Physio-Morphological and Biochemical Trait-Based Evaluation of Ethiopian and Chinese Wheat Germplasm for Drought Tolerance at the Seedling Stage
Abstract
1. Introduction
2. Material and Methods
2.1. Plant Material
2.1.1. Experimental Design and Growth Conditions
2.1.2. Drought Stress Treatment
2.2. Physio-Morphological Traits
2.2.1. Total Water Consumption
2.2.2. Leaf Relative Water Content (RWC)
2.2.3. Stomatal Conductance
2.2.4. Growth Traits
2.3. Biochemical Assay
2.3.1. Photosynthetic Pigments
2.3.2. Proline Content
2.4. Data Analysis
3. Results
3.1. Water Relations
3.2. Root Length, Shoot Length, and Their Biomasses
3.3. Photosynthesis Pigments
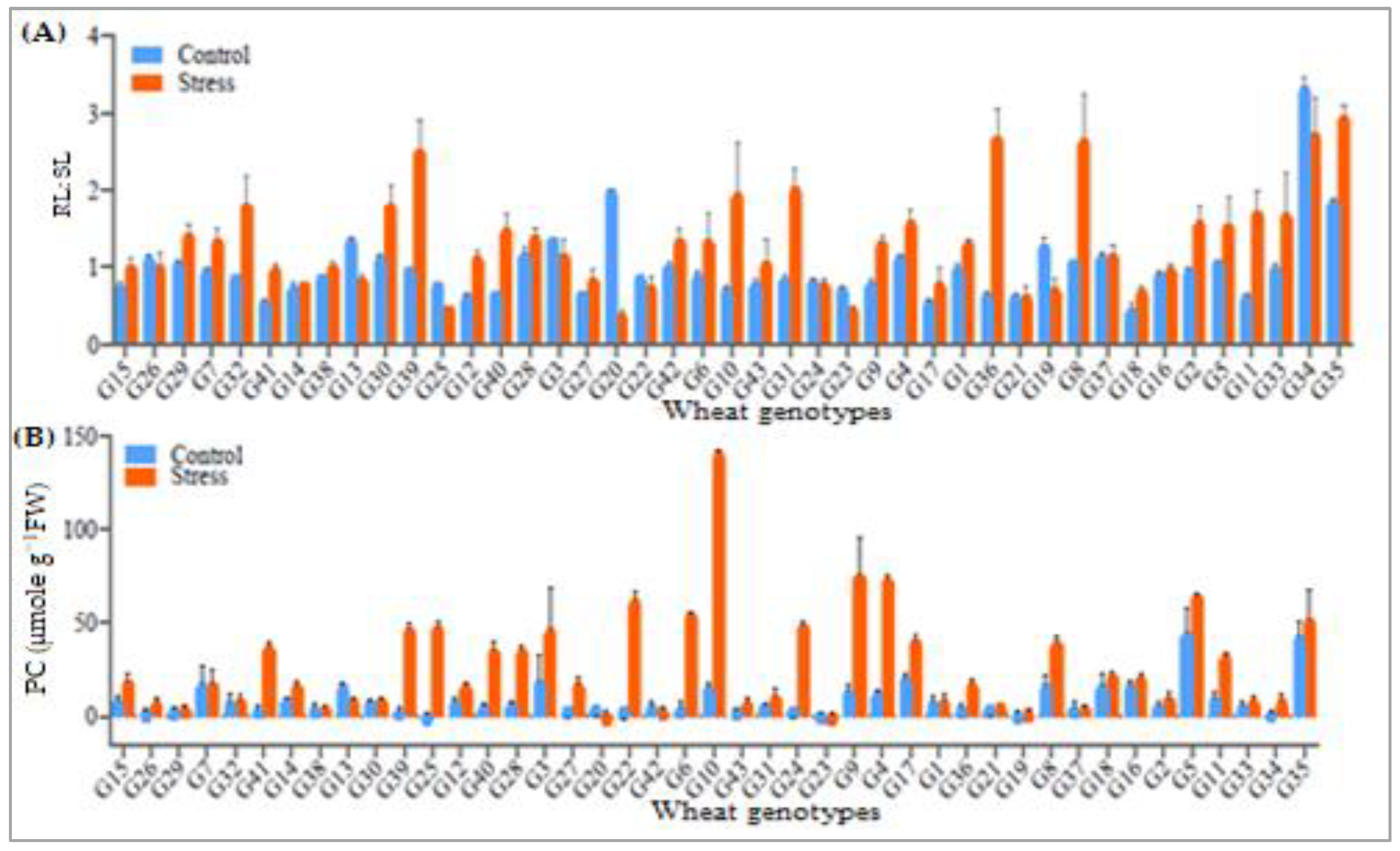
3.4. Proline Accumulation and Root Length to Shoot Length Ratio
3.5. Multivariate Analysis
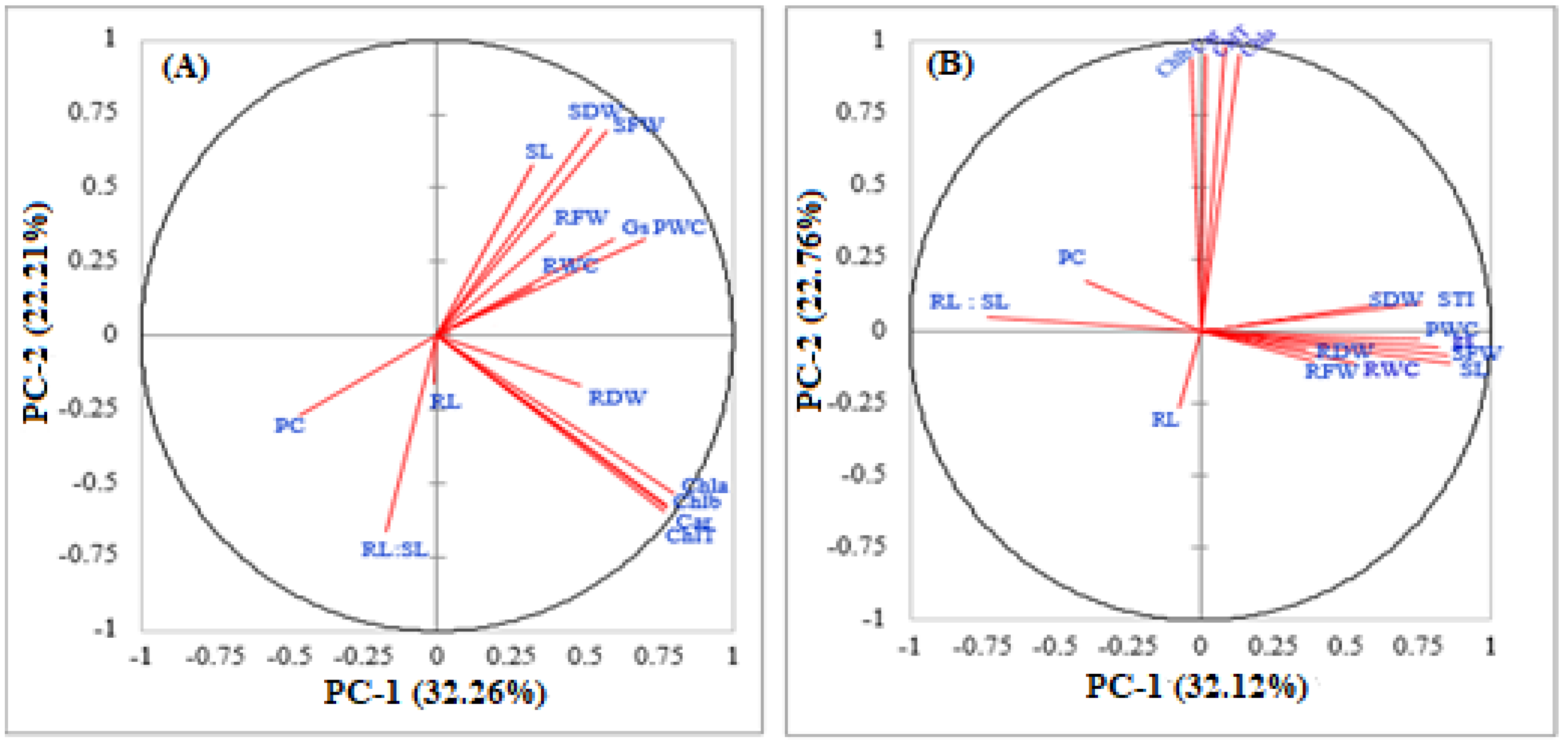
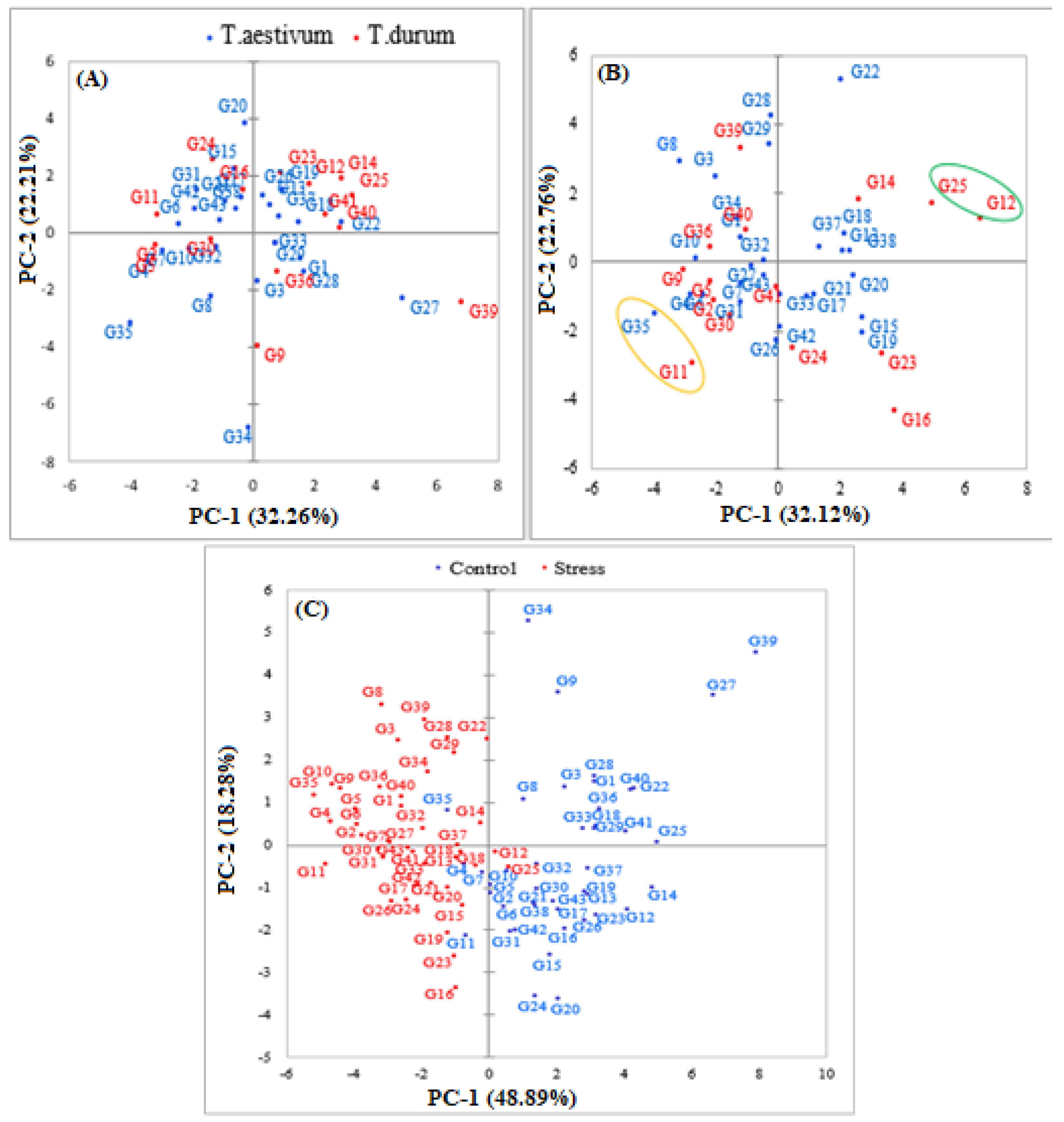
3.5.1. Principal Component Analysis (PCA)
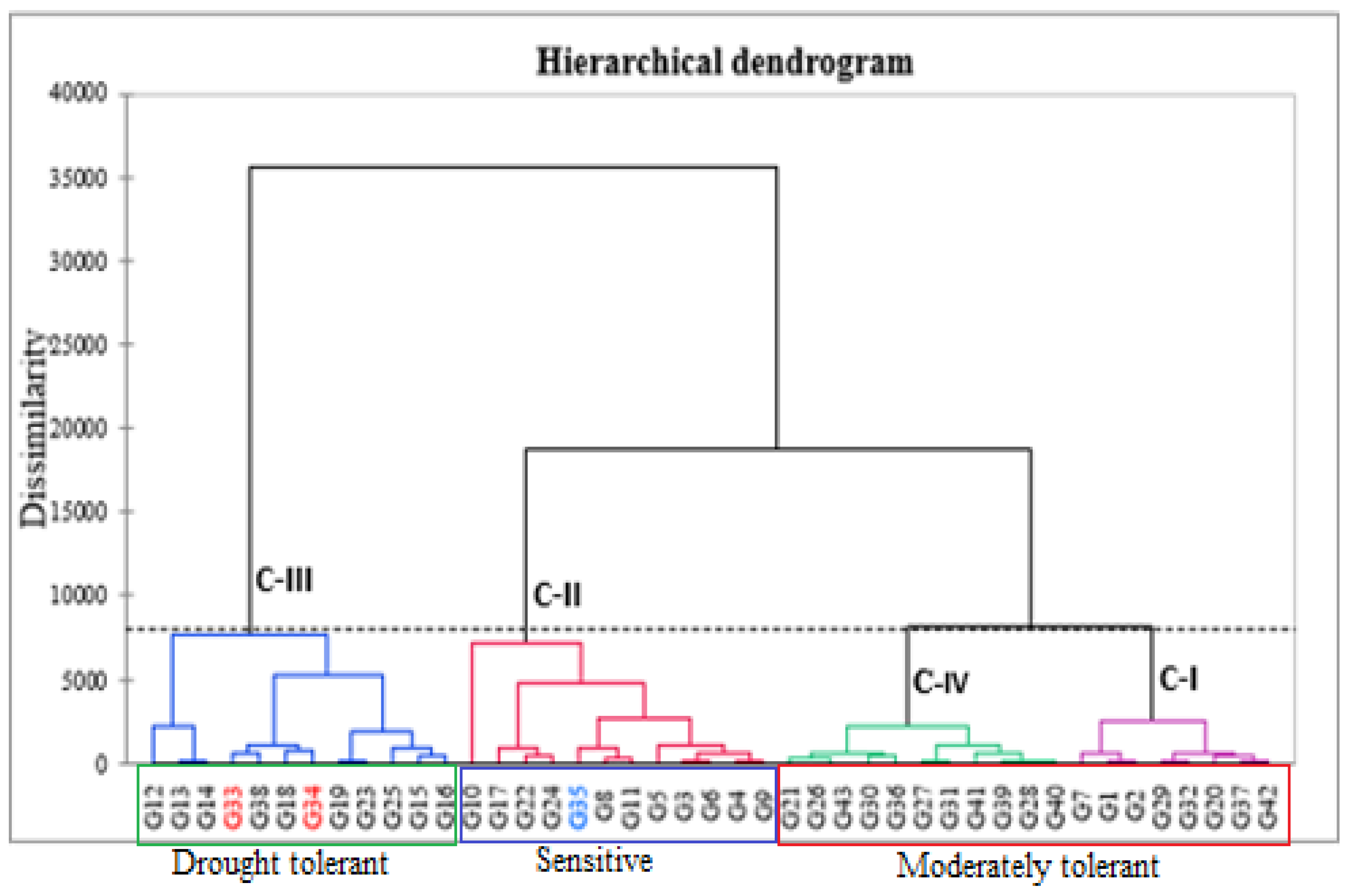
3.5.2. Agglomerative Hierarchical Clustering (AHC)
3.5.3. Correlation Analysis
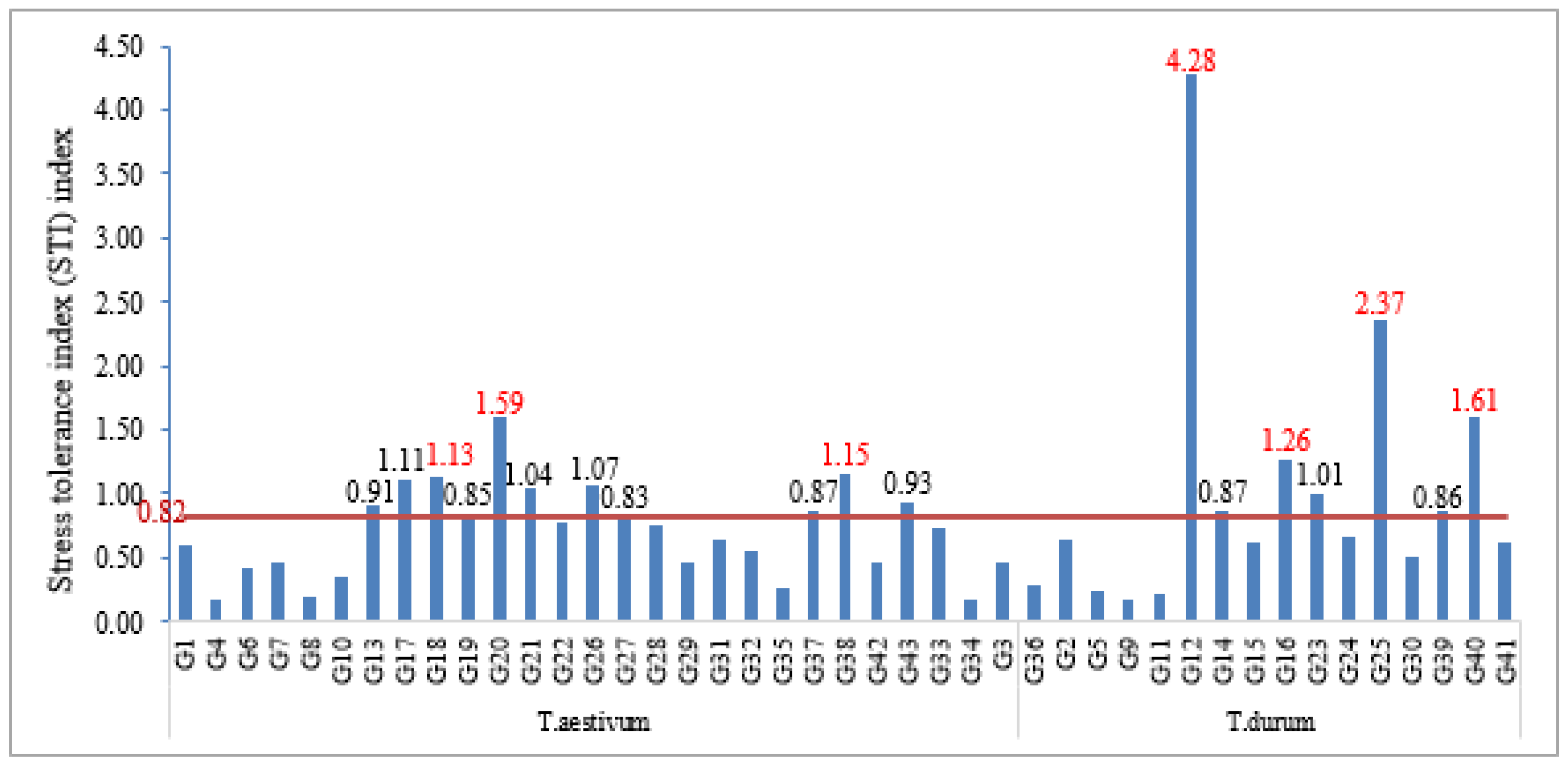
3.6. Screening of Wheat Genotypes Using Stress Tolerance Index (STI)
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Lev-Yadun, S.; Gopher, A.; Abbo, S. The cradle of agriculture. Science 2000, 288, 1602–1603. [Google Scholar] [CrossRef]
- Shiferaw, B.; Smale, M.; Braun, H.J.; Duveiller, E.; Reynolds, M.; Muricho, G. Crops that feed the world 10. Past successes and future challenges to the role played by wheat in global food security. Food Secur. 2013, 5, 291–317. [Google Scholar] [CrossRef]
- Shewry, P.R.; Hey, S.J. The contribution of wheat to human diet and health. Food Energy Secur. 2015, 4, 178–202. [Google Scholar] [CrossRef] [PubMed]
- Lobell, D.B.; Gourdji, S.M. The influence of climate change on global crop productivity. Plant. Physiol. 2012, 160, 1686–1697. [Google Scholar] [CrossRef]
- FAO. Crop Prospects and Food Situation-Quarterly Global Report No. 2; FAO: Quebec City, QC, Canada, 2020; Available online: http://www.fao.org/publications/card/en/c/b223a9f4-e425-4924-8478-6cf9e74647f7/ (accessed on 19 August 2020).
- Huaxia. China Evaluates 24,000 Files of Drought-Resistant Wheat Resources; Xuaxia: Beijing, China, 2020; Available online: http://www.xinhuanet.com/english/2020-06/10/c_139129191.html (accessed on 13 February 2021).
- He, Z.H. A History of Wheat Breeding in China; Cimmyt: Mexico City, Mexico, 2001. [Google Scholar]
- Li, H.; Zhou, Y.; Xin, W.; Wei, Y.; Zhang, J.; Guo, L. Wheat breeding in northern China: Achievements and technical advances. Crop J. 2019, 7, 718–729. [Google Scholar] [CrossRef]
- Hailu, G.-M.; Tanner, D.G.; Mengistu, H. Wheat Research in Ethiopia: A Historical Perspective; FAO: Quebec City, QC, Canada, 1991. [Google Scholar]
- Esterhuizen, D.; Bonsu, K. Grain and Feed Annual Report; USDA: Washington, DC, USA, 2020; pp. 1–19. [Google Scholar]
- Chesley, M.; Tefera, A. Ethiopia Grain and Feed; USDA: Washington, DC, USA, 2012. [Google Scholar]
- Tadesse, W.; Bishaw, Z.; Assefa, S. Wheat production and breeding in Sub-Saharan Africa: Challenges and opportunities in the face of climate change. Int. J. Clim. Chang. Strateg. Manag. 2019, 11, 696–715. [Google Scholar] [CrossRef]
- Alemu, A.; Feyissa, T.; Letta, T.; Abeyo, B. Genetic diversity and population structure analysis based on the high density SNP markers in Ethiopian durum wheat (Triticum turgidum ssp. durum). BMC Genet. 2020, 21, 1–12. [Google Scholar] [CrossRef]
- Mengistu, D.K.; Kiros, A.Y.; Pè, M.E. Phenotypic diversity in Ethiopian durum wheat (Triticum turgidum var. durum) landraces. Crop J. 2015, 3, 190–199. [Google Scholar] [CrossRef]
- Harlan, J.R. Ethiopia: A center of diversity. Econ. Bot. 1969, 23, 309–314. [Google Scholar] [CrossRef]
- Mengistu, D.K.; Kidane, Y.G.; Catellani, M.; Frascaroli, E.; Fadda, C.; Pè, M.E.; Dell’Acqua, M. High-density molecular characterization and association mapping in Ethiopian durum wheat landraces reveals high diversity and potential for wheat breeding. Plant Biotechnol. J. 2016, 14, 1800–1812. [Google Scholar] [CrossRef]
- Maccaferri, M.; Harris, N.S.; Twardziok, S.O.; Pasam, R.K.; Gundlach, H.; Spannagl, M.; Ormanbekova, D.; Lux, T.; Prade, V.M.; Milner, S.G.; et al. Durum wheat genome highlights past domestication signatures and future improvement targets. Nat. Genet. 2019, 51, 885–895. [Google Scholar] [CrossRef]
- Rao, I.U.; Pandey, B.K. Origin and introduction of crop plants, cereals, and pulses. Econ. Bot. 2007, 1–34. [Google Scholar]
- Hedberg, I.; Edwards, S. Flora os Ethiopia and Eritrea Poaceae. Natl. Herb. Ethiop. 1995, 7, 1–420. [Google Scholar]
- Kabbaj, H.; Sall, A.T.; Al-Abdallat, A.; Geleta, M.; Amri, A.; Filali-Maltouf, A.; Belkadi, B.; Ortiz, R.; Bassi, F.M. Genetic diversity within a global panel of durum wheat (Triticum durum) landraces and modern germplasm reveals the history of alleles exchange. Front. Plant. Sci. 2017, 8, 1–13. [Google Scholar] [CrossRef]
- Vavilov, N.I. Phytogeographic basis of plant breeding. The origin, variation, immunity, and breeding of cultivated plants. Agron. J. 1952, 44, 102. [Google Scholar]
- Adhikari, U.; Nejadhashemi, A.P.; Woznicki, S.A. Climate change and eastern Africa: A review of impact on major crops. Food Energy Secur. 2015, 4, 110–132. [Google Scholar] [CrossRef]
- Al-Othman, Z.A.; Ali, R.; Al-Othman, A.M.; Ali, J.A.; Habila, M. Assessment of toxic metals in wheat crops grown on selected soils, irrigated by different water sources. Arab. J. Chem. 2016, 9, S1555–S1562. [Google Scholar] [CrossRef]
- Duan, H.; Zhu, Y.; Li, J.; Ding, W.; Wang, H.; Jiang, L.; Zhou, Y. Effects of drought stress on growth and development of wheat seedlings. Int. J. Environ. Res. Public Health 2017, 19, 1119–1124. [Google Scholar]
- Abhinandan, K.; Skori, L.; Stanic, M.; Hickerson, N.M.N.; Jamshed, M.; Samuel, M.A. Abiotic stress signaling in wheat–an inclusive overview of hormonal interactions during abiotic stress responses in wheat. Front. Plant Sci. 2018, 9, 1–25. [Google Scholar] [CrossRef]
- Lamaoui, M.; Jemo, M.; Datla, R.; Bekkaoui, F. Heat and drought stresses in crops and approaches for their mitigation. Front. Chem. 2018, 6, 1–14. [Google Scholar] [CrossRef]
- DalCorso, G.; Fasani, E.; Manara, A.; Visioli, G.; Furini, A. Heavy metal pollutions: State of the art and innovation in phytoremediation. Int. J. Mol. Sci. 2019, 20, 3412. [Google Scholar] [CrossRef] [PubMed]
- Belay, A.; Recha, J.W.; Woldeamanuel, T.; Morton, J.F. Smallholder farmers’ adaptation to climate change and determinants of their adaptation decisions in the Central Rift Valley of Ethiopia. Agric. Food Secur. 2017, 6, 1–14. [Google Scholar] [CrossRef]
- Sultan, M.A.R.F.; Hui, L.; Yang, L.J.; Xian, Z.H. Assessment of drought tolerance of some triticum l. species through physiological indices. Czech J. Genet. Plant. Breed. 2012, 48, 178–184. [Google Scholar] [CrossRef]
- Rampino, P.; Pataleo, S.; Gerardi, C.; Mita, G.; Perrotta, C. Drought stress response in wheat: Physiological and molecular analysis of resistant and sensitive genotypes. Plant. Cell. Environ. 2006, 29, 2143–2152. [Google Scholar] [CrossRef] [PubMed]
- Polle, T.M.A.E.; Konzak, C.F. Screening spring wheat for drought tolerance. In Developments in Plant and Soil Sciences; Springer: Cham, Switzerland, 1987; pp. 79–87. [Google Scholar]
- Sharifi, P.; Mohammadkhani, N. Effects of drought stress on photosynthesis factors in wheat genotypes during anthesis. Cereal Res. Commun. 2016, 44, 229–239. [Google Scholar] [CrossRef]
- Lawlor, D.W.; Cornic, G. Photosynthetic carbon assimilation and associated metabolism in relation to water deficits in higher plants. Plant Cell Environ. 2002, 25, 275–294. [Google Scholar] [CrossRef] [PubMed]
- Szegletes, Z.; Erdei, L.; Tari, I.; Cseuz, L. Accumulation of osmoprotectants in wheat cultivars of different drought tolerance. Cereal Res. Commun. 2000, 28, 403–410. [Google Scholar] [CrossRef]
- Zhu, J.-K. Salt and drought stress signal transduction in plants. Annu. Rev. Plant. Biol. 2002, 53, 247–273. [Google Scholar] [CrossRef]
- Zivcak, M.; Brestic, M.; Balatova, Z.; Drevenakova, P.; Olsovska, K.; Kalaji, H.M.; Yang, X.; Allakhverdiev, S.I. Photosynthetic electron transport and specific photoprotective responses in wheat leaves under drought stress. Photosynth. Res. 2013, 117, 529–546. [Google Scholar] [CrossRef]
- Pour-Aboughadareh, A.; Omidi, M.; Naghavi, M.R.; Etminan, A.; Mehrabi, A.A.; Poczai, P.; Bayat, H. Effect of water deficit stress on seedling biomass and physio-chemical characteristics in different species of wheat possessing the D genome. Agronomy 2019, 9, 522. [Google Scholar] [CrossRef]
- Botyanszka, L.; Zivcak, M.; Chovancek, E.; Sytar, O.; Barek, V.; Hauptvogel, P.; Halabul, A.; Brestic, M. Chlorophyll fluorescence kinetics may be useful to identify early drought and irrigation effects on photosynthetic apparatus in field-grown wheat. Agronomy 2020, 10, 1275. [Google Scholar] [CrossRef]
- Sharma, D.K.; Andersen, S.B.; Ottosen, C.O.; Rosenqvist, E. Wheat cultivars selected for high Fv/Fm under heat stress maintain high photosynthesis, total chlorophyll, stomatal conductance, transpiration and dry matter. Physiol Plant. 2015, 153, 284–298. [Google Scholar] [CrossRef] [PubMed]
- Gonfa, A.B.; Tesfaye, K. Response of Ethiopian durum wheat genotypes to water deficit induced at various growth stages. Afr. J. Plant Sci. 2011, 5, 855–861. [Google Scholar]
- Mwadzingeni, L.; Shimelis, H.; Tesfay, S.; Tsilo, T.J. Screening of bread wheat genotypes for drought tolerance using phenotypic and proline analyses. Front. Plant Sci. 2016, 7, 1276. [Google Scholar] [CrossRef]
- Torabi, M.; Mokhtarzadeh, A.; Mahlooji, M. The role of hydroponics technique as a standard methodology in various aspects of plant biology researches. In Hydroponics a Standard Methodology for Plant Biological Researches; IntechOpen: London, UK, 2009. [Google Scholar]
- Ayalew, H.; Ma, X.; Yan, G. Screening wheat (triticum spp.) genotypes for root length under contrasting water regimes: Potential sources of variability for drought resistance breeding. J. Agron. Crop Sci. 2014, 201, 189–194. [Google Scholar] [CrossRef]
- Michel, B.E.; Kaufmann, M.R. The osmotic potential of polyethylene glycol 6000. Plant Physiol. 1973, 51, 914–916. [Google Scholar] [CrossRef]
- Mdluli, S.Y.; Shimelis, H.; Mashilo, J. Screening for pre- and post-anthesis drought responses in selected bread wheat (Triticum aestivum L.) genotypes. Acta Agric. Scand. Sect. B Soil Plant Sci. 2020, 70, 272–284. [Google Scholar] [CrossRef]
- Liwani, U.; Magwaza, L.S.; Odindo, A.O.; Sithole, N.J. Growth, morphological and yield responses of irrigated wheat (Triticum aestivum L.) genotypes to water stress. Acta Agric. Scand. Sect. B Soil Plant Sci. 2019, 69, 369–376. [Google Scholar] [CrossRef]
- Faisal, S.; Mujtaba, S.M.; Asma; Mahboob, W. Polyethylene glycol mediated osmotic stress impacts on growth and biochemical aspects of wheat (Triticum aestivum L.). J. Crop Sci. Biotechnol. 2019, 22, 213–223. [Google Scholar] [CrossRef]
- Ahmad, N.S.; Kareem, S.H.S.; Mustafa, K.M.; Ahmad, D.A. Early screening of some Kurdistan wheat (Triticum aestivum L.) cultivars under drought stress. J. Agric. Sci. 2017, 9, 88–103. [Google Scholar] [CrossRef]
- Bansal, R.; Pradheep, K.; Kumari, J.; Kumar, S.; Yadav, M.C.; Gurung, B.; Kumari, N.K.P.; Rana, J.C. Physiological and biochemical evaluation for drought tolerance in wheat germplasm collected from arid western plains of India. Indian J. Biochem. Biophys. 2016, 53, 212–217. [Google Scholar]
- Sallam, A.; Mourad, A.M.I.; Hussain, W.; Baenziger, P.S. Genetic variation in drought tolerance at seedling stage and grain yield in low rainfall environments in wheat (Triticum aestivum L.). Euphytica 2018, 214. [Google Scholar] [CrossRef]
- Khan, A.S.; Allah, S.U.; Sadique, S. Genetic variability and correlation among seedling traits of wheat (Triticum aestivum) under water stress. Int. J. Agric. Biol. 2010, 12, 247–250. [Google Scholar]
- Tripathi, P.; Rushton, P.J. Understanding water-stress responses in soybean using hydroponics system—A systems biology perspective. Front. Plant Sci. 2015, 6, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Toit, D.; Adriaan, A.G. Hydroponics as a Tool in Wheat Breeding. 2005. Available online: http://etd.uovs.ac.za/ETD-db/theses/available/etd-02142006-081835/unrestricted/DUTOITAGA.pdf (accessed on 30 November 2020).
- Shavrukov, Y.; Genc, Y.; Hayes, J. The use of hydroponics in abiotic stress tolerance research. hydroponics-a stand. In Hydroponics. A Standard Methodology for Plant Biological Researches; IntechOpen: London, UK, 2012. [Google Scholar]
- Liu, H.; Able, A.J.; Able, J.A. Genotypic water-deficit stress responses in durum wheat: Association between physiological traits, microRNA regulatory modules and field components. Funct. Plant Biol. 2017, 44, 538–551. [Google Scholar] [CrossRef]
- Figueroa-Bustos, V.; Palta, J.A.; Chen, Y.; Siddique, K.H.M. Early season drought largely reduces grain yield in wheat cultivars with smaller root systems. Plants 2019, 8, 305. [Google Scholar] [CrossRef]
- Szira, F.; Bálint, A.F.; Börner, A.; Galiba, G. Evaluation of drought-related traits and screening methods at different developmental stages in spring barley. J. Agron. Crop Sci. 2008, 194, 334–342. [Google Scholar] [CrossRef]
- Ahmed, H.G.M.D.; Khan, A.S.; LI, M.-ju.; Khan, S.H.; Kashif, M. Early selection of bread wheat genotypes using morphological and photosynthetic attributes conferring drought tolerance. J. Integr. Agric. 2019, 18, 2483–2491. [Google Scholar] [CrossRef]
- Ayalew, H.; Dessalegn, T.; Liu, H.; Yan, G.; Markos, D. Performance of Ethiopian bread wheat (Tritium aestivum L.) genotypes under contrasting water regimes: Potential sources of variability for drought resistance breeding. Aust. J. Crop Sci. 2016, 10, 370–376. [Google Scholar] [CrossRef]
- Sewak, R.; Tomar, S.; Tiwari, S.; Naik, B.K. Molecular and morpho-agronomical characterization of root architecture at seedling and reproductive stages for drought tolerance in wheat. PLoS ONE 2016, 11, e0156528. [Google Scholar] [CrossRef]
- Ahmed, H.G.M.D.; Zeng, Y.; Yang, X.; Anwaar, H.A.; Mansha, M.Z.; Hanif, C.M.S.; Ikram, K.; Ullah, A.; Alghanem, S.M.S. Conferring drought-tolerant wheat genotypes through morpho-physiological and chlorophyll indices at seedling stage. Saudi J. Biol. Sci. 2020, 27, 2116–2123. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, K.; Shabbir, G.; Ahmed, M.; Nawaz, K. Phenotyping for drought resistance in bread wheat using physiological and biochemical traits. Sci. Total Environ. 2020, 729, 139082. [Google Scholar] [CrossRef] [PubMed]
- Pour, A.; Alireza, A.; Mostafa, E.; Siddique, K.H.M. Assessment of biochemical and physiological parameters of durum wheat genotypes at the seedling stage during polyethylene glycol- induced water stress. Plant Growth Regul. 2020, 92, 81–93. [Google Scholar] [CrossRef]
- Liu, H.; Searle, I.R.; Mather, D.E.; Able, A.J.; Able, J.A. Morphological, physiological and yield responses of durum wheat to pre-anthesis water-deficit stress are genotype-dependent. Crop Pasture Sci. 2015, 66, 1024–1038. [Google Scholar] [CrossRef]
- Lin, Y.; Yi, X.; Tang, S.; Chen, W.; Wu, F.; Yang, X.; Jiang, X.; Shi, H.; Ma, J.; Chen, G.; et al. Dissection of phenotypic and genetic variation of drought-related traits in diverse Chinese wheat landraces. Plant Genome 2019, 12, 190025. [Google Scholar] [CrossRef]
- Lopes, M.S.; El-Basyoni, I.; Baenziger, P.S.; Singh, S.; Royo, C.; Ozbek, K.; Aktas, H.; Ozer, E.; Ozdemir, F.; Manickavelu, A.; et al. Exploiting genetic diversity from landraces in wheat breeding for adaptation to climate change. J. Ex. Bot. 2015, 66, 3477–3486. [Google Scholar] [CrossRef]
- Hoagland, D.R.; Arnon, D.I. The water-culture method for growing plants without soil. Circ. Calif. Agric. Exp. Stn. 1950, 347. [Google Scholar]
- Cao, H.X.; Zhang, Z.B.; Sun, C.X.; Shao, H.B.; Song, W.Y.; Xu, P. Chromosomal location of traits associated with wheat seedling water and phosphorus use efficiency under different water and phosphorus stresses. Int. J. Mol. Sci. 2009, 10, 4116–4136. [Google Scholar] [CrossRef]
- Qaseem, M.F.; Qureshi, R.; Shaheen, H. Effects of pre-anthesis drought, heat and their combination on the growth, yield and physiology of diverse wheat (triticum aestivum l.) genotypes varying in sensitivity to heat and drought stress. Sci. Rep. 2019, 9, 1–12. [Google Scholar] [CrossRef]
- Turner, N.C. Crop water deficits: A decade of progress. Adv. Agron. 1986, 39, C1–C51. [Google Scholar]
- Barrs, H.D.; Weatherley, P.E. A re-examination of the relative turgidity technique for estimating water deficits in leaves. Aust. J. Biol. Sci. 1962, 15, 413–428. [Google Scholar] [CrossRef]
- Decagon Device Inc. Operator’s Manual; Decagon Devices Inc.: Pullman, WA, USA, 2002; Available online: www.decagon.com (accessed on 30 July 2020).
- Lichtenthaler, H.K. Chlorophylls and carotenoids: Pigments of photosynthetic biomembranes. Methods Enzymol. 1987, 148, C350–C382. [Google Scholar]
- Bates, L.S.; Waldren, R.P.; Teare, I.D. Rapid determination of free proline for water-stress studies. Plant Soil. 1973, 39, 205–207. [Google Scholar] [CrossRef]
- de Mendiburu, F. CRAN-Package Agricolae. Available online: https://cran.r-project.org/web/packages/agricolae/index.html (accessed on 17 February 2021).
- Pour-Aboughadareh, A.; Yousefian, M.; Moradkhani, H.; Moghaddam Vahed, M.; Poczai, P.; Siddique, K.H.M. iPASTIC: An online toolkit to estimate plant abiotic stress indices. Appl. Plant Sci. 2019, 7, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Arteaga, S.; Yabor, L.; Díez, M.J.; Prohens, J.; Boscaiu, M.; Vicente, O. The use of proline in screening for tolerance to drought and salinity in common bean (Phaseolus vulgaris L.) genotypes. Agronomy 2020, 10, 817. [Google Scholar] [CrossRef]
- De Carvalho, M.A.A.P.; Bebeli, P.J.; Bettencourt, E.; Costa, G.; Dias, S.; Santos, T.M.M.D.; Slaski, J. Cereal landraces genetic resources in worldwide GeneBanks. A review. Agron. Sustain. Dev. 2013, 33, 177–203. [Google Scholar] [CrossRef]
- Sall, A.T.; Chiari, T.; Legesse, W.; Seid-Ahmed, K.; Ortiz, R.; van Ginkel, M.; Bassi, F.M. Durum wheat (Triticum durum Desf.): Origin, cultivation and potential expansion in Sub-Saharan Africa. Agronomy 2019, 9, 263. [Google Scholar] [CrossRef]
- Tshikunde, N.M.; Mashilo, J.; Shimelis, H.; Odindo, A. Agronomic and Physiological Traits, and Associated Quantitative Trait Loci (QTL) Affecting Yield Response in Wheat (Triticum aestivum L.): A Review. Front. Plant Sci. 2019, 10, 1428. [Google Scholar] [CrossRef]
- Ahmed, H.G.M.; Sajjad, M.; Li, M.; Azmat, M.A.; Rizwan, M.; Maqsood, R.H. Selection criteria for drought-tolerant bread wheat genotypes at seedling stage. Sustainability 2019, 11, 2584. [Google Scholar] [CrossRef]
- Baloch, M.J.; Dunwell, J.; Khan, N.U.; Jatoi, W.A.; Khakhwani, A.A.; Vessar, N.F.; Gul, S. Morpho-physiological characterization of spring wheat genotypes under drought stress. Int. J. Agric. Biol. 2013, 15, 945–950. [Google Scholar]
- Djanaguiraman, M.; Prasad, P.V.V.; Kumari, J.; Rengel, Z. Root length and root lipid composition contribute to drought tolerance of winter and spring wheat. Plant Soil 2019, 439, 57–73. [Google Scholar] [CrossRef]
- Jain, N.; Singh, G.P.; Yadav, R.; Pandey, R.; Ramya, P.; Shine, M.B.; Prabhu, K.V. Root trait characteristics and genotypic response in wheat under different water regimes. Cereal Res. Commun. 2014, 42, 426–438. [Google Scholar] [CrossRef]
- Pour-Aboughadareh, A.; Mohammadi, R.; Etminan, A.; Shooshtari, L.; Maleki-Tabrizi, N.; Poczai, P. Effects of drought stress on some agronomic and morpho-physiological traits in durum wheat genotypes. Sustainability 2020, 12, 5610. [Google Scholar] [CrossRef]
- Pour-aboughadareh, A.; Ahmadi, J.; Mehrabi, A.A.; Etminan, A. Physiological responses to drought stress in wild relatives of wheat: Implications for wheat improvement Physiological responses to drought stress in wild relatives of wheat: Implications for wheat improvement. Acta Physiol. Plant. 2017, 39, 106. [Google Scholar] [CrossRef]
- Xu, W.; Cui, K.; Xu, A.; Nie, L.; Huang, J.; Peng, S. Drought stress condition increases root to shoot ratio via alteration of carbohydrate partitioning and enzymatic activity in rice seedlings. Acta Physiol. Plant. 2015, 37, 9. [Google Scholar] [CrossRef]
- Grzesiak, M.T.; Hordyńska, N.; Maksymowicz, A.; Grzesiak, S.; Szechyńska-Hebda, M. Variation among spring wheat (Triticum aestivum L.) genotypes in response to the drought stress. II—Root system structure. Plants 2019, 8, 584. [Google Scholar] [CrossRef] [PubMed]
- Ratzmann, G.; Zakharova, L.; Tietjen, B. Optimal leaf water status regulation of plants in drylands. Sci. Rep. 2019, 9, 1–9. [Google Scholar] [CrossRef]
- Agathokleous, E.; Belz, R.G.; Kitao, M.; Koike, T.; Calabrese, E.J. Does the root to shoot ratio show a hormetic response to stress? An ecological and environmental perspective. J. For. Res. 2019, 30, 1569–1580. [Google Scholar] [CrossRef]
- Dryden, G. Water use and requirements. Anim. Nutr. Sci. 2015, 115–129. [Google Scholar]
- Wang, L.; Wang, S.; Chen, W.; Li, H.; Deng, X. Physiological mechanisms contributing to increased water-use efficiency in winter wheat under organic fertilization. PLoS ONE 2017, 12, e0180205. [Google Scholar] [CrossRef]
- Cajero-Sanchez, W.; Aceves-Garcia, P.; Fernández-Marcos, M.; Gutiérrez, C.; Rosas, U.; García-Ponce, B.; Alverez-Buylla, E.; de la Paz Sanchez, M.; Garay-Arroyo, A. Natural root cellular variation in responses to osmotic stress in arabidopsis thaliana accessions. Genes 2019, 10, 983. [Google Scholar] [CrossRef]
- Nakhforoosh, A.; Grausgruber, H.; Kaul, H.P.; Bodner, G. Dissection of drought response of modern and underutilized wheat varieties according to passioura’s yield-water framework. Front. Plant Sci. 2015, 6, 1–13. [Google Scholar] [CrossRef]
- Blum, A. Osmotic adjustment is a prime drought stress adaptive engine in support of plant production. Plant Cell Environ. 2017, 40, 4–10. [Google Scholar] [CrossRef]
- Kim, Y.; Chung, Y.S.; Lee, E.; Tripathi, P.; Heo, S.; Kim, K.H. Root response to drought stress in rice (Oryza sativa L.). Int. J. Mol. Sci. 2020, 21, 1513. [Google Scholar] [CrossRef]
- Nakhforoosh, A.; Bodewein, T.; Fiorani, F.; Bodner, G. Identification of water use strategies at early growth stages in durum wheat from shoot phenotyping and physiological measurements. Front. Plant Sci. 2016, 7, 1–13. [Google Scholar] [CrossRef]
- Kerstiens, G. Cuticular water permeability and its physiological significance. J. Exp. Bot. 1996, 47, 1813–1832. [Google Scholar] [CrossRef]
- Gašparovič, K.; Živčák, M.; Brestič, M.; Hauptvogel, P. Diversity of leaf cuticular transpiration and growth traits in field-grown wheat and aegilops genetic resources. Agronomy 2021, 11, 522. [Google Scholar] [CrossRef]
- Boyer, J.S.; James, R.A.; Munns, R.; Condon, T.; Passioura, J.B. Osmotic adjustment leads to anomalously low estimates of relative water content in wheat and barley. Funct. Plant Biol. 2008, 35, 1172–1182. [Google Scholar] [CrossRef] [PubMed]
- Shivakrishna, P.; Reddy, K.A.; Rao, D.M. Effect of PEG-6000 imposed drought stress on RNA content, relative water content (RWC), and chlorophyll content in peanut leaves and roots. Saudi J. Biol. Sci. 2018, 25, 285–289. [Google Scholar] [CrossRef]
- Bota, J.; Medrano, H.; Flexas, J. Is photosynthesis limited by decreased Rubisco activity and RuBP content under progressive water stress? New Phytol. 2004, 162, 671–681. [Google Scholar] [CrossRef]
- Use, W.; Zhao, W.; Liu, L.; Shen, Q.; Yang, J.; Han, X. Effects of water stress on photosynthesis, yield, and water use efficiency in winter wheat. Water 2020, 12, 2127. [Google Scholar]
- Laffray, D.; Louguet, P. Stomatal responses and drought resistance. Bull. la Soc. Bot. Fr. Actual. Bot. 1990, 137, 47–60. [Google Scholar] [CrossRef]
- Ahmad, Z.; Waraich, E.A.; Akhtar, S.; Anjum, S.; Ahmad, T.; Mahboob, W.; Hafeez, O.B.A.; Tapera, T.; Labuschagne, M.; Rizwan, M. Physiological responses of wheat to drought stress and its mitigation approaches. Acta Physiol. Plant 2018, 40. [Google Scholar] [CrossRef]
- Hussain, H.A.; Men, S.; Saddam, H.; Chen, Y.; Ali, S.; Zhang, S.; Zhang, K.; Li, Y.; Xu, Q.; Liao, C.; et al. Interactive effects of drought and heat stresses on morpho- physiological attributes, yield, nutrient uptake and oxidative status in maize hybrids. Sci. Rep. 2019, 9, 3890. [Google Scholar] [CrossRef]
- Faisal, S.; Mujtaba, S.M.; Khan, M.A.; Mahboob, W. Morpho-physiological assessment of wheat (Triticum aestivum L.) genotypes for drought stress tolerance at seedling stage. Pak. J. Bot. 2017, 49, 445–452. [Google Scholar]
- Upadhyay, D.; Budhlakoti, N.; Kumar, A.S.; Bansal, R.; Kumari, J.; Chaudhary, N.; Padaria, J.C.; Sareen, S.; Kumar, S. Drought tolerance in Triticum aestivum L. genotypes associated with enhanced antioxidative protection and declined lipid peroxidation. 3 Biotech. 2020, 10, 1–17. [Google Scholar] [CrossRef]
- Ibarra-Caballero, J.; Villanueva-Verduzco, C.; Molina-Galán, J.; Sánchez-de-Jiménez, E. Proline accumulation as a symptom of drought stress in maize: A tissue differentiation requirement. J. Exp. Bot. 1988, 39, 889–897. [Google Scholar] [CrossRef]
- Geravandi, M.; Farshadfar, E.; Kahrizi, D. Evaluation of some physiological traits as indicators of drought tolerance in bread wheat genotypes. Russ. J. Plant Physiol. 2011, 58, 69–75. [Google Scholar] [CrossRef]
- Semahegn, Y.; Shimelis, H.; Laing, M.; Mathew, I. Evaluation of bread wheat (Triticum aestivum L.) genotypes for yield and related traits under drought stress conditions. Acta Agric. Scand. Sect. B Soil Plant. Sci. 2020, 70, 474–484. [Google Scholar] [CrossRef]
- Sallam, A.; Alqudah, A.M.; Dawood, M.F.A.; Baenziger, P.S.; Börner, A. drought stress tolerance in wheat and barley: Advances in physiology, breeding and genetics research. Int. J. Mol. Sci. 2019, 20, 3137. [Google Scholar] [CrossRef]
- De la Rosa, L.; Zambrana, E.; Ramirez-Parra, E. Molecular bases for drought tolerance in common vetch: Designing new molecular breeding tools. BMC Plant Biol. 2020, 20, 71. [Google Scholar] [CrossRef]
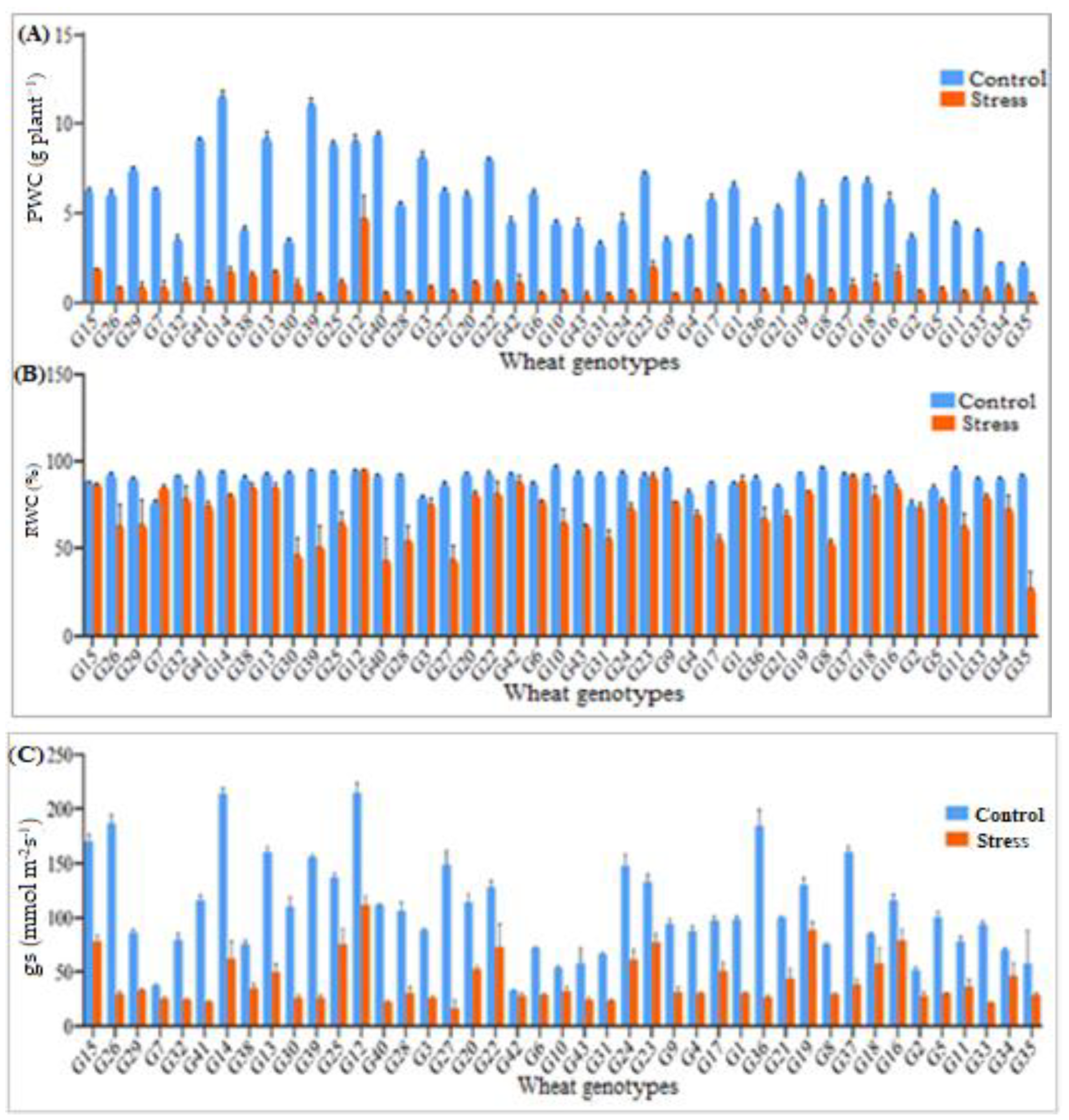
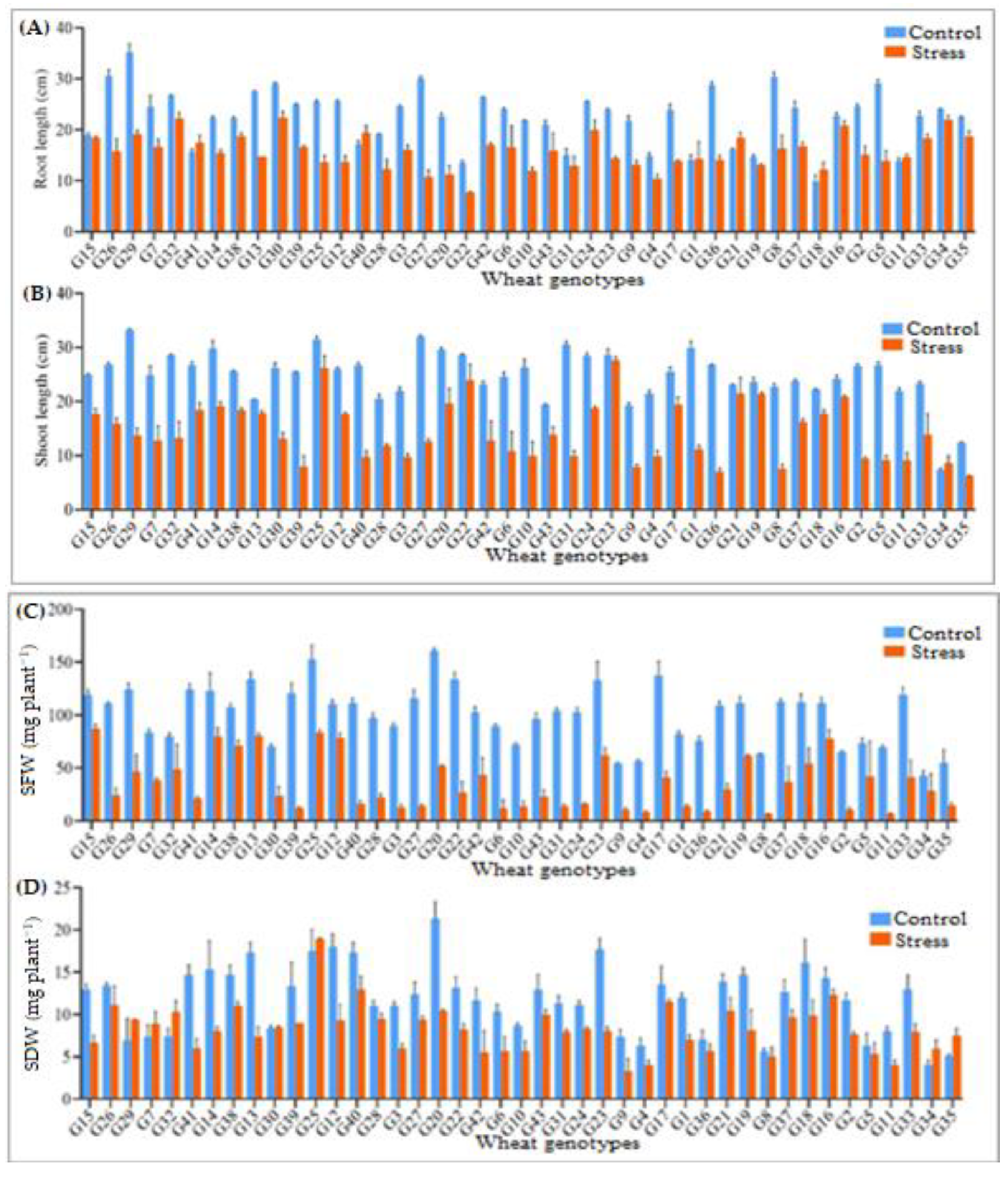
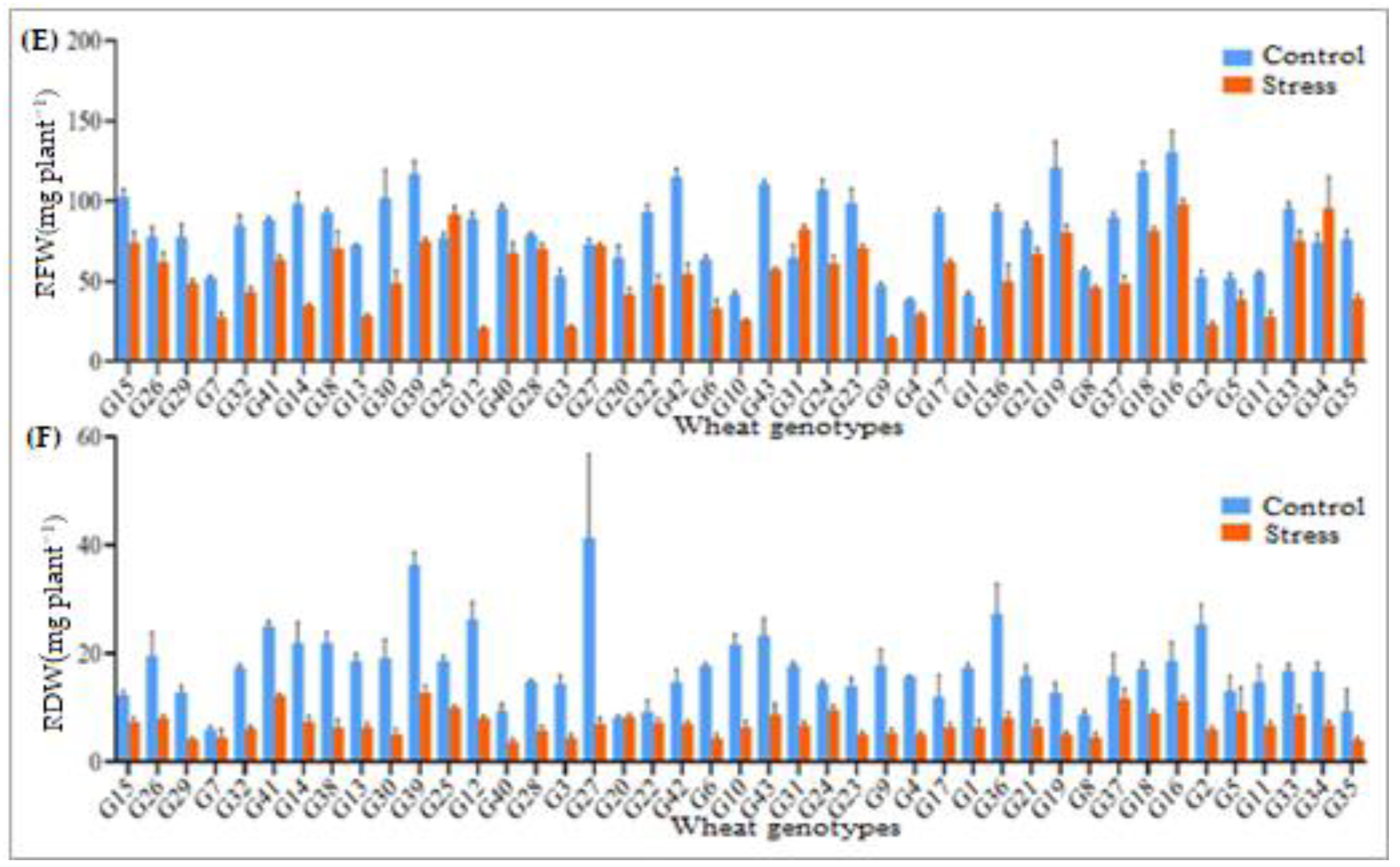
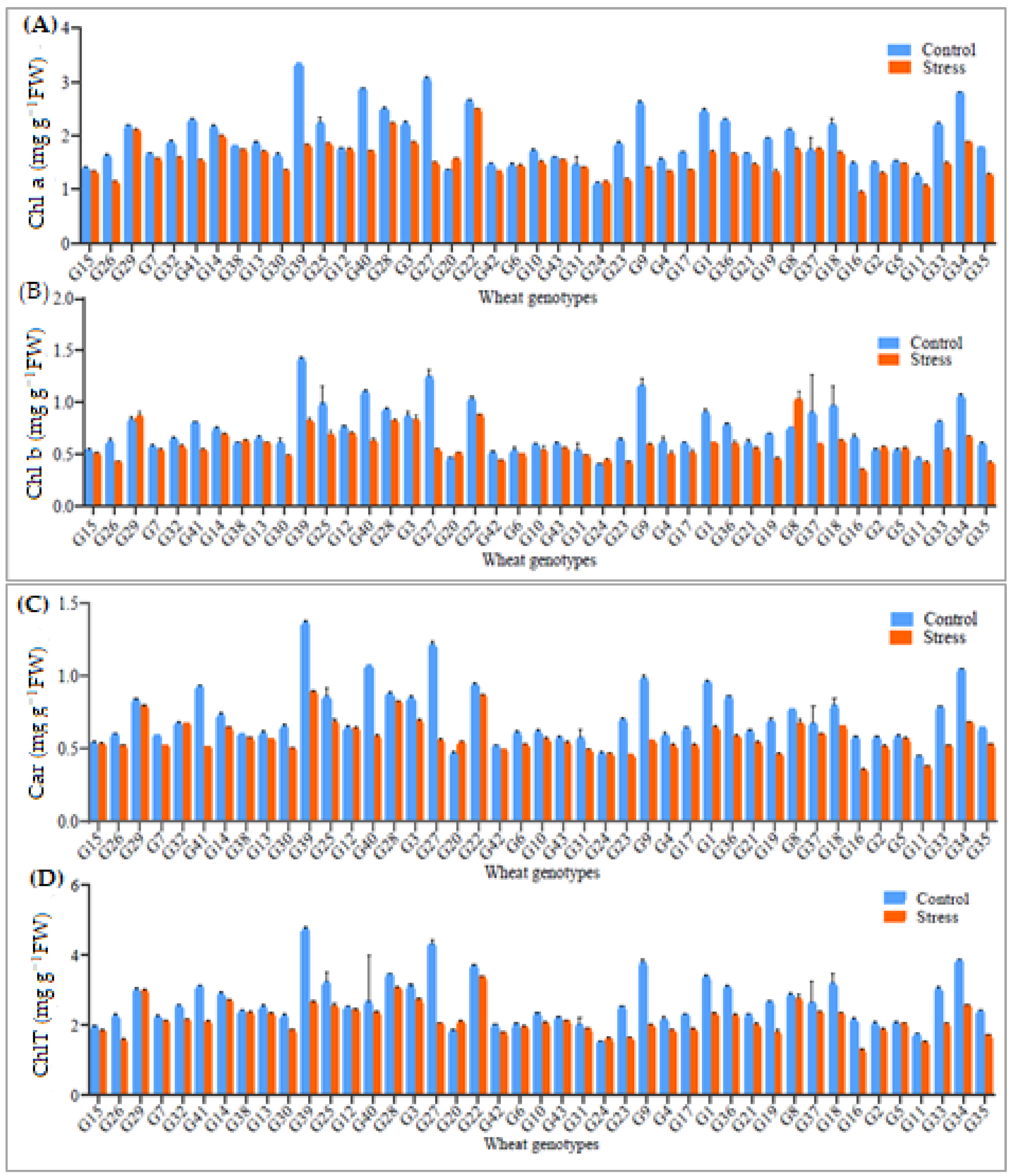
| Sources of Variation | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Control | Stress | ||||||||||||
| Traits | Trt | G | Trt X G | MSerr | CV (%) | LSD5% | Min | Max | Mean ± SD | Min | Max | Mean ± SD | RC (%) |
| DF | 1 | 42 | 42 | 172 | |||||||||
| PWC | 1613.85 *** | 419.5 *** | 278.6 *** | 0.16 | 11.3 | 17.47 | 2.13 | 11.6 | 6.03 ± 2.24 | 0.43 | 4.73 | 1.03 ± 0.71 | 83.0 |
| RWC | 24947.2 *** | 357.5 *** | 53.6 *** | 53.6 | 9.0 | 17.47 | 76.1 | 97.1 | 91.1 ± 4.8 | 27.1 | 95.2 | 71.4 ± 15.3 | 21.6 |
| Gs | 291312 *** | 5238 *** | 163 *** | 163 | 16.9 | 30.49 | 32.8 | 214.6 | 109.1 ± 44.8 | 16.1 | 112.0 | 41.9 ± 22.1 | 61.6 |
| RL | 2988.6 *** | 81.4 *** | 4.63 *** | 4.63 | 11.2 | 5.14 | 9.94 | 35.11 | 22.6 ± 5.53 | 7.70 | 22.4 | 15.7 ± 333 | 30.4 |
| SL | 7104.8 *** | 106.7 *** | 5.0 *** | 5.0 | 11.4 | 5.35 | 7.33 | 33.22 | 24.9 ± 4.8 | 6.30 | 27.6 | 14.4 ± 5.41 | 42.2 |
| RL:SL | 9.6 *** | 1.37*** | 0.09 *** | 0.09 | 25.7 | 0.73 | 0.45 | 3.29 | 0.97 ± 0.45 | 0.32 | 2.97 | 1.26 ± 0.56 | −30.7 |
| SFW | 273553 *** | 3291 *** | 208 *** | 208 | 21.3 | 34.4 | 42.7 | 160.3 | 100.4 ± 27.8 | 6.70 | 87.3 | 35.3 ± 25.0 | 64.9 |
| SDW | 568.8 *** | 88.3 *** | 24 ns | 24.01 | 47.3 | 5.14 | 4.07 | 21.33 | 11.8 ± 4.04 | 3.30 | 18.9 | 8.31 ± 2.82 | 25.1 |
| RFW | 51949 *** | 2636 *** | 106 *** | 106 | 15.3 | 24.5 | 38.3 | 130.3 | 81.7 ± 23.8 | 15.0 | 98.0 | 53.3 ± 22.5 | 34.8 |
| RDW | 3657.4 *** | 99.3 *** | 19.5 *** | 19.5 | 36.1 | 10.5 | 6.00 | 41.3 | 17.5 ± 6.91 | 3.67 | 12.7 | 7.03 ± 2.27 | 55.2 |
| Car | 1.47 *** | 0.13 *** | 0.001 *** | 0.001 | 4.8 | 0.07 | 0.45 | 1.37 | 0.74 ± 020 | 0.36 | 0.90 | 0.59 ± 0.12 | 20.5 |
| Chla | 10.05 *** | 0.82 *** | 0.003 *** | 0.003 | 3.1 | 0.14 | 1.13 | 3.35 | 1.97 ± 0.51 | 0.94 | 2.50 | 1.58 ± 0.31 | 20.0 |
| Chlb | 1.61 *** | 0.16 *** | 0.01 *** | 0.01 | 14.9 | 0.21 | 0.41 | 1.42 | 0.75 ± 0.23 | 0.35 | 1.04 | 0.59 ± 0.14 | 21.0 |
| ChlT | 19.7 *** | 1.67 *** | 0.021 *** | 0.021 | 5.9 | 0.34 | 1.54 | 4.77 | 2.69 ± 0.70 | 1.30 | 3.38 | 2.17 ± 044 | 20.3 |
| PC | 23321.8 *** | 1605.5 *** | 70.2 *** | 70.2 | 44.6 | 20.01 | −1.09 | 45.00 | 9.27 ± 9.78 | 0.70 | 141.2 | 28.3 ± 27.7 | −205.1 |
| Stress | Control | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Traits | PC-1 | PC-2 | PC-3 | PC-4 | PC-5 | Traits | PC-1 | PC-2 | PC-3 | PC-4 | PC-5 |
| PWC | 0.323 | −0.014 | −0.219 | 0.356 | −0.234 | PWC | 0.310 | 0.173 | −0.118 | 0.052 | 0.397 |
| RWC | 0.227 | −0.058 | −0.356 | 0.222 | 0.529 | RWC | 0.143 | 0.086 | 0.440 | −0.141 | −0.282 |
| gs | 0.351 | −0.030 | −0.182 | 0.048 | −0.168 | gs | 0.264 | 0.173 | 0.184 | 0.275 | 0.097 |
| RL | −0.032 | −0.135 | 0.435 | 0.500 | 0.089 | RL | −0.005 | −0.086 | 0.236 | 0.737 | 0.171 |
| SL | 0.369 | −0.057 | −0.019 | −0.196 | 0.113 | SL | 0.142 | 0.306 | −0.383 | 0.344 | −0.056 |
| RL: SL | −0.313 | 0.024 | 0.237 | 0.367 | −0.158 | RL: SL | −0.075 | −0.351 | 0.477 | 0.150 | 0.121 |
| SFW | 0.366 | −0.041 | −0.006 | 0.193 | 0.056 | SFW | 0.252 | 0.368 | 0.047 | −0.012 | 0.173 |
| SDW | 0.254 | 0.044 | 0.387 | −0.211 | −0.271 | SDW | 0.227 | 0.371 | 0.029 | −0.136 | 0.058 |
| RFW | 0.166 | −0.052 | 0.504 | −0.241 | 0.150 | RFW | 0.175 | 0.184 | 0.453 | −0.138 | −0.206 |
| RDW | 0.157 | −0.031 | 0.151 | −0.324 | 0.390 | RDW | 0.212 | −0.090 | −0.072 | 0.381 | −0.480 |
| Car | 0.009 | 0.483 | 0.073 | −0.005 | 0.054 | Car | 0.341 | −0.307 | −0.138 | −0.041 | −0.003 |
| Chla | 0.056 | 0.486 | 0.009 | 0.033 | 0.117 | Chla | 0.341 | −0.306 | −0.104 | −0.099 | 0.038 |
| Chlb | −0.013 | 0.478 | 0.001 | 0.096 | 0.014 | Chlb | 0.350 | −0.285 | −0.091 | −0.095 | 0.012 |
| ChlT | 0.035 | 0.500 | 0.007 | 0.056 | 0.089 | ChlT | 0.337 | −0.317 | −0.080 | −0.044 | −0.002 |
| PC | −0.167 | 0.089 | −0.265 | −0.368 | −0.309 | PC | −0.206 | −0.142 | 0.011 | −0.016 | 0.555 |
| STI | 0.326 | 0.049 | −0.012 | 0.032 | −0.464 | ||||||
| E.value | 5.460 | 3.869 | 1.991 | 1.403 | 1.023 | E.value | 5.162 | 3.554 | 1.542 | 1.384 | 1.092 |
| Var. (%) | 32.120 | 22.759 | 11.715 | 8.254 | 6.019 | Var. (%) | 32.260 | 22.211 | 9.637 | 8.648 | 6.827 |
| Cum. (%) | 32.120 | 54.878 | 66.593 | 74.846 | 80.866 | Cum. (%) | 32.260 | 54.471 | 64.107 | 72.755 | 79.582 |
| Traits | PWC | RWC | Gs | RL | SL | RL:SL | SFW | SDW | RFW | RDW | Car | Chla | Chlb | ChlT | PC |
| PWC | 1 | 0.120 | 0.588 | −0.009 | 0.412 | −0.350 | 0.636 | 0.595 | 0.232 | 0.165 | 0.373 | 0.374 | 0.392 | 0.331 | −0.157 |
| RWC | 0.522 | 1 | 0.303 | −0.038 | 0.011 | −0.015 | 0.231 | 0.218 | 0.407 | 0.084 | 0.082 | 0.116 | 0.145 | 0.119 | −0.197 |
| Gs | 0.766 | 0.479 | 1 | 0.159 | 0.319 | −0.150 | 0.460 | 0.455 | 0.333 | 0.340 | 0.192 | 0.185 | 0.243 | 0.210 | −0.267 |
| RL | −0.014 | −0.075 | −0.157 | 1 | 0.157 | 0.447 | −0.026 | −0.234 | −0.045 | 0.196 | 0.014 | −0.018 | −0.027 | 0.023 | 0.089 |
| SL | 0.476 | 0.457 | 0.709 | −0.113 | 1 | −0.698 | 0.541 | 0.380 | 0.029 | 0.190 | 0.010 | −0.041 | −0.042 | −0.060 | −0.340 |
| RL:SL | −0.395 | −0.506 | −0.540 | 0.493 | −0.827 | 1 | −0.440 | −0.461 | −0.081 | −0.019 | 0.140 | 0.158 | 0.119 | 0.186 | 0.193 |
| SFW | 0.712 | 0.537 | 0.703 | 0.099 | 0.697 | −0.530 | 1 | 0.839 | 0.461 | 0.030 | 0.040 | 0.055 | 0.087 | 0.050 | −0.403 |
| SDW | 0.164 | −0.113 | 0.280 | 0.125 | 0.552 | −0.365 | 0.453 | 1 | 0.425 | 0.121 | −0.017 | 0.010 | 0.059 | −0.035 | −0.399 |
| RFW | −0.039 | −0.138 | 0.213 | 0.225 | 0.386 | −0.157 | 0.288 | 0.515 | 1 | 0.143 | 0.052 | 0.061 | 0.095 | 0.049 | −0.289 |
| RDW | 0.104 | 0.222 | 0.178 | 0.001 | 0.279 | −0.265 | 0.206 | 0.251 | 0.375 | 1 | 0.430 | 0.362 | 0.399 | 0.446 | −0.290 |
| Car | −0.037 | −0.111 | −0.044 | −0.172 | −0.095 | 0.082 | −0.054 | 0.139 | −0.006 | 0.044 | 1 | 0.979 | 0.953 | 0.945 | −0.211 |
| Chla | 0.038 | 0.028 | 0.007 | −0.229 | 0.028 | −0.066 | 0.061 | 0.144 | −0.043 | −0.027 | 0.897 | 1 | 0.949 | 0.953 | −0.192 |
| Chlb | 0.007 | −0.095 | −0.049 | −0.161 | −0.142 | 0.109 | −0.086 | 0.017 | −0.109 | −0.089 | 0.868 | 0.849 | 1 | 0.944 | −0.205 |
| ChlT | 0.029 | −0.009 | −0.012 | −0.212 | −0.028 | −0.009 | 0.015 | 0.107 | −0.067 | −0.047 | 0.919 | 0.985 | 0.928 | 1 | −0.189 |
| PC | −0.267 | −0.257 | −0.108 | −0.333 | −0.284 | 0.135 | −0.366 | −0.283 | −0.313 | −0.047 | 0.137 | 0.043 | 0.128 | 0.072 | 1 |
| STI | 0.757 | 0.215 | 0.618 | −0.124 | 0.493 | −0.444 | 0.530 | 0.608 | 0.132 | 0.253 | 0.080 | 0.128 | 0.066 | 0.112 | −0.210 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Belay, G.A.; Zhang, Z.; Xu, P. Physio-Morphological and Biochemical Trait-Based Evaluation of Ethiopian and Chinese Wheat Germplasm for Drought Tolerance at the Seedling Stage. Sustainability 2021, 13, 4605. https://doi.org/10.3390/su13094605
Belay GA, Zhang Z, Xu P. Physio-Morphological and Biochemical Trait-Based Evaluation of Ethiopian and Chinese Wheat Germplasm for Drought Tolerance at the Seedling Stage. Sustainability. 2021; 13(9):4605. https://doi.org/10.3390/su13094605
Chicago/Turabian StyleBelay, Gizie Abeje, Zhengbin Zhang, and Ping Xu. 2021. "Physio-Morphological and Biochemical Trait-Based Evaluation of Ethiopian and Chinese Wheat Germplasm for Drought Tolerance at the Seedling Stage" Sustainability 13, no. 9: 4605. https://doi.org/10.3390/su13094605
APA StyleBelay, G. A., Zhang, Z., & Xu, P. (2021). Physio-Morphological and Biochemical Trait-Based Evaluation of Ethiopian and Chinese Wheat Germplasm for Drought Tolerance at the Seedling Stage. Sustainability, 13(9), 4605. https://doi.org/10.3390/su13094605







