A New Approach to Inform Restoration and Management Decisions for Sustainable Apiculture
Abstract
1. Introduction: What Is the Problem We Are Trying to Solve?
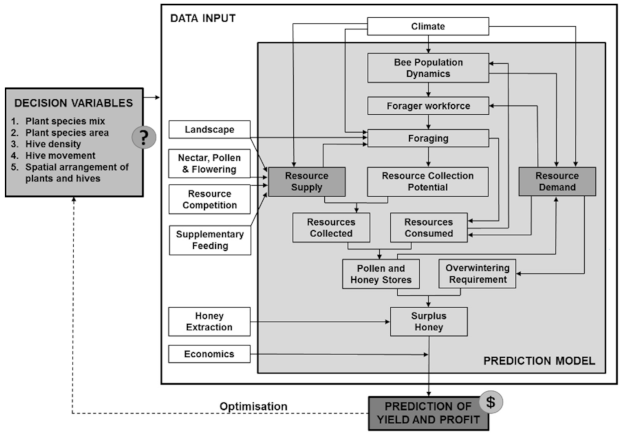
2. Overview: What Is Our Proposed Solution?
3. Decision Variables: What Kind of Decisions Will This Approach Help with and Why Are They Important?
3.1. Plant Species Mix
3.2. Plant Species Area and Hive Density
3.3. Hive Movement and Spatial Arrangement of Plants and Hives
4. The Model: What Do We Need the Model to Do?
5. Existing Models: Do Existing Models Already Do What We Need This Model to Do?
6. Input Data: What Data Will We Need and How Can We Get It?
6.1. The Swan Coastal Plain Case Study Area: What Is It and Why Is It Relevant?
6.2. Landscape Data
6.3. Nectar, Pollen, and Flowering Data
6.4. Resource Competition Data
6.5. Climate Data
6.6. Economic Data
6.7. Other Data
7. Optimisation: How Do We Determine the Best Design or Decision?
8. Additional Considerations: What Else Do We Need to Consider?
9. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- FAO. Value of Agricultural Production. FAOSTAT. Available online: http://www.fao.org/faostat/en/#data/QV (accessed on 7 June 2019).
- Clarke, M. Bushfire Recovery Plan: Understanding What Needs to Be Done to Ensure the Honey Bee and Pollination Industry Recovers from the 2019-20 Bushfire Crisis; AgriFutures Australia: Wagga Wagga, NSW, Australia, 2020; p. 37. [Google Scholar]
- Crane, E. When important honey plants are invasive weeds. Bee World 1981, 62, 28–30. [Google Scholar] [CrossRef]
- Smart, M.D.; Pettis, J.S.; Euliss, N.; Spivak, M.S. Land use in the Northern Great Plains region of the US influences the survival and productivity of honey bee colonies. Agric. Ecosyst. Environ. 2016, 230, 139–149. [Google Scholar] [CrossRef]
- Crenna, E.; Sala, S.; Polce, C.; Collina, E. Pollinators in life cycle assessment: Towards a framework for impact assessment. J. Clean. Prod. 2017, 140, 525–536. [Google Scholar] [CrossRef]
- Isaacs, R.; Williams, N.; Ellis, J.; Pitts-Singer, T.L.; Bommarco, R.; Vaughan, M. Integrated Crop Pollination: Combining strategies to ensure stable and sustainable yields of pollination-dependent crops. Basic Appl. Ecol. 2017, 22, 44–60. [Google Scholar] [CrossRef]
- Kovacs-Hostyanszki, A.; Espindola, A.; Vanbergen, A.J.; Settele, J.; Kremen, C.; Dicks, L.V. Ecological intensification to mitigate impacts of conventional intensive land use on pollinators and pollination. Ecol. Lett. 2017, 20, 673–689. [Google Scholar] [CrossRef] [PubMed]
- Aizen, M.A.; Harder, L.D. The global stock of domesticated honey bees is growing slower than agricultural demand for pollination. Curr. Biol. 2009, 19, 915–918. [Google Scholar] [CrossRef] [PubMed]
- Panța, N.D. A review of farm sustainability assessment methods: Are they applicable to the beekeeping sector? In Proceedings of the International Management Conference, Bucharest, Romania, 1–2 November 2018; Popa, I., Dobrin, C., Ciocoiu, C.N., Eds.; Faculty of Management, Academy of Economic Studies: Bucharest, Romania, 2018; Volume 12, pp. 48–55. [Google Scholar]
- Breeze, T.D.; Vaissiere, B.E.; Bommarco, R.; Petanidou, T.; Seraphides, N.; Kozak, L.; Scheper, J.; Biesmeijer, J.C.; Kleijn, D.; Gyldenkaerne, S.; et al. Agricultural policies exacerbate honeybee pollination service supply-demand mismatches across Europe. PLoS ONE 2014, 9, e91459. [Google Scholar] [CrossRef]
- Oldroyd, B.P. What’s killing American honey Bees? PLoS Biol. 2007, 5, 1195–1199. [Google Scholar] [CrossRef]
- Neumann, P.; Carreck, N.L. Honey bee colony losses. J. Apic. Res. 2010, 49, 1–6. [Google Scholar] [CrossRef]
- National Research Council. Status of Pollinators in North America; The National Academies Press: Washington, DC, USA, 2007. [Google Scholar] [CrossRef]
- Van Engelsdorp, D.; Hayes, J.J.; Underwood, R.M.; Pettis, J. A survey of honey bee colony losses in the U.S., fall 2007 to spring 2008. PLoS ONE 2008, 3, e4071. [Google Scholar] [CrossRef]
- Smith, K.M.; Loh, E.H.; Rostal, M.K.; Zambrana-Torrelio, C.M.; Mendiola, L.; Daszak, P. Pathogens, pests, and economics: Drivers of honey bee colony declines and losses. EcoHealth 2013, 10, 434–445. [Google Scholar] [CrossRef] [PubMed]
- Potts, S.G.; Biesmeijer, J.C.; Kremen, C.; Neumann, P.; Schweiger, O.; Kunin, W.E. Global pollinator declines: Trends, impacts and drivers. Trends Ecol. Evol. 2010, 25, 345–353. [Google Scholar] [CrossRef] [PubMed]
- Vanbergen, A.J.; Baude, M.; Biesmeijer, J.C.; Britton, N.F.; Brown, M.J.F.; Brown, M.; Bryden, J.; Budge, G.E.; Bull, J.C.; Carvell, C.; et al. Threats to an ecosystem service: Pressures on pollinators. Front. Ecol. Environ. 2013, 11, 251–259. [Google Scholar] [CrossRef]
- Goulson, D.; Nicholls, E.; Botias, C.; Rotheray, E.L. Bee declines driven by combined stress from parasites, pesticides, and lack of flowers. Science 2015, 347, 10. [Google Scholar] [CrossRef] [PubMed]
- Brown, M.J.F.; Paxton, R.J. The conservation of bees: A global perspective. Apidologie 2009, 40, 410–416. [Google Scholar] [CrossRef]
- Wright, G.A.; Nicolson, S.W.; Shafir, S. Nutritional physiology and ecology of honey bees. Annu. Rev. Entomol. 2018, 63, 327–344. [Google Scholar] [CrossRef] [PubMed]
- Baude, M.; Kunin, W.E.; Boatman, N.D.; Conyers, S.; Davies, N.; Gillespie, M.A.K.; Morton, R.D.; Smart, S.M.; Memmott, J. Historical nectar assessment reveals the fall and rise of floral resources in Britain. Nature 2016, 530, 85–88. [Google Scholar] [CrossRef]
- Rosenheim, J.A.; Nonacs, P.; Mangel, M. Sex ratios and multifaceted parental investment. Am. Nat. 1996, 148, 501–535. [Google Scholar] [CrossRef]
- Roulston, T.H.; Goodell, K. The role of resources and risks in regulating wild bee populations. Annu. Rev. Entomol. 2011, 56, 293–312. [Google Scholar] [CrossRef]
- Requier, F.; Odoux, J.F.; Henry, M.; Bretagnolle, V. The carry-over effects of pollen shortage decrease the survival of honeybee colonies in farmlands. J. Appl. Ecol. 2017, 54, 1161–1170. [Google Scholar] [CrossRef]
- Otis, G.W. Comments about colony collapse disorder. Am. Bee J. 2007, 147, 1033–1035. [Google Scholar]
- Van Engelsdorp, D.; Underwood, R.; Caron, D.; Hayes, J., Jr. An estimate of managed colony losses in the winter of 2006–2007: A report commissioned by the apiary inspectors of America. Am. Bee J. 2007, 147, 599–603. [Google Scholar]
- Naug, D. Nutritional stress due to habitat loss may explain recent honeybee colony collapses. Biol. Conserv. 2009, 142, 2369–2372. [Google Scholar] [CrossRef]
- Smart, M.D.; Pettis, J.S.; Rice, N.; Browning, Z.; Spivak, M. Linking measures of colony and individual honey bee health to survival among apiaries exposed to varying agricultural land use. PLoS ONE 2016, 11, 28. [Google Scholar] [CrossRef]
- Farrar, C.L. The influence of colony populations on honey production. J. Agric. Res. 1937, 54, 945–954. [Google Scholar]
- Harbo, J.R. Worker-bee crowding affects brood production, honey production, and longevity of honey-bees (Hymenoptera, Apidae). J. Econ. Entomol. 1993, 86, 1672–1678. [Google Scholar] [CrossRef]
- Eckert, J.E. The flight range of the honeybee. J. Agric. Res. 1933, 47, 257–285. [Google Scholar]
- Visscher, P.K.; Seeley, T.D. Foraging strategy of honeybee colonies in a temperate deciduous forest. Ecology 1982, 63, 1790–1801. [Google Scholar] [CrossRef]
- Dukas, R.; Edelstein-Keshet, L. The spatial distribution of colonial food provisioners. J. Theor. Biol. 1998, 190, 121–134. [Google Scholar] [CrossRef]
- Moritz, R.F.A.; Southwick, E.E. Bees as Superorganisms; Springer: Berlin/Heidelberg, Germany, 1992; p. 395. [Google Scholar]
- Tomlinson, S.; Dixon, K.W.; Didham, R.K.; Bradshaw, S.D. Landscape context alters cost of living in honeybee metabolism and feeding. Proc. Biol. Sci. 2017, 284. [Google Scholar] [CrossRef]
- Al-Ghamdi, A.; Adgaba, N.; Getachew, A.; Tadesse, Y. New approach for determination of an optimum honeybee colony’s carrying capacity based on productivity and nectar secretion potential of bee forage species. Saudi J. Biol. Sci. 2016, 23, 92–100. [Google Scholar] [CrossRef] [PubMed]
- Otto, C.R.V.; Zheng, H.; Gallant, A.L.; Iovanna, R.; Carlson, B.L.; Smart, M.D.; Hyberg, S. Past role and future outlook of the Conservation Reserve Program for supporting honey bees in the Great Plains. Proc. Natl. Acad. Sci. USA 2018, 115, 7629–7634. [Google Scholar] [CrossRef] [PubMed]
- Cane, J.H.; Tepedino, V.J. Gauging the effect of honey bee pollen collection on native bee communities. Conserv. Lett. 2017, 10, 205–210. [Google Scholar] [CrossRef]
- Manning, R. Honey Production Economic Value and Geographical Significance of Apiary Sites in Western Australia: Final Report; Western Australian Department of Agriculture: South Perth, WA, Australia, 1992; p. 132. [Google Scholar]
- Somerville, D. Forestry Plantations and Honeybees; RIRDC: Barton, ACT, Australia, 2010; p. 29. [Google Scholar]
- FAO. Live Animals. FAOSTAT. Available online: http://www.fao.org/faostat/en/#data/QA (accessed on 7 June 2019).
- Ricketts, T.H.; Regetz, J.; Steffan-Dewenter, I.; Cunningham, S.A.; Kremen, C.; Bogdanski, A.; Gemmill-Herren, B.; Greenleaf, S.S.; Klein, A.M.; Mayfield, M.M.; et al. Landscape effects on crop pollination services: Are there general patterns? Ecol. Lett. 2008, 11, 499–515. [Google Scholar] [CrossRef] [PubMed]
- Vaudo, A.D.; Tooker, J.F.; Grozinger, C.M.; Patch, H.M. Bee nutrition and floral resource restoration. Curr. Opin. Insect Sci. 2015, 10, 133–141. [Google Scholar] [CrossRef]
- Potts, S.G.; Imperatriz-Fonseca, V.; Ngo, H.T.; Aizen, M.A.; Biesmeijer, J.C.; Breeze, T.D.; Dicks, L.V.; Garibaldi, L.A.; Hill, R.; Settele, J.; et al. Safeguarding pollinators and their values to human well-being. Nature 2016, 540, 220–229. [Google Scholar] [CrossRef]
- Wratten, S.D.; Gillespie, M.; Decourtye, A.; Mader, E.; Desneux, N. Pollinator habitat enhancement: Benefits to other ecosystem services. Agric. Ecosyst. Environ. 2012, 159, 112–122. [Google Scholar] [CrossRef]
- Potts, S.G.; Vulliamy, B.; Dafni, A.; Ne’eman, G.; Willmer, P. Linking bees and flowers: How do floral communities structure pollinator communities? Ecology 2003, 84, 2628–2642. [Google Scholar] [CrossRef]
- Brosi, B.J.; Armsworth, P.R.; Daily, G.C. Optimal design of agricultural landscapes for pollination services. Conserv. Lett. 2008, 1, 27–36. [Google Scholar] [CrossRef]
- Haaland, C.; Naisbit, R.E.; Bersier, L.F. Sown wildflower strips for insect conservation: A review. Insect. Conserv. Divers. 2011, 4, 60–80. [Google Scholar] [CrossRef]
- Menz, M.H.M.; Phillips, R.D.; Winfree, R.; Kremen, C.; Aizen, M.A.; Johnson, S.D.; Dixon, K.W. Reconnecting plants and pollinators: Challenges in the restoration of pollination mutualisms. Trends Plant. Sci. 2011, 16, 4–12. [Google Scholar] [CrossRef]
- Burkle, L.A.; Delphia, C.M.; O’Neill, K.M. A dual role for farmlands: Food security and pollinator conservation. J. Ecol. 2017, 105, 890–899. [Google Scholar] [CrossRef]
- Iovanna, R.; Ando, A.; Swinton, S.; Kagan, J.; Hellerstein, D.; Mushet, D.; Otto, C. Chapter 1: Assessing Pollinator Habitat Services to Optimize Conservation Programs; The Council on Food, Agricultural and Resource Economics (C-FARE): Washington, DC, USA, 2017; p. 28. [Google Scholar] [CrossRef]
- M’Gonigle, L.K.; Williams, N.M.; Lonsdorf, E.; Kremen, C. A tool for selecting plants when restoring habitat for pollinators. Conserv. Lett. 2017, 10, 105–111. [Google Scholar] [CrossRef]
- Eisikowitch, D.; Masad, Y. Nectar-yielding plants during the dearth season in Israel. Bee World 1980, 61, 11–18. [Google Scholar] [CrossRef]
- Ayers, G.S.; Hoopingarner, R.A.; Howitt, A.J. Testing potential bee forage for attractiveness to bees. Am. Bee J. 1987, 127, 91–98. [Google Scholar]
- Kigatiira, K.I.; Kahenya, W.A.; Townsend, G.F. Prosopis spp. as multipurpose trees in dry zones. Bee World 1988, 69, 6–11. [Google Scholar] [CrossRef]
- Koltowski, Z.; Jabloñski, B. Attempt to develop an assortment of herbaceous honey-producing plants to be used for the improvement of bee pastures on idle lands. J. Apic. Sci. 2001, 45, 21–28. [Google Scholar]
- Keasara, T.; Shmida, A. An evaluation of Israeli forestry trees and shrubs as potential forage plants for bees. Isr. J. Plant. Sci. 2009, 57, 49–64. [Google Scholar] [CrossRef]
- Lonsdorf, E.; Kremen, C.; Ricketts, T.; Winfree, R.; Williams, N.; Greenleaf, S. Modelling pollination services across agricultural landscapes. Ann. Bot. 2009, 103, 1589–1600. [Google Scholar] [CrossRef]
- Jaric, S.; Macukanovic-Jocic, M.; Mitrovic, M.; Pavlovic, P. The melliferous potential of forest and meadow plant communities on Mount Tara (Serbia). Environ. Entomol. 2013, 42, 724–732. [Google Scholar] [CrossRef]
- Hicks, D.M.; Ouvrard, P.; Baldock, K.C.R.; Baude, M.; Goddard, M.A.; Kunin, W.E.; Mitschunas, N.; Memmott, J.; Morse, H.; Nikolitsi, M.; et al. Food for pollinators: Quantifying the nectar and pollen resources of urban flower meadows. PLoS ONE 2016, 11, 37. [Google Scholar] [CrossRef] [PubMed]
- Ausseil, A.G.E.; Dymond, J.R.; Newstrom, L. Mapping floral resources for honey bees in New Zealand at the catchment scale. Ecol. Appl. 2018, 28, 1182–1196. [Google Scholar] [CrossRef] [PubMed]
- Ion, N.; Odoux, J.F.; Vaissiere, B.E. Melliferous potential of weedy herbaceous plants in crop fields of Romania from 1949 to 2012. J. Apic. Sci. 2018, 62, 149–165. [Google Scholar] [CrossRef]
- Pamminger, T.; Becker, R.; Himmelreich, S.; Schneider, C.W.; Bergtold, M. The nectar report: Quantitative review of nectar sugar concentrations offered by bee visited flowers in agricultural and non-agricultural landscapes. PeerJ 2019, 7, 15. [Google Scholar] [CrossRef] [PubMed]
- Pamminger, T.; Becker, R.; Himmelreich, S.; Schneider, C.W.; Bergtold, M. Pollen report: Quantitative review of pollen crude protein concentrations offered by bee pollinated flowers in agricultural and non-agricultural landscapes. PeerJ 2019, 7, 13. [Google Scholar] [CrossRef] [PubMed]
- Janssens, X.; Bruneau, E.; Lebrun, P. Prediction of the potential honey production at the apiary scale using a Geographical Information System (GIS). Apidologie 2006, 37, 351–365. [Google Scholar] [CrossRef]
- Albayrak, A.; Duran, F.; Bayir, R. Development of intelligent decision support system using fuzzy cognitive maps for migratory beekeepers. Turk. J. Electr. Eng. Comput. Sci. 2018, 26, 2476–2488. [Google Scholar] [CrossRef]
- Isaacs, R.; Tuell, J.; Fiedler, A.; Gardiner, M.; Landis, D. Maximizing arthropod-mediated ecosystem services in agricultural landscapes: The role of native plants. Front. Ecol. Environ. 2009, 7, 196–203. [Google Scholar] [CrossRef]
- Leech, M. Bee Friendly: A planting Guide for European Honeybees and Australian Native Pollinators; RIRDC: Barton, ACT, Australia, 2012; p. 320. [Google Scholar]
- Williams, N.M.; Ward, K.L.; Pope, N.; Isaacs, R.; Wilson, J.; May, E.A.; Ellis, J.; Daniels, J.; Pence, A.; Ullmann, K.; et al. Native wildflower plantings support wild bee abundance and diversity in agricultural landscapes across the United States. Ecol. Appl. 2015, 25, 2119–2131. [Google Scholar] [CrossRef]
- Venturini, E.M.; Drummond, F.A.; Hoshide, A.K.; Dibble, A.C.; Stack, L.B. Pollination reservoirs for wild bee habitat enhancement in cropping systems: A review. Agroecol. Sustain. Food Syst. 2017, 41, 101–142. [Google Scholar] [CrossRef]
- Maia, K.P.; Vaughan, I.P.; Memmott, J. Plant species roles in pollination networks: An experimental approach. Oikos 2019, 128, 1446–1457. [Google Scholar] [CrossRef]
- Ayers, G.S.; Hoopingarner, R.A. Research imperatives for fixed-land honey production. Am. Bee J. 1987, 127, 39–41. [Google Scholar]
- Cunningham, S.A.; Fournier, A.; Neave, M.J.; Le Feuvre, D. Improving spatial arrangement of honeybee colonies to avoid pollination shortfall and depressed fruit set. J. Appl. Ecol. 2016, 53, 350–359. [Google Scholar] [CrossRef]
- Crane, E. Bees and Beekeeping: Science Practice and World Resources; Heinemann Newnes: Oxford, UK, 1990; p. 614. [Google Scholar]
- Somerville, D. Honey and Pollen Flora of South-Eastern Australia; Tocal College and NSW DPI: Paterson, NSW, Australia, 2019; p. 676. [Google Scholar]
- Crane, E.; Walker, P.; Day, R. Directory of Important World Honey Sources; International Bee Research Association: London, UK, 1984; p. 384. [Google Scholar]
- Haydak, M.H. Honey bee nutrition. Annu. Rev. Entomol. 1970, 15, 143–156. [Google Scholar] [CrossRef]
- Brodschneider, R.; Crailsheim, K. Nutrition and health in honey bees. Apidologie 2010, 41, 278–294. [Google Scholar] [CrossRef]
- De Groot, A.P. Protein and amino acid requirements of the honey bee. Physiol. Comp. Oecol. 1953, 3, 197–285. [Google Scholar]
- Basualdo, M.; Barragán, S.; Vanagas, L.; García, C.; Solana, H.; Rodríguez, E.; Bedascarrasbure, E. Conversion of high and low pollen protein diets into protein in worker honey bees (Hymenoptera Apidae). J. Econ. Entomol. 2013, 106, 1553–1558. [Google Scholar] [CrossRef]
- Alaux, C.; Ducloz, F.; Crauser, D.; Le Conte, Y. Diet effects on honeybee immunocompetence. Biol. Lett. 2010, 6, 562–565. [Google Scholar] [CrossRef]
- DeGrandi-Hoffman, G.; Chen, Y.P.; Huang, E.; Huang, M.H. The effect of diet on protein concentration, hypopharyngeal gland development and virus load in worker honey bees (Apis mellifera L.). J. Insect Physiol. 2010, 56, 1184–1191. [Google Scholar] [CrossRef]
- Antúnez, K.; Invernizzi, C.; Zunino, P. Why massive honeybee colony losses do not occur in Uruguay. In Bees: Biology Threats and Colonies; Florio, R.M., Ed.; Nova Science Publishers: New York, NY, USA, 2012; p. 343. [Google Scholar]
- Li, C.C.; Xu, B.H.; Wang, Y.X.; Yang, Z.B.; Yang, W.R. Protein content in larval diet affects adult longevity and antioxidant gene expression in honey bee workers. Entomol. Exp. Appl. 2014, 151, 19–26. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, S.W.; Zeng, Z.J.; Yan, W.Y. Nutrition affects longevity and gene expression in honey bee (Apis mellifera) workers. Apidologie 2014, 45, 618–625. [Google Scholar] [CrossRef]
- Müller, A.; Diener, S.; Schnyder, S.; Stutz, K.; Sedivy, C.; Dorn, S. Quantitative pollen requirements of solitary bees: Implications for bee conservation and the evolution of bee-flower relationships. Biol. Conserv. 2006, 130, 604–615. [Google Scholar] [CrossRef]
- Bozek, M. Pollen yield and pollen grain dimensions of some late-summer plant species of the Lamiaceae family. J. Apic. Sci. 2008, 52, 31–36. [Google Scholar]
- Denisow, B. Pollen Production of Selected Ruderal Plant Species in the Lublin Area; University of Life Sciences in Lublin Press: Lublin, Poland, 2011; p. 86. [Google Scholar]
- Gong, Y.B.; Huang, S.Q. Interspecific variation in pollen-ovule ratio is negatively correlated with pollen transfer efficiency in a natural community. Plant. Biol. 2014, 16, 843–847. [Google Scholar] [CrossRef]
- Roulston, T.H.; Cane, J.H. Pollen nutritional content and digestibility for animals. Plant Syst. Evol. 2000, 222, 187–209. [Google Scholar] [CrossRef]
- Roulston, T.H.; Cane, J.H.; Buchmann, S.L. What governs protein content of pollen: Pollinator preferences, pollen-pistil interactions, or phylogeny? Ecol. Monogr. 2000, 70, 617–643. [Google Scholar] [CrossRef]
- Donkersley, P.; Rhodes, G.; Pickup, R.W.; Jones, K.C.; Power, E.F.; Wright, G.A.; Wilson, K. Nutritional composition of honey bee food stores vary with floral composition. Oecologia 2017, 185, 749–761. [Google Scholar] [CrossRef]
- Filipiak, M.; Kuszewska, K.; Asselman, M.; Denisow, B.; Stawiarz, E.; Woyciechowski, M.; Weiner, J. Ecological stoichiometry of the honeybee: Pollen diversity and adequate species composition are needed to mitigate limitations imposed on the growth and development of bees by pollen quality. PLoS ONE 2017, 12, e0183236. [Google Scholar] [CrossRef]
- Thakur, M.; Nanda, V. Composition and functionality of bee pollen: A review. Trends Food Sci. Technol. 2020, 98, 82–106. [Google Scholar] [CrossRef]
- Herbert, E.W.; Shimanuki, H.; Caron, D. Optimum protein levels required by honey bees (Hymenoptera, Apidae) to initiate and maintain brood rearing. Apidologie 1977, 8, 141–146. [Google Scholar] [CrossRef]
- Zheng, B.L.; Wu, Z.F.; Xu, B.H. The effects of dietary protein levels on the population growth, performance, and physiology of honey bee workers during early spring. J. Insect Sci. 2014, 14, 191. [Google Scholar] [CrossRef][Green Version]
- Kleinschmidt, G.J.; Kondos, A.C. The effect of dietary protein on colony performance. Australas. Beekeep. 1978, 79, 251–257. [Google Scholar]
- Pernal, S.F.; Currie, R.W. Pollen quality of fresh and 1-year-old single pollen diets for worker honey bees (Apis mellifera L.). Apidologie 2000, 31, 387–409. [Google Scholar] [CrossRef]
- Roulston, T.H.; Cane, J.H. The effect of pollen protein concentration on body size in the sweat bee Lasioglossum zephyrum (Hymenoptera: Apiformes). Evol. Ecol. 2002, 16, 49–65. [Google Scholar] [CrossRef]
- Schmidt, J.O.; Thoenes, S.C.; Levin, M.D. Survival of honey bees, Apis mellifera (Hymenoptera: Apidae), fed various pollen sources. Ann. Entomol. Soc. Am. 1987, 80, 176–183. [Google Scholar] [CrossRef]
- Woyke, J. Correlations and interactions between population, length of worker life and honey production by honeybees in a temperate region. J. Apic. Res. 1984, 23, 148–156. [Google Scholar] [CrossRef]
- Seeley, T.D. Social foraging by honeybees: How colonies allocate foragers among patches of flowers. Behav. Ecol. Sociobiol. 1986, 19, 343–354. [Google Scholar] [CrossRef]
- Pirk, C.W.W.; Boodhoo, C.; Human, H.; Nicolson, S. The importance of protein type and protein to carbohydrate ratio for survival and ovarian activation of caged honeybees (Apis mellifera scutellata). Apidologie 2010, 41, 62–72. [Google Scholar] [CrossRef]
- Yang, W.C.; Tian, Y.Y.; Han, M.F.; Miao, X.Q. Longevity extension of worker honey bees (Apis mellifera) by royal jelly: Optimal dose and active ingredient. PeerJ 2017, 5, 15. [Google Scholar] [CrossRef]
- Human, H.; Nicolson, S.W.; Strauss, K.; Pirk, C.W.W.; Dietemann, V. Influence of pollen quality on ovarian development in honeybee workers (Apis mellifera scutellata). J. Insect Physiol. 2007, 53, 649–655. [Google Scholar] [CrossRef]
- Day, S.; Beyer, R.; Mercer, A.; Ogden, S. The nutrient composition of honeybee-collected pollen in Otago, New Zealand. J. Apic. Res. 1990, 29, 138–146. [Google Scholar] [CrossRef]
- Somerville, D.; Nicol, H.I. Crude protein and amino acid composition of honey bee-collected pollen pellets from south-east Australia and a note on laboratory disparity. Aust. J. Exp. Agric. 2006, 46, 141–149. [Google Scholar] [CrossRef]
- Nicolson, S.W.; Human, H. Chemical composition of the ‘low quality’ pollen of sunflower (Helianthus annuus, Asteraceae). Apidologie 2013, 44, 144–152. [Google Scholar] [CrossRef]
- Loper, G.M.; Cohen, A.C. Amino-acid content of dandelion pollen, a honey-bee (Hymenoptera, Apidae) nutritional-evaluation. J. Econ. Entomol. 1987, 80, 14–17. [Google Scholar] [CrossRef]
- Somerville, D. Fat Bees Skinny Bees: A Manual on Honey Bee Nutrition for Beekeepers; RIRDC: Barton, ACT, Australia, 2005; p. 142. [Google Scholar]
- Manning, R. Fatty acids in pollen: A review of their importance for honey bees. Bee World 2001, 82, 60–75. [Google Scholar] [CrossRef]
- Leach, M.E.; Drummond, F. A review of native wild bee nutritional health. Int. J. Ecol. 2018, 10. [Google Scholar] [CrossRef]
- Tihelka, E. The immunological dependence of plant-feeding animals on their host’s medical properties may explain part of honey bee colony losses. Arthropod Plant. Interact. 2018, 12, 57–64. [Google Scholar] [CrossRef]
- Asensio, I.; Vicente-Rubiano, M.; Muňoz, M.J.; Fernández-Carrión, E.; Sánchez-Vizcaíno, J.M.; Carballo, M. Importance of ecological factors and colony handling for optimizing health status of apiaries in mediterranean ecosystems. PLoS ONE 2016, 11, 18. [Google Scholar] [CrossRef]
- Shrader, C.; Adamson, N.; Sole, J.; Hensley, M. Kentucky Pollinator Handbook: A Field Office Technical Guide Reference for the Management of Pollinators and Their Habitats; USDA: Washington, DC, USA, 2016; p. 144.
- Waller, G.D. Evaluating responses of honey bees hymenoptera-apidae to sugar solutions using an artificial-flower feeder. Ann. Entomol. Soc. Am. 1972, 65, 857–862. [Google Scholar] [CrossRef]
- Bolten, A.B.; Feinsinger, P. Why do hummingbird flowers secrete dilute nectar? Biotropica 1978, 10, 307–309. [Google Scholar] [CrossRef]
- Roubik, D.W.; Buchmann, S.L. Nectar selection by Melipona and Apis Mellifera (Hymenoptera: Apidae) and the ecology of nectar intake by bee colonies in a tropical forest. Oecologia 1984, 61, 1–10. [Google Scholar] [CrossRef]
- Southwick, E.E.; Loper, G.M.; Sadwick, S.E. Nectar production composition energetics and pollinator attractiveness in spring flowers of western New York. Am. J. Bot. 1981, 68, 994–1002. [Google Scholar] [CrossRef]
- Kim, W.; Gilet, T.; Bush, J.W.M. Optimal concentrations in nectar feeding. Proc. Natl. Acad. Sci. USA 2011, 108, 16618–16621. [Google Scholar] [CrossRef]
- Nicholls, E.; de Ibarra, N.H. Assessment of pollen rewards by foraging bees. Funct. Ecol. 2017, 31, 76–87. [Google Scholar] [CrossRef]
- Van der Moezel, P.G.; Delfs, J.C.; Pate, J.S.; Loneragan, W.A.; Bell, D.T. Pollen selection by honeybees in shrublands of the northern sandplains of Western Australia. J. Apic. Res. 1987, 26, 224–232. [Google Scholar] [CrossRef]
- Wills, R.T. Management of the Flora Utilised by the European Honey Bee in Kwongan of the Northern Sandplain of Western Australia. Ph.D. Thesis, Department of Botany, University of Western Australia, Perth, WA, Australia, 1989. [Google Scholar]
- Liolios, V.; Tananaki, C.; Dimou, M.; Kanelis, D.; Goras, G.; Karazafiris, E.; Thrasyvoulou, A. Ranking pollen from bee plants according to their protein contribution to honey bees. J. Apic. Res. 2015, 54, 582–592. [Google Scholar] [CrossRef]
- Waddington, K.D.; Nelson, C.M.; Page, R.E. Effects of pollen quality and genotype on the dance of foraging honey bees. Anim. Behav. 1998, 56, 35–39. [Google Scholar] [CrossRef] [PubMed]
- Beekman, M.; Preece, K.; Schaerf, T.M. Dancing for their supper: Do honeybees adjust their recruitment dance in response to the protein content of pollen? Insect. Soc. 2016, 63, 117–126. [Google Scholar] [CrossRef]
- Zarchin, S.; Dag, A.; Salomon, M.; Hendriksma, H.P.; Shafir, S. Honey bees dance faster for pollen that complements colony essential fatty acid deficiency. Behav. Ecol. Sociobiol. 2017, 71, 11. [Google Scholar] [CrossRef]
- Detzel, A.; Wink, M. Attraction, deterrence or intoxication of bees (Apis mellifera) by plant allelochemicals. Chemoecology 1993, 4, 8–18. [Google Scholar] [CrossRef]
- Nepi, M.; Grasso, D.A.; Mancuso, S. Nectar in plant-insect mutualistic relationships: From food reward to partner manipulation. Front. Plant. Sci. 2018, 9, 14. [Google Scholar] [CrossRef]
- Wright, G.A.; Baker, D.D.; Palmer, M.J.; Stabler, D.; Mustard, J.A.; Power, E.F.; Borland, A.M.; Stevenson, P.C. Caffeine in floral nectar enhances a pollinator’s memory of reward. Science 2013, 339, 1202–1204. [Google Scholar] [CrossRef]
- Couvillon, M.; Riddell Pearce, F.C.; Accleton, C.; Fensome, K.A.; Quah, S.K.L.; Taylor, E.L.; Ratnieks, F.L.W. Honey bee foraging distance depends on month and forage type. Apidologie 2015, 46, 61–70. [Google Scholar] [CrossRef]
- Free, J.B. Factors determining the collection of pollen by honeybee foragers. Anim. Behav. 1967, 15, 134–144. [Google Scholar] [CrossRef]
- Dreller, C.; Page, R., Jr.; Fondrk, M. Regulation of pollen foraging in honeybee colonies: Effects of young brood, stored pollen, and empty space. Behav. Ecol. Sociobiol. 1999, 45, 227–233. [Google Scholar] [CrossRef]
- Scheiner, R.; Page, R.E.; Erber, J. Sucrose responsiveness and behavioral plasticity in honey bees (Apis mellifera). Apidologie 2004, 35, 133–142. [Google Scholar] [CrossRef]
- Danka, R.G.; Hellmich, R.L.; Rinderer, T.E.; Collins, A.M. Diet-selection ecology of tropically and temperately adapted honey bees. Anim. Behav. 1987, 35, 1858–1863. [Google Scholar] [CrossRef]
- Fewell, J.H.; Winston, M.L. Colony state and regulation of pollen foraging in the honey-bee, Apis mellifera L. Behav. Ecol. Sociobiol. 1992, 30, 387–393. [Google Scholar] [CrossRef]
- Altaye, S.Z.; Pirk, C.W.W.; Crewe, R.M.; Nicolson, S.W. Convergence of carbohydrate-biased intake targets in caged worker honeybees fed different protein sources. J. Exp. Biol. 2010, 213, 3311–3318. [Google Scholar] [CrossRef]
- Bodenheimer, F.S. Studies in animal populations II: Seasonal population-trends of the honey-bee. Q. Rev. Biol. 1937, 12, 406–425. [Google Scholar] [CrossRef]
- Somerville, D.; Annand, N. Healthy Bees: Managing Pests, Diseases and other Disorders of the Honey Bee; Department of Primary Industries: Paterson, NSW, Australia, 2014; p. 74. [Google Scholar]
- Schmickl, T.; Crailsheim, K. Cannibalism and early capping: Strategy of honeybee colonies in times of experimental pollen shortages. J. Comp. Physiol. A Sens. Neural Behav. Physiol. 2001, 187, 541–547. [Google Scholar] [CrossRef]
- Gavina, M.K.A.; Rabajante, J.F.; Cervancia, C.R. Mathematical programming models for determining the optimal location of beehives. Bull. Math. Biol. 2014, 76, 997–1016. [Google Scholar] [CrossRef] [PubMed]
- Waddington, K.D.; Herbert, T.J.; Visscher, P.K.; Richter, M.R. Comparisons of forager distributions from matched honey bee colonies in suburban environments. Behav. Ecol. Sociobiol. 1994, 35, 423–429. [Google Scholar] [CrossRef]
- Charnov, E.L. Optimal foraging, the marginal value theorem. Theor. Popul. Biol. 1976, 9, 129–136. [Google Scholar] [CrossRef]
- Pyke, G.H. Optimal foraging in bumblebees: Calculation of net rate of energy intake and optimal patch choice. Theor. Popul. Biol. 1980, 17, 232–246. [Google Scholar] [CrossRef]
- Hodges, C.M. Optimal foraging in bumblebees: Hunting by expectation. Anim. Behav. 1981, 29, 1166–1171. [Google Scholar] [CrossRef]
- Zimmerman, M. Optimal foraging, plant density and the marginal value theorem. Oecologia 1981, 49, 148–153. [Google Scholar] [CrossRef]
- Olsson, O.; Bolin, A. A model for habitat selection and species distribution derived from central place foraging theory. Oecologia 2014, 175, 537–548. [Google Scholar] [CrossRef]
- Steffan-Dewenter, I.; Kuhn, A. Honeybee foraging in differentially structured landscapes. Proc. R. Soc. B Biol. Sci. 2003, 270, 569–575. [Google Scholar] [CrossRef]
- Carvell, C.; Jordan, W.C.; Bourke, A.F.G.; Pickles, R.; Redhead, J.W.; Heard, M.S. Molecular and spatial analyses reveal links between colony-specific foraging distance and landscape-level resource availability in two bumblebee species. Oikos 2012, 121, 734–742. [Google Scholar] [CrossRef]
- Seeley, T.D. The Wisdom of the Hive: The Social Physiology of Honey Bee Colonies; Harvard University Press: Cambridge, MA, USA, 1995; p. 295. [Google Scholar]
- Amaya-Marquez, M. Floral constancy in bees: A revision of theories and a comparison with other pollinators. Rev. Colomb. Entomol. 2009, 35, 206–216. [Google Scholar]
- Somerville, D. Honey & Pollen Flora Suitable for Planting in South-Eastern NSW; NSW Agriculture: Orange, NSW, Australia, 2002; p. 4. [Google Scholar]
- Ohashi, K.; Yahara, T. How long to stay on, and how often to visit a flowering plant?: A model for foraging strategy when floral displays vary in size. Oikos 1999, 86, 386–392. [Google Scholar] [CrossRef]
- Goulson, D. Why do pollinators visit proportionally fewer flowers in large patches? Oikos 2000, 91, 485–492. [Google Scholar] [CrossRef]
- Spiesman, B.J.; Bennett, A.; Isaacs, R.; Gratton, C. Bumble bee colony growth and reproduction depend on local flower dominance and natural habitat area in the surrounding landscape. Biol. Conserv. 2017, 206, 217–223. [Google Scholar] [CrossRef]
- Rathcke, B. Competition and facilitation among plants for pollination. In Pollination Biology; Real, L., Ed.; Academic Press: Orlando, FL, USA, 1983; pp. 305–329. [Google Scholar]
- Williams, S.A. Beekeeper’s Guide to Australian Leptospermum Trees and Honey; School of Science and Engineering, University of the Sunshine Coast: Sippy Downs, QLD, Australia, 2018; p. 185. [Google Scholar]
- Ye, Z.M.; Dai, W.K.; Jin, X.F.; Gituru, R.W.; Wang, Q.F.; Yang, C.F. Competition and facilitation among plants for pollination: Can pollinator abundance shift the plant–plant interactions? Plant. Ecol. 2014, 215, 3–13. [Google Scholar] [CrossRef]
- Adeva, J.J.G. Simulation modelling of nectar and pollen foraging by honeybees. Biosyst. Eng. 2012, 112, 304–318. [Google Scholar] [CrossRef]
- Becher, M.A.; Grimm, V.; Thorbek, P.; Horn, J.; Kennedy, P.J.; Osborne, J.L. BEEHAVE: A systems model of honeybee colony dynamics and foraging to explore multifactorial causes of colony failure. J. Appl. Ecol. 2014, 51, 470–482. [Google Scholar] [CrossRef]
- Agatz, A.; Kuhl, R.; Miles, M.; Schad, T.; Preuss, T.G. An evaluation of the BEEHAVE model using honey bee field study data: Insights and recommendations. Environ. Toxicol. Chem. 2019, 38, 2535–2545. [Google Scholar] [CrossRef]
- Martin, S.J. The role of Varroa and viral pathogens in the collapse of honeybee colonies: A modelling approach. J. Appl. Ecol. 2001, 38, 1082–1093. [Google Scholar] [CrossRef]
- Schmickl, T.; Crailsheim, K. HoPoMo: A model of honeybee intracolonial population dynamics and resource management. Ecol. Model. 2007, 204, 219–245. [Google Scholar] [CrossRef]
- European Food Safety Authority. Statement on the suitability of the BEEHAVE model for its potential use in a regulatory context and for the risk assessment of multiple stressors in honeybees at the landscape level. EFSA J. 2015, 13, 92. [Google Scholar] [CrossRef]
- Sumpter, D.J.T.; Pratt, S.C. A modelling framework for understanding social insect foraging. Behav. Ecol. Sociobiol. 2003, 53, 131–144. [Google Scholar] [CrossRef]
- Becher, M.A.; Grimm, V.; Knapp, J.; Horn, J.; Twiston-Davies, G.; Osborne, J.L. BEESCOUT: A model of bee scouting behaviour and a software tool for characterizing nectar/pollen landscapes for BEEHAVE. Ecol. Model. 2016, 340, 126–133. [Google Scholar] [CrossRef] [PubMed]
- Timberlake, T.P.; Vaughan, I.P.; Memmott, J. Phenology of farmland floral resources reveals seasonal gaps in nectar availability for bumblebees. J. Appl. Ecol. 2019, 56, 1585–1596. [Google Scholar] [CrossRef]
- DeGrandi-Hoffman, G.; Roth, S.A.; Loper, G.L.; Erickson, E.H. BEEPOP: A honeybee population dynamics simulation model. Ecol. Model. 1989, 45, 133–150. [Google Scholar] [CrossRef]
- Khoury, D.S.; Myerscough, M.R.; Barron, A.B. A quantitative model of honey bee colony population dynamics. PLoS ONE 2011, 6, 6. [Google Scholar] [CrossRef]
- Khoury, D.S.; Barron, A.B.; Myerscough, M.R. Modelling food and population dynamics in honey bee colonies. PLoS ONE 2013, 8, 7. [Google Scholar] [CrossRef]
- Russell, S.; Barron, A.B.; Harris, D. Dynamic modelling of honey bee (Apis mellifera) colony growth and failure. Ecol. Model. 2013, 265, 158–169. [Google Scholar] [CrossRef]
- Betti, M.I.; Wahl, L.M.; Zamir, M. Effects of infection on honey bee population dynamics: A model. PLoS ONE 2014, 9, 12. [Google Scholar] [CrossRef] [PubMed]
- Torres, D.J.; Ricoy, U.M.; Roybal, S. Modeling honey bee populations. PLoS ONE 2015, 10, 28. [Google Scholar] [CrossRef] [PubMed]
- Paiva, J.; Paiva, H.M.; Esposito, E.; Morais, M.M. On the effects of artificial feeding on bee colony dynamics: A mathematical model. PLoS ONE 2016, 11, 18. [Google Scholar] [CrossRef]
- Bagheri, S.; Mirzaie, M. A mathematical model of honey bee colony dynamics to predict the effect of pollen on colony failure. PLoS ONE 2019, 14, 19. [Google Scholar] [CrossRef]
- Lonsdorf, E.; Ricketts, T.; Kremen, C.; Winfree, R.; Greenleaf, S.; Williams, N. Crop pollination services. In Natural Capital: Theory and Practice of Mapping Ecosystem Services; Kareiva, P., Daily, G.C., Polasky, S., Ricketts, T.H., Tallis, H., Eds.; Oxford University Press, Incorporated: Oxford, UK, 2011; pp. 168–187. [Google Scholar]
- Marchand, P.; Harmon-Threatt, A.N.; Chapela, I. Testing models of bee foraging behavior through the analysis of pollen loads and floral density data. Ecol. Model. 2015, 313, 41–49. [Google Scholar] [CrossRef]
- Olsson, O.; Bolin, A.; Smith, H.G.; Lonsdorf, E.V. Modeling pollinating bee visitation rates in heterogeneous landscapes from foraging theory. Ecol. Model. 2015, 316, 133–143. [Google Scholar] [CrossRef]
- Baveco, J.M.; Focks, A.; Belgers, D.; van der Steen, J.J.M.; Boesten, J.J.T.I.; Roessink, I. An energetics-based honeybee nectar-foraging model used to assess the potential for landscape-level pesticide exposure dilution. PeerJ 2016, 4, 25. [Google Scholar] [CrossRef]
- Gioia, P.; Hopper, S.D. A new phytogeographic map for the Southwest Australian Floristic Region after an exceptional decade of collection and discovery. Bot. J. Linn. Soc. 2017, 184, 1–15. [Google Scholar] [CrossRef]
- Beard, J.S.; Chapman, A.R.; Gioia, P. Species richness and endemism in the Western Australian flora. J. Biogeogr. 2000, 27, 1257–1268. [Google Scholar] [CrossRef]
- Smith, F.G. Honey Plants in Western Australia; Department of Agriculture and Food: Perth, WA, Australia, 1969; p. 78. [Google Scholar]
- Goodman, R.; Kaczynski, P. Australian Beekeeping Guide; Rural Industries Research and Development Corporation: Barton, ACT, Australia, 2015; p. 135. [Google Scholar]
- Sniderman, J.M.K.; Matley, K.A.; Haberle, S.G.; Cantrill, D.J. Pollen analysis of Australian honey. PLoS ONE 2018, 13, 24. [Google Scholar] [CrossRef]
- Blyth, J.D. Beekeeping and Land Management: Proceedings of a Workshop; Department of Conservation and Land Management: Como, WA, Australia, 1987; p. 96. [Google Scholar]
- Wardell-Johnson, G.; Wardell-Johnson, A.; Bradby, K.; Robinson, T.; Bateman, P.W.; Williams, K.; Keesing, A.; Braun, K.; Beckerling, J.; Burbridge, M. Application of a Gondwanan perspective to restore ecological integrity in the south-western Australian global biodiversity hotspot. Restor. Ecol. 2016, 24, 805–815. [Google Scholar] [CrossRef]
- Government of Western Australia. 2018 Statewide Vegetation Statistics: Full Report. DBCA Statewide Vegetation Statistics. Available online: https://catalogue.data.wa.gov.au/dataset/dbca-statewide-vegetation-statistics (accessed on 8 April 2019).
- Benecke, F.S. Commercial Beekeeping in Australia; RIRDC: Barton, ACT, Australia, 2007; p. 38. [Google Scholar]
- Keogh, R.C.; Robinson, A.P.W.; Mullins, I.J. Pollination Aware: The Real Value of Pollination in Australia; Rural Industries Research and Development Corporation: Barton, ACT, Australia, 2010; p. 60. [Google Scholar]
- Van Dijk, J.; Gomboso, J.; Levantis, C. Australian Honey Bee Industry: 2014–15 Survey Results; ABARES Research Report 16.18; ABARES: Canberra, ACT, Australia, 2016; p. 33. [Google Scholar]
- Rogers, S.R.; Staub, B. Standard use of Geographic Information System (GIS) techniques in honey bee research. J. Apic. Res. 2013, 52, 47. [Google Scholar] [CrossRef]
- Abou-Shaara, H.F. Geographical information system for beekeeping development. J. Apic. Sci. 2019, 63, 5–16. [Google Scholar] [CrossRef]
- Macintyre, P.; van Niekerk, A.; Mucina, L. Efficacy of multi-season Sentinel-2 imagery for compositional vegetation classification. Int. J. Appl. Earth Obs. Geoinf. 2020, 85, 10. [Google Scholar] [CrossRef]
- Ferreira, C. A Place in the Country; Fremantle Press: North Fremantle, WA, USA, 2018; p. 272. [Google Scholar]
- Ritchie, A.L.; Svejcar, L.N.; Ayre, B.M.; Bolleter, J.; Brace, A.; Craig, M.D.; Davis, B.; Davis, R.A.; Van Etten, E.J.B.; Fontaine, J.B.; et al. A threatened ecological community: Research advances and priorities for Banksia woodlands. Aust. J. Bot. 2021, 69, 111. [Google Scholar] [CrossRef]
- Prober, S.M.; Byrne, M.; McLean, E.H.; Steane, D.A.; Potts, B.M.; Vaillancourt, R.E.; Stock, W.D. Climate-adjusted provenancing: A strategy for climate-resilient ecological restoration. Front. Ecol. Evol. 2015, 3, 5. [Google Scholar] [CrossRef]
- Yates, C.J.; McNeill, A.; Elith, J.; Midgley, G.F. Assessing the impacts of climate change and land transformation on Banksia in the South West Australian Floristic Region. Divers. Distrib. 2010, 16, 187–201. [Google Scholar] [CrossRef]
- Law, B.; Mackowski, C.; Schoer, L.; Tweedie, T. Flowering phenology of myrtaceous trees and their relation to climatic, environmental and disturbance variables in northern New South Wales. Austral. Ecol. 2000, 25, 160–178. [Google Scholar] [CrossRef]
- Crane, E. What makes a good honey plant? Bee World 1975, 56, 32–34. [Google Scholar] [CrossRef]
- Ayers, G.S. How much honey can you get from an acre of land? Am. Bee J. 1992, 132, 583–585. [Google Scholar]
- Traynor, J. Evaluating pollen production of plants (with sample calculations for almonds). Am. Bee J. 2001, 141, 287–288. [Google Scholar]
- Lupo, A.; Eisikowitch, D. Eucalyptus erythrocoris: A source of nectar and pollen for honey bees in Israel. Apidologie 1990, 21, 25–33. [Google Scholar] [CrossRef]
- Corbet, S.A. Nectar sugar content: Estimating standing crop and secretion rate in the field. Apidologie 2003, 34, 1–10. [Google Scholar] [CrossRef]
- Masierowska, M.L. Floral nectaries and nectar production in brown mustard (Brassica juncea) and white mustard (Sinapis alba) (Brassicaceae). Plant. Syst. Evol. 2003, 238, 97–107. [Google Scholar] [CrossRef]
- Alqarni, A.S. Honeybee foraging, nectar secretion, and honey potential of wild jujube trees, Ziziphus nummularia. Neotrop. Entomol. 2015, 44, 232–241. [Google Scholar] [CrossRef]
- Woinarski, J.C.; Connors, G.; Franklin, D.C. Thinking honeyeater: Nectar maps for the Northern Territory, Australia. Pac. Conserv. Biol. 2000, 6, 61–80. [Google Scholar] [CrossRef]
- Opler, P.A. Nectar production in a tropical ecosystem. In The Biology of Nectaries; Bentley, B., Elias, T., Eds.; Columbia University Press: New York, NY, USA, 1983; pp. 30–79. [Google Scholar]
- Adams, G. Birdscaping Australian Gardens: A Guide to Native Plants and the Garden Birds They Attract; D&G Publishing: Vaucluse, NSW, Australia, 2011; p. 364. [Google Scholar]
- Paton, D.C. The diet of the New Holland honeyeater, Phylidonyris novaehollandiae. Aust. J. Ecol. 1982, 7, 279–298. [Google Scholar] [CrossRef]
- Arora, R.K. Optimization: Algorithms and Applications; CRC Press LLC: Boca Raton, FL, USA, 2015; p. 466. [Google Scholar]
- Kirkpatrick, S.; Gelatt, C.D.; Vecchi, M.P. Optimization by simulated annealing. Science 1983, 220, 671–680. [Google Scholar] [CrossRef]
- Colorni, A.; Dorigo, M.; Maffioli, F.; Maniezzo, V.; Righini, G.; Trubian, M. Heuristics from nature for hard combinatorial optimization problems. Int. Trans. Oper. Res. 1996, 3, 1–21. [Google Scholar] [CrossRef]
- Michalak, K. Evolutionary algorithm with a directional local search for multiobjective optimization in combinatorial problems. Optim. Methods Softw. 2016, 31, 392–404. [Google Scholar] [CrossRef]
- Kaim, A.; Cord, A.F.; Volk, M. A review of multi-criteria optimization techniques for agricultural land use allocation. Environ. Model. Softw. 2018, 105, 79–93. [Google Scholar] [CrossRef]
- Pannell, D.J. Sensitivity analysis of normative economic models: Theoretical framework and practical strategies. Agric. Econ. 1997, 16, 139–152. [Google Scholar] [CrossRef]
- Madavan, N.K. Multiobjective optimization using a Pareto differential evolution approach. In Proceedings of the 2002 Congress on Evolutionary Computation, Honolulu, HI, USA, 12–17 May 2002; pp. 1145–1150. [Google Scholar] [CrossRef]
- Hill, D.B.; Webster, T.C. Apiculture and forestry (bees and trees). Agrofor. Syst. 1995, 29, 313–320. [Google Scholar] [CrossRef]
- Dominati, E.J.; Maseyk, F.J.F.; Mackay, A.D.; Rendel, J.M. Farming in a changing environment: Increasing biodiversity on farm for the supply of multiple ecosystem services. Sci. Total Environ. 2019, 662, 703–713. [Google Scholar] [CrossRef]
- Ouvrard, P.; Transon, J.; Jacquemart, A.L. Flower-strip agri-environment schemes provide diverse and valuable summer flower resources for pollinating insects. Biodivers. Conserv. 2018, 27, 2193–2216. [Google Scholar] [CrossRef]
- Sugden, E.A.; Thorp, R.W.; Buchmann, S.L. Honey bee-native bee competition: Focal point for environmental change and apicultural response in Australia. Bee World 1996, 77, 26–44. [Google Scholar] [CrossRef]
- De-Miguel, S.; Pukkala, T.; Yeşil, A. Integrating pine honeydew honey production into forest management optimization. Eur. J. Res. 2014, 133, 423–432. [Google Scholar] [CrossRef]
- Ausseil, A.G.E.; Dymond, J.R.; Kirschbaum, M.U.F.; Andrew, R.M.; Parfitt, R.L. Assessment of multiple ecosystem services in New Zealand at the catchment scale. Environ. Model. Softw. 2013, 43, 37–48. [Google Scholar] [CrossRef]
- Gulliford, B. Beekeeping for Business and Pleasure; NSW Agriculture and Fisheries: Paterson, NSW, USA, 1989. [Google Scholar]
- Ghazoul, J. Floral diversity and the facilitation of pollination. J. Ecol. 2006, 94, 295–304. [Google Scholar] [CrossRef]
- Lawes, R.; Renton, M. Gaining insight into the risks, returns and value of perfect knowledge for crop sequences by comparing optimal sequences with those proposed by agronomists. Crop. Pasture Sci. 2015, 66, 622–633. [Google Scholar] [CrossRef]
- Ewel, J.J.; Putz, F.E. A place for alien species in ecosystem restoration. Front. Ecol. Environ. 2004, 2, 354–360. [Google Scholar] [CrossRef]
- Shackelford, N.; Hobbs, R.J.; Heller, N.E.; Hallett, L.M.; Seastedt, T.R. Finding a middle-ground: The native/non-native debate. Biol. Conserv. 2013, 158, 55–62. [Google Scholar] [CrossRef]
- Blaix, C.; Moonen, A.C.; Dostatny, D.F.; Izquierdo, J.; Le Corff, J.; Morrison, J.; Von Redwitz, C.; Schumacher, M.; Westerman, P.R. Quantification of regulating ecosystem services provided by weeds in annual cropping systems using a systematic map approach. Weed Res. 2018, 58, 151–164. [Google Scholar] [CrossRef]
- Kouchner, C.; Ferrus, C.; Blanchard, S.; Decourtye, A.; Basso, B.; Le Conte, Y.; Tchamitchian, M. Bee farming system sustainability: An assessment framework in metropolitan France. Agric. Syst. 2019, 176, 8. [Google Scholar] [CrossRef]
- Barbieri, C.; Mahoney, E. Why is diversification an attractive farm adjustment strategy?: Insights from Texas farmers and ranchers. J. Rural Stud. 2009, 25, 58–66. [Google Scholar] [CrossRef]
- Darnhofer, I.; Bellon, S.; Dedieu, B.; Milestad, R. Adaptiveness to enhance the sustainability of farming systems: A review. Agron. Sustain. Dev. 2010, 30, 545–555. [Google Scholar] [CrossRef]
- Martin, G.; Magne, M.A. Agricultural diversity to increase adaptive capacity and reduce vulnerability of livestock systems against weather variability—A farm-scale simulation study. Agric. Ecosyst. Environ. 2015, 199, 301–311. [Google Scholar] [CrossRef]
- Crane, E. Some multipurpose trees that are important honey sources in the tropics and subtropics. In Proceedings of the Third International Conference on Apiculture in Tropical Climates, Nairobi, Kenya, 5–9 November 1984; International Bee Research Association: Nairobi, Kenya, 1984; pp. 192–197. [Google Scholar]
- Ayers, G.S.; Ayers, S. Bee forages with other uses part II: Plants with marketable value. Am. Bee J. 1997, 137, 651–656. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Picknoll, J.L.; Poot, P.; Renton, M. A New Approach to Inform Restoration and Management Decisions for Sustainable Apiculture. Sustainability 2021, 13, 6109. https://doi.org/10.3390/su13116109
Picknoll JL, Poot P, Renton M. A New Approach to Inform Restoration and Management Decisions for Sustainable Apiculture. Sustainability. 2021; 13(11):6109. https://doi.org/10.3390/su13116109
Chicago/Turabian StylePicknoll, Joanne Lee, Pieter Poot, and Michael Renton. 2021. "A New Approach to Inform Restoration and Management Decisions for Sustainable Apiculture" Sustainability 13, no. 11: 6109. https://doi.org/10.3390/su13116109
APA StylePicknoll, J. L., Poot, P., & Renton, M. (2021). A New Approach to Inform Restoration and Management Decisions for Sustainable Apiculture. Sustainability, 13(11), 6109. https://doi.org/10.3390/su13116109





