 | H | J | D |
| Total nitrogen | 0.006 | −0.231 ** | 0.376 ** | 0.236 ** | −0.056 | 0.105 |
| Total phosphorus | −0.090 | −0.246 ** | 0.469 ** | 0.371 ** | −0.007 | 0.188 ** |
| Total potassium | −0.160 ** | −0.209 ** | 0.392 ** | 0.285 ** | −0.064 | 0.101 |
| N:P | 0.137 * | 0.013 | −0.249 ** | −0.279 ** | −0.064 | −0.164 ** |
| Soil organic carbon | 0.198 ** | 0.186 ** | −0.189 ** | −0.132 * | 0.087 | −0.052 |
| pH | −0.269 ** | 0.025 | −0.040 | −0.012 | −0.027 | 0.016 |
| soil bulk density | −0.121 * | −0.199 ** | 0.471 ** | 0.348 ** | −0.034 | 0.161 ** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, M.; Zhu, Y.; Wei, Y.; Zhao, N. Soil Nutrient and Vegetation Diversity Patterns of Alpine Wetlands on the Qinghai-Tibetan Plateau. Sustainability 2021, 13, 6221. https://doi.org/10.3390/su13116221
Ma M, Zhu Y, Wei Y, Zhao N. Soil Nutrient and Vegetation Diversity Patterns of Alpine Wetlands on the Qinghai-Tibetan Plateau. Sustainability. 2021; 13(11):6221. https://doi.org/10.3390/su13116221
Chicago/Turabian StyleMa, Muyuan, Yaojun Zhu, Yuanyun Wei, and Nana Zhao. 2021. "Soil Nutrient and Vegetation Diversity Patterns of Alpine Wetlands on the Qinghai-Tibetan Plateau" Sustainability 13, no. 11: 6221. https://doi.org/10.3390/su13116221
APA StyleMa, M., Zhu, Y., Wei, Y., & Zhao, N. (2021). Soil Nutrient and Vegetation Diversity Patterns of Alpine Wetlands on the Qinghai-Tibetan Plateau. Sustainability, 13(11), 6221. https://doi.org/10.3390/su13116221






