Abstract
Ashes from biomass heat (and power) plants that apply untreated woody biofuels may be suitable for use as fertilizers if certain requirements regarding pollutant and nutrient contents are met. The aim of this study was to examine if both bottom and cyclone ashes from 17 Bavarian heating plants and one ash collection depot are suitable as fertilizers (n = 50). The range and average values of relevant nutrients and pollutants in the ashes were analyzed and evaluated for conformity with the German Fertilizer Ordinance (DüMV). Approximately 30% of the bottom ashes directly complied with the heavy metal limits of the Fertilizer Ordinance. The limits were exceeded for chromium(VI) (62%), cadmium (12%) and lead (4%). If chromium(VI) could be reduced by suitable treatment, 85% of the bottom ashes would comply with the required limit values. Cyclone ashes were high in cadmium, lead, and zinc. The analysis of the main nutrients showed high values for potassium and calcium in bottom ashes, but also relevant amounts of phosphorus, making them suitable as fertilizers if pollutant limits are met. Quality assurance systems should be applied at biomass heating plants to improve ash quality if wood ashes are used as fertilizers in agriculture.
1. Introduction
Combustion of wood in heat (and power) plants generates solid residues in the form of ashes [1,2]. In the Federal State of Bavaria (i.e., Southeast Germany), a total of 30,000 to 60,000 t/a of wood ashes from untreated wood accumulates each year from plants with an installed capacity of more than 1 MWtherm (calculated from the 2018 Energy Wood Market Report of the Bavarian State Institute of Forestry (LWF)) [3]. Due to the physical and chemical properties of these combustion by-products, suitable utilization strategies might be recommended for their use as raw materials in the bioeconomy.
Depending on the point of origin of wood ashes in the heat (and power) plant, a distinction can be made between different ash fractions. The ash accumulating in the boiler is called “bottom ash” or “coarse ash”. In most cases, the ash from the heat exchangers is also considered as part of the bottom ash. After the hot flue gas passes through the heat exchanger, the air is usually cleaned by a cyclone in which the “cyclone ash” (also called “coarse fly ash”) is separated. If the plant has an electrostatic precipitator, a fabric filter or a flue gas condensation system, a third ash fraction, i.e., the so-called “filter ash” (also called “fine fly ash”) or the “condensate sludge” is generated [1]. The following article focuses on bottom ash and cyclone ash from grate-fired boilers as these are the most common ash fractions in Bavarian heat (and power) plants.
The chemical composition of individual ash fractions depends on the fuel quality and the plant technology [1,2]. Chemical elements such as plant nutrients (e.g., Ca, Mg) or pollutants such as heavy metals vary in wood fuels depending on the species, but also on bark content, the share of green biomass (i.e., needles/leaves), growing conditions, the degree of external contamination or dry or wet ash removal from the combustion unit [4,5,6,7,8,9]. Major and trace elements are volatile to varying degrees at temperatures that prevail within the combustion chamber [1,10,11,12]. For instance, heavy metals such as Cd, Pb, Zn, and Hg are highly volatile, while elements such as Cr or Cu have low volatility. Therefore, the elements accumulate differently in bottom, cyclone or filter ash [10,13,14,15].
The material use of wood ashes as raw materials for the bioeconomy poses certain challenges [16,17,18]. Complex legal frameworks, difficult ash logistics due to decentralized accumulation, fluctuating product qualities and aspects of storage and occupational safety are just some of the points that must be considered in this context [11,19,20,21]. In addition, many utilization pathways are still under development or at the pilot stage [14,18,22,23] and are not applied regularly. Consequently, ashes from biomass heat (and power) plants are usually not perceived as by-products of the energetic use of wood. Thus, it is often not regarded as a valuable intermediate for further processing but is classified as waste that must be disposed at significant costs [24]. A survey conducted under the AshUse-project showed that in the opinion of the heating plant operators the challenges for implementing recycling of a functioning material include legal uncertainties, fluctuating ash qualities and low economic revenues. In addition, operators often lack knowledge about quality management strategies, such as how a defined ash quality can be reliably maintained and verified [21]. The planned further work at TFZ will therefore focus on the area of quality management in the production of wood ash at biomass heating (power) plants.
Wood ashes are already used to some extent as raw materials for various purposes in Germany and in other European countries, and to some extent also in Northern America, depending on the technical, economic, and legal circumstances and considering environmental aspects. A relatively widespread application is the use of ashes as a fertilizer or as an additive for fertilizer production for agricultural and forestry applications [11,15,20,25,26,27,28,29]. In Germany and Austria, suitable ashes are also added to composts [30,31]. In Austria, this pathway is limited to a very low blending rate of 2% ash to the compost, which makes this process uneconomical and no relevant quantities of ash are recycled via this route [30]. Instead, approximately 40% of the annually produced ash in Austria is processed by the cement and building materials industry [30]. The use of ashes in road construction has successfully been tested in research projects in Austria and Finland [22,32,33]. Tejada et al. (2019) [34] investigated wood ashes as a source of raw materials in urban mining.
Heavy metal contamination, and in particular Cd in ashes, is seen as a major concern in the use of ashes as fertilizers [16,35,36]. Limit values in other European countries are considerably higher, especially for Cd [19]. There are regulations on specific parameters of ash in individual countries. For example, in Germany, there is a limit value for Cr(VI) for application on arable land [11,37], and in Denmark there is a conductivity limit value for the eluate from ashes [38].
Many authors document the chemical composition of ashes used as fertilizers or soil conditioners [10,11,19,20,24,26,28,29]. This is carried out by considering national conditions regarding prevailing plant technology and legal requirements for the application of the ashes. Due to the higher limits for heavy metals in ashes for fertilizer purposes in many countries [19], higher contaminated mixed bottom and fly ashes or ashes from fluidized bed combustion plants are also sometimes used as fertilizers [13,15,39,40]. In terms of composition, these are often not comparable to the predominantly pure bottom ashes from grate-fired furnaces, as they mainly occur in the study area [21] and are eligible for use as fertilizer and soil conditioner under the German fertilizer law. Ash qualities of German biomass heating plants are documented by Reichle et al. (2009) [16], Wilpert (2016) [11], and Schilling (2020) [10]. Wilpert (2016) [11] and Schilling (2020) [10] examined bottom ashes that were collected in the context of a quality assessment of bottom ashes for fertilizing purposes. Therefore, rather less contaminated ashes than average may have been used. The values of Reichle et al. (2009) [16] originate from before 2003, without giving any further details on the sampled plants. Data on the ordinary ash quality of combustion plants according to the current state of the art are therefore missing. However, these data are necessary to estimate the bioeconomic potential of an increased ash utilization.
Due to the high solubility of calcium oxide (CaO) in the ash, a pH shock is feared when spreading in the forest, which negatively affects the soil flora and soil fauna. Therefore, the ash often is pretreated before application. This process, the so-called “ash stabilisation” or “ash hardening”, includes the addition of water followed by a storage period of several months. Moistening and contact with atmospheric carbon dioxide causes a variety of chemical transformations. Most importantly, the easily soluble calcium oxide (CaO) transforms into the poorly soluble calcium carbonate (CaCO3) [11,12,24,38]. In large piles, this reaction occurs only on the surface if there is no mixing [12,28].
In Germany, wood ash is mixed with lime dolomite and is then used for soil improvement on arable and forestry land [11,41,42]. Wilpert (2020) [11] points out that the use of wood ash-lime mixtures is particularly recommended where improved potassium supply is desired and the alkaline effect is present. Since the solubility of the alkali salts remains high even after ash hardening, the hardened ash should be protected from rain during storage in order to prevent nutrient leaching. Another positive effect of humidifying the ash and storing it for several months is the conversion of any toxic chromium(VI) into the harmless chromium(III). Schilling (2020) [10] and Polandt-Schwandt (1999) [9] observed this effect in the case of ash from combustion plants with a wet ash discharge system where the hot ash was placed in a water bath and then discharged moist. Pelletizing and granulation of wood ash also serve to reduce the reactivity of the wood ash. Auxiliary materials such as cement or organic binders can be used in this process [28,43]. Pelletized or granulated ash can be applied with conventional fertilizer spreaders [40]. Moistening of the ash is not recommended if the ash is to be used as a substitute for quicklime— e.g., in road construction. In this case, the ash must be stored dry [33].
Many authors emphasize the liming effect of ashes and mixtures with ashes on agricultural and forest soils [11,24,28,44]. Katzensteiner et al. (2011) [45] describe the plant availability of calcium and potassium from wood ashes as “high”, magnesium availability as “medium” and phosphate availability as “low”. “Low” in this context means that less than 10% of the total phosphate from wood ashes is available to the plant in the year of application. In pot experiments, Kebli et al. (2017) [46] and Maltas et al. (2014) [47] demonstrated the uptake of potassium from wood ash in ryegrass and sunflowers. In the case of sunflowers, P uptake from the ashes was also observed.
An important prerequisite for approval as a fertilizer in Germany is compliance with the heavy metal limits in the German Fertilizer Ordinance (DüMV) [42] and, if applicable, the minimum nutrient contents required, depending on the fertilizer. If the ash is mixed with biowaste or compost, the limit values of the German Biowaste Ordinance (BioAbfV) [48] must also be complied with in certain cases [31]. Table 1 summarizes these limit values. The DüMV also contains limit values for organic compounds (perfluorinated tensides, dioxins and dioxin-like substances). These compounds are usually absent in ash from biomass heating (and power) plants [10] and were not investigated in this study. Currently, both bottom ashes and cyclone ashes (if the cyclone is not the last precipitation unit in the plant) may be used for fertilizer production according to DüMV. Compared to the application of wood ashes on farmland, 50% higher heavy metal limit values apply to the application on forestry land. The Cr(VI) limit only applies to ash fertilization on arable land.

Table 1.
Limit values or maximum contents (in brackets) for wood ashes according to the current German Fertilizer Ordinance (DüMV) and Biowaste Ordinance (BioAbfV) (d.b. = dry basis).
Much uncertainty remains around the variability of wood ashes among plants or within the same plant and which of these ashes might be suitable for application as fertilizers in agriculture or for liming of forest soils. The aim of this study was to assess the range and average values of nutrients and pollutants in ashes from individual Bavarian biomass heat (and power) plants. This is an important prerequisite for an increase in ash utilization as it is in in line with the Bavarian bioeconomy strategy [49].
For this purpose, mainly bottom ashes but also mixtures of bottom and cyclone ashes (due to individual ash handling at certain plants) and pure cyclone ashes from heat (and power) plants with a thermal output of more than 1 MW were sampled and analyzed. The cyclone ashes are used for comparison with the bottom ashes and for an estimate of how the distribution of ash constituents could be influenced by plant operation.
2. Materials and Methods
A total of 17 biomass heat (and power) plants with an installed thermal capacity between 0.8 and 31.6 MWtherm, as well as one centralized ash collection depot of several small heating plants (plant ID 18), were selected for sampling. Table 2 gives an overview of the sampled heat (and power) plants with thermal outputs, fuels used, ash samples and plant IDs. The quality of the ashes varied due to different fuels, plant types or operating parameters of the furnace. At 17 sites, pure bottom ash could be sampled. At one plant (plant ID 1, TFZ), pure cyclone ash was sampled too. At five points, mixtures of bottom ash and cyclone ash could be sampled, leading to a total of 50 ash samples (Table 2). Depending on the ash management procedure, the storage duration of bottom ashes at the heating plants varied considerably and ranged from a few days to several weeks. For ten plants, sampling took place in two different heating seasons (winter 2018/2019 and winter 2019/2020). Seven plants and the central ash collection depot were sampled only once (n = 1). At the heating plant of TFZ (plant ID 1), a series of a total of 20 ash samples was obtained over an entire heating period (12 × bottom ashes, 8 × cyclone ashes). For the general analysis of variability between plants, mean values on ash quality per plant were calculated, while individual samples were used to assess heterogeneity within one plant.

Table 2.
Overview of sampled heating (power) plants with thermal output, fuels (a = wood chips, b = wood pellets both from untreated wood) and composition of bottom ashes and mixtures of bottom and cyclone ashes.
Sampling was carried out directly at the heating plants in accordance with LAGA PN 98 [50]. Thereby, it was necessary to prepare a representative sample for laboratory analysis from several individual samples of the ashes stored at their respective locations. The minimum volume of an individual on-site sample and of the laboratory sample prepared by sample combination, homogenization and sample division depends on the maximum grain size of the ash and was between 0.5 and 10 L. Fine-grained ashes have a lower required minimum volume than coarse-grained ashes. The minimum number of incremental samples results from the basic quantity of stored bottom ash or cyclone ash. For example, up to a volume of 30 m3, at least eight individual samples should be taken according to LAGA PN 98. During sampling, the individual samples were recorded photographically (Figure 1).

Figure 1.
Sampling of bottom ash according to LAGA PN 98 [50].
To obtain the laboratory sample from the individual samples, the samples were combined and thoroughly mixed with a shovel. After that, the mixed sample was divided into four even parts. Two of the four parts were discarded. The two remaining quarters were combined again, carefully mixed and the laboratory sample of approx. 8 L was taken from the mixture. Each sampling was documented on a sampling protocol.
The TFZ heating plant was an exception regarding sampling. Here, twelve individual samples of bottom ash and eight individual samples of cyclone ash were collected to assess variability of this plant over a complete heating season. To compare variation among heating plants, results from the bottom ash analyses were combined mathematically by calculating a theoretical mixed sample for the entire heating season.
The chemical analyses were performed by Wessling GmbH, Neuried, Germany. The analysis included the following ash components with a fertilizing effect—the macronutrients calcium (Ca), phosphorus (P), potassium (K) and sulfur (S), as well as the micronutrients cobalt (Co), iron (Fe), manganese (Mn), molybdenum (Mo), sodium (Na) and selenium (Se). The following heavy metals were analyzed: arsenic (As), lead (Pb), cadmium (Cd), chromium (Cr), both as total content and as chromium(VI), copper (Cu), nickel (Ni), mercury (Hg), thallium (Th), and zinc (Zn). In addition, pH, moisture content and loss of ignition of the ashes were measured. Elemental concentrations of the ash were determined mostly according to ISO standards. The dry residue was determined according to DIN EN 12879 [51]. The ash samples were dissolved with aqua regia (DIN ISO 11466 1997-06) [52] and analyzed by plasma mass spectrometry (ICP-MS) (DIN EN ISO 17294-2 (2005-02) [53]. Cr(VI) was determined according to DIN 19734 (1999-01) [54] The pH value in the solid was analyzed according to DIN ISO 10390 (2005-12) [55] and the alkaline active components according to VDLUFA Method Book Volume II.2, Method 4.5.1 [56].
3. Results and Discussion
Table 2 summarizes the results for the bottom ashes and the mixtures of bottom and cyclone ashes. The results are given per heating plant and ordered from left to right with ascending boiler output. In this order, the different combustion plants were also provided with IDs. All plants except one used wood chips from natural wood as fuel, and one plant used wood pellets. The analysis of the ashes included heavy metals, nutrients, pH, moisture content and loss of ignition. The results refer to the dry mass and are given either as concentrations (mg/kg d.b.) or as mass fractions (wt% d.b).
3.1. Quality of Bottom Ash
First, the results of the heavy metals in bottom ashes were evaluated in more detail (Figure 2), followed by analysis of the nutrient contents (Figure 3). The mean values for the relevant chemical elements and the physical ash properties per plant are given in Table 2.
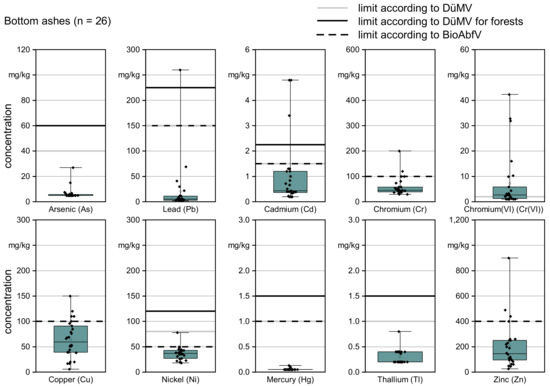
Figure 2.
Heavy metal contents in mg/kg (on dry basis) of the 26 bottom ash samples as point clouds and as boxplots with 25% and 75% quantiles (box) and minimum to maximum values (whisker). Horizontal lines show the respective limit values according to the German DüMV and BioAbfV.
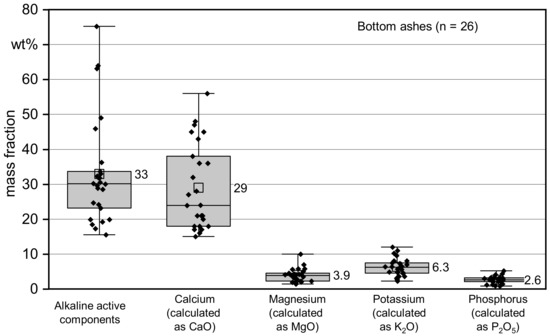
Figure 3.
Main nutrients of the 26 bottom ashes (based on dry matter) as point clouds as well as boxplots with 25% and 75% quantiles (box) and minimum to maximum values (whisker). The numbers next to the boxplots are the mean values.
The results refer to dry basis (d.b.) and, for each element, the individual results are shown as a cloud of points and as a boxplot with a minimum and maximum. The twelve ash samples from the TFZ heating plant are included in the evaluation as one mean value to avoid weighting effects. This results in a total number of n = 26 for the evaluation of the variation of bottom ashes among plants. In addition, the limit values for agricultural and forestry use according to the German DüMV and the limit values of the German BioAbfV are indicated in Figure 2. Table 3 gives the results in numbers.

Table 3.
Analytical results of heavy metal contents of 26 bottom ashes from Bavarian biomass heat (and power) plants with an installed capacity of >1 MW (d.b. = dry basis).
The limit values of the DüMV were exceeded in one sample for Pb (3%) and in three samples for Cd (8%). These exceedances apply to the DüMV limit values of both agricultural and forestry applications, although a 50% higher heavy metal content is permissible for forestry applications (Table 1). Schilling (2020) [10] examined 334 ash samples from 12 plants. He found exceedances for Pb in 1.9% of cases and for Cd in 1.6% of cases. The author documented exceedances for Cr(VI) in just 6% of cases. This deviates strongly from the values in the present study. For an application on agricultural land, a limit value for Cr(VI) of 2.0 mg/kg applies, according to DüMV. This limit is exceeded by 62% of the examined bottom ashes. Additionally, Reichle et al. (2009) [16] point out that the Cr(VI) limit is frequently exceeded in bottom ash from wood combustion. The authors recommend paying particular attention to Cr(VI) during the recycling of wood ash.
Ten heating plants were sampled twice in the current study, i.e., during winter 2018/2019 and during winter 2019/2020, and only two heating plants complied with the limit value for Cr(VI) in both samples. These were plants with a wet ash removal system (plant IDs 4, 14, 16), whereas all other plants used dry ash removal systems. For the plants that used dry ash removal, at least one sample per heating plant exceeded the limit value for Cr(VI). In three plants, the limit value was exceeded both times. Moistening of bottom ashes provides the conditions for a chemical reduction of Cr(VI) into Cr(III) [9]. Pohlandt-Schwandt (1999) [9] and Schilling (2020) [10] state that wet bottom ashes are low in Cr(VI). Therefore, moistening of bottom ashes is already often applied as a quality management tool to improve bottom ash quality [9,57].
In contrast to DüMV, there is no limit value for Cr(VI) in the BioAbfV. However, some of the other limit values in the BioAbfV are lower compared to DüMV and some bottom ashes exceeded the values for copper (19%, n = 5), nickel (8%, n = 2) and zinc (15%, n = 4).
The DüMV limit value for Cd was exceeded by two plants (plant IDs 6 and 11). Nickel and copper limits of BioAbfV were exceeded in each of the heating plants that were sampled twice in one of the samples, while the BioAbfV limit value for zinc was exceeded by both samples at one heating plant. Thereby, Zn was exceeded in all three ash samples with exceeded Cd. Kovacs et al. (2018) [8] and Schilling (2020) [10] show that there is a negative correlation between the concentration of volatile metals such as Cd, Pb or Zn and the temperature in the combustion chamber. Therefore, higher temperature combustion could probably solve the problem of Cd in bottom ash. Schilling (2020) [10] observed a complete volatilization of Cd at an average temperature of above 750 °C in the combustion chamber. The boiling temperature of Cd is 767 °C.
In total, only eight of the bottom ashes sampled complied with all heavy metal limit values according to the DüMV and the BioAbfV (Table 1), directly. Assuming that Cr(VI) can be sufficiently reduced by suitable treatments, e.g., by moistening the ashes [9,10], 85% of the ashes (n = 22) complied with the limit values of the DüMV. A total of 54% of the ashes (n = 14) also complied with the requirements of the BioAbfV regarding the maximum permissible heavy metal concentrations.
Bottom ashes contain many nutrients that are relevant for plant growth [11,24,26,28]. The sum of the basic components (metal oxides and carbonates [24]) and the individual values for calcium (calculated as CaO), potassium (calculated as potassium oxide K2O), magnesium (calculated as magnesium oxide MgO) and phosphorus (calculated as phosphate P2O5) are shown in Figure 3 as point clouds and box plots. Table 4 shows the results in figures together with the contents of the additional trace nutrients and other parameters.

Table 4.
Analytical results of trace nutrients and other parameters of 26 bottom ashes from Bavarian biomass heat (and power) plants with an installed capacity of >1 MW (d.b. = dry basis).
First, a comparison is made with publications on ash quality from Germany and Austria. Since here the wood qualities and the technology of the CHP plants are quite similar to the plants investigated. Reichle et al. (2009) [16] reported average nutrient contents for bottom ash of 25 to 45 wt% for calcium oxide (CaO), 3 to 6 wt% for magnesium oxide (MgO) and potassium oxide (K2O), each, and of 2 to 3 wt% for phosphate (P2O5). In the current study, higher values were measured, especially for potassium oxide. Here, the mean value is 6.3 wt% (d.b.) and 50% of the analytical results were between 4.5 and 7.5 wt% (d.b.). Obernberger (1997) [58] also gives a higher value for K2O than Reichle et al. (2009) [16] with 6.7 wt% d.b. as the average value for the content of potassium oxide in 12 bottom ashes from the combustion of wood chips. The mean phosphate content in Obernberger (1997) [58] is 3.6 wt% (d.b.) and thus about one percentage point higher than results in this study.
The results indicate that the nutrient contents in bottom ash from wood combustion can fluctuate over a wide range of values. The pH values of the ashes examined vary between pH 12.3 (minimum) and pH 13.3 (maximum) (Table 2). They thus fluctuate quite closely around the mean value of pH 12.8 and lie within the range of pH 11 to pH 13 given by Reichle et al. (2009) [16] for wood ashes.
Most of the ashes were very dry, the median value of the moisture content is 0.5 wt%. Nurmesniemi et al. (2012) [15] also notes this value for bottom ashes. Only the two plants with wet ash removal raised the mean moisture content to 6.2 wt%. For the plants with a wet ash removal system, the moisture content varied between 21 and 33 wt%.
Most of the ashes were completely combusted and showed only a low loss of ignition, which amounted to 0.6 wt% on average and reached a maximum of 3.6 wt%. Thus, all ashes remained below the value of 5 wt%. Therefore, it can be assumed that there are no organic pollutants in the ash [16].
Looking at ash qualities that have been published beyond Germany and Austria, similar contents for CaO, MgO, P2O5 and K2O have been reported by Okmanis et al. (2015) [40] and Ingerslev et al. (2011) [20]. Considerably lower nutrient levels have been published by Nurmesniemi et al. (2012) [13] and Hannam et al. (2018) [16] for bottom ashes. Except for Cr (partly originating from the steels in the combustion chamber [20], the ash constituents originate from the fuels [7,20]. These differences can therefore be partly due to different fuel compositions. However, the main causes are differences in combustion technology and different temperatures in the combustion chamber.
Table 5 correlates the bottom ash contents of the present study with the thermal power of the combustion unit classified in <1 MW, 1 to 10 MW and >10 MW. The nutrient levels of alkaline active substances (CaO), MgO, P2O5 and K2O decrease with increasing furnace power due to higher temperatures in the combustion chamber. This is consistent with the research of Okmanis et al. (2015) [40] who examined the ash from heating plants in Lithuania. Additionally, Wilpert et al. (2016) [11] shares this observation and suggests a mixture of ashes from large and smaller heating plants to increase the nutrient content in fertilizers from wood ash.

Table 5.
Chemical composition (mean value and range) of the bottom ash according to thermal power of the combustion unit.
The bottom ashes which, apart from Cr(VI), do not exceed any other limit values of the DüMV, all contain more than 15 wt% (d.b.) CaO and thus meet the requirement for a “lime fertilizer made from ash from the combustion of vegetable matter”. A recycling path established in Bavaria and Baden-Württemberg consists of mixing ashes of this quality with lime or lime dolomite to form “carbonic acid lime”. The ash content may not exceed 30 wt%. Theoretically, it would also be possible to mix this lime fertilizer from ash with biowaste. However, minimum nutrient content limits in the finished product of 3 wt% N, 3 wt% P2O5 or 3 wt% K2O in the dry matter would then have to be met. According to Kehres (2016) [31], these contents are generally not achieved by mixtures of bottom ash and biowaste.
For a large part of the bottom ash (69%), the classification as “PK fertilizer from ash from the incineration of vegetable matter” would be possible, since at least 2 wt% P2O5 and 3 wt% K2O are contained in their dry matter.
Four of the ashes (corresponding to approximately 15%) contain at least 10 wt% (d.b.) K2O and would thus fulfil the requirement for a “potassium fertilizer from ashes of the combustion of vegetable matter”.
Wood ash can also be used in composting. If the resulting “organic-mineral fertilizers” are to be spread on agricultural land in accordance with DüMV, the limit values of the BioAbfV must also be met. Taking into account the exceedances of Cr(VI) according to the DüMV, a total of 54% of the bottom ash examined also complies with the limit values of the BioAbfV. However, the limit values of the BioAbfV do not have to be met if the application takes place on land, for which the BioAbfV does not apply, such as in gardening and landscaping or if substrates or topsoil materials are produced from the mixture of ash and compost [31]. This latter recovery path would thus be possible for 85% of the bottom ash investigated, as long as a reduction in the Cr(VI) content can be assumed.
3.2. Distribution of Element Loads between Bottom Ash to Cyclone Ash (TFZ Heating Plant)
At the TFZ heating plant, the distribution of the element loads between bottom ash and cyclone ash was investigated. For this purpose, individual samples of bottom ash and cyclone ash were sampled simultaneously at eight points during the same heating period.
Volatile ash components, such as Cd, Pb, Zn and Hg, evaporate at the high temperatures in the combustion chamber [8,10,13,15,16]. For this reason, volatile components can be discharged from the hot ash bed and accumulate in the cyclone ash through condensation. This results in increased concentrations of these elements in the cyclone ash compared to the bottom ash. By using the data set of samples obtained at the TFZ heating plant, this correlation should be directly verifiable.
Table 6 shows the heavy metal and nutrient concentrations in the bottom ash in direct comparison with the corresponding cyclone ash. The mean value and the standard deviation of the eight samples taken in pairs are given in each case. Pairs of mean values that differ significantly are printed in bold. Means were compared using the Wilcoxon signed-rank test. At the points where there is no standard deviation, all samples had fallen below the detection or determination limit with respect to this element. The specified detection or quantification limit was then used as the concentration. For the elements As and Hg, which also occur at very low concentrations in the cyclone ash, this can lead to a distortion in the calculation of the element loads, since this procedure means that a similarly high value must be assumed in both the bottom ash and the cyclone ash. In fact, it can be assumed that the proportion of the two volatile elements As and Hg is higher in the cyclone ash than in the bottom ash. However, the detection limit of the analysis via the external laboratory does not allow this conclusion to be drawn.

Table 6.
Mean heavy metal and nutrient concentrations (including standard deviation) in bottom ashes and in the associated cyclone ashes from eight paired samplings at the TFZ heating plant.
The interpretation of the results in Table 4 is based on the calculated absolute element loads related to the total mass of the respective element in the ash (Figure 4). In order to make quantitative statements about how the actual loads of the individual elements are distributed between the bottom ash and the cyclone ash, it is first necessary to make reasonable assumptions about the mass ratio between bottom ash and the associated cyclone ash. For fixed-bed furnaces, a proportion of 10 to 30 wt% of cyclone ash is usually reported [45,58,59,60]. Fine fly ash is not considered in the following analysis.
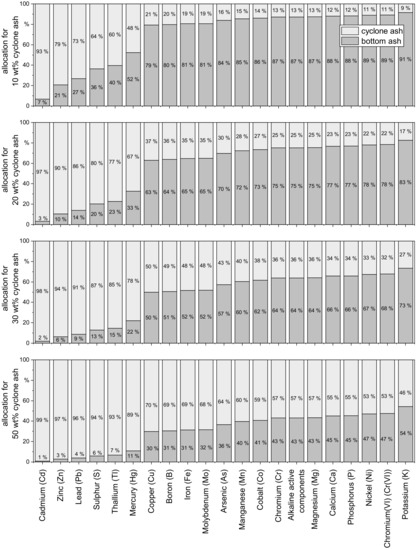
Figure 4.
Ratio of element loads in bottom ash and cyclone ash of the TFZ heating plant at hypothetical mixing ratios with cyclone ash contents of 10, 20, 30 and 50 wt%.
The actual proportion of cyclone ash depends on various factors, such as the turbulence of the primary air in the combustion bed or the fineness of the fuel, for comparison of the ash fractions from wood chips or sawdust shows [1]. With these assumptions, it is possible to derive from the eight pairwise analyses of the bottom ash and the cyclone ash at the TFZ heating plant how the fractions of heavy metals and nutrients are distributed between the ash fractions. In addition to the 1:1 mixing ratio (bottom bar chart), Figure 4 shows the distribution of the loads at 10, 20 and 30 wt% cyclone ashes of the total ash.
Heavy metal compounds containing Pb, Cd, Tl, Hg and Zn, are highly volatile [8,13] and are predominantly found in the cyclone ash in all calculations. Consequently, even at the lowest assumed cyclone ash content of 10 wt% of total ash, Cd accumulates in the cyclone ash of up to 93 wt%. Should high concentrations of highly volatile elements be observed in bottom ashes that are considered for utilization as fertilizer, an increase in the temperature in the combustion bed could result in a reduction in these elements in bottom ashes and an increase in cyclone ashes.
For As, no clear effect could be seen in the data presented here. As the concentrations of As in the investigated bottom and cyclone ashes were overall very low, the limit of determination often had to be used as the concentration in the ash fractions. Cu, Cr, Ni and the main nutrients Mg, P, Ca and K are less volatile and, depending on the calculation performed here, are found in only 11 to 50 wt% in the cyclone ash. Therefore, they predominantly remain in the bottom ash.
Obernberger (1997) [58] shows basically similar element ratios between bottom ash and cyclone ash for wood chips. However, the reported concentrations in the cyclone ash were consistently lower compared to the results presented here (with exception of K and P), which may be due to different combustion chamber and cyclone temperatures of the heating plants investigated. The combustion chamber temperatures near the combustion bed are not known for the TFZ heating plant. Lanzerstorfer (2017) [13] observed that at combustion chamber temperatures between 830 and 920 °C, Cd, Pb and Zn accumulate in the fly ashes, while most nutrients (Ca, Mg, P2O5) remain in the bottom ash. Both Lanzerstorfer (2017) [13] and Schilling (2020) [10] note a higher volatility for potassium, which leads to K losses from the bottom ash. These increased K losses could not be observed at the TFZ heating plant, suggesting that the combustion temperatures are sufficiently high to remove the volatile heavy metals and at the same time low enough to avoid high potassium losses.
3.3. Quality of Mixtures of Bottom Ash with Cyclone Ash
In some heating plants, the bottom ash and the cyclone ash are collected in the same container due to the plant design. The composition of these ashes is shown in Table 2 (right columns). All five samples of these mixed ashes exceed the DüMV limit value for Cd. Further exceedances occurred for Cr(VI) (n = 4), thallium (n = 1) and lead (n = 1). None of the bottom ashes mixed with cyclone ash can meet the requirements regarding the heavy metal limit values of the DüMV or the BioAbfV. They are therefore not eligible as a source material for fertilizers. These ashes are excluded from being spread on agricultural and forestry land in Germany. If the aim is to recycle bottom ashes, it is recommended that these ash fractions are collected and reused separately. When using other fuels, e.g., when firing agricultural fuels such as straw, a mixture of bottom ash and cyclone ash can often comply with the limit values of the DüMV [42]. This is due to the generally lower heavy metal content of agricultural fuels compared to wood fuels.
4. Conclusions
The energetic use of untreated wood in biomass heat (and power) plants produces combustion residues in the form of ash. The increased use of by-products and residues contributes to the conservation of natural resources. It has been shown that the bottom ashes produced are basically suitable for use as fertilizers or as a raw materials for fertilizers despite the low pollutant limits in the German DüMV.
On average, the bottom ashes examined contained 33 wt% alkaline active components, 29 wt% Calcium (calculated as CaO), 3.9 wt% Magnesium (calculated as MgO), 6.3 wt% Potassium (calculated as K2O) and 2.6 wt% of phosphorus (calculated as P2O5).
However, quality assurance of the ashes and compliance with the relevant legal requirements due to possible exceedances of the heavy metal limits prescribed by the German Fertilizer Ordinance are crucial. The limits were exceeded in the bottom ashes for chromium(VI) (62%), cadmium (12%) and lead (4%). Mixing of the bottom ashes with cyclone ashes led, in all cases, to the heavy metal limit values being exceeded, especially for cadmium. The following measures contribute to the quality assurance of ashes for fertilization purposes:
- -
- As prescribed by the German Fertilizer Ordinance, only untreated wood should be used in biomass heat (and power) plants, since waste wood can contain elevated concentrations of heavy metals.
- -
- Fluctuations in fuel quality or the combustion conditions can change the heavy metal contents in bottom ash. Regular sampling and chemical analysis of the ash is therefore necessary.
- -
- Since some of the heavy metals are volatile under the usual combustion conditions in biomass heating (power) plants, care should be taken to ensure that the combustion temperatures in the boiler are constantly high enough. An average temperature of over 750 °C, for example, reliably leads to sufficiently low cadmium contents in the bottom ash.
- -
- As has been shown, mixing of bottom ash and cyclone ash leads to an increase in heavy metals. Separate collection of these ash fractions is essential.
The frequently exceeded limit value for chromium(VI) in the German Fertilizer Ordinance can be reduced by moistening and storing the bottom ashes. In this process, chromium(VI) converts into the harmless chromium(III).
The present study maps the ash quality of typical biomass heating plants according to the state of the art in Germany. The evaluation of the results is carried out according to the regulations applicable in Germany for the use of biomass ash for fertilizer purposes. Other combustion techniques, other fuels and other legal regulations may lead to different assessments.
Author Contributions
H.B. designed and performed the experiments, derived the models and analyzed the data. Both D.K. and H.H. contributed to the final version of the manuscript. D.K. supervised the project. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Bavarian State Ministry of Food, Agriculture and Forestry, grant number G2/KS/17/02.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
BioAbfV = German Biowaste Ordinance; d.b. = on dry basis; DIN = German Institute for Standardization; DüMV = German Fertilizer Ordinance; ISO = International Organization for Standardization; LAGA = German Federal/State Working Group on Waste; TFZ = Technology and Support Centre in the Centre of Excellence for Renewable Resources; VDLUFA = Association of German Agricultural Analytic and Research Institutes.
References
- Kaltschmitt, M.; Hartmann, H.; Hofbauer, H. Energie aus Biomasse. Grundlagen, Techniken und Verfahren; Springer: Berlin/Heidelberg, Germany, 2016; p. 1755. [Google Scholar]
- Vassilev, S.V.; Baxter, D.; Vassileva, C.G. An overview of the behaviour of biomass during combustion: Part II. Ash fusion and ash formation mechanisms of biomass types. Fuel 2014, 117, 152–183. [Google Scholar] [CrossRef]
- Gößwein, S.; Hiendlmeier, S. Energieholzmarkt Bayern 2016. Untersuchung des Energieholzmarktes in Bayern hinsichtlich Aufkommen und Verbrauch; LWF: Freising, Germany, 2018; p. 131. [Google Scholar]
- Dietz, E.; Kuptz, D.; Blum, U.; Schulmeyer, F.; Borchert, H.; Hartmann, H. Qualität von Holzhackschnitzeln in Bayern. Gehalte ausgewählter Elemente, Heizwert und Aschegehalt, Berichte aus dem TFZ, Straubing, Nr. 46; Technologie- und Förderzentrum im Kompetenzzentrum für Nachwachsende Rohstoffe (TFZ); Bayerische Landesanstalt für Wald und Forstwirtschaft (LWF): Freising-Weihenstephan, Germany, 2016; p. 141. [Google Scholar]
- Kuchler, C.; Zimmermann, D.; Kuptz, D.; Dietz, E.; Rist, E.; Riebler, M.; Schön, C.; Mack, R.; Blum, U.; Borchert, H.; et al. Contamination of wood chips with mineral soils–Fuel quality and combustion behaviour. In Proceedings of the 52nd International Symposium on Forestry Mechanization (FORMEC), Sopron, Hungary/Forchtenstein, Austria, 6–9 October 2019; University of Sopron Press: Sopron, Hungary, 2019; pp. 320–329. [Google Scholar]
- FRED Feste Regenerative Energieträger Datenbank. Straubing: Technologie- und Förderzentrum im Kompetenzzentrum für Nachwachsende Rohstoffe (TFZ). Available online: https//:www.fred.bayern.de/ (accessed on 10 June 2020).
- Werkelin, J.; Skrifvars, B.-J.; Hupa, M. Ash-forming elements in four Scandinavian wood species: Part 1: Summer harvest. Biomass Bioenergy 2005, 29, 451–466. [Google Scholar] [CrossRef]
- Kovacs, H.; Dobo, Z.; Koos, T.; Gyimesi, A.; Nagy, G. Influence of the flue gas temperature on the behavior of metals during biomass combustion. Energy Fuels 2018, 32, 7851–7856. [Google Scholar] [CrossRef]
- Pohlandt-Schwandt, K. Treatment of wood ash containing soluble chromate. Biomass Bioenergy 1999, 16, 447–462. [Google Scholar] [CrossRef]
- Schilling, S. Steuerungsmöglichkeiten der Qualität und Eignung von Holzaschen für deren Einsatz bei der Waldkalkung. Master’s Thesis, Albert-Ludwigs-Universität Freiburg, Freiburg, Germany, 2020. [Google Scholar]
- Wilpert, K.V.; Hartmann, P.; Schäffer, J. Quality control in a wood ash re-cycling concept for forests. VGB Powertech 2016, 96, 67–72. [Google Scholar]
- Steenari, B.-M.; Karlsson, L.G.; Lindqvist, O. Evaluation of the leaching characteristics of wood ash and the influence of ash agglomeration. Biomass Bioenergy 1999, 16, 119–136. [Google Scholar] [CrossRef]
- Lanzerstorfer, C. Grate-fired biomass combustion plants using forest residues as fuel. Enrichment factors for components in the fly ash. Waste Biomass Valorization 2017, 8, 235–240. [Google Scholar] [CrossRef]
- Lienemann, P.; Vock, W. Elementgehalte in Holzaschen und Validierung der Holzaschenkontrolle; ZHAW: Wädenswil, Switzerland, 2013; p. 96. [Google Scholar]
- Nurmesniemi, H.; Manskinen, K.; Pöykiö, R.; Dahl, O. Forest fertilizer properties of the bottom ash and fly ash from a large-sized (115 MW) industrial power plant incinerating wood-based biomass residues. J. Univ. Chem. Technol. Metall. 2012, 47, 43–52. [Google Scholar]
- Reichle, E.; Müller, R.; Schmoeckel, G.; Müller, C.; Wendland, M.; Geiger, H.; Stetter, U.; Zormaier, F. Verwertung und Beseitigung von Holzaschen. Merkblatt. Stand: 01.08.2009; Bayerisches Landesamt für Umwelt (LfU); Bayerische Landesanstalt für Wald und Forstwirtschaft (LWF); Bayerische Landesanstalt für Landwirtschaft (LfL): Augsburg, Germany, 2009; p. 19. [Google Scholar]
- Schrägle, R. Innovative Ansätze und Perspektiven der Verwertung von Holzaschen. In Proceedings of the 12th Internationaler BBE-Fachkongress Holzenergie, Augsburg, Germany, 27–28 September 2012; pp. 1–41. [Google Scholar]
- Stetter, U.; Zormaier, F. Verwertung und Beseitigung von Holzaschen. Neues LfU-Merkblatt Greift Altes Thema auf; LWF aktuell: Geneva, Switzerland, 2010; Volume 74, pp. 28–30. [Google Scholar]
- Hannam, K.D.; Venier, L.; Allen, D.; Deschamps, C.; Hope, E.; Jull, M.; Kwiaton, M.; McKenney, D.; Rutherford, P.M.; Hazlett, P.W. Wood ash as a soil amendment in Canadian forests: What are the barriers to utilization? Can. J. For. Res. 2018, 48, 442–450. [Google Scholar] [CrossRef]
- Lama, I.; Sain, D. A case study review of wood ash land application programs in North America, TAPPI Journal February 2021. TAPPI J. 2021, 20, 111–120. [Google Scholar]
- Bachmaier, H.; Kuptz, D.; Hartmann, H. Ash management at biomass heating plants in Southern Germany. In Setting the Course for a Biobased Economy–Papers of the 27th European Biomass Conference, Proceedings of the 27th European Biomass Conference, Lisbon, Portugal, 27–30 May 2019; Carvalho, M.D.G., Scarlat, N., Grassi, A., Helm, P., Eds.; ETA-Florence Renewable Energies: Florence, Italy, 2019; pp. 1814–1817. [Google Scholar]
- Bohrn, G.; Stampfer, K. Untreated wood ash as a structural stabilizing material in forest roads. Croat. J. For. Eng. 2014, 35, 81–89. [Google Scholar]
- Lahtinen, P. Utilization of biomass ashes in infrastructure construction in Finland. In Proceedings of the 4th Central European Biomass Conference, Graz, Austria, 15–18 January 2014; pp. 1–36. [Google Scholar]
- Ingerslev, M.; Hansen, M.; Pedersen, L.B.; Skov, S. Effects of wood chip ash fertilization on soil chemistry in a Norway spruce plantation on a nutrient-poor soil. For. Ecol. Manag. 2014, 334, 10–17. [Google Scholar] [CrossRef]
- Hansen, M.T. Options for increased use of ash from biomass combustion. In Proceedings of the Scandinavian Bio Mass Ash Workshop 2019. BMA Workshop, Copenhagen, Denmark, 25 March 2019; pp. 1–17. [Google Scholar]
- Saarsalmi, A.; Smolander, A.; Kukkola, M.; Moilanen, M.; Saramäki, J. 30-Year effects of wood ash and nitrogen fertilization on soil chemical properties, soil microbial processes and stand growth in a Scots pine stand. For. Ecol. Manag. 2012, 278, 63–70. [Google Scholar] [CrossRef]
- Brais, S.; Bélanger, N.; Guillemette, T. Wood ash and N fertilization in the Canadian boreal forest: Soil properties and response of jack pine and black spruce. For. Ecol. Manag. 2015, 348, 1–14. [Google Scholar] [CrossRef]
- Karltun, E.; Saarsalmi, A.; Ingerslev, M.; Mandre, M.; Andersson, S.; Gaitnieks, T.; Ozolincius, R.; Varnagiryte-Kabasinskiene, I. Wood ash recycling–possibilities and risks: Chapter 4. In Sustainable Use of Forest Biomass for Energy: A Synthesis with Focus on the Baltic and Nordic Region; Röser, D., Asikainen, A., Raulund-Rasmussen, K., Stupak, I., Eds.; Springer: Berlin/Heidelberg, Germany, 2008; pp. 79–108. ISBN 978-1-4020-5054-1. [Google Scholar]
- Ernfors, M.; Sikström, U.; Nilsson, M.; Klemedtsson, L. Effects of wood ash fertilization on forest floor greenhouse gas emissions and tree growth in nutrient poor drained peatland forests. Sci. Total Environ. 2010, 408, 4580–4590. [Google Scholar] [CrossRef]
- Walter, B.; Mostbauer, P.; Karigl, B. Biomasse–Aschenströme in Österreich; Umweltbundesamt–Report, Nr. REP-0561; Umweltbundesamt GMBH: Wien, Austria, 2016; p. 56. [Google Scholar]
- Kehres, B. Zumischung von Holzasche bei der Kompostierung. H&K Aktuell 2016, 5, 1–3. [Google Scholar]
- Korpjiarvi, K. The development in the use of ashes in Finland. In Proceedings of the Scandinavian Biomass Ash Workshop 2019. BMA Workshop, Copenhagen, Denmark, 25 March 2019; pp. 1–22. [Google Scholar]
- Obernberger, I.; Supancic, K. Fact-Sheet: Einsatz von Holzasche als Bindemittel zur Bodenstabilisierung z.B. im Straßenbau; BIOS BIOENERGIESYSTEME GmbH: Wien, Austria, 2015; p. 8. [Google Scholar]
- Tejada, J.; Grammer, P.; Kappler, A.; Thorwarth, H. Trace element concentrations in firewood and corresponding stove ashes. Energy Fuels 2019, 33, 2236–2247. [Google Scholar] [CrossRef]
- Mortensen, L.H.; Rønn, R.; Vestergård, M. Bioaccumulation of cadmium in soil organisms—With focus on wood ash application. Ecotoxicol. Environ. Saf. 2018, 156, 452–462. [Google Scholar] [CrossRef]
- Saarsalmi, A.; Mälkönen, E.; Piirainen, S. Efffects of Wood Ash Fertilization on Forest Soil Chemical Properties. Filva Fenn. 2001, 35, 355–368. [Google Scholar]
- Kehres, B. Verwertung von Holzaschen auf Flächen; 08.03.2013. 2., überarb. Fassung; Bundesgütegemeinschaft Kompost e. V., Ed.; BGK Information: Köln-Gremberghoven, Gernamy, 2013; p. 14. [Google Scholar]
- Maresca, A.; Krüger, O.; Herzel, H.; Adam, C.; Kalbe, U.; Astrup, T.F. Influence of wood ash pre-treatment on leaching behaviour, liming and fertilising potential. Waste Manag. 2019, 83, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Ingerslev, M.; Skov, S.; Sevel, L.; Pedersen, L.B. Element budgets of forest biomass combustion and ash fertilization–A Danish case-study. Biomass Bioenergy 2011, 35, 2697–2704. [Google Scholar] [CrossRef]
- Okmanis, M.; Lazdiņa, D.; Lazdiņš, A. The composition and use value of tree biomass ash. Rural Sustain. Res. 2015, 34, 32–37. [Google Scholar] [CrossRef][Green Version]
- Bundesverband Mineralischer Rohstoffe e. V. (MIRO). Deutscher Nachhaltigkeitspreis 2019–Preisträger und Projekte. Die deutsche Gesteinsindustrie–modern, Effizient, Nachhaltig; Bundesverband Mineralischer Rohstoffe e. V. (MIRO): Duisburg, Germany, 2019; p. 39. [Google Scholar]
- Bundesministerium für Ernährung, Landwirtschaft und Verbraucherschutz. Verordnung über das Inverkehrbringen von Düngemitteln, Bodenhilfsstoffen, Kultursubstraten und Pflanzenhilfsmitteln. BGBI, 2017; Part I; Volume 68, pp. 1305–1349. Available online: https://www.gesetze-im-internet.de/d_mv_2012/BJNR248200012.html (accessed on 16 June 2020).
- Ettl, R.; Weis, W.; Göttlein, A. Laborversuch zur Bewertung von Organo-Asche-Presslingen und einem Kalk-Asche-Gemisch als mögliche Produkte für eine nährstoffliche Kreislaufwirtschaft in Wäldern. Forstarchiv 2010, 81, 12–20. [Google Scholar]
- Saarsalmi, A.; Smolander, A.; Moilanen, M.; Kukkola, M. Wood ash in boreal, low-productive pine stands on upland and peatland sites: Long-term effects on stand growth and soil properties. For. Ecol. Manag. 2014, 327, 86–95. [Google Scholar] [CrossRef]
- Katzensteiner, K.; Holzner, H.; Obernberger, I. Richtlinien für den sachgerechten Einsatz von Pflanzenaschen zur Verwertung auf land- und forstwirtschaftlich genutzten Flächen; Bundesministerium für Land- und Forstwirtschaft, Umwelt und Wasserwirtschaft, Eds.; Fachbeirat für Bodenfruchtbarkeit und Bodenschutz: Wien, Austria, 2011; 74p. [Google Scholar]
- Kebli, H.; Maltas, A.; Sinaj, S. Landwirtschaftlisches Potenzial von Asche aus rezykliertem Holz. Agrar. Schweiz 2017, 8, 30–37. [Google Scholar]
- Maltas, A.; Sinaj, S. Holzasche: Ein neuer Dünger für die Landwirtschaft. Agrar. Schweiz 2014, 5, 232–239. [Google Scholar]
- Bundesministerium für Umwelt, Naturschutz und Reaktorsicherheit (BMU); Bundesministerium für Ernährung, Landwirtschaft und Forsten (BMELF); Bundesministerium für Gesundheit (BMG). Verordnung über die Verwertung von Bioabfällen auf landwirtschaftlich, forstwirtschaftlich und gärtnerisch genutzten Böden). Bioabfallverordnung—BioAbfV, in der Fassung vom 1. August, Vorschriftensammlung Version 02; Stuttgart: Gewerbeaufsicht Baden-Württemberg, Germany, 2012; pp. 1–58. [Google Scholar]
- Sachverständigenrat Bioökonomie Bayern; Empfehlungen zur Förderung der Bioökonomie in Bayern: Straubing, Germany, 2017.
- Länderarbeitsgemeinschaft Abfall. LAGA PN 98—Grundregeln für die Entnahme von Proben aus festen und stichfesten Abfällen sowie abgelagerten Materialien Richtlinie für das Vorgehen bei physikalischen, chemischen und biologischen Untersuchungen im Zusammenhang mit der Verwertung/Beseitigung von Abfällen; LAGA: Potsdam, Germany, 2019; p. 69. [Google Scholar]
- Deutsches Institut für Normung (DIN). Characterization of Sludges–Determination of the Loss on Ignition of Dry Mass; German Version (EN 12879:2000); Beuth-Verlag: Berlin, Germany, 2001. [Google Scholar]
- Deutsches Institut für Normung (DIN). Soil Quality–Extraction of Trace Elements Soluble in Aqua Regia (ISO 11466:1995); Beuth-Verlag: Berlin, Germany, 1997. [Google Scholar]
- Deutsches Institut für Normung (DIN). Water Quality–Application of Inductively Coupled Plasma Mass Spectrometry (ICP-MS)–Part 2: Determination of 62 Elements (ISO 17294-2:2003), German version (EN ISO 17294-2:2004); Beuth-Verlag: Berlin, Germany, 2005. [Google Scholar]
- Deutsches Institut für Normung (DIN). Soil Quality–Determination of Chromium(VI) in Phosphate Extract (DIN 19734:1999); Beuth-Verlag: Berlin, Germany, 1999. [Google Scholar]
- Deutsches Institut für Normung (DIN). Soil quality–Determination of pH (ISO 10390:2005); Beuth-Verlag: Berlin, Germany, 2005. [Google Scholar]
- Verband deutscher landwirtschaftlicher Untersuchungs- und Forschungsanstalten (VDLUFA). Methodenbuch II. 2 Die Untersuchung von Sekundärrohstoffdüngern, Kultursubstraten und Bodenhilfsstoffen; VDLUFA: Darmstadt, Germany, 2000. [Google Scholar]
- Eberl, G. Veränderung von Chrom(VI)-Gehalten in Holzaschen durch Bewässerung. Vortrag. In Proceedings of the 21th Österreichischer Biomassetag, Kufstein, Austria, 6–7 November 2018; pp. 1–14. [Google Scholar]
- Obernberger, I. Aschen aus Biomassefeuerungen–Zusammensetzung und Verwertung. In Proceedings of the Thermische Biomassenutzung–Technik und Realisierung. Internationale Tagung, Salzburg, Austria, 23–24 April 1997; pp. 199–222. [Google Scholar]
- Ministerium für Umwelt und Verkehr Baden-Württemberg. Schadstoffströme bei der Entsorgung von Holzasche. Schadstoffströme bei der Verbrennung naturbelassener Hölzer und holzartiger Biomassen im Hinblick auf die Ascheentsorgung. Reihe Abfall 2003, 76, 79. [Google Scholar]
- Zimmermann, S.; Hässig, J.; Landolt, W. Literaturreview Holzasche–Wald. Nährstoffentzug durch Holzernte, abiotische und biotische Wirkungen; Eidgenössische Forschungsanstalt für Wald, Schnee und Landschaft (WSL): Birmensdorf, Switzerland, 2010; p. 80. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).