Assessment of Drinking Water Purification Plant Efficiency in Al-Hassa, Eastern Region of Saudi Arabia
Abstract
1. Introduction
2. Materials and Methods
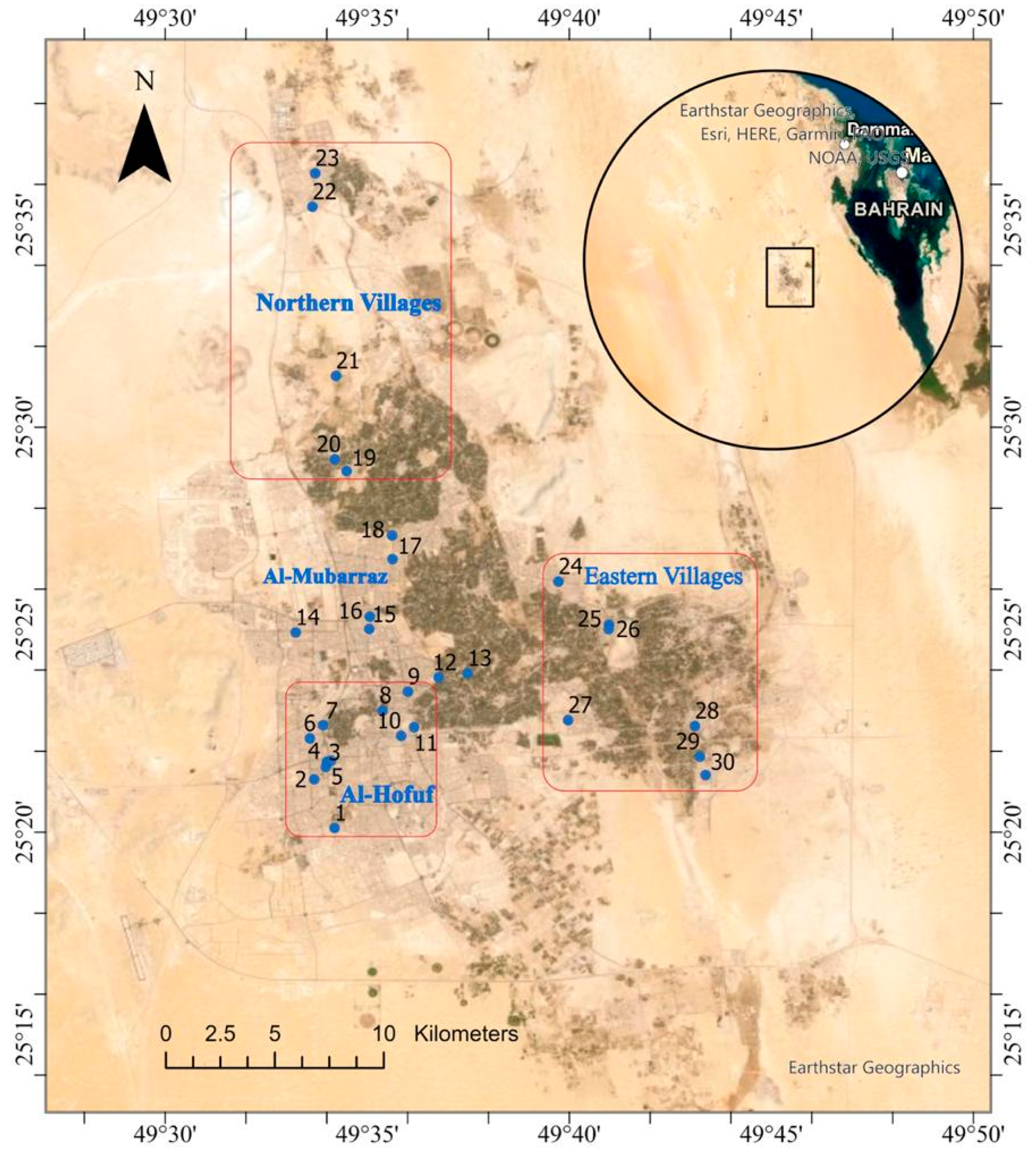
2.1. Study Area
2.2. Sample Collection
2.3. Water Analysis
2.4. Water Quality Index Computing
3. Results and Discussion
3.1. Groundwater Quality
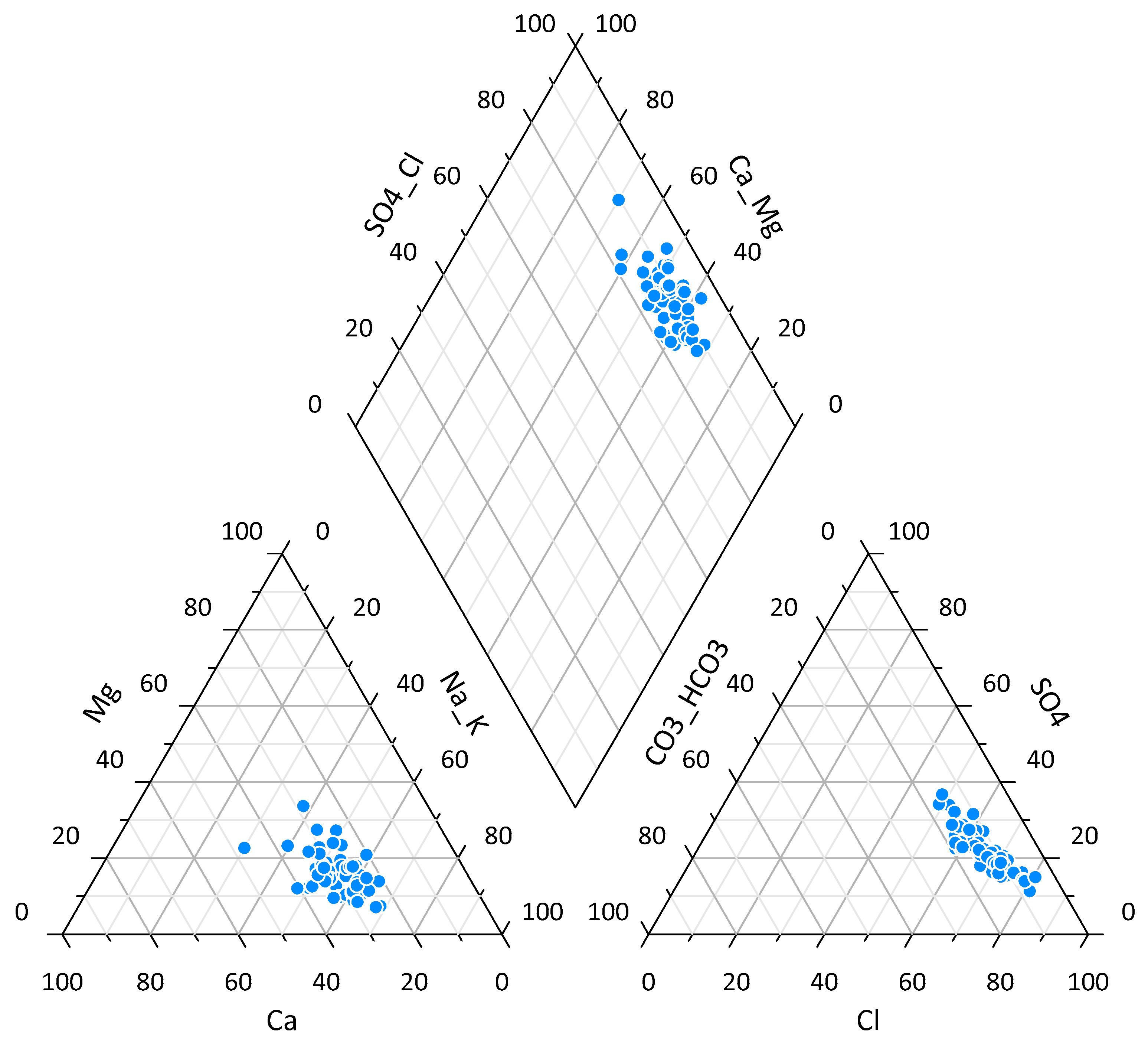
3.1.1. Groundwater Anions
3.1.2. Groundwater Cations
3.1.3. Overall Groundwater Quality
3.2. Purified Drinking Water Quality
3.2.1. Drinking Water Anions
3.2.2. Drinking Water Cations
3.2.3. Overall Drinking Water Quality
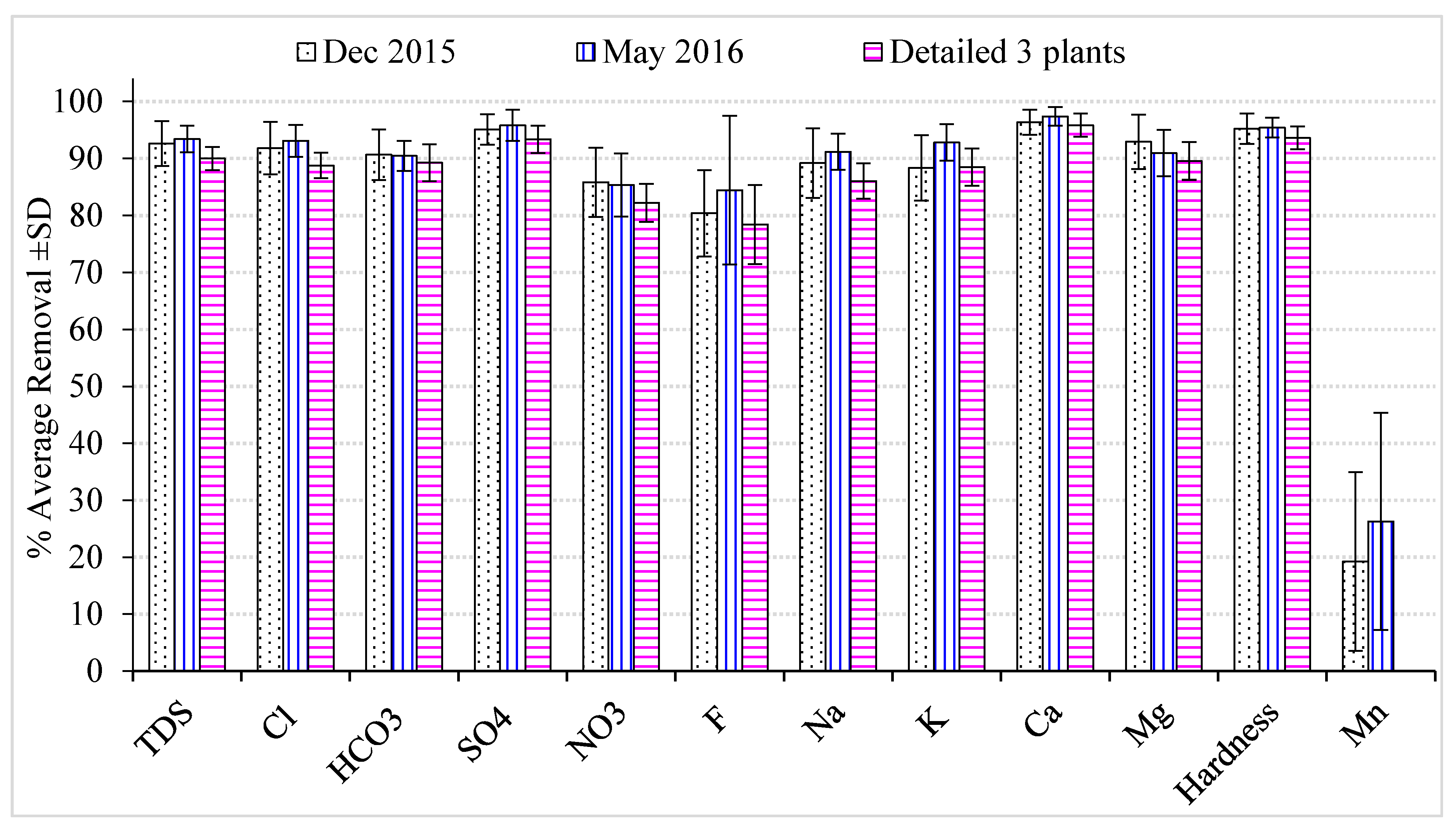
3.3. Water Purification Plants’ Efficiency
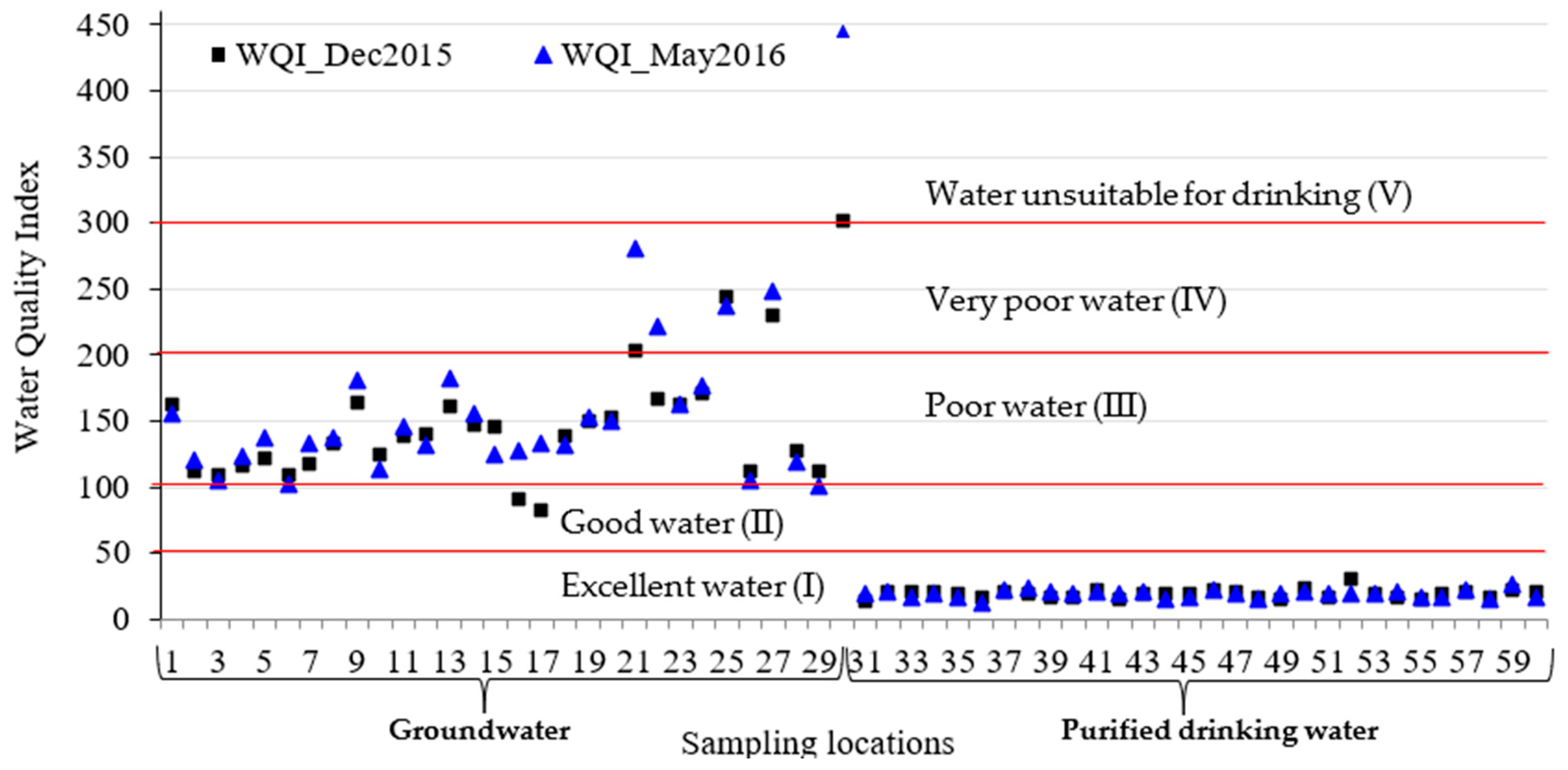
3.4. Water Quality Index
3.5. Correlation between Water Quality Variables
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rojas, L.V.; Santos, J.A.; Corcho-Alvarado, J.A.; Amaral, R.S.; Rollin, S.; Milan, M.O.; Fernandez, Z.H.; Francis, K.; Cavalcanti, M.; Santos, J.M.N. Quality and management status of the drinking water supplies in a semiarid region of Northeastern Brazil. J. Environ. Sci. Health Part A Toxic/Hazard. Subst. Environ. Eng. 2020, 55, 1247–1256. [Google Scholar] [CrossRef] [PubMed]
- Badr, E.A.; El-Sonbati, M.A.; Nassef, H.M. Water Quality Assessment in the Nile River, Damietta Branch, Egypt. Int. J. Environ. Sci. 2013, 8, 43–53. [Google Scholar] [CrossRef]
- Aly, A.A.; Al Omran, A.M.; Alharby, M.M. The water quality index and hydrochemical characterization of groundwater resources in Hafar Albatin, Saudi Arabia. Arab. J. Geosci. 2015, 8, 4177–4190. [Google Scholar] [CrossRef]
- Ghanim, A. Water-Resources-Crisis-in-Saudi-Arabia-Challenges-and-Possible-Management-Options-An-Analytic-Review. Int. J. Environ. Ecol. Eng. 2019, 13, 51–56. [Google Scholar]
- Al Omran, A.; Al Barakah, F.; Altuquq, A.; Aly, A.; Nadeem, M. Drinking water quality assessment and water quality index of Riyadh, Saudi Arabia. Water Qual. Res. J. Can. 2015, 50, 287–296. [Google Scholar] [CrossRef]
- Al-Naeem, A.A.; Al-Barrak, K.M. Assessment and evaluation of hydro-chemical and elemental analysis for Ain Al-Khadoud, Al-Hassa Oasis, Eastern Province, Saudi Arabia. J. Soil Sci. Agric. Eng. Mansoura Univ. 2010, 1, 815–826. [Google Scholar]
- Alghamdi, A.G.; Aly, A.A.; Aldhumri, S.A.; Al Barakaha, F.N. Hydrochemical and Quality Assessment of Groundwater Resources in Al-Madinah City, Western Saudi Arabia. Sustainability 2020, 12, 3106. [Google Scholar] [CrossRef]
- Al-Redhaiman, K.N.; Abdel Magid, H.M. The applicability of the local and international water quality guidelines to Al-Gassim region of central Saudi Arabia. Water Air Soil Pollut. 2002, 137, 235–246. [Google Scholar] [CrossRef]
- Alabdula’aly, A.I.; Al Rehaili, A.M.; Al Zarah, A.I.; Khan, M.A. Assessment of nitrate concentration in groundwater in Saudi Arabia. Environ. Monit. Assess. 2010, 161, 1–9. [Google Scholar] [CrossRef]
- Mohebbi, M.R.; Saeedi, R.; Montazeri, A.; Azam Vaghefi, K.; Labbafi, S.; Oktaie, S.; Abtahi, M.; Mohagheghian, A. Assessment of water quality in groundwater resources of Iran using a modified drinking water quality index (DWQI). Ecol. Indic. 2013, 30, 28–34. [Google Scholar] [CrossRef]
- Badr, E.S.A.; Al Naeem, A.A. Potable Water Quality Assessment in Al-Hassa, Eastern Region of Saudi Arabia. Fresenius Environ. Bull. 2016, 25, 4118–4129. [Google Scholar]
- Perez-Gonzalez, A.; Urtiaga, A.M.; Ibanez, R.; Ortiz, I. State of the art and review on the treatment technologies of water reverse osmosis concentrates. Water Res. 2012, 46, 267–283. [Google Scholar] [CrossRef] [PubMed]
- SASO. Bottled and Unbottled Drinking Water; Standard No. 409; SASO (Saudi Arabian Standards Organization): Riyadh, Saudi Arabia, 2009.
- WHO. Guidelines for Drinking Water Quality, 4th ed.; World Health Organization: Geneva, Switzerland, 2011. [Google Scholar]
- Massoud, M.A.; Al-Dakheel, Y.Y.; Hussein, A.H.; El-Mahmoudi, A.S. Spatial Decision Support System for Drinking Water Quality Monitoring and Evaluation in Al-Hassa. Int. J. Water Resour. Arid Environ. 2011, 1, 457–468. [Google Scholar]
- Ma, J.Z.; Ding, Z.Y.; Wei, G.X.; Zhao, H.; Huang, T.M. Sources of water pollution and evolution of water quality in the Wuwei basin of Shiyang river, Northwest China. J. Environ. Manag. 2009, 90, 1168–1177. [Google Scholar] [CrossRef] [PubMed]
- Zaidi, F.K.; Mogren, S.; Mukhopadhyay, M.; Ibrahim, E. Evaluation of groundwater chemistry and its impact on drinking and irrigation water quality in the eastern part of the Central Arabian graben and trough system, Saudi Arabia. J. Afr. Earth Sci. 2016, 120, 208–219. [Google Scholar] [CrossRef]
- Ikem, A.; Osibanjo, O.; Sridhar, M.K.C.; Sobande, A. Evaluation of groundwater quality characteristics near two waste sites in Ibadan and Lagos, Nigeria. Water Air Soil Pollut. 2002, 140, 307–333. [Google Scholar] [CrossRef]
- Soltan, M.E. Evaluation of ground water quality in Dakhla Oasis (Egyptian Western Desert). Environ. Monit. Assess. 1999, 57, 157–168. [Google Scholar] [CrossRef]
- Al-Naeem, A.A. Monitoring of Groundwater Salinity for Water Resources Management in Irrigated Areas of Al-Jouf Region, Saudi Arabia. Res. J. Environ. Sci. 2015, 9, 256–269. [Google Scholar] [CrossRef][Green Version]
- Al-Otaibi, E.L.; Zaki, M.S. Physico-chemical Quality of Drinking Water at Mushait, Aseer, South-Western, Saudi Arabia. Afr. J. Clin. Exp. Microbiol. 2009, 10, 117–127. [Google Scholar] [CrossRef]
- Al-Zarah, A.I. Elemental Composition of Groundwater and Spring Waters in Al-Ahsa Oasis, Eastern Region Saudi Arabia. Trends Appl. Sci. Res. 2011, 6, 1–18. [Google Scholar] [CrossRef][Green Version]
- Karavoltsos, S.; Sakellari, A.; Mihopoulos, N.; Dassenakis, M.; Scoullos, M.J. Evaluation of the quality of drinking water in regions of Greece. Desalination 2008, 224, 317–329. [Google Scholar] [CrossRef]
- Gupta, A.K.; Gupta, S.K.; Patil, R.S. A comparison of water quality indices for coastal water. J. Environ. Sci. Health Part A-Toxic/Hazard. Subst. Environ. Eng. 2003, 38, 2711–2725. [Google Scholar] [CrossRef]
- Lateef, K.H. Evaluation of groundwater quality for drinking purpose for tikrit and samarra cities using water quality index. Eur. J. Sci. Res. 2011, 58, 472–481. [Google Scholar]
- Rahaman, M.F.; Ali, M.S.; Arefin, R.; Mazumder, Q.H.; Majumder, R.K.; Jahan, C.S. Assessment of drinking water quality characteristics and quality index of Rajshahi city, Bangladesh. Environ. Dev. Sustain. 2020, 22, 3957–3971. [Google Scholar] [CrossRef]
- Saeedi, M.; Abessi, O.; Sharifi, F.; Meraji, H. Development of groundwater quality index. Environ. Monit. Assess. 2010, 163, 327–335. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, J.; Wong, L.P.; Chua, Y.P.; Channa, N. Drinking Water Quality Mapping Using Water Quality Index and Geospatial Analysis in Primary Schools of Pakistan. Water 2020, 12, 3382. [Google Scholar] [CrossRef]
- Stambuk-Giljanovic, N. Water quality evaluation by index in Dalmatia. Water Res. 1999, 33, 3423–3440. [Google Scholar] [CrossRef]
- Almuhanna, E.A. Atmospheric aerosol characterization and element composition at Al-Ahsa Oasis of Saudi Arabia. Sci. J. King Faisal Univ. Basic Appl. Sci. 2017, 18, 35–47. [Google Scholar]
- Al Omran, A.M.; El Maghraby, S.E.; Aly, A.A.; Al Wabel, M.I.; Al Asmari, Z.A.; Nadeem, M.E. Quality assessment of various bottled waters marketed in Saudi Arabia. Environ. Monit. Assess. 2013, 185, 6397–6406. [Google Scholar] [CrossRef]
- APHA. Standard Methods for the Examination of Waters and Wastewaters, 20th ed.; American Public Health Association (APHA): Washington, DC, USA, 2005. [Google Scholar]
- Adams, V.D. Water & Wastewater Examination Manual; Lewis Publishers: Chelsea, MI, USA, 1990. [Google Scholar]
- Hanrahan, G.; Gardolinski, P.; Gledhill, M.; Worsfold, P. Environmental monitoring of nutrients. In Environmental Monitoring Handbook; Burden, F.R., Mckelvie, I.D., Forstner, U., Guenther, A., Eds.; McGraw-Hill: New York, NY, USA, 2002; pp. 8.1–8.16. [Google Scholar]
- Simoes, F.d.S.; Moreira, A.B.; Bisinoti, M.C.; Gimenez, S.M.N.; Yabe, M.J.S. Water quality index as a simple indicator of aquaculture effects on aquatic bodies. Ecol. Indic. 2008, 8, 476–484. [Google Scholar] [CrossRef]
- Yidana, S.M.; Yidana, A. Assessing water quality using water quality index and multivariate analysis. Environ. Earth Sci. 2010, 59, 1461–1473. [Google Scholar] [CrossRef]
- Sanchez, E.; Colmenarejo, M.F.; Vicente, J.; Rubio, A.; Garcia, M.G.; Travieso, L.; Borja, R. Use of the water quality index and dissolved oxygen deficit as simple indicators of watersheds pollution. Ecol. Indic. 2007, 7, 315–328. [Google Scholar] [CrossRef]
- Ramakrishnaiah, C.R.; Sadashivaiah, C.; Ranganna, G. Assessment of Water Quality Index for the Groundwater in Tumkur Taluk, Karnataka State, India. E-J. Chem. 2009, 6, 523–530. [Google Scholar] [CrossRef]
- Sadiq, M.; Hussain, G. Drinking water quality in Saudi Arabia—An overview. Arab. J. Sci. Eng. 1997, 22, 153–164. [Google Scholar]
- Al-Ghanim, K.A.; Abd El-Salam, M.M.; Mahboob, S. Assessment of Water Quality for Some Roof Tanks in Alkharj Governorate, KSA. Pak. J. Zool. 2014, 46, 1003–1012. [Google Scholar]
- Nouri, D.A.; Abdulkarim, B.A.; Arzoo, S.; Bakeet, Z.A.N. Quality Characteristics of Commonly Consumed Drinking Water in Riyadh and Effect of Domestic Treatments on Its Chemical Constituents. J. Food Nutr. Res. 2014, 2, 25–33. [Google Scholar]
- Alabdulaaly, A.I. Nitrate concentrations in Riyadh, Saudi Arabia drinking water supplies. Environ. Monit. Assess. 1997, 47, 315–324. [Google Scholar] [CrossRef]
- Piper, A.M. A graphic procedure in the geochemical interpretation of water-analyses. Trans. Am. Geophys. Union 1944, 25, 914–923. [Google Scholar] [CrossRef]
| Parameters | Weight (wi) | Relative Weight (Wi) | WHO (2011) and SASO (2009) Standards |
|---|---|---|---|
| pH | 3 | 0.061 | 6.5–8.5 |
| TDS (mg/L) | 5 | 0.102 | 500 |
| Turbidity (NTU) | 3 | 0.061 | 5 |
| Free Cl2 (mg/L) | 2 | 0.041 | 0.2–0.5 |
| Total Hardness (mg/L) | 3 | 0.061 | 500 |
| Chloride (mg/L) | 4 | 0.082 | 250 |
| Bicarbonate (mg/L) | 2 | 0.041 | 125 |
| Sulphate (mg/L) | 3 | 0.061 | 250 |
| Nitrate (mg/L as N) | 4 | 0.082 | 10 |
| Fluoride (mg/L) | 3 | 0.061 | 1.5 |
| Sodium (mg/L) | 4 | 0.082 | 200 |
| Potassium (mg/L) | 3 | 0.061 | 12 |
| Calcium (mg/L) | 3 | 0.061 | 100 |
| Magnesium (mg/L) | 3 | 0.061 | 50 |
| Iron (µg/L) | 2 | 0.041 | 300 |
| Manganese (µg/L) | 2 | 0.041 | 100 |
| 49 | 1 |
| Water Class | Type of Water | WQI Value Range |
|---|---|---|
| I | Excellent water | <50 |
| II | Good water | 50–100 |
| III | Poor water | 100–200 |
| IV | Very poor water | 200–300 |
| V | Water unsuitable for drinking | >300 |
| Parameters | Groundwater Quality, All Data (n = 60) | December 2015 (n = 30) | May 2016 (n = 30) | |||
|---|---|---|---|---|---|---|
| Average ± SD | Min | Max | ANOVA F | Average ± SD | Average ± SD | |
| Temperature | 24.3 ± 4.39 | 19.5 | 33.5 | 4.06 | 21.0 ± 2.17 | 27.7 ± 3.35 |
| pH | 7.15 ± 0.17 | 6.77 | 7.69 | 0.91 | 7.22 ± 0.19 | 7.08 ± 0.11 |
| TDS (mg/L) | 1916 ± 806 | 850 | 5513 | 6.63 | 1868 ± 706 | 1965 ± 905 |
| EC (µS/cm) | 3191 ± 1341 | 1416 | 9173 | 6.74 | 3110 ± 1170 | 3273 ± 1508 |
| Turbidity | 0.288 ± 0.14 | 0.100 | 0.745 | 0.94 | 0.263 ± 0.16 | 0.312 ± 0.11 |
| Free Cl2 (mg/L) | 0.083 ± 0.03 | 0.030 | 0.160 | 0.45 | 0.078 ± 0.03 | 0.089 ± 0.02 |
| Cl (mg/L) | 675 ± 370 | 253 | 2357 | 6.55 | 644 ± 317 | 707 ± 419 |
| HCO3 (mg/L) | 191 ± 20.0 | 130 | 228 | 4.98 | 185 ± 22.9 | 198 ± 14.6 |
| SO4 (mg/L) | 276 ± 70.4 | 167 | 596 | 4.00 | 276 ± 57.8 | 276 ± 82.2 |
| NO3 (mg N/L) | 6.30 ± 2.27 | 0.635 | 14.6 | 1.10 | 6.24 ± 2.24 | 6.35 ± 2.33 |
| F (mg/L) | 0.999 ± 0.28 | 0.538 | 1.94 | 1.06 | 1.03 ± 0.26 | 0.973 ± 0.29 |
| Na (mg/L) | 323 ± 132 | 117 | 988 | 4.30 | 291 ± 101 | 356 ± 151 |
| K (mg/L) | 50.0 ± 30.7 | 16.1 | 217 | 2.67 | 39.8 ± 16.1 | 60.1 ± 38.0 |
| Ca (mg/L) | 158 ± 68.9 | 82.5 | 459 | 10.33 | 172 ± 74.9 | 143 ± 60.0 |
| Mg (mg/L) | 52.7 ± 24.9 | 19.3 | 141 | 3.77 | 59.9 ± 19.0 | 45.5 ± 28.2 |
| Hardness (mg/L) | 611 ± 254 | 344 | 1503 | 8.83 | 677 ± 232 | 545 ± 261 |
| Fe (µg/L) | 114 ± 92.2 | 26.2 | 311 | 2.78 | 82.3 ± 68.0 | 146 ± 103 |
| Mn (µg/L) | 35.9 ± 18.9 | 6.49 | 89.9 | 0.29 | 34.0 ± 18.3 | 37.8 ± 19.6 |
| WQI | 155 ± 59.2 | 82.3 | 445 | 6.01 | 148 ± 46.8 | 161 ± 69.7 |
| Parameters | Purified Water Quality, All Data (n = 60) | December 2015 (n = 30) | May 2016 (n = 30) | |||
|---|---|---|---|---|---|---|
| Average ± SD | Min | Max | ANOVA F | Average ± SD | Average ± SD | |
| Temperature | 24.1 ± 4.27 | 19.4 | 33.4 | 3.13 | 20.8 ± 1.75 | 27.5 ± 3.26 |
| pH | 7.54 ± 0.27 | 6.74 | 8.08 | 1.14 | 7.46 ± 0.30 | 7.61 ± 0.21 |
| TDS (mg/L) | 118 ± 32.9 | 50.6 | 265 | 1.43 | 122 ± 40.8 | 115 ± 22.7 |
| EC (µS/cm) | 197 ± 54.8 | 82.8 | 442 | 1.41 | 203 ± 68.1 | 191 ± 37.4 |
| Turbidity | 0.239 ± 0.13 | 0.100 | 0.783 | 0.31 | 0.247 ± 0.16 | 0.230 ± 0.08 |
| Free Cl2 (mg/L) | 0.106 ± 0.11 | 0.040 | 0.840 | 1.73 | 0.090 ± 0.08 | 0.122 ± 0.14 |
| Cl (mg/L) | 42.6 ± 14.8 | 17.4 | 130 | 2.30 | 44.3 ± 18.8 | 40.9 ± 9.30 |
| HCO3 (mg/L) | 17.9 ± 6.61 | 6.83 | 33.4 | 4.32 | 16.9 ± 7.56 | 18.9 ± 5.46 |
| SO4 (mg/L) | 11.8 ± 6.70 | 2.33 | 29.6 | 5.05 | 13.2 ± 7.28 | 10.5 ± 5.88 |
| NO3 (mg N/L) | 0.881 ± 0.43 | 0.173 | 1.96 | 1.70 | 0.848 ± 0.42 | 0.914 ± 0.45 |
| F (mg/L) | 0.177 ± 0.12 | 0.036 | 0.579 | 1.85 | 0.197 ± 0.08 | 0.156 ± 0.14 |
| Na (mg/L) | 28.3 ± 9.99 | 8.33 | 83.6 | 2.81 | 28.1 ± 12.5 | 28.6 ± 6.84 |
| K (mg/L) | 3.72 ± 1.08 | 1.60 | 9.82 | 2.42 | 3.99 ± 1.23 | 3.45 ± 0.84 |
| Ca (mg/L) | 4.35 ± 2.31 | 1.78 | 15.1 | 0.84 | 5.41 ± 2.54 | 3.29 ± 1.44 |
| Mg (mg/L) | 3.46 ± 1.41 | 1.02 | 9.02 | 4.58 | 3.67 ± 1.84 | 3.25 ± 0.75 |
| Hardness(mg/L) | 25.1 ± 8.87 | 13.6 | 54.8 | 3.16 | 28.6 ± 10.9 | 21.6 ± 3.92 |
| Fe (µg/l) | 75.7 ± 43.3 | 20.5 | 187 | 1.19 | 66.2 ± 39.0 | 85.3 ± 45.9 |
| Mn (µg/l) | 27.7 ± 16.1 | 5.13 | 79.3 | 0.33 | 27.5 ± 13.7 | 27.9 ± 18.5 |
| WQI | 18.9 ± 2.78 | 12.7 | 30.9 | 0.63 | 19.1 ± 3.06 | 18.8 ± 2.51 |
| pH | TDS | TUR | Cl2 | Cl | HCO3 | SO4 | NO3 | F | Na | K | Ca | Mg | Fe | Mn | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| pH | 1.00 | ||||||||||||||
| TDS | −0.33 | 1.00 | |||||||||||||
| TUR | 0.14 | 0.18 | 1.00 | ||||||||||||
| Cl2 | 0.04 | −0.12 | −0.10 | 1.00 | |||||||||||
| Cl | −0.28 | 0.99 | 0.17 | −0.11 | 1.00 | ||||||||||
| HCO3 | −0.41 | 0.89 | 0.19 | −0.15 | 0.82 | 1.00 | |||||||||
| SO4 | −0.45 | 0.87 | 0.09 | −0.15 | 0.79 | 0.92 | 1.00 | ||||||||
| NO3 | −0.33 | 0.84 | 0.11 | −0.12 | 0.80 | 0.83 | 0.82 | 1.00 | |||||||
| F | −0.46 | 0.70 | 0.17 | −0.14 | 0.60 | 0.85 | 0.85 | 0.54 | 1.00 | ||||||
| Na | −0.32 | 0.99 | 0.19 | −0.12 | 0.98 | 0.88 | 0.85 | 0.84 | 0.68 | 1.00 | |||||
| K | −0.29 | 0.94 | 0.13 | −0.10 | 0.94 | 0.78 | 0.81 | 0.80 | 0.56 | 0.95 | 1.00 | ||||
| Ca | −0.36 | 0.96 | 0.14 | −0.13 | 0.93 | 0.89 | 0.90 | 0.82 | 0.74 | 0.92 | 0.85 | 1.00 | |||
| Mg | −0.34 | 0.92 | 0.11 | −0.14 | 0.89 | 0.84 | 0.87 | 0.77 | 0.75 | 0.88 | 0.83 | 0.91 | 1.00 | ||
| Fe | −0.26 | 0.19 | 0.08 | 0.07 | 0.18 | 0.28 | 0.23 | 0.26 | 0.19 | 0.23 | 0.17 | 0.20 | 0.08 | 1.00 | |
| Mn | −0.14 | 0.26 | 0.05 | −0.05 | 0.25 | 0.24 | 0.23 | 0.27 | 0.15 | 0.26 | 0.30 | 0.24 | 0.19 | 0.12 | 1.00 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Badr, E.-S.A.; Al-Naeem, A.A. Assessment of Drinking Water Purification Plant Efficiency in Al-Hassa, Eastern Region of Saudi Arabia. Sustainability 2021, 13, 6122. https://doi.org/10.3390/su13116122
Badr E-SA, Al-Naeem AA. Assessment of Drinking Water Purification Plant Efficiency in Al-Hassa, Eastern Region of Saudi Arabia. Sustainability. 2021; 13(11):6122. https://doi.org/10.3390/su13116122
Chicago/Turabian StyleBadr, El-Sayed A., and Ahmed A. Al-Naeem. 2021. "Assessment of Drinking Water Purification Plant Efficiency in Al-Hassa, Eastern Region of Saudi Arabia" Sustainability 13, no. 11: 6122. https://doi.org/10.3390/su13116122
APA StyleBadr, E.-S. A., & Al-Naeem, A. A. (2021). Assessment of Drinking Water Purification Plant Efficiency in Al-Hassa, Eastern Region of Saudi Arabia. Sustainability, 13(11), 6122. https://doi.org/10.3390/su13116122






