Forage Species Identity Shapes Soil Biota in a Temperate Agroecosystem
Abstract
1. Introduction
2. Materials and Methods
2.1. Site Description
2.2. Experimental Design
2.3. Data Collection
2.4. Data Analysis
3. Results
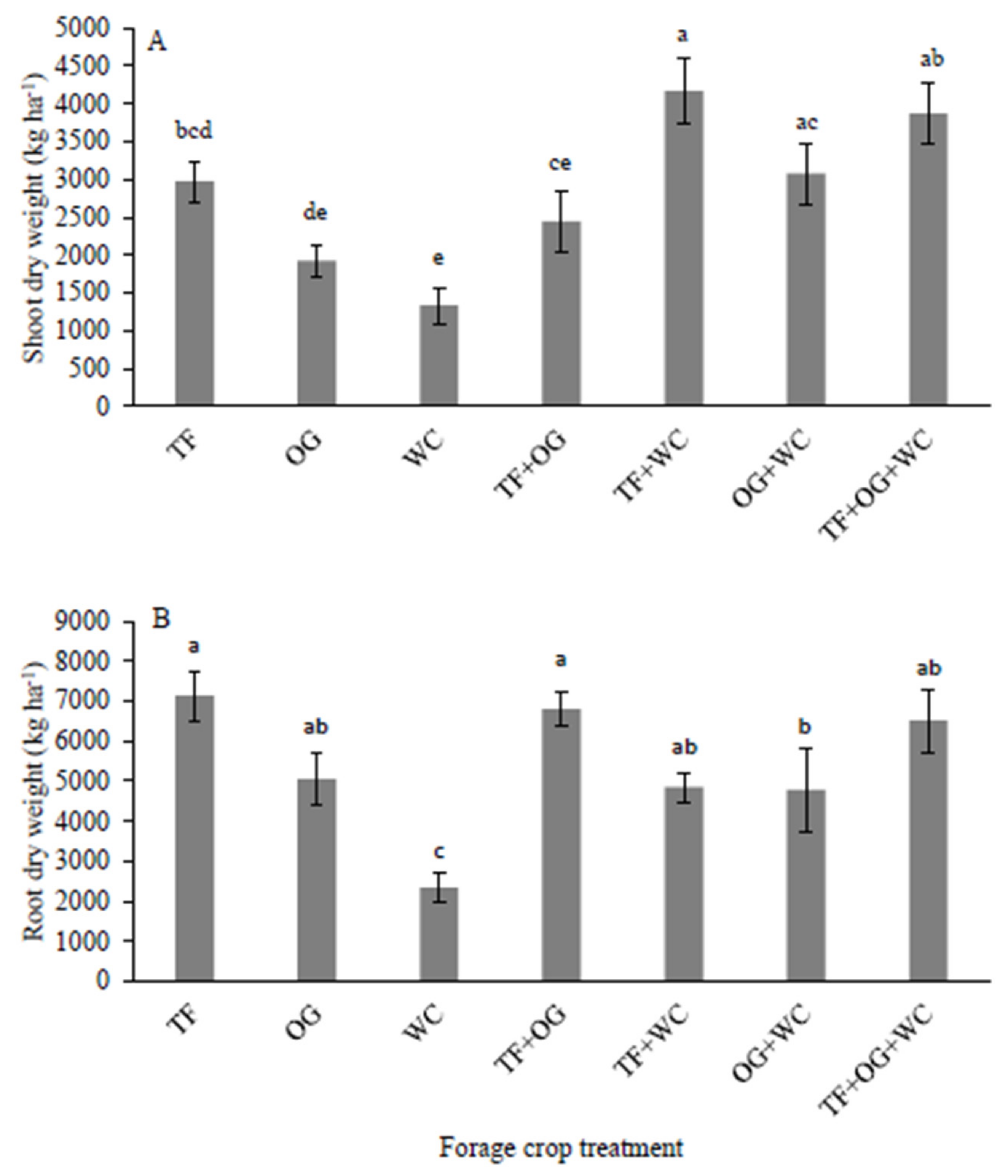
3.1. Above- and Below-Ground Biomass
3.2. Microbial Community Size, Structure, and Activity
3.3. Carbon Pools
4. Discussion
4.1. Intercropping Effects on Productivity
4.2. Crop Selection Effects on Microbial Communities and Carbon Pools
4.3. Implications for Agroecosystem Services
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Foley, J.A.; Ramankutty, N.; Brauman, K.A.; Cassidy, E.S.; Gerber, J.S.; Johnston, M.; Mueller, N.D.; O’Connell, C.; Ray, D.K.; West, P.C.; et al. Solutions for a cultivated planet. Nature 2011, 478, 337–342. [Google Scholar] [CrossRef] [PubMed]
- Robertson, G.P.; Gross, K.L.; Hamilton, S.K.; Landis, D.A.; Schmidt, T.; Snapp, S.S.; Swinton, S.M. Farming for Ecosystem Services: An Ecological Approach to Production Agriculture. Bioscience 2014, 64, 404–415. [Google Scholar] [CrossRef] [PubMed]
- Franzluebbers, A.J.; Sawchik, J.; Taboada, M.A. Agronomic and environmental impacts of pasture-crop rotations in temperate North and South America. Agric. Ecosyst. Environ. 2014, 190, 18–26. [Google Scholar] [CrossRef]
- Barrios, E. Soil biota, ecosystem services and land productivity. Ecol. Econ. 2007, 64, 269–285. [Google Scholar] [CrossRef]
- King, A.E.; Blesh, J. Crop rotations for increased soil carbon: Perenniality as a guiding principle. Ecol. Appl. 2018, 28, 249–261. [Google Scholar] [CrossRef] [PubMed]
- Paustian, K.; Andren, O.; Janzen, H.H.; Lal, R.; Smith, P.; Tian, G.; Tiessen, H.; Van Noordwijk, M.; Woomer, P.L. Agricultural soils as a sink to mitigate CO2 emissions. Soil Use Manag. 1997, 13, 230–244. [Google Scholar] [CrossRef]
- Sokol, N.W.; Kuebbing, S.E.; Karlsen-Ayala, E.; Bradford, M.A. Evidence for the primacy of living root inputs, not root or shoot litter, in forming soil organic carbon. New Phytol. 2019, 221, 233–246. [Google Scholar] [CrossRef]
- Bardgett, R.D.; Bowman, W.D.; Kaufmann, R.; Schmidt, S.K. A temporal approach to linking aboveground and belowground ecology. Trends Ecol. Evol. 2005, 20, 634–641. [Google Scholar] [CrossRef] [PubMed]
- Jackson, R.B.; Mooney, H.A.; Schulze, E.-D. A global budget for fine root biomass, surface area, and nutrient contents. Proc. Natl. Acad. Sci. USA 1997, 94, 7362–7366. [Google Scholar] [CrossRef]
- Chen, S.; Wang, W.; Xu, W.; Wang, Y.; Wan, H.; Chen, D.; Tang, Z.; Tang, X.; Zhou, G.; Xie, Z.; et al. Plant diversity enhances productivity and soil carbon storage. Proc. Natl. Acad. Sci. USA 2018, 115, 4027–4032. [Google Scholar] [CrossRef]
- Poirier, V.; Roumet, C.; Munson, A.D. The root of the matter: Linking root traits and soil organic matter stabilization processes. Soil Biol. Biochem. 2018, 120, 246–259. [Google Scholar] [CrossRef]
- Chenu, C.; Angers, D.A.; Barré, P.; Derrien, D.; Arrouays, D.; Balesdent, J. Increasing organic stocks in agricultural soils: Knowledge gaps and potential innovations. Soil Tillage Res. 2019, 188, 41–52. [Google Scholar] [CrossRef]
- Wiesmeier, M.; Urbanski, L.; Hobley, E.; Lang, B.; von Lützow, M.; Marin-Spiotta, E.; van Wesemael, B.; Rabot, E.; Ließ, M.; Garcia-Franco, N.; et al. Soil organic carbon storage as a key function of soils—A review of drivers and indicators at various scales. Geoderma 2019, 333, 149–162. [Google Scholar] [CrossRef]
- Ye, C.; Hall, S.J. Mechanisms underlying limited soil carbon gains in perennial and cover-cropped bioenergy systems revealed by stable isotopes. GCB Bioenergy 2020, 12, 101–117. [Google Scholar] [CrossRef]
- Sanderson, M.A.; Brink, G.; Ruth, L.; Stout, R. Grass-Legume Mixtures Suppress Weeds during Establishment Better than Monocultures. Agron. J. 2012, 104, 36–42. [Google Scholar] [CrossRef]
- Tracy, B.F.; Albrecht, K.; Flores, J.; Hall, M.; Islam, A.; Jones, G.; Lamp, W.; Macadam, J.W.; Skinner, H.; Teutsch, C. Evaluation of Alfalfa-Tall Fescue Mixtures across Multiple Environments. Crop Sci. 2016, 56, 2026–2034. [Google Scholar] [CrossRef]
- Picasso, V.D.; Brummer, E.C.; Liebman, M.; Dixon, P.M.; Wilsey, B.J. Diverse perennial crop mixtures sustain higher productivity over time based on ecological complementarity. Renew. Agric. Food Syst. 2011, 26, 317–327. [Google Scholar] [CrossRef]
- Picasso, V.D.; Brummer, E.C.; Liebman, M.; Dixon, P.M.; Wilsey, B.J. Crop Species Diversity Affects Productivity and Weed Suppression in Perennial Polycultures under Two Management Strategies. Crop Sci. 2008, 48, 331–342. [Google Scholar] [CrossRef]
- Hooper, D.U.; Adair, E.C.; Cardinale, B.J.; Byrnes, J.E.K.; Hungate, B.A.; Matulich, K.L.; Gonzalez, A.; Duffy, J.E.; Gamfeldt, L.; O’Connor, M.I. A global synthesis reveals biodiversity loss as a major driver of ecosystem change. Nat. Cell Biol. 2012, 486, 105–108. [Google Scholar] [CrossRef] [PubMed]
- Mueller, K.E.; Tilman, D.; Fornara, D.A.; Hobbie, S.E. Root depth distribution and the diversity–productivity relationship in a long-term grassland experiment. Ecology 2013, 94, 787–793. [Google Scholar] [CrossRef]
- Skinner, R.H.; Gustine, D.L.; Sanderson, M.A. Growth, Water Relations, and Nutritive Value of Pasture Species Mixtures under Moisture Stress. Crop Sci. 2004, 44, 1361–1369. [Google Scholar] [CrossRef]
- Skinner, R.H.; Sanderson, M.A.; Tracy, B.F.; Dell, C.J. Above- and Belowground Productivity and Soil Carbon Dynamics of Pasture Mixtures. Agron. J. 2006, 98, 320–326. [Google Scholar] [CrossRef]
- Skinner, R.H. Yield, Root Growth, and Soil Water Content in Drought-Stressed Pasture Mixtures Containing Chicory. Crop Sci. 2008, 48, 380–388. [Google Scholar] [CrossRef]
- McNally, S.R.; Laughlin, D.C.; Rutledge, S.; Dodd, M.B.; Six, J.; Schipper, L.A. Root carbon inputs under moderately diverse sward and conventional ryegrass-clover pasture: Implications for soil carbon sequestration. Plant Soil 2015, 392, 289–299. [Google Scholar] [CrossRef]
- Van Eekeren, N.; Bos, M.; De Wit, J.; Keidel, H.; Bloem, J. Effect of individual grass species and grass species mixtures on soil quality as related to root biomass and grass yield. Appl. Soil Ecol. 2010, 45, 275–283. [Google Scholar] [CrossRef]
- Cardinale, B.J.; Matulich, K.L.; Hooper, D.U.; Byrnes, J.E.; Duffy, E.; Gamfeldt, L.; Balvanera, P.; O’Connor, M.I.; Gonzalez, A. The functional role of producer diversity in ecosystems. Am. J. Bot. 2011, 98, 572–592. [Google Scholar] [CrossRef]
- Mallarino, A.P.; Wedin, W.F.; Perdomo, C.H.; Goyenola, R.S.; West, C.P. Nitrogen Transfer from White Clover, Red Clover, and Birdsfoot Trefoil to Associated Grass. Agron. J. 1990, 82, 790–795. [Google Scholar] [CrossRef]
- Trenbath, B.R. Plant Interactions in Mixed Crop Communities. In Multiple Cropping; American Society of Agronomy: Madison, WI, USA, 1976. [Google Scholar]
- Hooper, D.U.; Shadmehr, R.; Holcomb, H.H. The Effects of Plant Composition and Diversity on Ecosystem Processes. Science 1997, 277, 1302–1305. [Google Scholar] [CrossRef]
- Skinner, R.H.; Comas, L.H. Root Distribution of Temperate Forage Species Subjected to Water and Nitrogen Stress. Crop Sci. 2010, 50, 2178–2185. [Google Scholar] [CrossRef]
- Schmid, B.; Hector, A.; Saha, P.; Loreau, M. Biodiversity effects and transgressive overyielding. J. Plant Ecol. 2008, 1, 95–102. [Google Scholar] [CrossRef]
- Crush, J.R.; Waller, J.E.; Care, D.A. Root distribution and nitrate interception in eleven temperate forage grasses. Grass Forage Sci. 2005, 60, 385–392. [Google Scholar] [CrossRef]
- Postma, J.A.; Lynch, J.P. Complementarity in root architecture for nutrient uptake in ancient maize/bean and maize/bean/squash polycultures. Ann. Bot. 2012, 110, 521–534. [Google Scholar] [CrossRef] [PubMed]
- Finney, D.; Buyer, J.; Kaye, J. Living cover crops have immediate impacts on soil microbial community structure and function. J. Soil Water Conserv. 2017, 72, 361–373. [Google Scholar] [CrossRef]
- Groffman, P.M.; Eagan, P.; Sullivan, W.M.; Lemunyon, J.L. Grass species and soil type effects on microbial biomass and activity. Plant Soil 1996, 183, 61–67. [Google Scholar] [CrossRef]
- Zak, D.R.; Holmes, W.E.; White, D.C.; Peacock, A.D.; Tilman, D. Plant Diversity, Soil Microbial Communities, and Ecosystem Function: Are There Any Links? Ecology 2003, 84, 2042–2050. [Google Scholar] [CrossRef]
- Maul, J.; Drinkwater, L. Short-term plant species impact on microbial community structure in soils with long-term agricultural history. Plant Soil 2009, 330, 369–382. [Google Scholar] [CrossRef]
- Six, J.; Frey, S.D.; Thiet, R.K.; Batten, K.M. Bacterial and Fungal Contributions to Carbon Sequestration in Agroecosystems. Soil Sci. Soc. Am. J. 2006, 70, 555–569. [Google Scholar] [CrossRef]
- Malik, A.A.; Chowdhury, S.; Schlager, V.; Oliver, A.; Puissant, J.; Vazquez, P.G.M.; Jehmlich, N.; Von Bergen, M.; Griffiths, R.I.; Gleixner, G. Soil Fungal: Bacterial Ratios Are Linked to Altered Carbon Cycling. Front. Microbiol. 2016, 7, 1247. [Google Scholar] [CrossRef]
- Scherber, C.; Eisenhauer, N.; Weisser, W.W.; Schmid, B.; Voigt, W.; Fischer, M.; Schulze, E.-D.; Roscher, C.; Weigelt, A.; Allan, E.; et al. Bottom-up effects of plant diversity on multitrophic interactions in a biodiversity experiment. Nature 2010, 468, 553–556. [Google Scholar] [CrossRef]
- Lange, M.; Habekost, M.; Eisenhauer, N.; Roscher, C.; Bessler, H.; Engels, C.; Oelmann, Y.; Scheu, S.; Wilcke, W.; Schulze, E.-D.; et al. Biotic and abiotic properties mediating plant diversity effects on soil microbial communities in an experimental grassland. PLoS ONE 2014, 9. [Google Scholar] [CrossRef]
- Chen, C.; Chen, H.Y.H.; Chen, X.; Huang, Z. Meta-analysis shows positive effects of plant diversity on microbial biomass and respiration. Nat. Commun. 2019, 10, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Van Eekeren, N.; Van Liere, D.; de Vries, F.; Rutgers, M.; de Goede, R.; Brussaard, L. A mixture of grass and clover combines the positive effects of both plant species on selected soil biota. Appl. Soil Ecol. 2009, 42, 254–263. [Google Scholar] [CrossRef]
- Dhakal, D.; Islam, M.A. Grass-Legume Mixtures for Improved Soil Health in Cultivated Agroecosystem. Sustainability 2018, 10, 2718. [Google Scholar] [CrossRef]
- Goslee, S.C.; Veith, T.L.; Skinner, R.H.; Comas, L.H. Optimizing ecosystem function by manipulating pasture community composition. Basic Appl. Ecol. 2013, 14, 630–641. [Google Scholar] [CrossRef]
- Sprunger, C.D.; Culman, S.W.; Peralta, A.L.; DuPont, S.T.; Lennon, J.T.; Snapp, S.S. Perennial grain crop roots and nitrogen management shape soil food webs and soil carbon dynamics. Soil Biol. Biochem. 2019, 137, 107573. [Google Scholar] [CrossRef]
- Miller, R.; Horneck, D. Determination of Total Nitrogen in Plant Tissue. In Handbook of Reference Methods for Plant Analysis; CRC Press: New York, NY, USA, 1998. [Google Scholar]
- Buyer, J.S.; Sasser, M. High throughput phospholipid fatty acid analysis of soils. Appl. Soil Ecol. 2012, 61, 127–130. [Google Scholar] [CrossRef]
- Zelles, L. Fatty acid patterns of phospholipids and lipopolysaccharides in the characterisation of microbial communities in soil: A review. Biol. Fertil. Soils 1999, 29, 111–129. [Google Scholar] [CrossRef]
- Zelles, L. Phospholipid fatty acid profiles in selected members of soil microbial communities. Chemosphere 1997, 35, 275–294. [Google Scholar] [CrossRef]
- Olsson, P.A. Signature fatty acids provide tools for determination of the distribution and interactions of mycorrhizal fungi in soil. FEMS Microbiol. Ecol. 1999, 29, 303–310. [Google Scholar] [CrossRef]
- Frostegård, A.; Bååth, E. The use of phospholipid fatty acid analysis to estimate bacterial and fungal biomass in soil. Biol. Fertil. Soils 1996, 22, 59–65. [Google Scholar] [CrossRef]
- Culman, S.W.; Snapp, S.S.; Freeman, M.A.; Schipanski, M.E.; Beniston, J.; Lal, R.; Drinkwater, L.E.; Franzluebbers, A.J.; Glover, J.D.; Grandy, A.S.; et al. Permanganate Oxidizable Carbon Reflects a Processed Soil Fraction that is Sensitive to Management. Soil Sci. Soc. Am. J. 2012, 76, 494–504. [Google Scholar] [CrossRef]
- Hurisso, T.T.; Culman, S.W.; Horwath, W.R.; Wade, J.; Cass, D.; Beniston, J.W.; Bowles, T.M.; Grandy, A.S.; Franzluebbers, A.J.; Schipanski, M.E.; et al. Comparison of Permanganate-Oxidizable Carbon and Mineralizable Carbon for Assessment of Organic Matter Stabilization and Mineralization. Soil Sci. Soc. Am. J. 2016, 80, 1352–1364. [Google Scholar] [CrossRef]
- Islam, K.R.; Stine, M.A.; Gruver, J.B.; Samson-Liebig, S.E.; Weil, R.R. Estimating active carbon for soil quality assessment: A simplified method for laboratory and field use. Am. J. Altern. Agric. 2003, 18, 3–17. [Google Scholar] [CrossRef]
- Jenkinson, D.S. Chemical tests for potentially available nitrogen in soil. J. Sci. Food Agric. 1968, 19, 160–168. [Google Scholar] [CrossRef]
- R Development Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2013. [Google Scholar]
- Loreau, M.; Hector, A.L. Partitioning selection and complementarity in biodiversity experiments. Nat. Cell Biol. 2001, 412, 72–76. [Google Scholar] [CrossRef] [PubMed]
- Cadotte, M.W.; Cavender-Bares, J.; Tilman, D.; Oakley, T.H. Using Phylogenetic, Functional and Trait Diversity to Understand Patterns of Plant Community Productivity. PLoS ONE 2009, 4, e5695. [Google Scholar] [CrossRef]
- Finney, D.M.; Kaye, J.P. Functional diversity in cover crop polycultures increases multifunctionality of an agricultural system. J. Appl. Ecol. 2017, 54, 509–517. [Google Scholar] [CrossRef]
- Cong, W.-F.; Van Ruijven, J.; Mommer, L.; De Deyn, G.B.; Berendse, F.; Hoffland, E. Plant species richness promotes soil carbon and nitrogen stocks in grasslands without legumes. J. Ecol. 2014, 102, 1163–1170. [Google Scholar] [CrossRef]
- Tilman, D.; Lehman, C.L.; Thomson, K.T. Plant diversity and ecosystem productivity: Theoretical considerations. Proc. Natl. Acad. Sci. USA 1997, 94, 1857–1861. [Google Scholar] [CrossRef]
- Wardle, D.A.; Peltzer, D.A. Interspecific interactions and biomass allocation among grassland plant species. Oikos 2003, 100, 497–506. [Google Scholar] [CrossRef]
- Paustian, K.; Easter, M.; Brown, K.; Chambers, A.; Eve, M.; Huber, A.; Marx, E.; Layer, M.; Stermer, M.; Sutton, B.; et al. Field- and farm-scale assessment of soil greenhouse gas mitigation using COMET-Farm. In Precision Conservation: Geospatial Teachniques for Agricultural and Natural Resources Conservation; Access Publishing: North Delhi, India, 2018; pp. 341–359. [Google Scholar]
- Strecker, T.; Macé, O.G.; Scheu, S.; Eisenhauer, N. Functional composition of plant communities determines the spatial and temporal stability of soil microbial properties in a long-term plant diversity experiment. Oikos 2016, 125, 1743–1754. [Google Scholar] [CrossRef]
- Bossuyt, H.; Denef, K.; Six, J.; Frey, S.; Merckx, R.; Paustian, K. Influence of microbial populations and residue quality on aggregate stability. Appl. Soil Ecol. 2001, 16, 195–208. [Google Scholar] [CrossRef]
- Mawdsley, R.D.B.J.L. Continuous defoliation of perennial ryegrass (Lolium perenne) and white clover (Trifolium repens) and associated changes in the composition and activity of the microbial population of an upland grassland soil. Biol. Fertil. Soils 1996, 24, 52–58. [Google Scholar] [CrossRef]
- Wardle, D.A.; Bardgett, R.D.; Klironomos, J.N.; Setälä, H.; Van Der Putten, W.H.; Wall, D.H. Ecological Linkages Between Aboveground and Belowground Biota. Science 2004, 304, 1629–1633. [Google Scholar] [CrossRef]
- De Vries, F.T.; Hoffland, E.; Van Eekeren, N.; Brussaard, L.; Bloem, J. Fungal/bacterial ratios in grasslands with contrasting nitrogen management. Soil Biol. Biochem. 2006, 38, 2092–2103. [Google Scholar] [CrossRef]
- Van Der Heijden, M.G.A.; Bardgett, R.D.; Van Straalen, N.M. The unseen majority: Soil microbes as drivers of plant diversity and productivity in terrestrial ecosystems. Ecol. Lett. 2008, 11, 296–310. [Google Scholar] [CrossRef] [PubMed]
- Strickland, M.S.; Rousk, J. Considering fungal:bacterial dominance in soils—Methods, controls, and ecosystem implications. Soil Biol. Biochem. 2010, 42, 1385–1395. [Google Scholar] [CrossRef]
- Bradford, M.A.; Keiser, A.D.; Davies, C.A.; Mersmann, C.A.; Strickland, M.S. Empirical evidence that soil carbon formation from plant inputs is positively related to microbial growth. Biogeochemistry 2013, 113, 271–281. [Google Scholar] [CrossRef]
- Kallenbach, C.; Grandy, A.; Frey, S.; Diefendorf, A. Microbial physiology and necromass regulate agricultural soil carbon accumulation. Soil Biol. Biochem. 2015, 91, 279–290. [Google Scholar] [CrossRef]
- Sprunger, C.D.; Culman, S.W.; Robertson, G.P.; Snapp, S.S. Perennial grain on a Midwest Alfisol shows no sign of early soil carbon gain. Renew. Agric. Food Syst. 2018, 33, 360–372. [Google Scholar] [CrossRef]
- Carney, K.M.; Hungate, B.A.; Drake, B.G.; Megonigal, J.P. Altered soil microbial community at elevated CO2 leads to loss of soil carbon. Proc. Natl. Acad. Sci. USA 2007, 104, 4990–4995. [Google Scholar] [CrossRef] [PubMed]
- Bybee-Finley, K.; Ryan, M. Advancing Intercropping Research and Practices in Industrialized Agricultural Landscapes. Agriculture 2018, 8, 80. [Google Scholar] [CrossRef]
| Parameter | 2017 | 2018 | 2019 |
|---|---|---|---|
| Annual precipitation, mm | 1066.8 | 1828.5 | 1269.0 |
| Average annual temperature, °C | 11.8 | 11.5 | 11.4 |
| Average annual maximum temperature, °C | 17.2 | 16.3 | 16.8 |
| Average annual minimum temperature, °C | 6.4 | 6.5 | 6.0 |
| Total growing degree days | 3357 | 3496 | 3435 |
| Treatment Category | Aboveground Biomass (kg ha−1) | Belowground Biomass (kg ha−1) |
|---|---|---|
| Monocultures | 2080 ± 948 b † | 4740 ± 512 |
| Bicultures | 3229 ± 273 a | 5408 ± 396 |
| Triculture | 3866 ± 410 a | 6510 ± 790 |
| 0–15 cm Soil Depth | ||||
| Treatment | Total Root Dry Weight (kg ha−1) | % of Total Root Biomass in This Layer | Fine Root Dry Weight ‡ (kg ha−1) | Root C:N * |
| Tall fescue (TF) | 6230.8 ± 595.7 a † | 87 | 2153.2 ± 321.5 | 44.2 ± 1.6 a |
| Orchardgrass (OG) | 4460.1 ± 571.2 ab | 88 | 1512.5 ± 242.4 | 38.8 ± 2.6 ab |
| White clover (WC) | 2286.5 ± 416.0 c | 90 | 1315.3 ± 392.4 | 20.4 ± 1.9 c |
| TF + OG | 5873.7 ± 415.2 ab | 86 | 1944.8 ± 331.9 | 45.6 ± 1.9 a |
| TF + WC | 4048.8 ± 320.1 bc | 84 | 1452.1 ± 221.3 | 40.5 ± 2.5 ab |
| OG + WC | 4244.4 ± 933.4 abc | 89 | 1334.2 ± 354.4 | 35.7 ± 2.8 b |
| TF + OG + WC | 5774.7 ± 686.3 ab | 89 | 1557.5 ± 246.8 | 40.0 ± 3.7 ab |
| 15–30 cm Soil Depth | ||||
| Treatment | Total Root Dry Weight (kg ha−1) | % of Total Root Biomass in This Layer | Fine Root Dry Weight (kg ha−1) | Root C:N |
| Tall fescue (TF) | 865.8 ± 84.0 a | 13 | 310.4 ± 36.2 | 60.5 ± 5.0 |
| Orchardgrass (OG) | 588.7 ± 90.8 a | 12 | 345.5 ± 56.9 | 52.6 ± 6.3 |
| White clover (WC) | 188.6 ± 51.7 b | 10 | 152.7 ± 50.4 | 21.3 ± 1.1 |
| TF + OG | 930.0 ± 95.8 a | 14 | 378.8 ± 44.1 | 60.2 ± 5.3 |
| TF + WC | 798.2 ± 111.2 a | 16 | 352.9 ± 50.7 | 50.7 ± 5.1 |
| OG + WC | 638.4 ± 152.1 a | 11 | 335.0 ± 99.3 | 50.9 ± 1.4 |
| TF + OG + WC | 735.2 ± 125.8 a | 11 | 248.0 ± 63.4 | 48.2 ± 5.6 |
| Treatment | Total PLFAs (nmol g−1) | Bacterial Biomarkers (nmol g−1) | Fungal Biomarkers (nmol g−1) | F:B Ratio | Basal Respiration (μg CO2-C g–1 soil h–1) |
|---|---|---|---|---|---|
| Tall fescue (TF) | 100.3 ± 5.8 ab † | 74.1 ± 4.0 ab | 7.7 ± 0.7 | 0.102 ± 0.007 a | 11.5 ± 0.5 |
| Orchardgrass (OG) | 86.6 ± 5.4 b | 64.2 ± 3.9 b | 6.4 ± 0.5 | 0.099 ± 0.004 a | 12.7 ± 2.0 |
| White clover (WC) | 100.6 ± 7.5 ab | 76.5 ± 6.0 a | 6.9 ± 0.9 | 0.089 ± 0.005 b | 11.0 ± 1.0 |
| TF + OG | 91.9 ± 6.6 ab | 68.5 ± 4.6 ab | 6.7 ± 0.8 | 0.095 ± 0.007 ab | 11.7 ± 1.3 |
| TF + WC | 103.6 ± 5.3 a | 77.6 ± 3.8 a | 7.2 ± 0.6 | 0.092 ± 0.004 ab | 12.4 ± 1.4 |
| OG + WC | 105.3 ± 6.1 a | 78.6 ± 4.4 a | 7.7 ± 0.7 | 0.096 ± 0.005 ab | 14.2 ± 2.2 |
| TF + OG + WC | 102.7 ± 6.6 a | 76.4 ± 4.6 a | 7.4 ± 0.8 | 0.095 ± 0.006 ab | 13.0 ± 0.9 |
| POX-C (mg C kg−1 Soil) | TOC (g C kg−1 Soil) | |
|---|---|---|
| Tall fescue (TF) | 454.1 ± 12.1 | 15.4 ± 0.2 |
| Orchardgrass (OG) | 402.8 ± 22.1 | 14.1 ± 0.3 |
| White clover (WC) | 414.7 ± 16.8 | 15.5 ± 0.3 |
| TF + OG | 412.3 ± 22.2 | 15.6 ± 0.5 |
| TF + WC | 454.8 ± 27.5 | 15.8 ± 0.5 |
| OG + WC | 471.6 ± 26.4 | 15.8 ± 0.6 |
| TF + OG + WC | 466.2 ± 44.0 | 15.0 ± 0.7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Finney, D.M.; Garritano, S.; Kenwood, M. Forage Species Identity Shapes Soil Biota in a Temperate Agroecosystem. Sustainability 2021, 13, 5689. https://doi.org/10.3390/su13105689
Finney DM, Garritano S, Kenwood M. Forage Species Identity Shapes Soil Biota in a Temperate Agroecosystem. Sustainability. 2021; 13(10):5689. https://doi.org/10.3390/su13105689
Chicago/Turabian StyleFinney, Denise M., Samantha Garritano, and Matthew Kenwood. 2021. "Forage Species Identity Shapes Soil Biota in a Temperate Agroecosystem" Sustainability 13, no. 10: 5689. https://doi.org/10.3390/su13105689
APA StyleFinney, D. M., Garritano, S., & Kenwood, M. (2021). Forage Species Identity Shapes Soil Biota in a Temperate Agroecosystem. Sustainability, 13(10), 5689. https://doi.org/10.3390/su13105689





