Effect of Intensifier Additives on the Performance of Butanolic Extract of Date Palm Leaves against the Corrosion of API 5L X60 Carbon Steel in 15 wt.% HCl Solution
Abstract
1. Introduction
2. Experimental Descriptions
2.1. Leaves Collection, Preparation, and Extraction
2.2. Metal Specimen Composition, Preparation, and Corrosive Medium
2.3. Corrosion Testing Experiments
3. Results and Discussion
3.1. Corrosion Inhibition of BUT
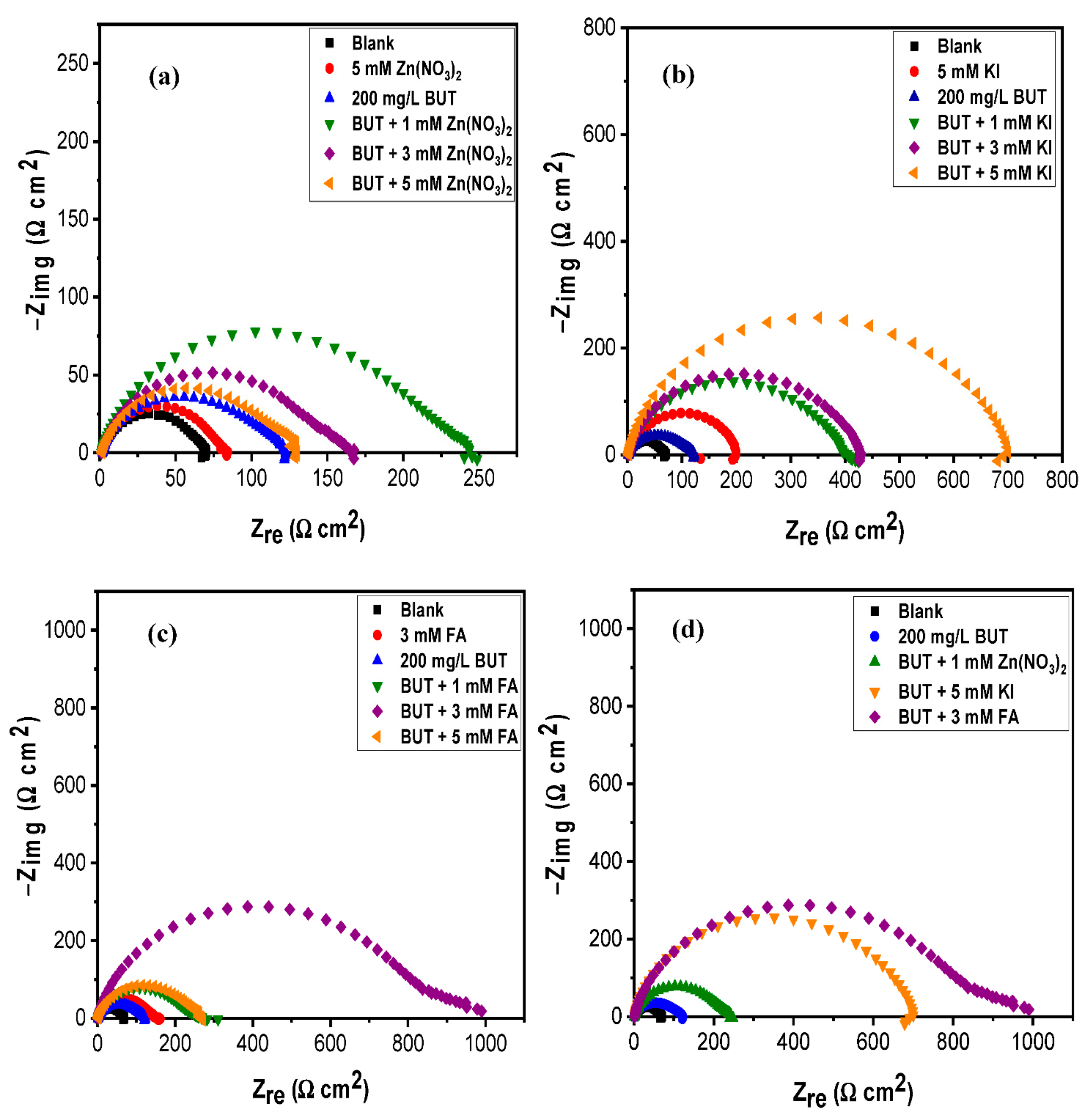
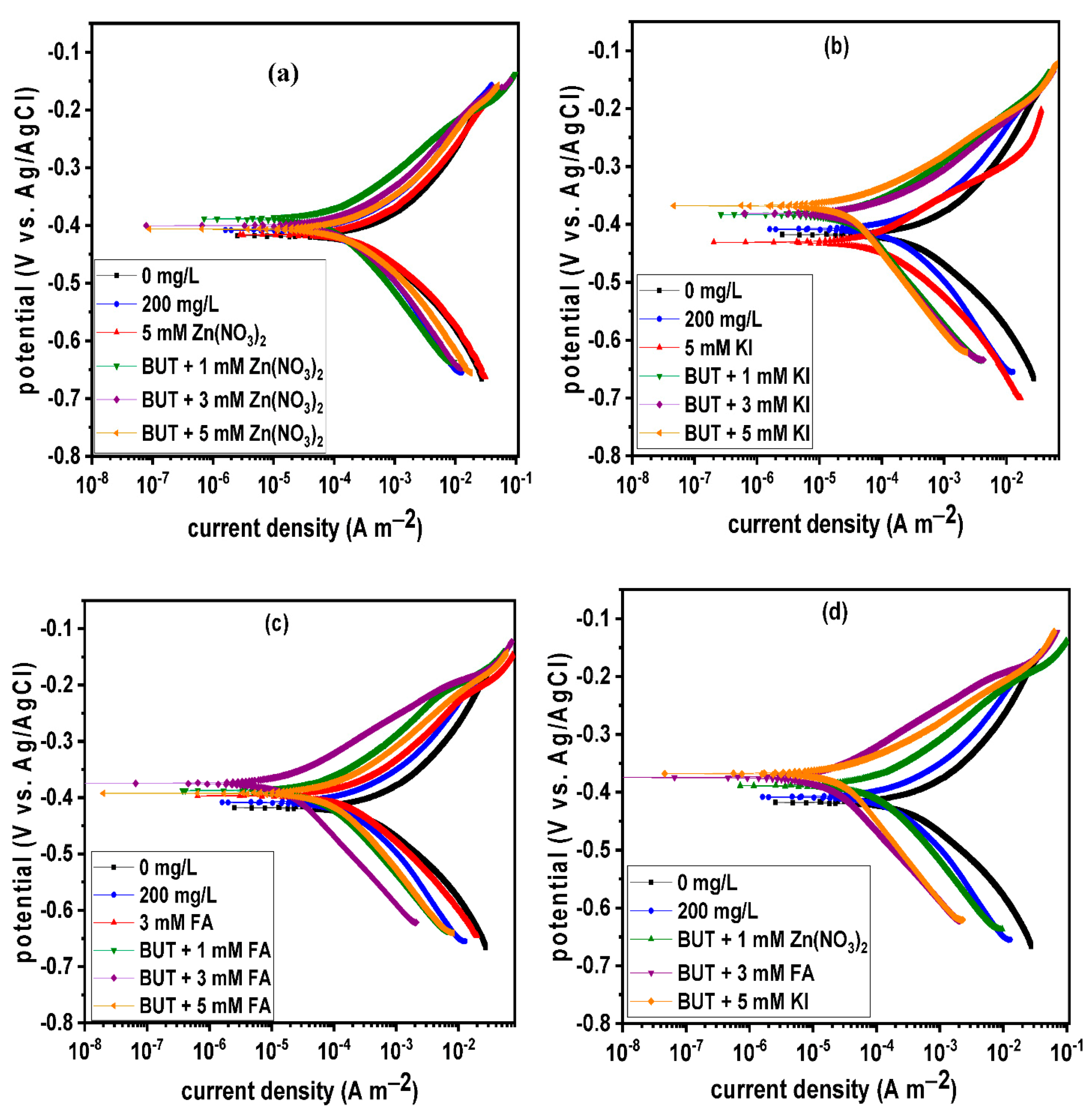
3.2. Effect of Intensifier Additives on the Corrosion Inhibition of BUT
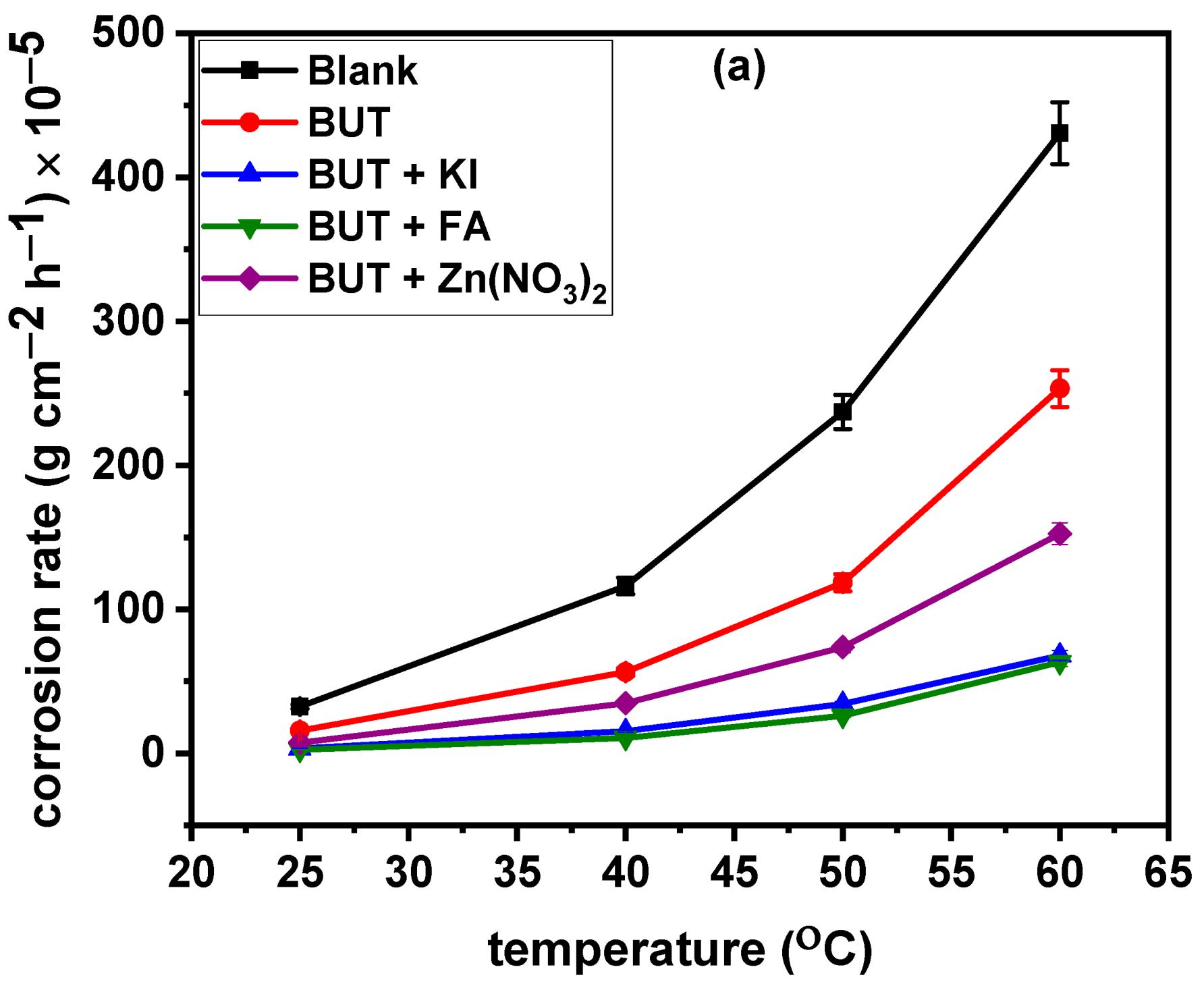
3.3. Effect of Temperature On Corrosion Rate and Corrosion Inhibition
3.4. Surface Analysis
3.4.1. SEM and EDAX
3.4.2. AFM
4. Conclusions and Outlook
- BUT has the potential to serve as an active in corrosion inhibitor package developed for carbon steel protection in an acidizing environment;
- FA and KI at appropriate concentration can be utilized as intensifier for BUT. With 200 mg/L BUT + 3 mM FA and 200 mg/L BUT + 5 mM KI, inhibition efficiency of 97% and 95%, respectively can be realized at normal temperature;
- Zn(NO3)2 is not a suitable intensifier for BUT under acidizing conditions;
- The adsorption of BUT + FA and BUT + KI is synergistic in nature;
- BUT alone and in combination with the selected intensifiers act as a mixed-type corrosion inhibition inhibiting both the anodic and cathodic corrosion reactions;
- Increase in the temperature of the acid solution would cause a slight decline in the inhibition efficiency of BUT + FA and BUT + KI but inhibition efficiency of above 85% is achievable at 60 °C;
- The SEM and AFM confirm that BUT + FA and BUT + KI are effective in inhibiting the corrosion of API 5L X60 carbon steel in 15 wt.% HCl solution.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Global Oil & Gas Exploration & Production—Industry Data, Trends, Stats|IBISWorld. Available online: https://www.ibisworld.com/global/market-research-reports/global-oil-gas-exploration-production-industry/ (accessed on 3 February 2021).
- Oil & Gas|Blackmer. Available online: https://www.psgdover.com/blackmer/markets/oil-gas (accessed on 3 February 2021).
- Obot, I.B.; Solomon, M.M.; Umoren, S.A.; Suleiman, R.; Elanany, M.; Alanazi, N.M.; Sorour, A.A. Progress in the development of sour corrosion inhibitors: Past, present, and future perspectives. J. Ind. Eng. Chem. 2019, 79, 1–18. [Google Scholar] [CrossRef]
- Umoren, S.A.; Solomon, M.M. Protective polymeric films for industrial substrates: A critical review on past and recent applications with conducting polymers and polymer composites/nanocomposites. Prog. Mater. Sci. 2019, 104, 380–450. [Google Scholar] [CrossRef]
- Cao, S.; Liu, D.; Ding, H.; Wang, J.; Lu, H.; Gui, J. Task-specific ionic liquids as corrosion inhibitors on carbon steel in 0.5 M HCl solution: An experimental and theoretical study. Corros. Sci. 2019, 153, 301–313. [Google Scholar] [CrossRef]
- Onyeachu, I.B.; Obot, I.B.; Sorour, A.A.; Abdul-Rashid, M.I. Green corrosion inhibitor for oilfield application I: Electrochemical assessment of 2-(2-pyridyl) benzimidazole for API X60 steel under sweet environment in NACE brine ID196. Corros. Sci. 2019, 150, 183–193. [Google Scholar] [CrossRef]
- Umoren, S.A.; Solomon, M.M. Polymeric Corrosion Inhibitors for Oil and Gas Industry. In Corrosion Inhibitors in the Oil and Gas Industry; Umoren, S.A., Saji, V.S., Eds.; Wiley: Hoboken, NJ, USA, 2020; pp. 303–320. [Google Scholar]
- Singh, A.; Quraishi, M.A. Acidizing Corrosion Inhibitors: A Review. J. Mater. Environ. Sci 2015, 6, 224–235. [Google Scholar]
- Alhaffar, M.T.; Umoren, S.A.; Obot, I.B.; Ali, S.A.; Solomon, M.M. Studies of the anticorrosion property of a newly synthesized Green isoxazolidine for API 5L X60 steel in acid environment. J. Mater. Res. Technol. 2019, 8, 4399–4416. [Google Scholar] [CrossRef]
- Zhang, Q.H.; Hou, B.S.; Li, Y.Y.; Zhu, G.Y.; Liu, H.F.; Zhang, G.A. Two novel chitosan derivatives as high efficient eco-friendly inhibitors for the corrosion of mild steel in acidic solution. Corros. Sci. 2019, 164, 108346. [Google Scholar] [CrossRef]
- Umoren, S.A.; Solomon, M.M. Synergistic corrosion inhibition effect of metal cations and mixtures of organic compounds: A Review. J. Environ. Chem. Eng. 2016, 5, 246–273. [Google Scholar] [CrossRef]
- Obot, I.B.; Solomon, M.M.; Onyeachu, I.B.; Umoren, S.A.; Meroufel, A.; Alenazi, A.; Sorour, A.A. Development of a green corrosion inhibitor for use in acid cleaning of MSF desalination plant. Desalination 2020, 495, 114675. [Google Scholar] [CrossRef]
- Marciales, A.; Haile, T.; Ahvazi, B.; Ngo, T.D.; Wolodko, J. Performance of green corrosion inhibitors from biomass in acidic media. Corros. Rev. 2018, 36, 239–266. [Google Scholar] [CrossRef]
- Lerner, S. Europeans Aim to Phase Out Toxic PFAS Chemicals by 2030. Available online: https://theintercept.com/2019/12/19/pfas-chemicals-europe-phase-out/ (accessed on 6 October 2020).
- Haque, J.; Verma, C.; Srivastava, V.; Nik, W.B.W. Corrosion inhibition of mild steel in 1M HCl using environmentally benign Thevetia peruviana flower extracts. Sustain. Chem. Pharm. 2021, 19, 100354. [Google Scholar] [CrossRef]
- Haldhar, R.; Prasad, D.; Mandal, N.; Benhiba, F.; Bahadur, I.; Dagdag, O. Colloids and Surfaces A: Physicochemical and Engineering Aspects Anticorrosive properties of a green and sustainable inhibitor from leaves extract of Cannabis sativa plant: Experimental and theoretical approach. Colloids Surf. A Physicochem. Eng. Asp. 2021, 614, 126211. [Google Scholar] [CrossRef]
- Zuo, X.; Li, W.; Luo, W.; Zhang, X.; Qiang, Y.; Zhang, J.; Li, H.; Tan, B. Research of Lilium brownii leaves extract as a commendable and green inhibitor for X70 steel corrosion in hydrochloric acid. J. Mol. Liq. 2021, 321, 114914. [Google Scholar] [CrossRef]
- Tan, B.; He, J.; Zhang, S.; Xu, C.; Chen, S.; Liu, H.; Li, W. Insight into anti-corrosion nature of Betel leaves water extracts as the novel and eco-friendly inhibitors. J. Colloid Interface Sci. 2021, 585, 287–301. [Google Scholar] [CrossRef] [PubMed]
- Farhadian, A.; Rahimi, A.; Safaei, N.; Shaabani, A.; Abdouss, M.; Alavi, A. A theoretical and experimental study of castor oil-based inhibitor for corrosion inhibition of mild steel in acidic medium at elevated temperatures. Corros. Sci. 2020, 175, 108871. [Google Scholar] [CrossRef]
- Umoren, S.A.; Solomon, M.M.; Obot, I.B.; Suleiman, R.K. A critical review on the recent studies on plant biomaterials as corrosion inhibitors for industrial metals. J. Ind. Eng. Chem. 2019, 76, 91–115. [Google Scholar] [CrossRef]
- Umoren, S.A.; Solomon, M.M. Effect of halide ions on the corrosion inhibition efficiency of different organic species—A review. J. Ind. Eng. Chem. 2015, 21, 81–100. [Google Scholar] [CrossRef]
- Finšgar, M.; Jackson, J. Application of corrosion inhibitors for steels in acidic media for the oil and gas industry: A review. Corros. Sci. 2014, 86, 17–41. [Google Scholar] [CrossRef]
- Feng, L.; Zhang, S.; Lu, Y.; Tan, B.; Chen, S.; Guo, L. Synergistic corrosion inhibition effect of thiazolyl-based ionic liquids between anions and cations for copper in HCl solution. Appl. Surf. Sci. 2019, 483, 901–911. [Google Scholar] [CrossRef]
- Loto, C.A.; Loto, R.T.; Popoola, A.P.I. Synergistic effect of tobacco and kola tree extracts on the corrosion inhibition of mild steel in acid chloride. Int. J. Electrochem. Sci. 2011, 6, 3830–3843. [Google Scholar]
- Obot, I.B.; Madhankumar, A. Synergistic effect of iodide ion addition on the inhibition of mild steel corrosion in 1 M HCl by 3-amino-2-methylbenzylalcohol. Mater. Chem. Phys. 2016, 177, 266–275. [Google Scholar] [CrossRef]
- Al-Katheeri, M.I.; Nasr-El-Din, H.A.; Taylor, K.C.; Al-Grainees, A.H. Determination and Fate of Formic Acid in High Temperature Acid Stimulation Fluids. In Proceedings of the International Symposium and Exhibition on Formation Damage Control, Lafayette, LA, USA, 20–21 February 2002; pp. 469–476. [Google Scholar]
- Cassidy, J.M.; McNeil, R.I.; Kiser, C.E. Understanding formic acid decomposition as a corrosion inhibitor intensifier in strong acid environments. In Proceedings of the International Symposium on Oilfield Chemistry, Houston, TX, USA, 28 February–2 March 2007; pp. 422–430. [Google Scholar]
- Farag, A.A.; Hegazy, M.A. Synergistic inhibition effect of potassium iodide and novel Schiff bases on X65 steel corrosion in 0.5 M H2SO4. Corros. Sci. 2013, 74, 168–177. [Google Scholar] [CrossRef]
- Zhang, W.; Li, H.J.; Liu, Y.; Wang, D.; Chen, L.; Xie, L.; Li, L.; Zhang, W.; Wu, Y.C. Stevioside–Zn2+ system as an eco-friendly corrosion inhibitor for C1020 carbon steel in hydrochloric acid solution. Colloids Surf. A Physicochem. Eng. Asp. 2021, 612, 126010. [Google Scholar] [CrossRef]
- Alinejad, S.; Naderi, R.; Mahdavian, M. Effect of inhibition synergism of zinc chloride and 2-mercaptobenzoxzole on protective performance of an ecofriendly silane coating on mild steel. J. Ind. Eng. Chem. 2017, 48, 88–98. [Google Scholar] [CrossRef]
- Al-Redhaiman, K.N. Date Palm cultivation in Saudi Arabia: Current status and future prospects for development. In Proceedings of the ASHS Annual Conference, Orlando, FL, USA, 28–31 July 2014. [Google Scholar]
- Nabili, A.; Fattoum, A.; Passas, R.; Elaloui, E. Extraction and characterization of cellulose from Date Palm seeds (Phoenix dactylifera L.). Cellul. Chem. Technol. 2016, 50, 1015–1023. [Google Scholar]
- Nasser, R.A.; Salem, M.Z.M.; Hiziroglu, S.; Al-Mefarrej, H.A.; Mohareb, A.S.; Alam, M.; Aref, I.M. Chemical analysis of different parts of date palm (Phoenix dactylifera L.) using ultimate, proximate and thermo-gravimetric techniques for energy production. Energies 2016, 9, 374. [Google Scholar] [CrossRef]
- ASTM. G.-90. Standard Practice for Preparing, Cleaning, and Evaluating Corrosion Test Specimens; ASTM: West Conshohocken, PA, USA, 2017. [Google Scholar]
- NACE International. TM0193-2016-SG Laboratory Corrosion Testing of Metals in Static Chemical Cleaning Solutions at Temperatures below 93 °C (200 °F); NACE International: Houston, TX, USA, 2016. [Google Scholar]
- Odewunmi, N.A.; Solomon, M.M.; Umoren, S.A.; Ali, S.A. Comparative studies of the corrosion inhibition efficacy of a dicationic monomer and its polymer against API X60 steel corrosion in simulated acidizing fluid under static and hydrodynamic conditions. ACS Omega 2020, 5, 27057–27071. [Google Scholar] [CrossRef] [PubMed]
- ASTM G3-89 Conventions applicable to electrochemical measurements in corrosion testing. In Annual Book of ASTM Standard; ASTM: West Conshohocken, PA, USA, 1994; Volume 30.
- ASTM G3-94 Making potentiostatic and potentiodynamic anodic polarization measurements. In Annual Book of ASTM Standard; ASTM: West Conshohocken, PA, USA, 1994; Volume 48.
- Solomon, M.M.; Umoren, S.A.; Obot, I.B.; Sorour, A.A.; Gerengi, H. Exploration of Dextran for Application as Corrosion Inhibitor for Steel in Strong Acid Environment: Effect of Molecular Weight, Modification, and Temperature on Efficiency. ACS Appl. Mater. Interfaces 2018, 10, 28112–28129. [Google Scholar] [CrossRef] [PubMed]
- Dickson, O.; Goodluck, O.; Onyekpe, B.O. Corrosion inhibition for alloy 304L (UNS S30403) in H2SO4 1 M solution by Centrosema pubescens leaves extract. Appl. Surf. Sci. Adv. 2021, 3, 100061. [Google Scholar]
- Kousar, K.; Walczak, M.S.; Ljungdahl, T.; Wetzel, A.; Oskarsson, H.; Restuccia, P.; Ahmad, A.; Harrison, N.M.; Lindsay, R. Corrosion inhibition of carbon steel in hydrochloric acid: Elucidating the performance of an imidazoline-based surfactant. Corros. Sci. 2021, 180, 109195. [Google Scholar] [CrossRef]
- Brug, G.J.; van den Eeden, A.L.G.; Sluyters-Rehbach, M.; Sluyters, J.H. The analysis of electrode impedances complicated by the presence of a constant phase element. J. Electroanal. Chem. 1984, 176, 275–295. [Google Scholar] [CrossRef]
- Solomon, M.M.; Umoren, S.A.; Quraishi, M.A.; Tripathi, D.; Abai, E.J. Effect of akyl chain length, flow, and temperature on the corrosion inhibition of carbon steel in a simulated acidizing environment by an imidazoline-based inhibitor. J. Pet. Sci. Eng. 2020, 187, 106801. [Google Scholar] [CrossRef]
- Luo, X.; Ci, C.; Li, J.; Lin, K.; Du, S.; Zhang, H.; Li, X.; Cheng, Y.F.; Zang, J.; Liu, Y. 4-aminoazobenzene modified natural glucomannan as a green eco-friendly inhibitor for the mild steel in 0.5 M HCl solution. Corros. Sci. 2019, 151, 132–142. [Google Scholar] [CrossRef]
- Gerengi, H.; Sen, N.; Uygur, I.; Solomon, M.M.M. Corrosion response of ultra-high strength steels used for automotive applications. Mater. Res. Express 2019, 6, 0865a6. [Google Scholar] [CrossRef]
- Arellanes-Lozada, P.; Díaz-Jiménez, V.; Hernández-Cocoletzi, H.; Nava, N.; Olivares-Xometl, O.; Likhanova, N.V. Corrosion inhibition properties of iodide ionic liquids for API 5L X52 steel in acid medium. Corros. Sci. 2020, 175, 108888. [Google Scholar] [CrossRef]
- Honarvar Nazari, M.; Shihab, M.S.; Havens, E.A.; Shi, X. Mechanism of corrosion protection in chloride solution by an apple-based green inhibitor: Experimental and theoretical studies. J. Infrastruct. Preserv. Resil. 2020, 1, 7. [Google Scholar] [CrossRef]
- Cao, C. On electrochemical techniques for interface inhibitor research. Corros. Sci. 1996, 38, 2073–2082. [Google Scholar] [CrossRef]
- Umoren, S.A.; Solomon, M.M.; Udosoro, I.I.; Udoh, A.P. Synergistic and antagonistic effects between halide ions and carboxymethyl cellulose for the corrosion inhibition of mild steel in sulphuric acid solution. Cellulose 2010, 17, 635–648. [Google Scholar] [CrossRef]
- Solomon, M.M.; Umoren, S.A.; Quraishi, M.A.; Salman, M. Myristic acid based imidazoline derivative as effective corrosion inhibitor for steel in 15% HCl medium. J. Colloid Interface Sci. 2019, 551, 47–60. [Google Scholar] [CrossRef]
- Petrucci, R.; Herring, F.; Madura, J.; Bissonnette, C. General Chemistry: Principles and Modern Applications, Loose Leaf Version, 10th ed.; Pearson Prentice Hall: Hoboken, NJ, USA, 2010; ISBN 978-0132064521. [Google Scholar]
- Paul, P.K.; Yadav, M. Investigation on corrosion inhibition and adsorption mechanism of triazine-thiourea derivatives at mild steel/HCl solution interface: Electrochemical, XPS, DFT and Monte Carlo simulation approach. J. Electroanal. Chem. 2020, 877, 114599. [Google Scholar] [CrossRef]
- Tao, Z.; He, W.; Wang, S.; Zhang, S.; Zhou, G. A study of differential polarization curves and thermodynamic properties for mild steel in acidic solution with nitrophenyltriazole derivative. Corros. Sci. 2012, 60, 205–213. [Google Scholar] [CrossRef]
- Solomon, M.M.; Gerengi, H.; Kaya, T.; Umoren, S.A. Enhanced corrosion inhibition effect of chitosan for St37 in 15% H2SO4 environment by silver nanoparticles. Int. J. Biol. Macromol. 2017, 104, 638–649. [Google Scholar] [CrossRef] [PubMed]

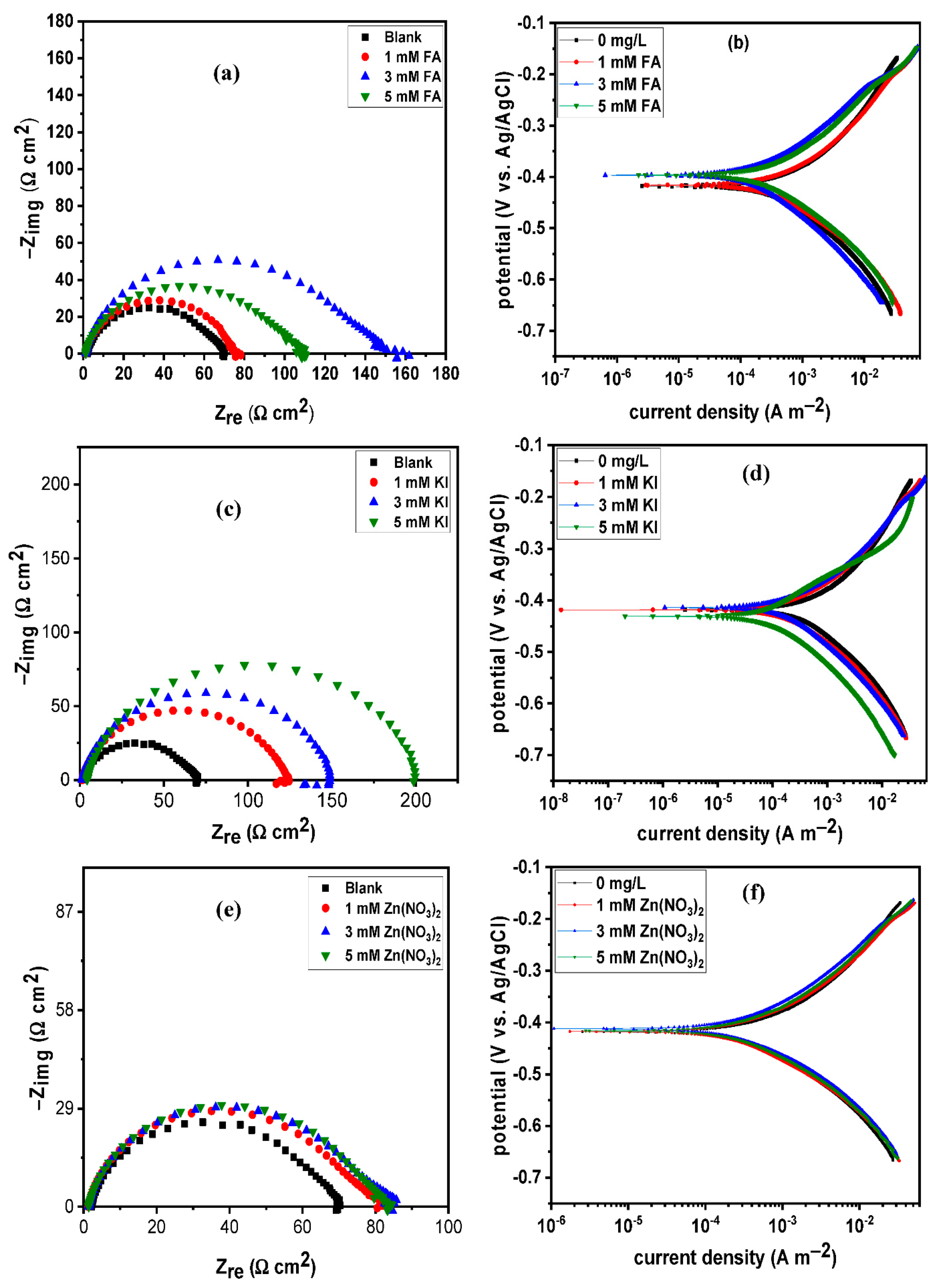





| System | Rs (Ω cm2) | CPEdl | Rct (Ω cm2) | Cdl (µF cm−2) | x2 (×10−4) | % ηEIS | |
|---|---|---|---|---|---|---|---|
| Ydl (µF cm−2 sn−1) | ndl | ||||||
| BUT extract | |||||||
| 0 ppm | 2.07 ± 0.03 | 222.00 ± 0.00 | 0.87 ± 0.01 | 69.36 ± 2.44 | 70.05 | 3.31 | – |
| 200 ppm | 1.61 ± 0.02 | 151.00 ± 0.00 | 0.82 ± 0.01 | 117.65 ± 1.51 | 24.20 | 8.66 | 41.00 |
| 500 ppm | 1.34 ± 0.02 | 54.38 ± 0.00 | 0.87 ± 0.01 | 258.70 ± 0.59 | 13.08 | 3.98 | 73.20 |
| 700 ppm | 1.92 ± 0.03 | 55.50 ± 0.00 | 0.86 ± 0.01 | 394.20 ± 2.40 | 12.51 | 1.56 | 82.40 |
| Synergist | |||||||
| 1 mM FA | 1.20 ± 0.12 | 231.70 ± 0.00 | 0.84 ± 0.01 | 74.29 ± 4.18 | 48.55 | 2.38 | 6.64 |
| 3 mM FA | 1.20 ± 0.17 | 125.90 ± 0.00 | 0.90 ± 0.17 | 157.69 ± 0.61 | 41.49 | 3.28 | 56.01 |
| 5 mM FA | 1.20 ± 0.18 | 123.60 ± 0.00 | 0.89 ± 0.11 | 109.85 ± 1.00 | 47.32 | 2.33 | 36.86 |
| 1 mM KI | 1.36 ± 0.14 | 141.70 ± 0.00 | 0.88 ± 0.00 | 120.20 ± 0.63 | 44.07 | 5.02 | 42.30 |
| 3 mM KI | 1.30 ± 0.01 | 116.40 ± 0.00 | 0.88 ± 0.00 | 141.70 ± 0.73 | 35.03 | 13.94 | 51.05 |
| 5 mM KI | 4.30 ± 0.03 | 87.40 ± 0.00 | 0.88 ± 0.00 | 173.60 ± 0.92 | 29.72 | 10.04 | 60.05 |
| 1 mM Zn(NO3)2 | 1.23 ± 0.01 | 183.70 ± 0.00 | 0.87 ± 0.00 | 84.22 ± 9.33 | 52.26 | 1.34 | 17.64 |
| 3 mM Zn(NO3)2 | 1.41 ± 0.01 | 195.20 ± 0.00 | 0.88 ± 0.00 | 82.98 ± 7.08 | 63.64 | 2.09 | 16.41 |
| 5 mM Zn(NO3)2 | 1.40 ± 0.01 | 220.80 ± 0.00 | 0.86 ± 0.00 | 86.90 ± 6.98 | 59.06 | 1.47 | 20.18 |
| Conc. (ppm) | LPR | PDP | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Rp (Ω cm2) | % ηLPR | −Ecorr (mV/Ag/AgCl) | icorr (µA cm−2) | βa (mV dec−1) | −βc (mV dec−1) | fa | fc | % ηPDP | |
| BUT extract | |||||||||
| 0 | – | – | 418 | 514.85 | 106.80 | 123.80 | - | - | – |
| 200 | – | – | 416 | 255.60 | 107.00 | 122.10 | 0.51 | 0.50 | 50.35 |
| 500 | – | – | 384 | 102.07 | 85.80 | 153.60 | 0.29 | 0.24 | 80.17 |
| 700 | – | – | 381 | 62.16 | 82.20 | 154.60 | 0.19 | 0.15 | 87.93 |
| Synergist | |||||||||
| 1 mM FA | 70.01 ± 2.20 | 11.06 | 416 | 431.30 | 117.70 | 122.40 | 0.85 | 0.85 | 16.23 |
| 3 mM FA | 152.10 ± 0.60 | 59.06 | 397 | 230.24 | 100.40 | 120.10 | 0.55 | 0.53 | 55.28 |
| 5 mM FA | 106.70 ± 2.60 | 41.64 | 397 | 296.37 | 115.00 | 124.00 | 0.69 | 0.68 | 42.44 |
| 1 mM KI | 107.80 ± 1.40 | 42.24 | 418 | 292.14 | 108.00 | 122.40 | 0.57 | 0.57 | 43.26 |
| 3 mM KI | 124.50 ± 1.70 | 49.98 | 414 | 247.03 | 87.20 | 112.90 | 0.50 | 0.50 | 52.02 |
| 5 mM KI | 131.00 ± 3.21 | 52.46 | 411 | 238.10 | 67.90 | 88.60 | 0.51 | 0.50 | 53.75 |
| 1 mM Zn(NO3)2 | 75.82 ± 0.09 | 17.87 | 417 | 420.86 | 109.30 | 122.60 | 0.82 | 0.82 | 18.26 |
| 3 mM Zn(NO3)2 | 77.55 ± 1.22 | 19.70 | 412 | 415.14 | 129.40 | 121.70 | 0.84 | 0.85 | 19.37 |
| 5 mM Zn(NO3)2 | 74.90 ± 1.72 | 16.86 | 417 | 422.69 | 130.40 | 130.50 | 0.83 | 0.83 | 17.90 |
| System | Rs (Ω cm2) | CPEdl | Rct (Ω cm2) | Cdl (µF cm−2) | x2 (×10−4) | % ηEIS | |
|---|---|---|---|---|---|---|---|
| Ydl (µF cm−2 sn−1) | ndl | ||||||
| 200 ppm BUT | 1.61 ± 0.02 | 151.00 ± 0.00 | 0.82 ± 0.01 | 117.65 ± 1.51 | 24.20 | 8.66 | 41.00 |
| BUT + 1 mM FA | 1.22 ± 0.03 | 129.90 ± 0.00 | 0.82 ± 0.00 | 227.00 ± 3.17 | 19.01 | 27.46 | 73.37 |
| BUT + 3 mM FA | 1.18 ± 0.02 | 52.72 ± 0.00 | 0.85 ± 0.00 | 810.10 ± 10.88 | 9.54 | 47.39 | 92.54 |
| BUT + 5 mM FA | 1.23 ± 0.03 | 124.30 ± 0.00 | 0.82 ± 0.00 | 242.00 ± 3.46 | 18.05 | 17.18 | 75.02 |
| BUT + 1 mM KI | 1.61 ± 0.03 | 75.54 ± 0.00 | 0.85 ± 0.00 | 387.40 ± 5.06 | 15.38 | 25.42 | 84.40 |
| BUT + 3 mM KI | 1.57 ± 0.03 | 64.19 ± 0.00 | 0.86 ± 0.00 | 408.90 ± 4.55 | 14.34 | 22.23 | 85.22 |
| BUT + 5 mM KI | 1.65 ± 0.03 | 43.39 ± 0.00 | 0.87 ± 0.00 | 667.30 ± 7.32 | 10.42 | 21.47 | 90.94 |
| BUT + 1 mM Zn(NO3)2 | 1.00 ± 0.02 | 139.20 ± 0.00 | 0.82 ± 0.00 | 220.70 ± 3.14 | 19.80 | 17.34 | 72.61 |
| BUT + 3 mM Zn(NO3)2 | 1.03 ± 0.02 | 204.00 ± 0.00 | 0.81 ± 0.00 | 146.00 ± 2.01 | 27.94 | 12.12 | 58.60 |
| BUT + 5 mM Zn(NO3)2 | 1.31 ± 0.02 | 226.00 ± 0.00 | 0.81 ± 0.00 | 120.30 ± 1.91 | 33.52 | 12.49 | 49.76 |
| System | LPR | PDP | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Rp (Ω cm2) | % ηLPR | −Ecorr (mV/Ag/AgCl) | icorr (µA cm−2) | βa (mV dec−1) | −βc (mV dec−1) | fa | fc | % ηPDP | |
| 200 ppm BUT | 122.40 ± 1.40 | 49.12 | 416 | 255.60 | 107.00 | 122.10 | 0.51 | 0.50 | 50.35 |
| BUT + 1 mM FA | 341.10 ± 1.05 | 81.74 | 388 | 82.04 | 92.40 | 136.50 | 0.22 | 0.20 | 84.07 |
| BUT + 3 mM FA | 1035.00 ± 2.75 | 93.98 | 374 | 15.96 | 66.60 | 119.00 | 0.06 | 0.04 | 96.90 |
| BUT + 5 mM FA | 269.00 ± 1.56 | 76.85 | 393 | 104.55 | 89.50 | 138.90 | 0.27 | 0.24 | 79.69 |
| BUT + 1 mM KI | 473.30 ± 1.57 | 86.84 | 384 | 33.12 | 58.30 | 128.40 | 0.12 | 0.08 | 93.57 |
| BUT + 3 mM KI | 376.70 ± 3.02 | 83.47 | 383 | 34.13 | 48.60 | 137.50 | 0.14 | 0.09 | 93.37 |
| BUT + 5 mM KI | 641.90 ± 2.90 | 90.30 | 368 | 25.96 | 55.30 | 139.20 | 0.12 | 0.07 | 94.96 |
| BUT + 1 mM Zn(NO3)2 | 240.90 ± 4.44 | 74.15 | 388 | 112.16 | 84.80 | 137.30 | 0.31 | 0.27 | 78.22 |
| BUT + 3 mM Zn(NO3)2 | 155.70 ± 2.31 | 60.01 | 400 | 209.14 | 92.20 | 141.10 | 0.49 | 0.46 | 59.37 |
| BUT + 5 mM Zn(NO3)2 | 120.10 ± 1.21 | 48.15 | 407 | 306.17 | 108.70 | 136.10 | 0.66 | 0.64 | 40.53 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Umoren, S.A.; Solomon, M.M.; Obot, I.B.; Suleiman, R.K. Effect of Intensifier Additives on the Performance of Butanolic Extract of Date Palm Leaves against the Corrosion of API 5L X60 Carbon Steel in 15 wt.% HCl Solution. Sustainability 2021, 13, 5569. https://doi.org/10.3390/su13105569
Umoren SA, Solomon MM, Obot IB, Suleiman RK. Effect of Intensifier Additives on the Performance of Butanolic Extract of Date Palm Leaves against the Corrosion of API 5L X60 Carbon Steel in 15 wt.% HCl Solution. Sustainability. 2021; 13(10):5569. https://doi.org/10.3390/su13105569
Chicago/Turabian StyleUmoren, Saviour A., Moses M. Solomon, Ime B. Obot, and Rami K. Suleiman. 2021. "Effect of Intensifier Additives on the Performance of Butanolic Extract of Date Palm Leaves against the Corrosion of API 5L X60 Carbon Steel in 15 wt.% HCl Solution" Sustainability 13, no. 10: 5569. https://doi.org/10.3390/su13105569
APA StyleUmoren, S. A., Solomon, M. M., Obot, I. B., & Suleiman, R. K. (2021). Effect of Intensifier Additives on the Performance of Butanolic Extract of Date Palm Leaves against the Corrosion of API 5L X60 Carbon Steel in 15 wt.% HCl Solution. Sustainability, 13(10), 5569. https://doi.org/10.3390/su13105569









