Waste-Derived Green Nanocatalyst for Biodiesel Production: Kinetic-Mechanism Deduction and Optimization Studies
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Catalyst Preparation
2.2.1. Catalyst Prepared via Calcination Method
2.2.2. Catalyst Prepared via Thermal Hydration and Dehydration Method
2.3. Parameter Effects
2.4. Transesterification Reaction
2.5. Gas-Chromatography Analysis for Biodiesel
2.6. Catalysts Characterization
3. Results
3.1. Catalyst-Characterization Results
3.1.1. Thermogravimetric (TGA) Analysis
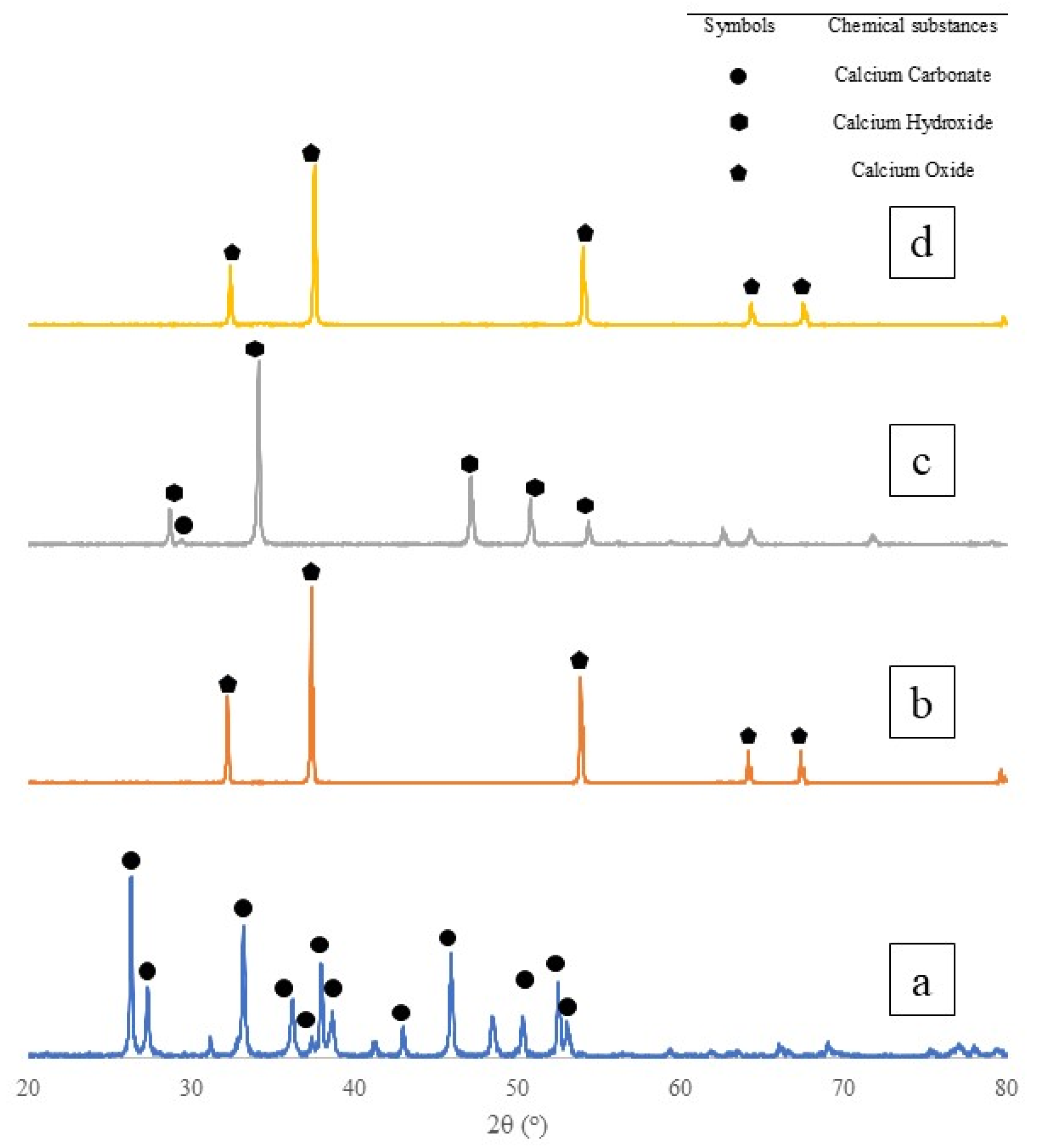
3.1.2. X-ray Diffraction (XRD) Analysis
3.1.3. Scanning Electron Microscopy (SEM) Analysis
3.1.4. Energy-Dispersive X-ray (EDX) Analysis
3.1.5. Temperature-Programmed Desorption (TPD) Analysis
3.1.6. Nitrogen Physisorption
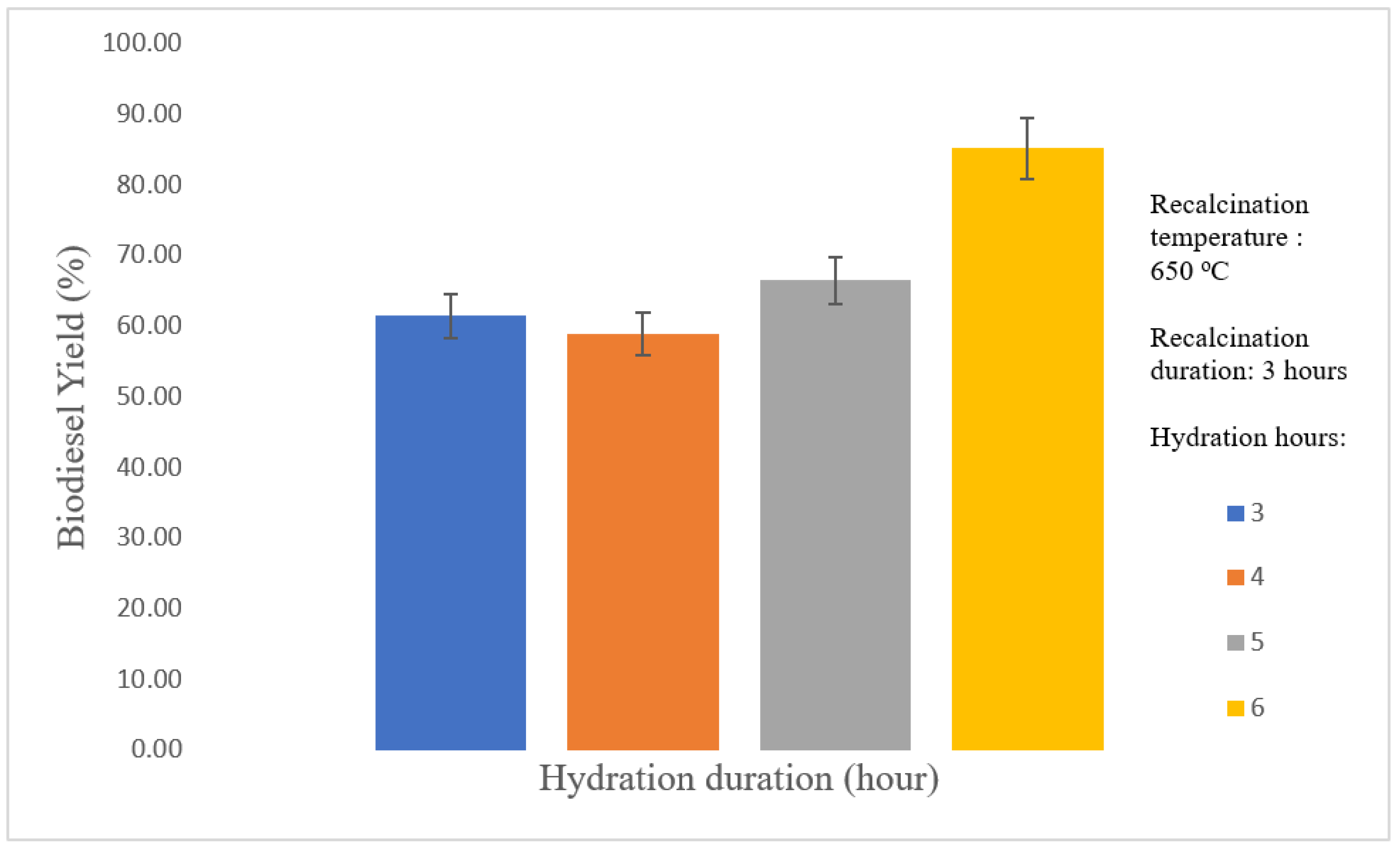
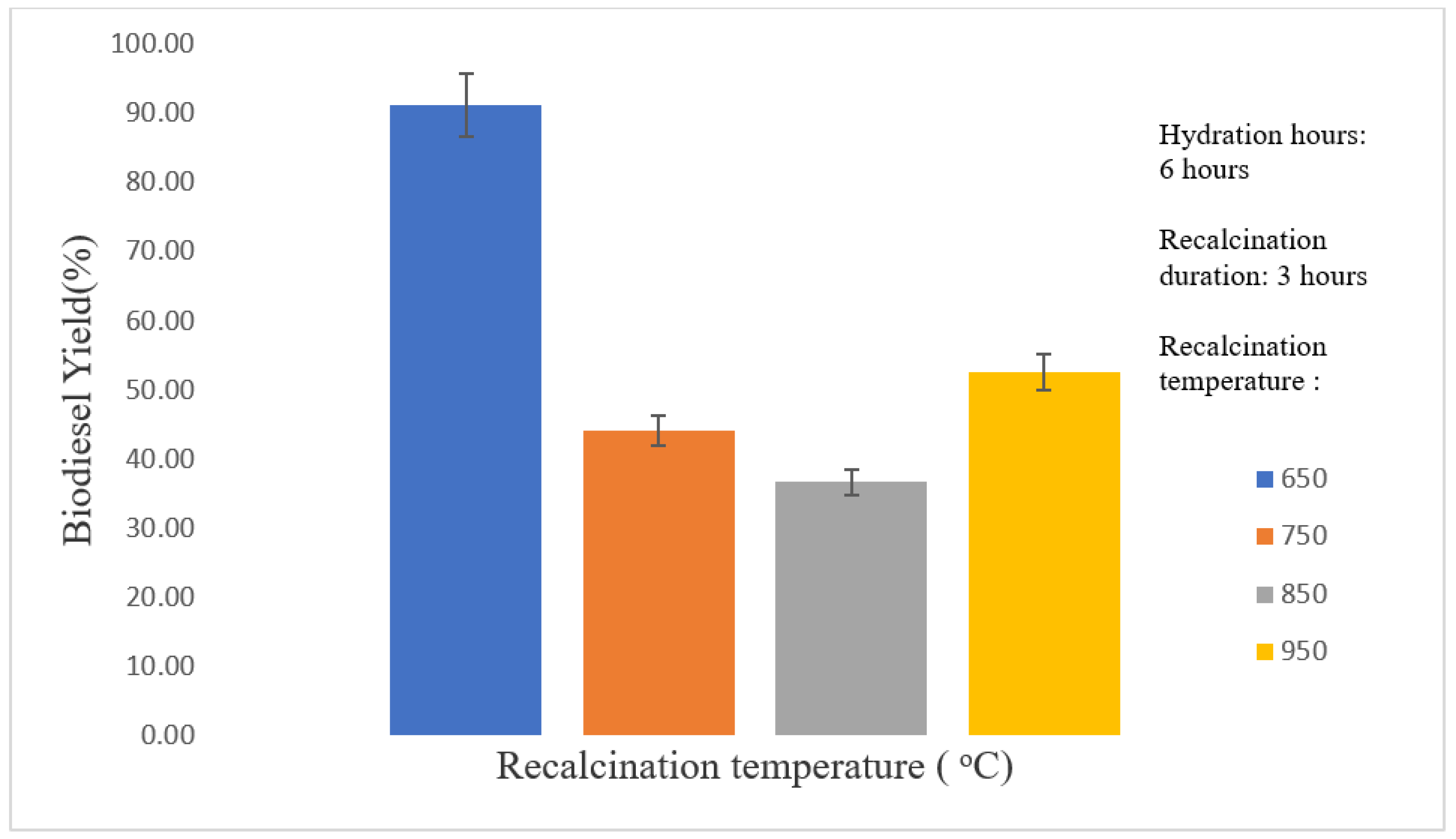
3.2. Optimization of Thermal Hydration–Dehydration Treatment
3.2.1. Effect of Hydration Duration
3.2.2. Effect of Recalcination Temperature
3.2.3. Effect of Recalcination Duration
3.3. Kinetic Mechanism
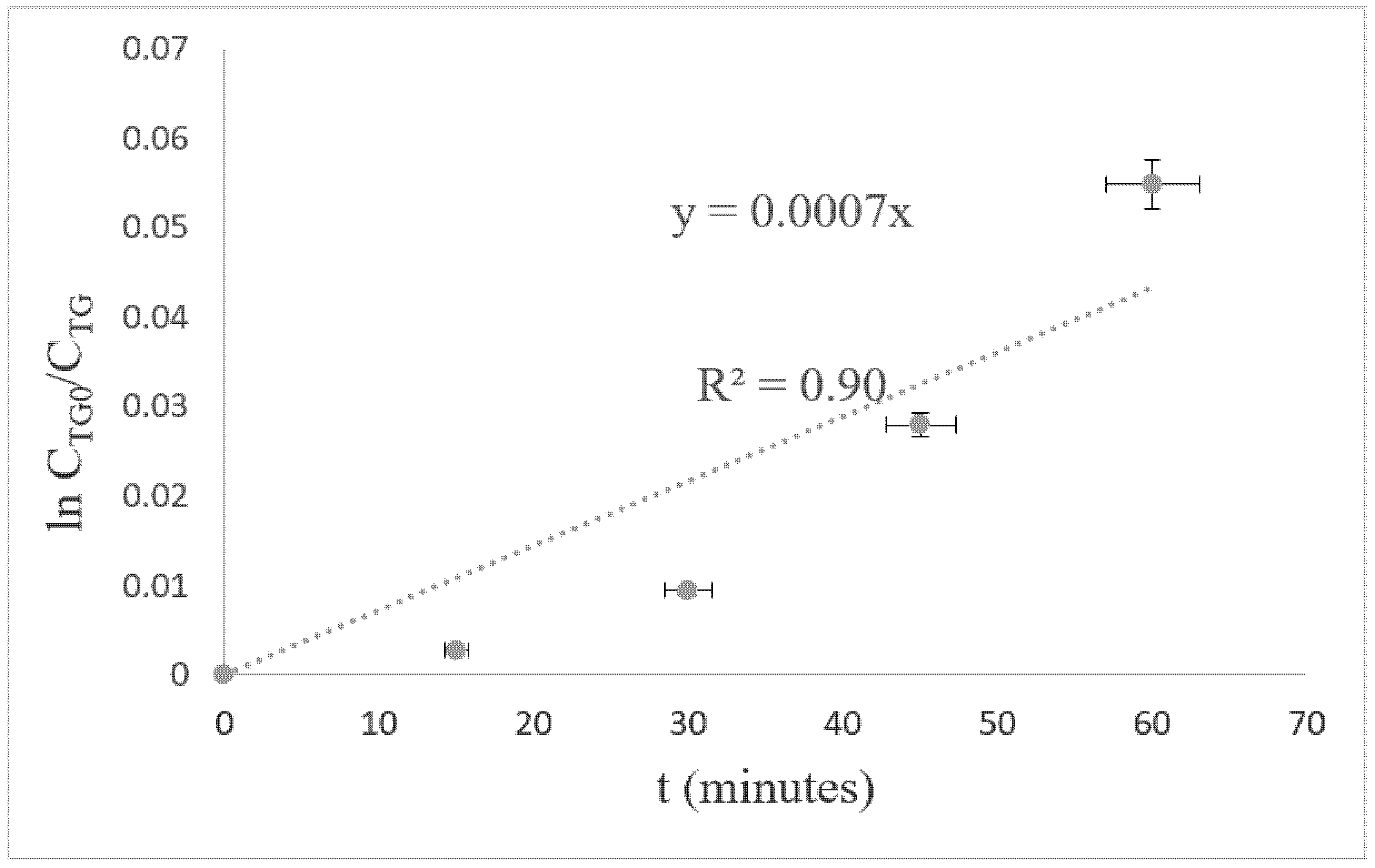
3.3.1. Derivation of Kinetic-Rate Equation
- Triglycerides were the limiting reactant, whereas methanol was the excess reactant.
- Triglyceride molecules were attached to the active sites and reacted with free-moving methanol molecules.
- Glycerol was free-moving after transesterification, while FAME molecules remained attached to the vacant sites.
- The surface reaction was assumed to be a rate-limiting step.
3.3.2. Kinetic Equation and Experiment Data
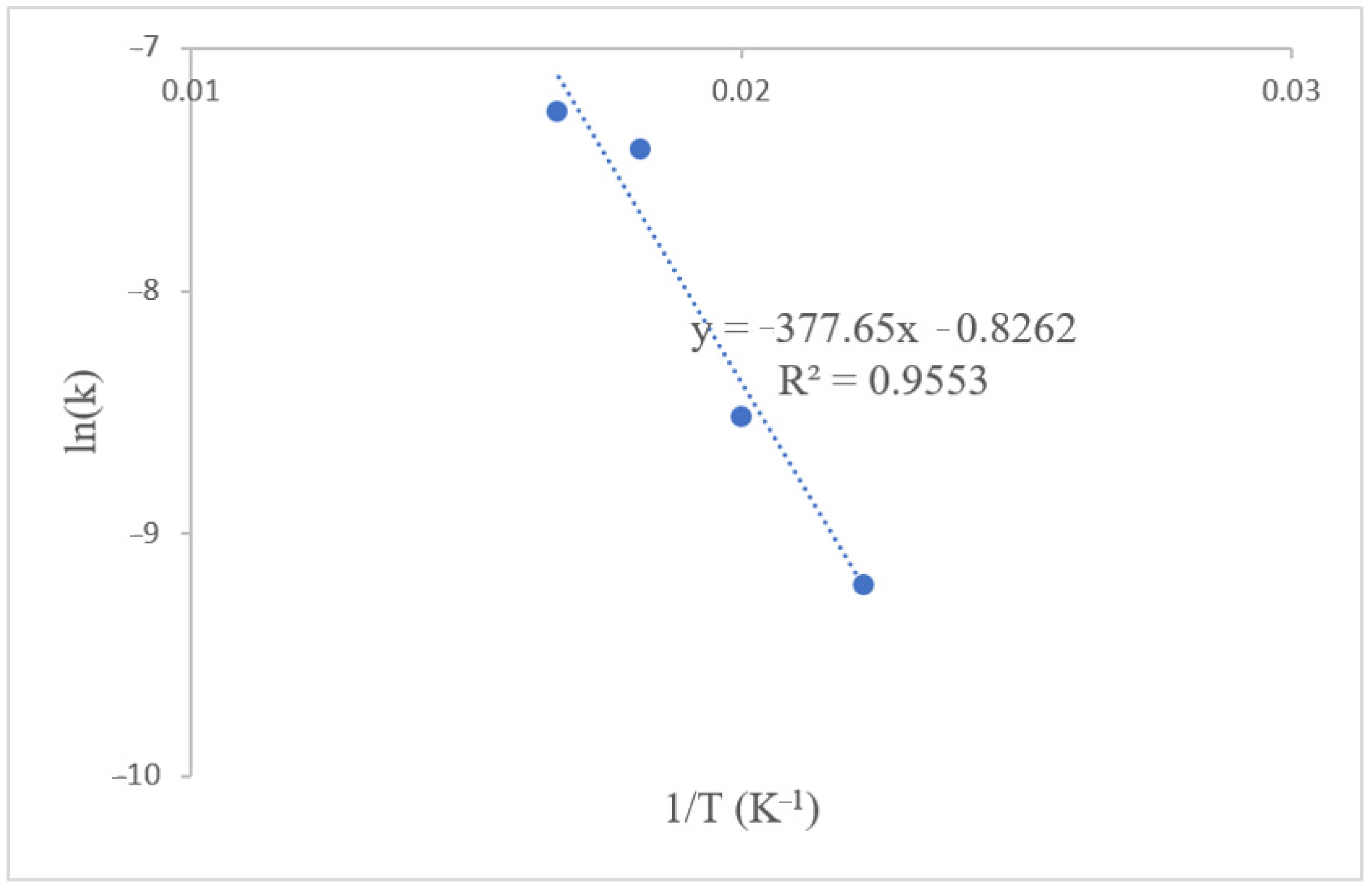
3.3.3. Activation Energy and Prefrequency Factor
4. Conclusions
Author Contributions
Funding
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Looney, B. Statistical Review of World Energy, 2020|69th Edition. BP. 2020. Available online: https://www.bp.com/content/dam/bp/business-sites/en/global/corporate/pdfs/energy-economics/statistical-review/bp-stats-review-2020-full-report.pdf. (accessed on 3 February 2021).
- Leung, D.Y.C.; Wu, X.; Leung, M.K.H. A review on biodiesel production using catalyzed transesterification. Appl. Energy 2010, 87, 1083–1095. [Google Scholar] [CrossRef]
- Ahmad, A.L.; Yasin, N.H.M.; Derek, C.J.C.; Lim, J.K. Microalgae as a sustainable energy source for biodiesel production: A review. Renew. Sustain. Energy Rev. 2011, 15, 584–593. [Google Scholar] [CrossRef]
- Borges, M.E.; Díaz, L. Recent developments on heterogeneous catalysts for biodiesel production by oil esterification and transesterification reactions: A review. Renew. Sustain. Energy Rev. 2012, 16, 2839–2849. [Google Scholar] [CrossRef]
- Ezebor, F.; Khairuddean, M.; Abdullah, A.Z.; Boey, P.L. Oil palm trunk and sugarcane bagasse derived solid acid catalysts for rapid esterification of fatty acids and moisture-assisted transesterification of oils under pseudo-infinite methanol. Bioresour. Technol. 2014, 157, 254–262. [Google Scholar] [CrossRef]
- Cheirsilp, B.; Louhasakul, Y. Industrial wastes as a promising renewable source for production of microbial lipid and direct transesterification of the lipid into biodiesel. Bioresour. Technol. 2013, 142, 329–337. [Google Scholar] [CrossRef]
- Dehkhoda, A.M.; West, A.H.; Ellis, N. Biochar based solid acid catalyst for biodiesel production. Appl. Catal. A Gen. 2010, 382, 197–204. [Google Scholar] [CrossRef]
- Balat, M. Production of biodiesel from vegetable oils: A survey. Energy Sources Part A Recover. Util. Environ. Eff. 2007, 29, 895–913. [Google Scholar] [CrossRef]
- Talebian-Kiakalaieh, A.; Amin, N.A.S.; Mazaheri, H. A review on novel processes of biodiesel production from waste cooking oil. Appl. Energy 2013, 104, 683–710. [Google Scholar] [CrossRef]
- Canakci, M.; Van Gerpen, J. Biodiesel production from oils and fats with high free fatty acids. Trans. Am. Soc. Agric. Eng. 2001, 44, 1429–1436. [Google Scholar] [CrossRef]
- Karmakar, A.; Karmakar, S.; Mukherjee, S. Properties of various plants and animals feedstocks for biodiesel production. Bioresour. Technol. 2010, 101, 7201–7210. [Google Scholar] [CrossRef]
- Borugadda, V.B.; Goud, V.V. Biodiesel production from renewable feedstocks: Status and opportunities. Renew. Sustain. Energy Rev. 2012, 16, 4763–4784. [Google Scholar] [CrossRef]
- Kumar, N.; Varun; Chauhan, S.R. Performance and emission characteristics of biodiesel from different origins: A review. Renew. Sustain. Energy Rev. 2013, 21, 633–658. [Google Scholar] [CrossRef]
- Yaakob, Z.; Mohammad, M.; Alherbawi, M.; Alam, Z.; Sopian, K. Overview of the production of biodiesel from Waste cooking oil. Renew. Sustain. Energy Rev. 2013, 18, 184–193. [Google Scholar] [CrossRef]
- Atadashi, I.M.; Aroua, M.K.; Aziz, A.A. Biodiesel separation and purification: A review. Renew. Energy 2011, 36, 437–443. [Google Scholar] [CrossRef]
- Sharma, Y.C.; Singh, B.; Korstad, J. Latest developments on application of heterogenous basic catalysts for an efficient and eco friendly synthesis of biodiesel: A review. Fuel 2011, 90, 1309–1324. [Google Scholar] [CrossRef]
- Mosaddegh, E.; Hassankhani, A. Preparation and characterization of nano-CaO based on eggshell waste: Novel and green catalytic approach to highly efficient synthesis of pyrano[4,3-b]pyrans. Chin. J. Catal. 2014, 35, 351–356. [Google Scholar] [CrossRef]
- Chen, G.-Y.; Shan, R.; Yan, B.-B.; Shi, J.-F.; Li, S.-Y.; Liu, C.-Y. Remarkably enhancing the biodiesel yield from palm oil upon abalone shell-derived CaO catalysts treated by ethanol. Fuel Process. Technol. 2016, 143, 110–117. [Google Scholar] [CrossRef]
- Lesbani, A.; Okta, S.; Sitompul, C.; Mohadi, R.; Hidayati, N. Characterization and Utilization of Calcium Oxide (CaO) Thermally Decomposed from Fish Bones as a Catalyst in the Production of Biodiesel from Waste Cooking Oil. Chem. Eng. 2016, 20, 121–126. [Google Scholar] [CrossRef]
- Reddy, C.; Reddy, V.; Oshel, R.; Verkade, J.G. Room-temperature conversion of soybean oil and poultry fat to biodiesel catalyzed by nanocrystalline calcium oxides. Energy Fuels 2006, 20, 1310–1314. [Google Scholar] [CrossRef]
- Kesic, Z.; Lukic, I.; Zdujic, M.; Mojovic, L.; Skala, D. Calcium oxide based catalysts for biodiesel production: A review. Chem. Ind. Chem. Eng. Q. 2016, 22, 391–408. [Google Scholar] [CrossRef]
- Islam, N.; Miyazaki, K. Nanotechnology systems of innovation: Investigation of scientific disciplines’ fusion trend into nanotech. In Proceedings of the PICMET’07—2007 Portland International Conference on Management of Engineering & Technology, Portland, OR, USA, 5–9 August 2007; pp. 2922–2931. [Google Scholar]
- Yilmaz, B.; Müller, U. Catalytic Applications of Zeolites in Chemical Industry. Top. Catal. 2009, 52, 888–895. [Google Scholar] [CrossRef]
- Schmidt, F. New catalyst preparation technologies—Observed from an industrial viewpoint. Appl. Catal. A Gen. 2001, 221, 15–21. [Google Scholar] [CrossRef]
- Zhang, Y.; Dubé, M.A.; McLean, D.D.; Kates, M. Biodiesel production from waste cooking oil: 1. Process design and technological assessment. Bioresour. Technol. 2003, 89, 1–16. [Google Scholar] [CrossRef]
- Garrigue, P.; Delville, M.H.; Labrugère, C.; Cloutet, E.; Kulesza, P.J.; Morand, J.P.; Kuhn, A. Top-down approach for the preparation of colloidal carbon nanoparticles. Chem. Mater. 2004, 16, 2984–2986. [Google Scholar] [CrossRef]
- Nadagouda, M.N.; Varma, R.S. Green and controlled synthesis of gold and platinum nanomaterials using vitamin B2: Density-assisted self-assembly of nanospheres, wires and rods. Green Chem. 2006, 8, 516–518. [Google Scholar] [CrossRef]
- Kumar, D.; Ali, A. Nanocrystalline K–CaO for the transesterification of a variety of feedstocks: Structure, kinetics and catalytic properties. Biomass Bioenergy 2012, 46, 459–468. [Google Scholar] [CrossRef]
- Zhao, L.; Qiu, Z.; Stagg-Williams, S.M. Transesterification of canola oil catalyzed by nanopowder calcium oxide. Fuel Process. Technol. 2013, 114, 154–162. [Google Scholar] [CrossRef]
- Latchubugata, C.S.; Kondapaneni, R.V.; Patluri, K.K.; Virendra, U.; Vedantam, S. Kinetics and optimization studies using Response Surface Methodology in biodiesel production using heterogeneous catalyst. Chem. Eng. Res. Des. 2018, 135, 129–139. [Google Scholar] [CrossRef]
- Ljupkovic, R.; Micic, R.; Tomic, M.; Radulovic, N.; Bojic, A.; Zarubica, A. Significance of the structural properties of CaO catalyst in the production of biodiesel: An effect on the reduction of greenhouse gases emission. Hem. Ind. 2014, 68, 399–412. [Google Scholar] [CrossRef]
- Vujicic, D.; Comic, D.; Zarubica, A.; Micic, R.; Boskovic, G. Kinetics of biodiesel synthesis from sunflower oil over CaO heterogeneous catalyst. Fuel 2010, 89, 2054–2061. [Google Scholar] [CrossRef]
- Micic, R.D.; Bosnjak Kiralj, M.S.; Panic, S.N.; Tomic, M.D.; Jovic, B.D.; Boskovic, G.C. Activation temperature imposed textural and surface synergism of CaO catalyst for sunflower oil transesterification. Fuel 2015, 159, 638–645. [Google Scholar] [CrossRef]
- Chong, K.Y.; Chia, C.H.; Zakaria, S. Polymorphs calcium carbonate on temperature reaction. AIP Conf. Proc. 2014, 1614, 52–56. [Google Scholar]
- Cho, Y.B.; Seo, G.; Chang, D.R. Transesterification of tributyrin with methanol over calcium oxide catalysts prepared from various precursors. Fuel Process. Technol. 2009, 90, 1252–1258. [Google Scholar] [CrossRef]
- Roschat, W.; Phewphong, S.; Thangthong, A.; Moonsin, P. Catalytic performance enhancement of CaO by hydration-dehydration process for biodiesel production at room temperature. Energy Convers. Manag. 2018, 165, 1–7. [Google Scholar] [CrossRef]
- Saoud, K.; Ibala, I.; Ladki, D.; Ezzeldeen, O.; Saeed, S. Microwave Assisted Preparation of Calcium Hydroxide and Barium Hydroxide Nanoparticles and Their Application for Conservation of Cultural Heritage; Springer: Cham, Switzerland, 2014. [Google Scholar]
- Widayat, W.; Darmawan, T.; Hadiyanto, H.; Rosyid, R.A. Preparation of Heterogeneous CaO Catalysts for Biodiesel Production. J. Phys. Conf. Ser. 2017, 877, 012018. [Google Scholar] [CrossRef]
- Tangboriboon, N.; Kunanuruksapong, R.; Srivat, A. Meso-porosity and phase transformation of bird eggshells via pyrolysis. J. Ceram. Process. Res. 2012, 13, 413–419. [Google Scholar]
- Asikin-Mijan, N.; Taufiq-Yap, Y.H.; Lee, H.V. Synthesis of clamshell derived Ca(OH)2 nano-particles via simple surfactant-hydration treatment. Chem. Eng. J. 2015, 262, 1043–1051. [Google Scholar] [CrossRef]
- Putra, R.S.; Liyanita, A.; Arifah, N.; Puspitasari, E.; Sawaludin; Hizam, M.N. Enhanced Electro-Catalytic Process on the Synthesis of FAME Using CaO from Eggshell. Energy Procedia 2017, 105, 289–296. [Google Scholar] [CrossRef]
- Yoosuk, B.; Udomsap, P.; Puttasawat, B.; Krasae, P. Modification of calcite by hydration–dehydration method for heterogeneous biodiesel production process: The effects of water on properties and activity. Chem. Eng. J. 2010, 162, 135–141. [Google Scholar] [CrossRef]
- Smith, S.M.; Oopathum, C.; Weeramongkhonlert, V.; Smith, C.B.; Chaveanghong, S.; Ketwong, P.; Boonyuen, S. Transesterification of soybean oil using bovine bone waste as new catalyst. Bioresour. Technol. 2013, 143, 686–690. [Google Scholar] [CrossRef]
- Galván-Ruiz, M.; Hernández, J.; Baños, L.; Noriega-Montes, J.; Rodríguez-García, M.E. Characterization of Calcium Carbonate, Calcium Oxide, and Calcium Hydroxide as Starting Point to the Improvement of Lime for Their Use in Construction. J. Mater. Civ. Eng. 2009, 21, 694–698. [Google Scholar] [CrossRef]
- Menad, K.; Feddag, A.; Rubenis, K. Synthesis and study of calcination temperature influence on the change of structural properties of the LTA zeolite. Rasayan J. Chem. 2016, 9, 788–797. [Google Scholar]
- Reli, M.; Kočí, K.; Matějka, V.; Kovář, P.; Obalová, L. Effect of calcination temperature and calcination time on the kaolinite/TiO2 composite for photocatalytic reduction of CO2. Geosci. Eng. 2012, 58, 10–22. [Google Scholar] [CrossRef]
- Chang, H.J.; Crynes, B.L. Effect of Catalyst Pore and Pellet Sizes on Deactivation in SRC Oil Hydrotreatment. AIChE J. 1986, 32, 224–232. [Google Scholar] [CrossRef]
- Shuit, S.H.; Ng, E.P.; Tan, S.H. A facile and acid-free approach towards the preparation of sulphonated multi-walled carbon nanotubes as a strong protonic acid catalyst for biodiesel production. J. Taiwan Inst. Chem. Eng. 2015, 52, 100–108. [Google Scholar] [CrossRef]
- Ten Elshof, J.E.; Abadal, C.R.; Sekulić, J.; Chowdhury, S.R.; Blank, D.H.A. Transport mechanisms of water and organic solvents through microporous silica in the pervaporation of binary liquids. Microporous Mesoporous Mater. 2003, 65, 197–208. [Google Scholar] [CrossRef]
- Jahn, D.A.; Wong, J.; Bachler, J.; Loerting, T.; Giovambattista, N. Glass polymorphism in glycerol-water mixtures: I. A computer simulation study. Phys. Chem. Chem. Phys. 2016, 18, 11042–11057. [Google Scholar] [CrossRef] [PubMed]
- Yoosuk, B.; Udomsap, P.; Puttasawat, B. Hydration–dehydration technique for property and activity improvement of calcined natural dolomite in heterogeneous biodiesel production: Structural transformation aspect. Appl. Catal. A Gen. 2011, 395, 87–94. [Google Scholar] [CrossRef]
- Freedman, B.; Butterfield, R.O.; Pryde, E.H. Transesterification kinetics of soybean oil 1. J. Am. Oil Chem. Soc. 1986, 63, 1375–1380. [Google Scholar] [CrossRef]







| FAME Types | Molecular Weight (g/mol) | Retention Time (min) |
|---|---|---|
| Methyl heptadecanoate (IS) | 284.48 | 13.27 |
| Methyl linoleate | 294.47 | 15.88 |
| Methyl oleate | 296.49 | 15.02 |
| Methyl palmitate | 270.45 | 12.17 |
| Methyl stearate | 298.50 | 14.65 |
| Samples | Average Weightage (wt %) | ||
|---|---|---|---|
| C | O | Ca | |
| Waste cockle shells | 12.48 | 44.64 | 41.20 |
| CaO catalyst prepared via calcination treatment | 0.00 | 16.72 | 83.28 |
| Hydrated CaO | 4.23 | 35.31 | 60.47 |
| Nano CaO prepared via thermal hydration–dehydration treatment | 2.29 | 16.06 | 75.65 |
| Catalyst | Total Basicity (μmol CO2/g) |
|---|---|
| CaO prepared via calcination method | 464 |
| Nano CaO prepared via thermal hydration–dehydration treatment | 1046 |
| Sample | Multipoint BET (m2/g) | Total Pore Volume (cm3/g) | Average Pore Diameter (nm) | Biodiesel Yield at 3 h Reaction Time (%) |
|---|---|---|---|---|
| CaO catalyst prepared via calcination treatment | 0.710 ± 0.004 | 0.006 | 124 | 10 |
| Nano CaO catalyst prepared via thermal hydration–dehydration treatment | ||||
| 650 °C recalcination temperature | 13.9 ± 0.2 | 0.032 | 33.2 | 94 |
| 950 °C recalcination temperature | 10.2 ± 0.08 | 0.022 | 41.8 | 49 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chooi, C.Y.; Sim, J.H.; Tee, S.F.; Lee, Z.H. Waste-Derived Green Nanocatalyst for Biodiesel Production: Kinetic-Mechanism Deduction and Optimization Studies. Sustainability 2021, 13, 5849. https://doi.org/10.3390/su13115849
Chooi CY, Sim JH, Tee SF, Lee ZH. Waste-Derived Green Nanocatalyst for Biodiesel Production: Kinetic-Mechanism Deduction and Optimization Studies. Sustainability. 2021; 13(11):5849. https://doi.org/10.3390/su13115849
Chicago/Turabian StyleChooi, Chee Yoong, Jia Huey Sim, Shiau Foon Tee, and Zhi Hua Lee. 2021. "Waste-Derived Green Nanocatalyst for Biodiesel Production: Kinetic-Mechanism Deduction and Optimization Studies" Sustainability 13, no. 11: 5849. https://doi.org/10.3390/su13115849
APA StyleChooi, C. Y., Sim, J. H., Tee, S. F., & Lee, Z. H. (2021). Waste-Derived Green Nanocatalyst for Biodiesel Production: Kinetic-Mechanism Deduction and Optimization Studies. Sustainability, 13(11), 5849. https://doi.org/10.3390/su13115849







