Abstract
The objective of this study was to analyze the effects of calcium chloride (CaCl2) used as a de-icing agent on growth and physiological responses of three ground cover plants, Hosta longipes, Iris ensata, and Iris pseudacorus. CaCl2 concentration was set to be 0 mM (control), 9 mM (0.1%), 18 mM (0.2%), and 54 mM (0.6%). After treatment with CaCl2 for 3 months, plant heights, fresh weights of shoots and roots, photosynthetic rates, chlorophyll fluorescence, chlorophyll content (SPAD), and leaf water potential were measured. After Hosta longipes was treated with CaCl2 for 60 days, physiological damage began to occur in all treatment groups except for the control group. Those treated with 54 mM CaCl2 completely withered. At 90 days after treatment (DAT), there were significant differences for all measured parameters for Hosta longipes compared to the control (p < 0.001). The higher the treatment concentration, the lower the photosynthetic rate, the SPAD, and the leaf water potential. There was no significant difference in plant height in Iris ensata after 60 DAT (p > 0.05), but in other physiological responses, there was a significant difference by concentration (p < 0.001). Iris ensata had a visually healthier state at 90 DAT, showing the smallest reduction in photosynthetic rate at 60 DAT and 90 DAT. Its SPAD value was increased more at 90 DAT than at 60 DAT, indicating its higher resistance to CaCl2. Iris pseudacorus showed similar results as Iris ensata. Over time, the 54 mM treatment resulted in significant damage. Among these three plants, Iris ensata showed the highest tolerance to CaCl2. Therefore, they are considered to be highly beneficial ground cover plants for green infrastructure in urban areas with high CaCl2 concentrations.
1. Introduction
In order to restore the natural circulation of a city, urban parks, urban forests, vertical gardens, street trees, and so on are created. A network with existing green spaces or close to nature is called green infrastructure (GI). GI has been proven to be a more economically, socially, and environmentally sustainable option for an urban environment than traditional gray infrastructure solutions [1]. These multiple benefits of GI have been found in over 65% of 447 projects case studies from the American Society of Landscape Architects. A multi-functionality-based GI application will be able to advance highly acceptable climate change adaptation [2]. GI is being introduced in many countries around the world. However, because of technical, institutional, policy, and economic difficulties, it is mainly being applied in some advanced countries [3,4,5]. These countries are actively accepting the development of various GI-related technologies. As for the method of its application, the most appropriate method is selected for each country and region. The application of GI has been tried in a wide variety of ways. Various GI-related technologies and methods are being actively developed and tried in various ways to find the most suitable method for each country and region [6]. The frequency of cold waves in Korea has risen because of recent climate change. In the Cheongju area in 2015, there were 16 snow days, and the average amount of snowfall was 0.47 cm. In 2016, there were 22 snow days, and the average amount of snowfall was 0.24 cm. In 2017, there were 27 snow days, and the average amount of snowfall was 0.19 cm [7]. Therefore, the use of de-icing agents for the safety of vehicles and pedestrians has continuously risen because of the increase in snow days and heavy snowfall. Salt sprayed for road management following a snowfall harms the local ecosystem. It is mixed with other pollutants, causing various kinds of damages to plants in the city [8].
Low-impact development (LID) refers to land use planning and urban development technology used to minimize the destruction of aquatic ecosystems caused by urbanization through infiltration and storage from rainfall runoff sources to make them as close as possible to the city before development. LID technology is being applied throughout urban areas. In particular, various plants including street trees are recognized as critical factors that play an essential role in GI composition, such as connecting urban parks and urban forests, improving air quality, and enhancing thermal comfort in urban areas [9]. For the planting pattern of street trees, environmental ecological design using multi-layer planting of grasses, shrubs, and trees is applied based on the natural form of a forest. Such multi-layer planting is an effective technology in GI. Among multi-layer planting components, herbaceous plants are greatly affected by environmental conditions such as road conditions, soil, and microclimate [10,11,12].
De-icing agents currently used in urban areas can be divided into chloride-based (Cl) and non-chloride-based. Salt stress is one of the most important abiotic stresses [13,14]. As snow melts, chloride ions and alkaline substances from chloride de-icing agents can increase the pH of the soil [15]. For plant growth, salt accumulated in the soil can cause water stress, nutritional imbalance, and specific ionic effects (salt stress) because of a low osmotic pressure in the soil. A combination of these factors can impair the growth of plants physiologically and biochemically [16,17].
Ju et al. [18] have evaluated the effects of CaCl2 treatment on growth and physiological responses of three evergreen shrubs (Euonymus japonica, Rhodoendron indicum, and Buxus koreana). Sung et al. [19] have reported effects of CaCl2 on characteristics of photosynthetic apparatus, stomatal conductance, and fluorescence image of leaves of Cornus kousa. Swoczyna and Latocha [20] have determined seasonal damage of photosynthetic apparatus of these three types of street trees exposed to roadside salinity caused by heavy traffic. These studies were chosen to select salinity-tolerant trees. Because of increases in natural disasters such as heavy rain and heavy snow caused by climate change, many efforts are being made to establish sustainable measures using plants. Recently, plants have been widely used in LIDs to establish water circulation based on infiltration, storage, and evapotranspiration of rainwater in urban areas. Herbaceous plants applied to LID are more damaged than woody plants because their shallow roots can lead to greater exposure to CaCl2 [21]. However, studies on salinity tolerance of herbaceous plants that can be applied to LID facilities are insufficient. It is necessary to select plants with great tolerance to CaCl2 to diversify the landscape using ground cover plants. Therefore, our objective in this study was to obtain basic data for creating sustainable urban green areas in the future by analyzing the growth and physiological responses of three groundcover plants according to the concentration of CaCl2, a widely used de-icing agent.
2. Materials and Methods
2.1. Plant Materials and Treatments
Hosta longipes, Iris ensata, and Iris pseudacorus were selected as plant materials in this study because of their relatively high resistance to salt based on previous studies [18,19,20]. Hosta longipes belongs to Liliaceae. It is mainly distributed in East Asia. Globally, over 100 varieties and cultivars of Hosta longipes are growing naturally and over 1000 varieties have been horticulturally improved. Iris ensatais, a plant belonging to Iridaceae, has larger leaves than those of Acorus calamus. It is native to Korea and distributed nationwide. Iris pseudacorus also belongs to Iridaceae. It is planted in wetlands and ponds. It can also be planted on the roadside for ornamental purposes. These three kinds of plants were purchased from Yangchon Flower Shop (Seowon-gu, Cheongju-si, Korea) in August 2017 and planted in plastic pots with a diameter of 22.5 cm and a height of 18.5 cm. The soil used in this study was horticultural substrate (Doobaena gold, Nongkyung, Korea). Its raw materials were: cocopeat 47.4%, peatmoss 20.0%, zeolite 11.0%, pearlite 10.0%, vermiculite 7.0%, bottom ash 4.0%, fertilizer 0.49%, dolomite 0.1%, and wetting agent 0.01%. After purchasing the young plants, they were sufficiently grown in a greenhouse at Chungbuk National University for experimentation. Sodium chloride (NaCl) and calcium chloride (CaCl2) are commonly used as de-icing agents. CaCl2 is more effective for melting ice with less damage to plants [22,23]. In May 2018, we selected 24 pots each of Hosta longipes, Iris ensata, and Iris pseudacorus. CaCl2 was then prepared at 0 mM (control), 9 mM (0.1%), 18 mM (0.2%), and 54 mM (0.6%) using 74% industrial calcium chloride (DCRE Co Ltd., Incheon, Korea). Ju et al. [18] have treated these three species of shrubs with 0% to 5% CaCl2 and found that two of the species could survive only under a concentration of 1%. Therefore, in this study, we did the experiments using CaCl2 at a concentration under 1%.
On 22 May 2018, 29 May 2018, 25 June 2018, and 2 July 2018, 500 mL of each pot was treated a total of four times. During the experiment, bottom watering was performed using tap water twice a week to prevent water stress. The experiment was repeated five times for each concentration and each plant in a greenhouse to avoid the effects of rainfall. During the measurement period, the temperature and humidity of the greenhouse were measured at 1 h intervals using a temperature and humidity data logger (SK-L200TH II, SATO, Tokyo, Japan). The average temperature and the average humidity of the greenhouse were 30.5 ± 7.6 °C and 56.9 ± 23.4%, respectively.
2.2. Measurements and Analyses
During the experiment, we evaluated growth and physiological responses at regular intervals of 30, 60, and 90 days after treatment (DAT). The first, second, and third measurements were taken on 19 June (30 DAT), 17 July (60 DAT), and 15 August (90 DAT), respectively. We measured plant height from the shoot to the end of the undead leaf using a ruler. The photosynthetic rate was measured using a photosynthesis system (LCpro+, ADC BioScientific Ltd., Hoddesdon, UK). Data was collected from 10:00 a.m. to 12:00 p.m. before a marked reduction in photosynthesis occurred after mid-day. Chlorophyll fluorescence was measured using a chlorophyll fluorometer (OS30p+, Opti-Sciences, Hudson, NH, USA) between 9:00 a.m. and 12:00 p.m. We measured SPAD using a chlorophyll meter (SPAD-502Plus, Konica Minolta, Tokyo, Japan). We measured leaf water potential of a fresh leaf sample with a PSYPRO water potential system (WESCOR, Inc, Logan, UT, USA) and fresh weights of shoots and roots with an electronic balance (SW-1S, CAS, Seoul, Korea) after we shook off the soil, washed the plants with water, and dried them with paper towels. For statistical analysis, we used the SAS 9.3 program (SAS Institute Inc., Cary, NC, USA). Differences between treatment means were tested for significance by Duncan’s multiple range test (DMRT, p < 0.05).
3. Results and Discussion
3.1. Effects of CaCl2 on Growth of Three Plant Species
Hosta longipes began to show light leaf blight symptoms at 10 days after CaCl2 treatment. At 60 DAT, dry leaf tips or spots in the 9 mM group and leaf blight symptoms in the 18 mM group were clearly observed, but plants in the 54 mM group almost withered. At 90 DAT, there was severe damage in all CaCl2 treatment plots except for the control, resulting in near death (Figure 1). Iris ensata did not show any damage at 30 DAT, although slight browning was observed in the 54 mM group from 33 DAT. At 60 DAT, in the 54 mM group, slight browning continued at the tip of the plant. A similar trend was observed at 90 DAT. Iris pseudacorus showed slight browning at the tips of leaves in the 54 mM group from 24 DAT. At 60 DAT, the browning appeared in the 9 mM and 18 mM groups. Visually significant damage appeared in the 54 mM group. Plants in the 9 mM and 18 mM groups at 90 DAT showed results similar to those at 60 DAT. However, in the 54 mM group, more than half the leaves dried up and almost all leaves withered at 90 DAT, whereas relatively few parts of Iris ensata withered in the 54mM group at 90 DAT.
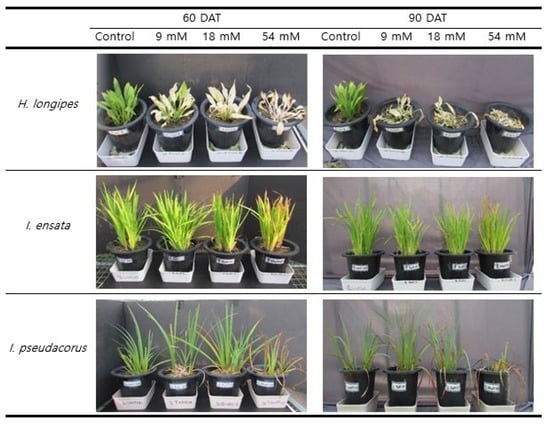
Figure 1.
Effects on saline stress on growth and morphology of three species exposed to different levels of CaCl2 at 60 DAT and 90 DAT.
For crops, the yield is the most important characteristic of salt tolerance, whereas visible aesthetic quality is the most important aspect for ornamental plants [24]. CaCl2 as a de-icing agent can inhibit plants’ growth, increases their mortality, and affect the growth of plants and microorganisms by changing the soil structure [21]. Je and Kim [25] have studied the growth and physiological response of Pinus strobus to CaCl2 and found that browning, dropping of leaves, and leaf blight are visible damages. They also found that higher CaCl2 concentration and longer treatment periods resulted in more significant damage.
Plant heights of Hosta longipes showed significant (p < 0.001) differences by concentration after 60 DAT. At 90 DAT, shoots completely withered in the 54 mM group (Table 1). For Iris ensata and Iris pseudacorus, there was no significant difference in plant height between the CaCl2 concentrations until 60 DAT (p > 0.05). However, there was a significant difference in plant height at 90 DAT (Iris ensata, p < 0.05; Iris pseudacorus, p < 0.001). In particular, there was a marked difference in plant height for the 54 mM group at 90 DAT.

Table 1.
Plant heights of three plant species exposed to different CaCl2 levels at 30, 60, and 90 DAT (n = 5).
By measuring fresh weights of shoots and roots after the experiment, we found that shoots were more sensitive to CaCl2 treatment than roots (Figure 2). Fresh weights of shoots of Hosta longipes were significantly different (p < 0.001) between control and CaCl2 treatment plots. There was no significant difference in fresh shoot weight between the 9 mM and 18 mM groups. Shoot measurement for the 54 mM group was impossible, because they were completely dead. There was no significant difference in fresh root weight among control, 9 mM, and 18 mM plots. In the 54 mM group, shoots withered, showing relatively low root weights. Fresh shoot weights of Iris ensata decreased significantly (p < 0.05) with increasing treatment concentrations (0, 9 mM, 18 mM, and 54 mM) of CaCl2. Weights of fresh shoots and roots of Iris pseudacorus were not significantly different between control and CaCl2 treatment plots. For root measurements of Iris ensata and Iris pseudacorus, there were significant differences by plots. Although there were differences in average weights, such differences were insignificant. Hooks and Niu [24] have studied salt (NaCl) tolerance of four types of perennial ornamental herbaceous plants. By measuring weights of shoots and roots, they found that shoots were more sensitive to salt than were roots. Munns and Tester [26] have also reported that shoots are more sensitive to salt than roots are. In the present study, growth of shoots and roots for all plants showed similar relative decreases, identical to the results of a previous study.
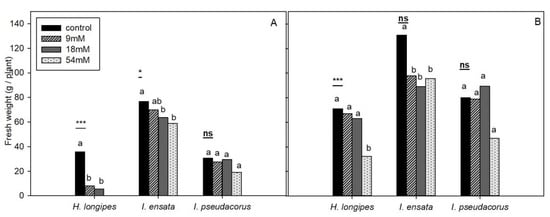
Figure 2.
Shoot fresh weights (A) and root fresh weights (B) of three plant species exposed to different CaCl2 levels at 90 DAT (n = 5). Different letters indicate significant difference according to DMRT at p < 0.05. ns, * and ***, nonsignificant, significant at p < 0.05, and p < 0.001, respectively.
3.2. Effects of CaCl2 on Physiological Responses of Three Plant Species
Gałuszka et al. [15] have confirmed that high concentrations of chloride and sodium could inhibit cell membrane function, induce osmotic stress, and decrease chlorophyll content, leading to a photosynthesis disorder. Results of photosynthetic rate measurements revealed a significant difference in photosynthetic rate of Hosta longipes at 30 DAT after CaCl2 treatment (p < 0.001) compared to the control. Measurement was impossible for the 54 mM group at 60 DAT or for the 18 mM group at 90 DAT (Table 2). Iris ensata could be measured up to 90 DAT. Its photosynthetic rate decreased as treatment concentration increased during the period from 30 DAT up to 60 DAT. There was a significant difference by concentration (p < 0.001). However, at 90 DAT, there was no difference between the groups in photosynthetic rate between 18 mM and 54 mM, indicating that Hosta longipes was resistant to CaCl2. The photosynthetic rate of Iris pseudacorus tended to be similar to that of Iris ensata. For Iris ensata, its photosynthetic rate at 60 DAT was 100%, and its photosynthetic rates in 18 mM and 54 mM groups at 90 DAT were 59.6% and 55.2%, respectively. For Iris pseudacorus, its photosynthetic rate at 60 DAT was 100%, and its photosynthetic rates in 18 mM and 54 mM groups at 90 DAT were 22.9% and 34.1%, respectively. Therefore, the photosynthetic rate of Iris ensata decreased less after treatment with CaCl2 at 18 mM and 54 mM.

Table 2.
Photosynthetic rates (µmolCO2m−2s−1) of three plant species exposed to different levels of CaCl2 at 30, 60, and 90 DAT (n = 5).
Chlorophyll fluorescence analysis is widely used in plant physiology as it can provide important information about the state of photosystem II [27]. Chlorophyll fluorescence of Hosta longipes showed a tendency to decrease with increasing concentration of CaCl2 from 30 DAT (p < 0.05). At 60 DAT, the measurement was impossible for plots treated at 54 mM (p < 0.001). At 90 DAT, chlorophyll fluorescence measurement was impossible for experimental plots except for the control ones because of the stress effect of CaCl2 treatment (Table 3). Iris ensata chlorophyll fluorescence showed significant differences between control and CaCl2 treatment plots at 30 DAT. However, it showed no significant difference between different CaCl2 treatment concentrations (p < 0.001). At 60 and 90 DAT, there were significant differences in chlorophyll fluorescence between control and CaCl2 treatment plots (p < 0.001). With increasing concentration of CaCl2, chlorophyll fluorescence tended to decrease. For Iris ensata, chlorophyll fluorescence increased at 90 DAT compared to that at 60 DAT in the 9 mM group, because the plant had adapted to the concentration of CaCl2. Je and Kim [25] have reported that when 3-year-old Pinus strobus is treated with CaCl2, the higher the concentration, the smaller the stomata length, the higher the stomatal density, and the smaller the leaf size as an adaptation strategy for the survival of the tree. There was no significant difference in chlorophyll fluorescence of Iris pseudacorus between control and 9 mM treatment plots at 30 or 60 DAT. However, significant difference appeared in the 18 mM group. At 90 DAT, there was a significant difference in chlorophyll fluorescence by CaCl2 treatment concentration (p < 0.001). One study has identified the biochemical, physiological, and anatomical mechanisms of adaptation of Callistemon citrinus and Viburnum lucidum to NaCl and CaCl2 salinization [28]. It was found that during the adaptation of these two plants to salt, stomata sizes were smaller, the number of stomata was increased, and leaves were thinner [28]. We found that the Fv/Fm value of Iris ensata was higher at 90 DAT than at 60 DAT in the 9 mM group. We determined that Iris ensata exhibited an adaptation mechanism to salt. This result needs to be confirmed in the future.

Table 3.
Chlorophyll fluorescence (Fv/Fm) values of three plant species exposed to different CaCl2 levels at 30, 60, and 90 DAT (n = 5).
Chlorophyll content showed a tendency to decrease with increasing CaCl2 treatment concentrations after 30 DAT for all three plants (Table 4). However, the SPAD value for Iris ensata was increased by 1.4 times for each CaCl2 treatment group (of 9, 18, or 54 mM) at 90 DAT compared to that at 60 DAT. Therefore, we believe that Iris ensata develops resistance to CaCl2 over time, as seen from the photosynthetic rate (Table 2) and SPAD value (Table 4) increase and growth photos (Figure 1) at 90 DAT.

Table 4.
Chlorophyll content (SPAD) values of three plant species exposed to different CaCl2 levels at 30, 60, and 90 DAT (n = 10).
The decrease in chlorophyll content indicates a decrease in chlorophyll synthesis caused by the accumulation of ionic toxicity after CaCl2 treatment as well as by damage from oxidative stress resulting from chlorophyll decomposition, which causes a decrease in the photosynthesis rate [29]. Cheng et al. [30] have reported that chlorophyll a and chlorophyll b contents of Sedum kamtschaticum and Hosta longipes are decreased compared to those with controlled influx ozone through stomata. Photosynthetic rates of Sedum kamtschaticum and Hosta longipes were also decreased [31]. This fact suggests that Sedum kamtschaticum and Hosta longipes can be used as indicator plants for ozone among air pollutants. Among the three plants tested, Hosta longipes tended to respond to CaCl2 the most sensitively. Thus, it can be used as an indicator plant for roadside CaCl2 damage.
CaCl2 is composed of a strong base and a strong acid. In water, it is ionized into calcium cations and chloride ions, generating heat and causing snow to melt. It can also absorb moisture well. When it is mixed with soil, it can inhibit plants’ ability to absorb moisture and nutrients from the soil, thereby increasing drying [32]. After analyzing the pH of roadside soil by studying the effect of snow removal agents on the environment, the research found acid soil to be gradually alkalized by a large amount of salt, with pH values being higher in November than in January when a de-icing agent was sprayed [33].
Leaf water potential decreased as the concentration of CaCl2 increased in all three plants (Table 5). Leaf water potential was significantly lowered by salt stress because the increase in salt ions caused by the accumulation of organic or inorganic solutes in cells could increase the osmotic pressure and inhibit water absorption [34]. A decrease in leaf water can also potentially decrease plant cells’ growth and enzyme activity and close the stomata, thereby lowering the photosynthesis rate [35].

Table 5.
Leaf water potential (Mpa) of three plant species exposed to different levels of CaCl2 at 60, and 90 DAT (n = 5).
4. Conclusions
In this study, we analyzed the growth and physiological responses of Hosta longipes, Iris ensata, and Iris pseudacorus according to CaCl2 concentration. We found that Iris ensata and Iris pseudacorus showed higher resistance to CaCl2 than did Hosta longipes. Therefore, it would be desirable to plant Iris ensata or Iris pseudacorus on the roadside where there is concern about damage caused by a de-icing agent. On the other hand, Hosta longipes can be planted relatively far from the roadside when selecting planting locations. Additionally, the effect of CaCl2 treatment on plants tends to increase over time. To reduce damage to plant growth, methods to dilute the concentration of CaCl2 accumulated in the soil should be studied if there is heavy use of de-icing agents during winter.
In general, Iris ensata and Iris pseudacorus are groundcover plants mainly used for wetland greening. However, they were tolerant to CaCl2 in this study. In the future, sustainable urban greenery can be created by using them as groundcover plants on the roadside if large amounts of de-icing agents are used when constructing GI in urban areas.
Author Contributions
Conceptualization, B.-J.P.; Methodology, S.-Y.K., N.-R.J., and B.-J.P.; Investigation, K.-J.K. and J.C.; Formal analysis, K.-J.K., J.C., S.-Y.K., and N.-R.J.; Writing—original draft preparation, K.-J.K., J.C., S.-Y.K., and N.-R.J.; Writing—review and editing, J.C., S.-Y.K., N.-R.J., and B.-J.P.; Supervision, B.-J.P.; Project administration, N.-R.J. and B.-J.P.; Funding acquisition, B.-J.P. All authors have read and agreed to the published version of the manuscript.
Funding
This work was financially supported by an Education Program for Urban Regeneration Expert funded by the Ministry of Land, Infrastructure and Transport (MOLIT), Korea.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors have no conflict of interest relevant to this study to disclose.
References
- Law, E.P.; Diemont, S.A.; Toland, T.R. A sustainability comparison of green infrastructure interventions using emergy evaluation. J. Clean Prod. 2017, 145, 374–385. [Google Scholar] [CrossRef]
- Kim, D.; Song, S.K. The multifunctional benefits of green infrastructure in community development: An analytical review based on 447 cases. Sustainability 2019, 11, 3917. [Google Scholar] [CrossRef]
- Kato, S. Green infrastructure for Asian cities: The spatial concepts and planning strategies. In Journal of the 2011 International Symposium on City Planning; Korea Planners Association: Seoul, Korea, 2011; pp. 161–170. [Google Scholar]
- Dhakal, K.P.; Chevalier, L.R. Managing urban stormwater for urban sustainability: Barriers and policy solutions for green infrastructure application. J. Environ. Manag. 2017, 203, 171–181. [Google Scholar] [CrossRef] [PubMed]
- Venter, Z.S.; Krog, N.H.; Barton, D.N. Linking green infrastructure to urban heat and human health risk mitigation in Oslo, Norway. Sci. Total Environ. 2020, 709, 136193. [Google Scholar] [CrossRef]
- Mell, I.C. Aligning fragmented planning structures through a green infrastructure approach to urban development in the UK and USA. Urban For. Urban Green. 2014, 13, 612–620. [Google Scholar] [CrossRef]
- Korea Meteorological Administration. Available online: https://www.weather.go.kr (accessed on 27 April 2021).
- Mullins, A.R.; Bain, D.J.; Pfeil-McCullough, E.; Hopkins, K.G.; Lavin, S.; Copeland, E. Seasonal drivers of chemical and hydrological patterns in roadside infiltration-based green infrastructure. Sci. Total Environ. 2020, 714, 136503. [Google Scholar] [CrossRef]
- Saaroni, H.; Amorim, J.H.; Hiemstra, J.A.; Pearlmutter, D. Urban green infrastructure as a tool for urban heat mitigation: Survey of research methodologies and findings across different climatic regions. Urban. Clim. 2018, 24, 94–110. [Google Scholar] [CrossRef]
- Kim, M.K.; Sim, W.K. Suggestion for multi-layer planting model in Seoul area based on a cluster analysis and interspecific association. J. Korean Inst. Landsc. Archit. 2010, 38, 106–127. [Google Scholar]
- Prihatmanti, R.; Taib, N. Multi-layer planting as a strategy of greening the transitional space in high-rise buildings: A review. In IOP Conference Series: Earth and Environmental Science; IOP Publishing: Philadelphia, PA, USA, 2018; Volume 126. [Google Scholar] [CrossRef]
- Benton-Short, L.; Keeley, M.; Rowland, J. Green infrastructure, green space, and sustainable urbanism: geography’s important role. Urban. Geogr. 2019, 40, 330–351. [Google Scholar] [CrossRef]
- Slama, I.; Abdelly, C.; Bouchereau, A.; Flowers, T.; Savoure, A. Diversity, distribution and roles of osmoprotective compounds accumulated in halophytes under abiotic stress. Ann. Bot. 2015, 115, 433–447. [Google Scholar] [CrossRef]
- Himabindu, Y.; Chakradhar, T.; Reddy, M.C.; Kanygin, A.; Redding, K.E.; Chandrasekhar, T. Salt-tolerant genes from halophytes are potential key players of salt tolerance in glycophytes. Environ. Exp. Bot. 2016, 124, 39–63. [Google Scholar] [CrossRef]
- Gałuszka, A.; Migaszewski, Z.M.; Podlaski, R.; Dołęgowska, S.; Michalik, A. The influence of chloride deicers on mineral nutrition and the health status of roadside trees in the city of Kielce, Poland. Environ. Monit. Assess. 2011, 176, 451–464. [Google Scholar] [CrossRef] [PubMed]
- Shannon, M.C. Adaption of plants to salinity. Adv. Agron. 1998, 60, 75–120. [Google Scholar] [CrossRef]
- Parida, A.K.; Das, A.B. Salt tolerance and salinity effects on plants: A review. Ecotoxicol. Environ. Saf. 2005, 60, 324–349. [Google Scholar] [CrossRef] [PubMed]
- Ju, J.H.; Park, J.Y.; Hui, X.; Lee, E.Y.; Hyun, K.H.; Jung, J.S.; Choi, E.Y.; Yoon, Y.H. Growth and physiological response of three evergreen shrubs to de-icing salt (CaCl2) at different concentrations in winter-Focusing on Euonymus japonica, Rhodoendron indicum, and Buxus koreana. J. Korean Inst. Landsc. Archit. 2016, 44, 122–129. [Google Scholar] [CrossRef]
- Sung, J.H.; Je, S.M.; Kim, S.H.; Kim, Y.K. Effect of calcium chloride (CaCl2) on the characteristics of photosynthetic apparatus, stomatal conductance, and fluorescence image of the leaves of Cornus kousa. Korean J. Agric. For. Meteorol. 2009, 11, 143–150. [Google Scholar] [CrossRef]
- Swoczyna, T.; Latocha, P. Monitoring seasonal damage of photosynthetic apparatus in mature street trees exposed to road-side salinity caused by heavy traffic. Photosynthetica 2020, 58, 388–399. [Google Scholar] [CrossRef]
- Choi, H.S.; Hong, J.S.; Geronimo, F.K.F.; Kim, L.H. Implications of CaCl2 application to plants in LID facilities. Water Sci. Technol. 2018, 78, 1045–1053. [Google Scholar] [CrossRef]
- Nixon, W.A. Economics of Using Calcium Chloride vs. Sodium Chloride for Deicing/Anti-Icing; Final Report TR488; University of Iowa: Iowa City, IA, USA, 2008. [Google Scholar]
- Trajkova, F.; Papadantonakis, N.; Savvas, D. Comparative effects of NaCl and CaCl2 salinity on cucumber grown in a closed hydroponic system. HortScience 2006, 41, 437–441. [Google Scholar] [CrossRef]
- Hooks, T.; Niu, G. Relative salt tolerance of four herbaceous perennial ornamentals. Horticulturae 2019, 5, 36. [Google Scholar] [CrossRef]
- Je, S.M.; Kim, S.H. Growth and physiological responses of Pinus strobus to CaCl2. J. Korean Inst. Landsc. Archit. 2017, 45, 1–8. [Google Scholar] [CrossRef]
- Munns, R.; Tester, M. Mechanisms of salinity tolerance. Annu. Rev. Plant. Biol. 2008, 59, 651–681. [Google Scholar] [CrossRef] [PubMed]
- Maxwell, K.; Johnson, G.N. Chlorophyll fluorescence: A practical guide. J. Exp. Bot. 2000, 51, 659–668. [Google Scholar] [CrossRef]
- Cirillo, C.; De Micco, V.; Arena, C.; Carillo, P.; Pannico, A.; De Pascale, S.; Rouphael, Y. Biochemical, physiological and anatomical mechanisms of adaptation of Callistemon citrinus and Viburnum lucidum to NaCl and CaCl2 salinization. Front. Plant. Sci. 2019, 10, 742. [Google Scholar] [CrossRef] [PubMed]
- Jaleel, C.A.; Sankar, B.; Sridharan, R.; Panneerselvam, R. Soil salinity alters growth, chlorophyll content, and secondary metabolite accumulation in Catharanthus roseus. Turk. J. Biol. 2008, 32, 79–83. [Google Scholar]
- Cheng, H.C.; Woo, S.Y.; Lee, S.H.; Kwak, M.J.; Kim, K.N. Biochemical responses of Sedum kamtschaticum and Hosta longipes to ozone stress. Korean J. Hort. Sci. Technol. 2013, 31, 1–7. [Google Scholar] [CrossRef][Green Version]
- Cheng, H.C.; Woo, S.Y.; Lee, S.H.; Baek, S.G. Photosynthesis, antioxidant enzyme and anatomical difference of Sedum kamtschaticum and Hosta longipes to ozone. Korean J. Hort. Sci. Technol. 2010, 8, 394–402. [Google Scholar]
- White, P.J.; Broadley, M.R. Chloride in soils and its uptake and movement within the plant: A review. Ann. Bot. 2001, 88, 967–988. [Google Scholar] [CrossRef]
- Shin, J.H.; Heo, H.R.; Shin, J.S.; Kim, M.Y.; Shin, J.Y. A study of effects on environment from road deicings. Korean J. Sanit. 2001, 16, 31–37. [Google Scholar]
- Nam, Y.I.; Woo, Y.H. Influence of Chilling stress on photosynthetic and physiological responses of cucumber (Cucumis sativus L.) Seedlings. J. Bio. Environ. Control 2001, 10, l59–l164. [Google Scholar]
- Park, Y.M. Characteristic of matter allocation of Calystegia soldanella under water stress. J. Environ. Sci. Int. 2013, 22, 187–193. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).