Long-Term Enclosure Can Benefit Grassland Community Stability on the Loess Plateau of China
Abstract
1. Introduction
2. Material and Methods
2.1. Site Description
2.2. Experimental Design
2.3. Plant Community Composition Monitoring
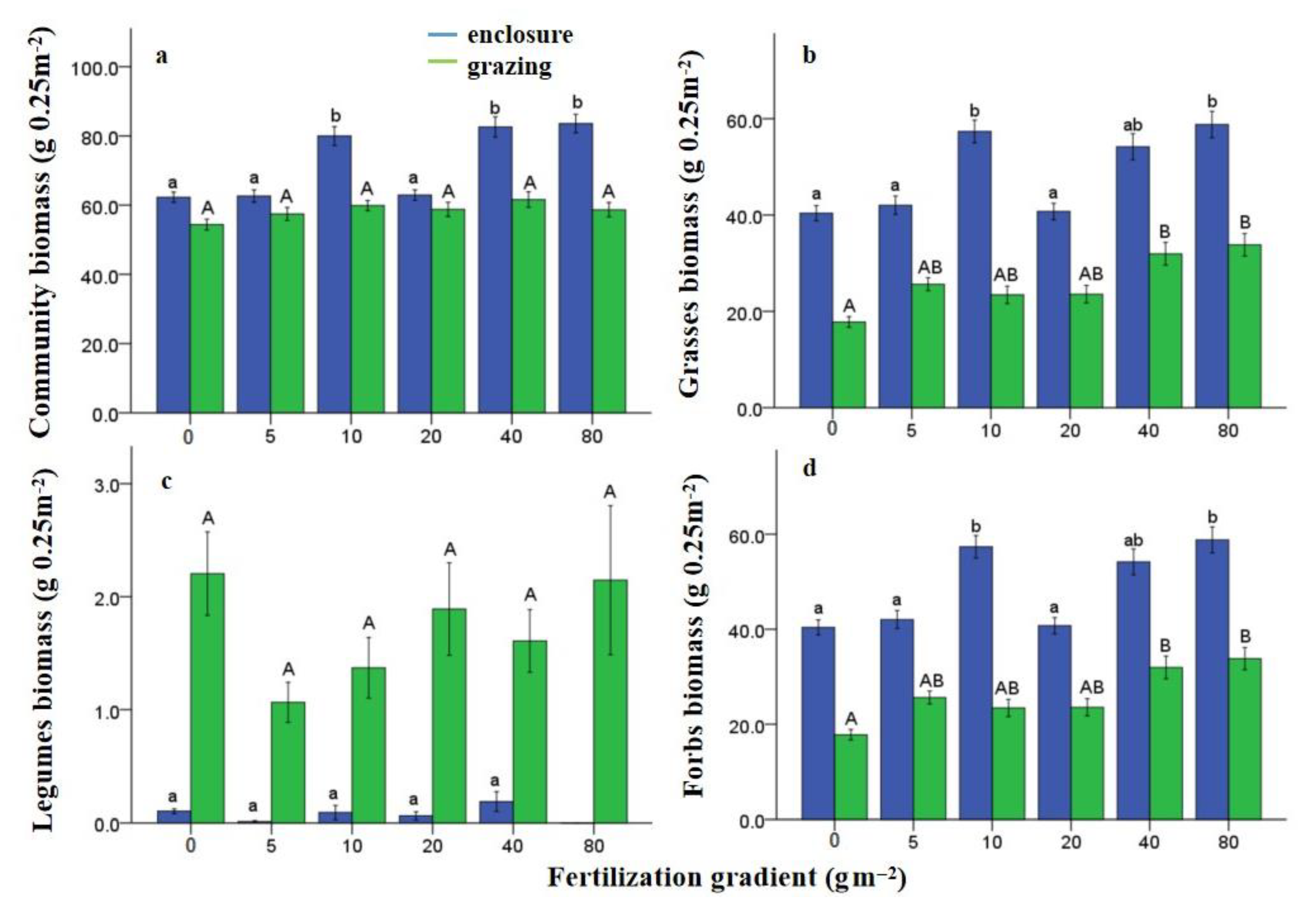
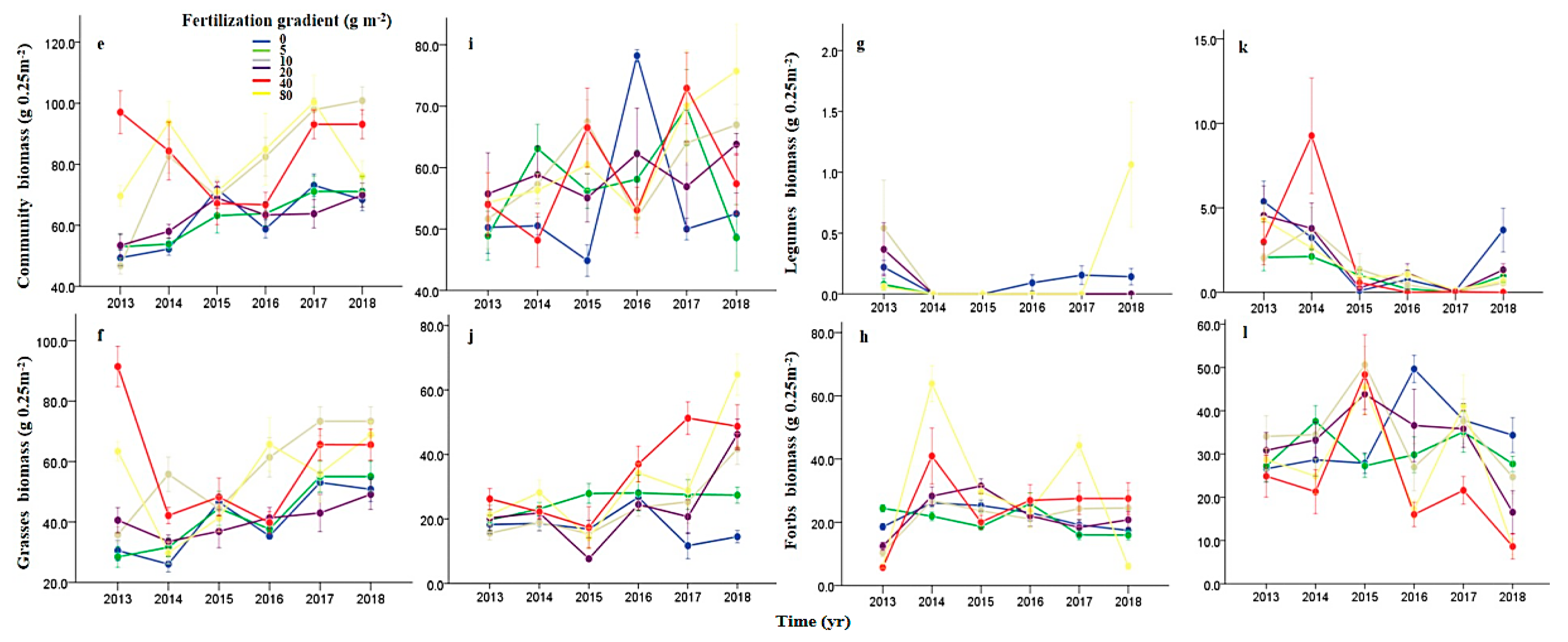
2.4. Plant Aboveground Productivity Measurement
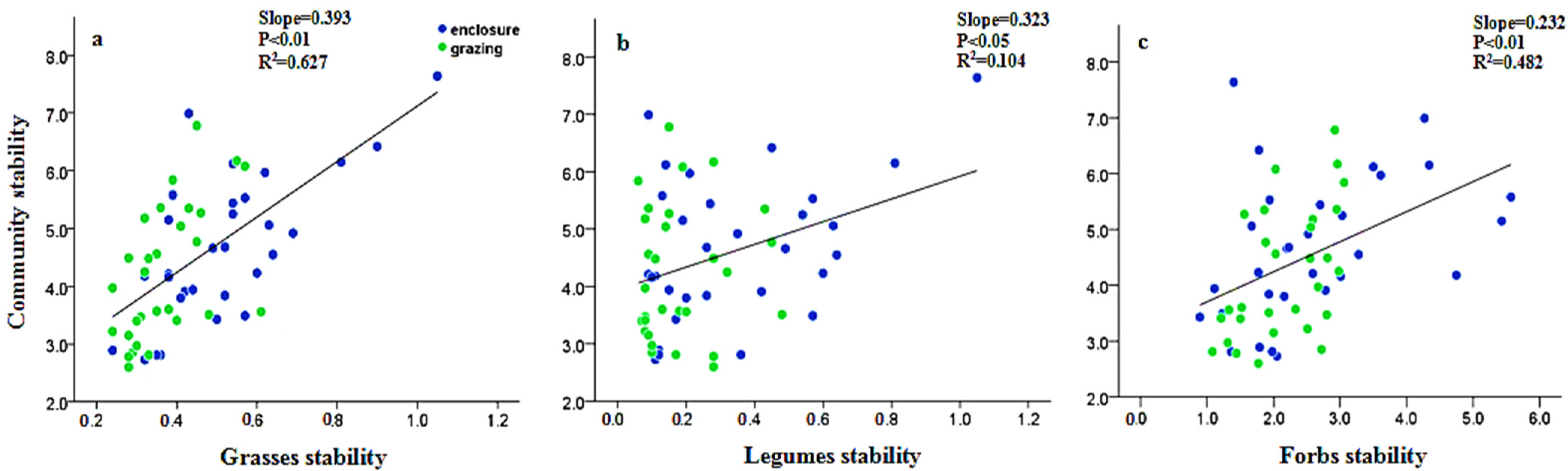
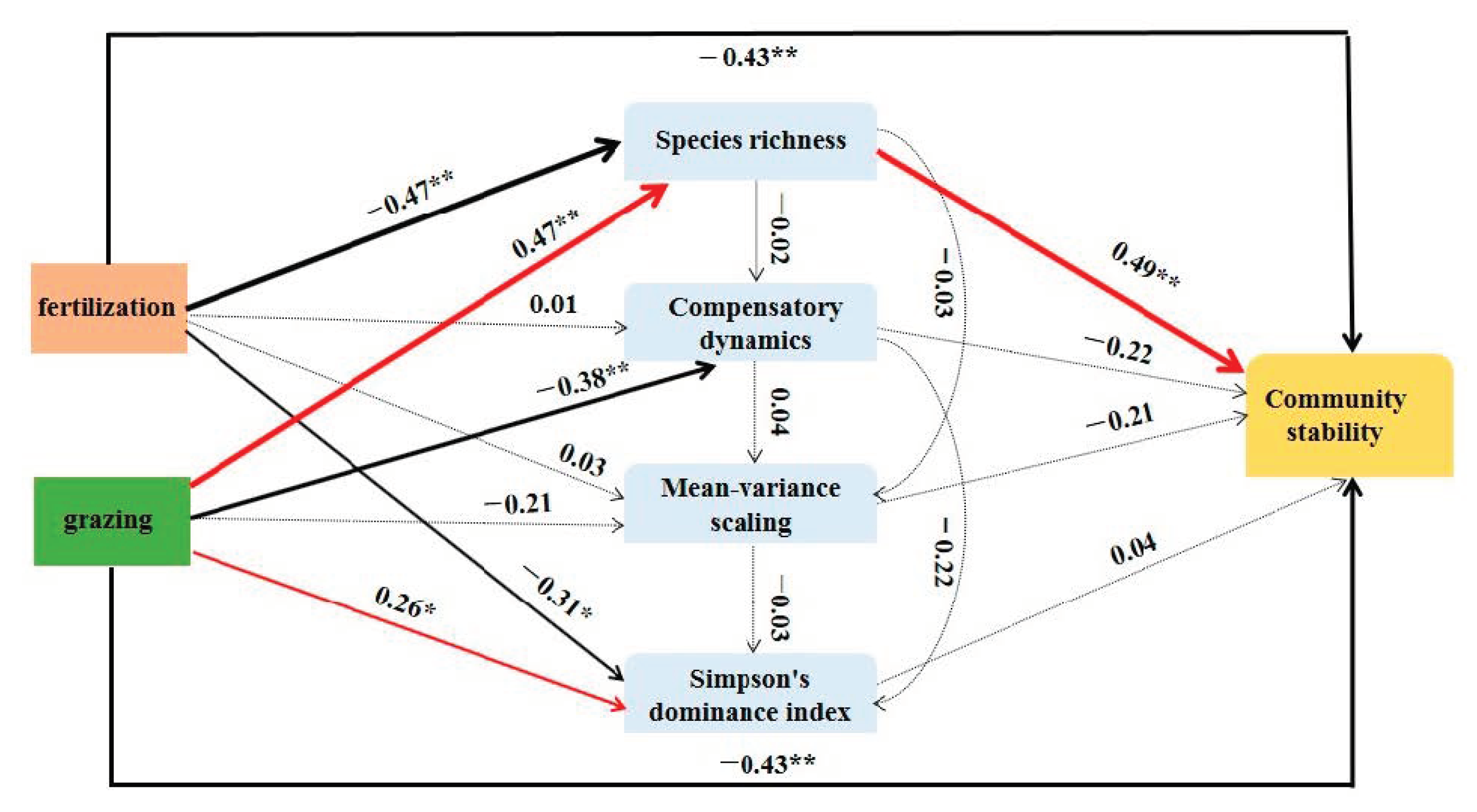
2.5. Relationships between Community Stability and the Underlying Mechanisms
2.6. Statistical Analysis
3. Results
3.1. Effects of N Fertilization and Grazing on Community Stability
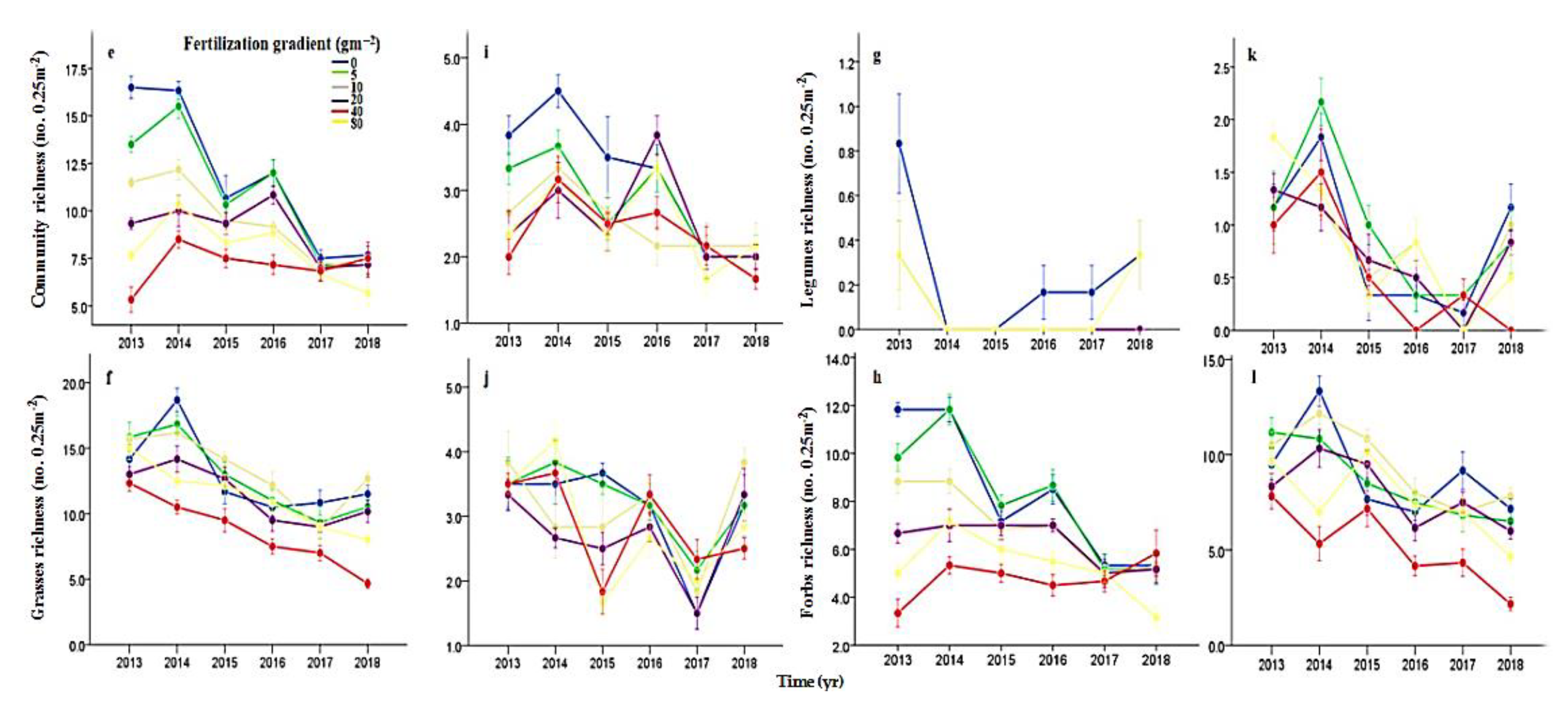
3.2. Effects of N Fertilization and Grazing on Species Diversity
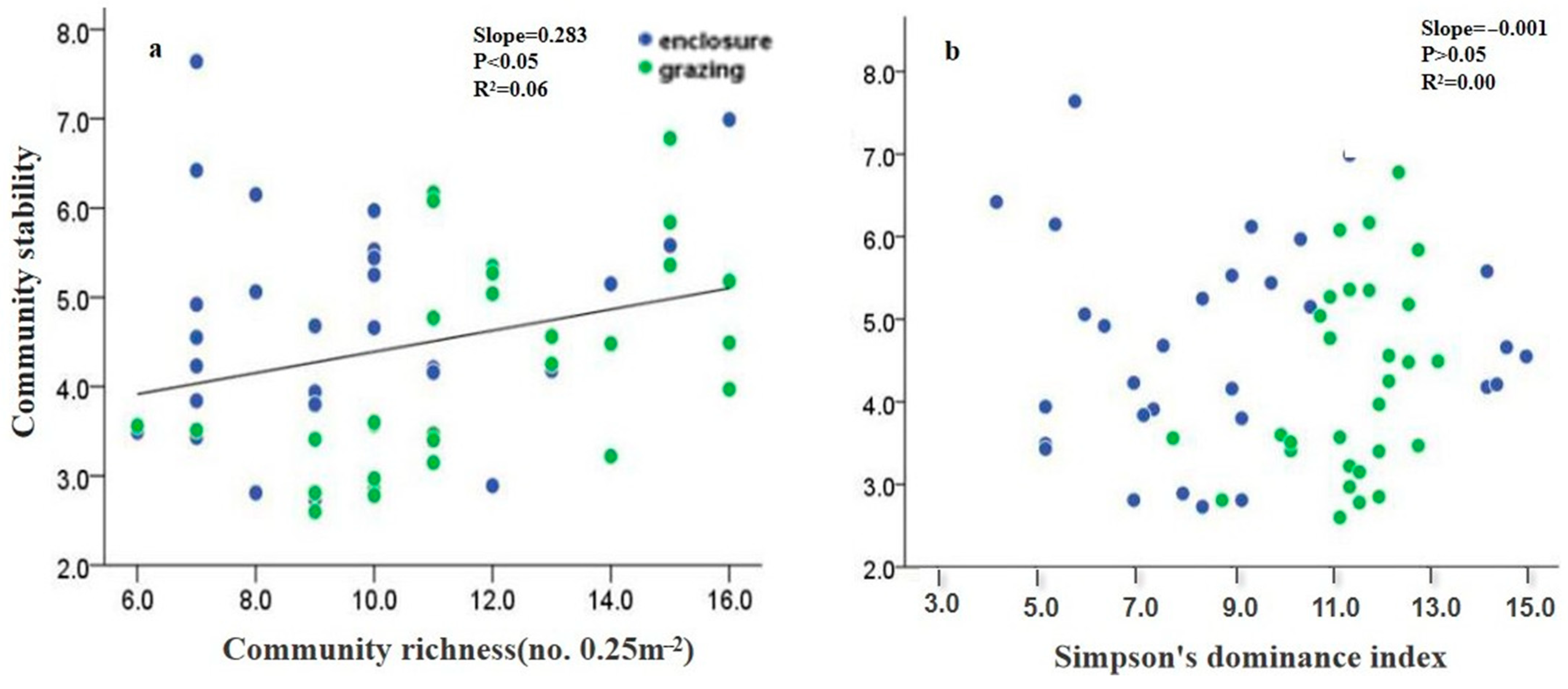
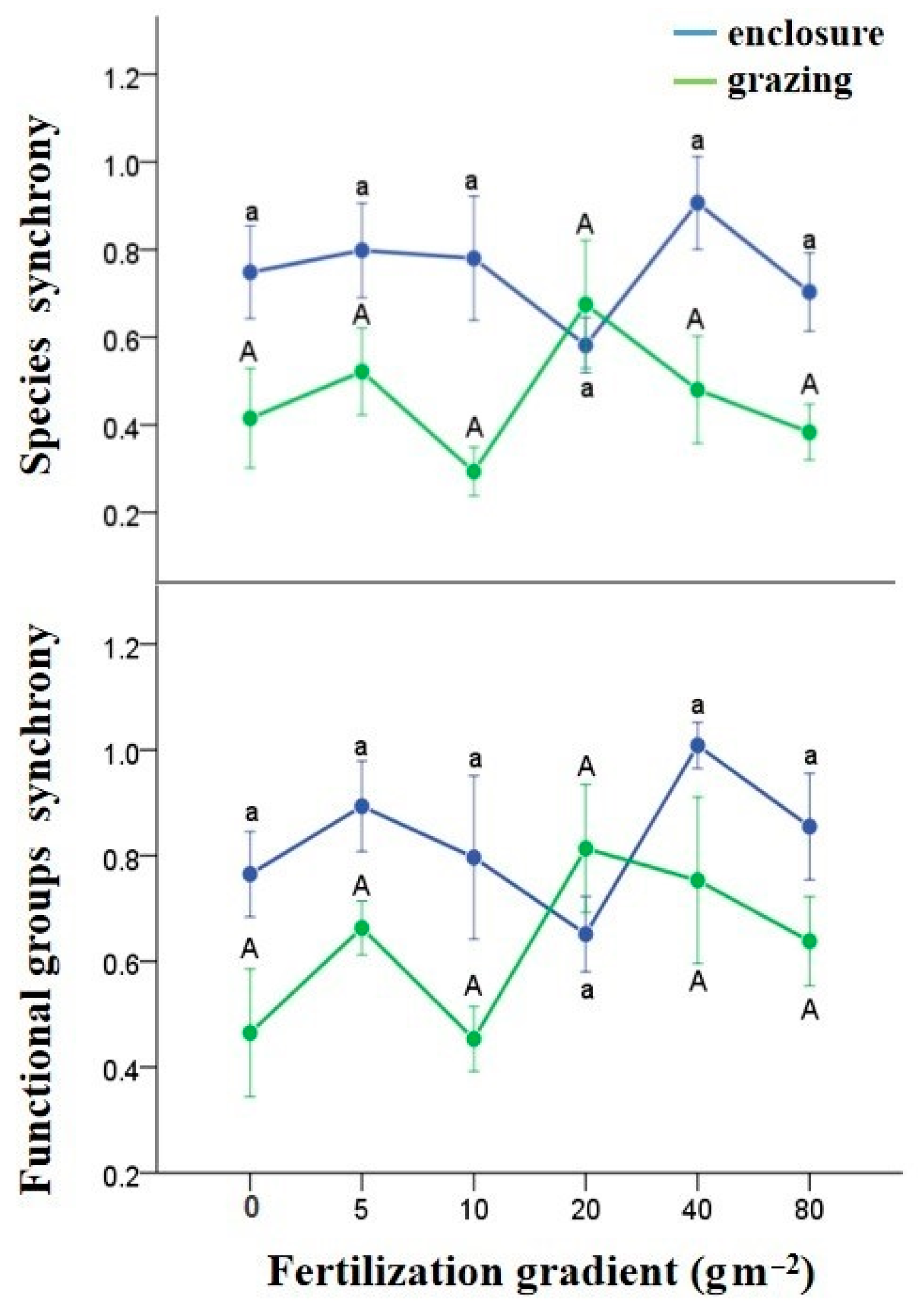
3.3. Effects of N Fertilization and Grazing on Compensatory Dynamics
3.4. Effects of N Fertilization and Grazing on Mean–Variance Scaling
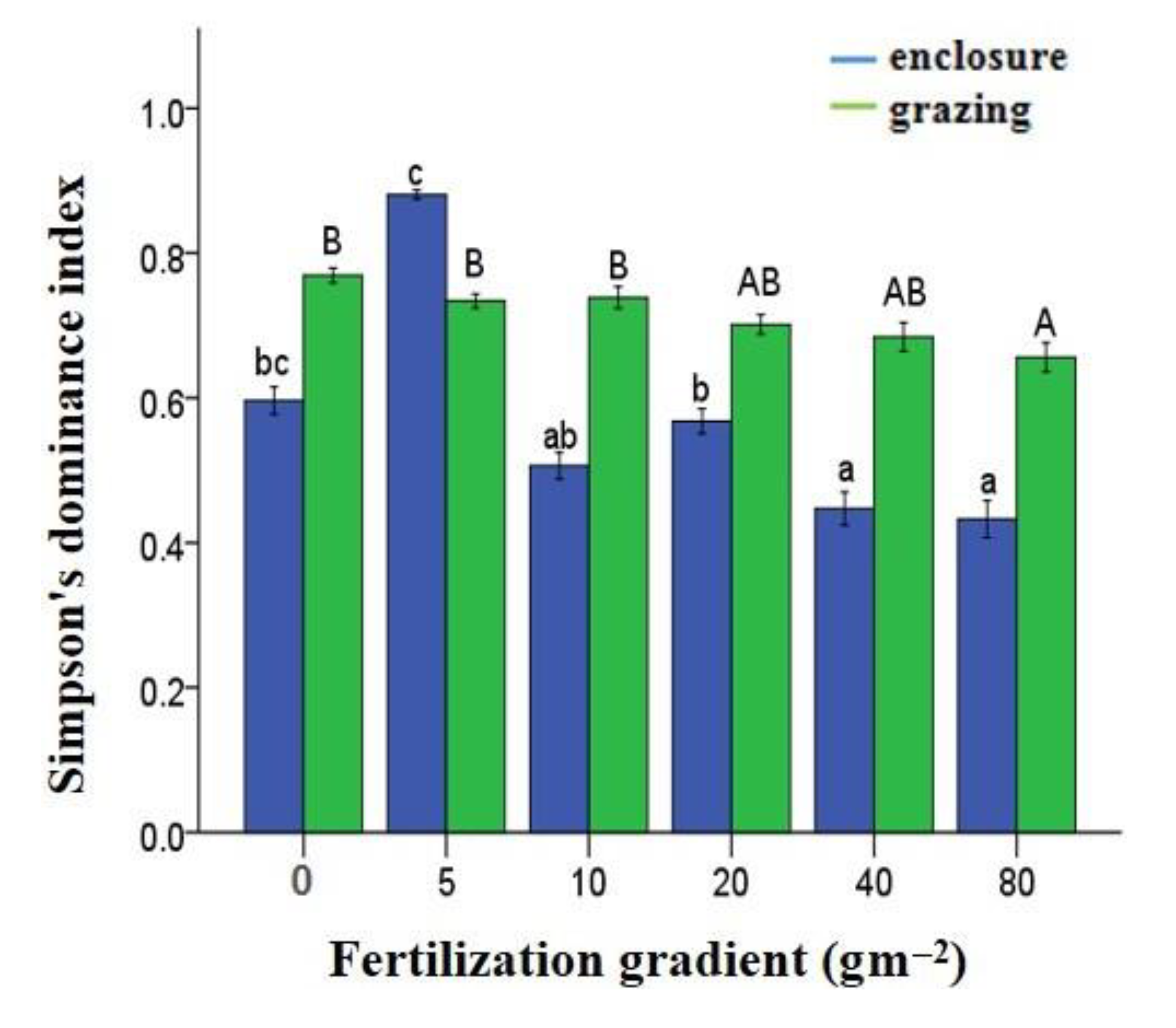
3.5. Effects of N Fertilization and Grazing on Dominance
4. Discussion
4.1. Effects of Fertilization and Grazing on Community Stability
4.2. Effects of Fertilization and Grazing on the Underlying Mechanisms Contributed to Community Stability
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
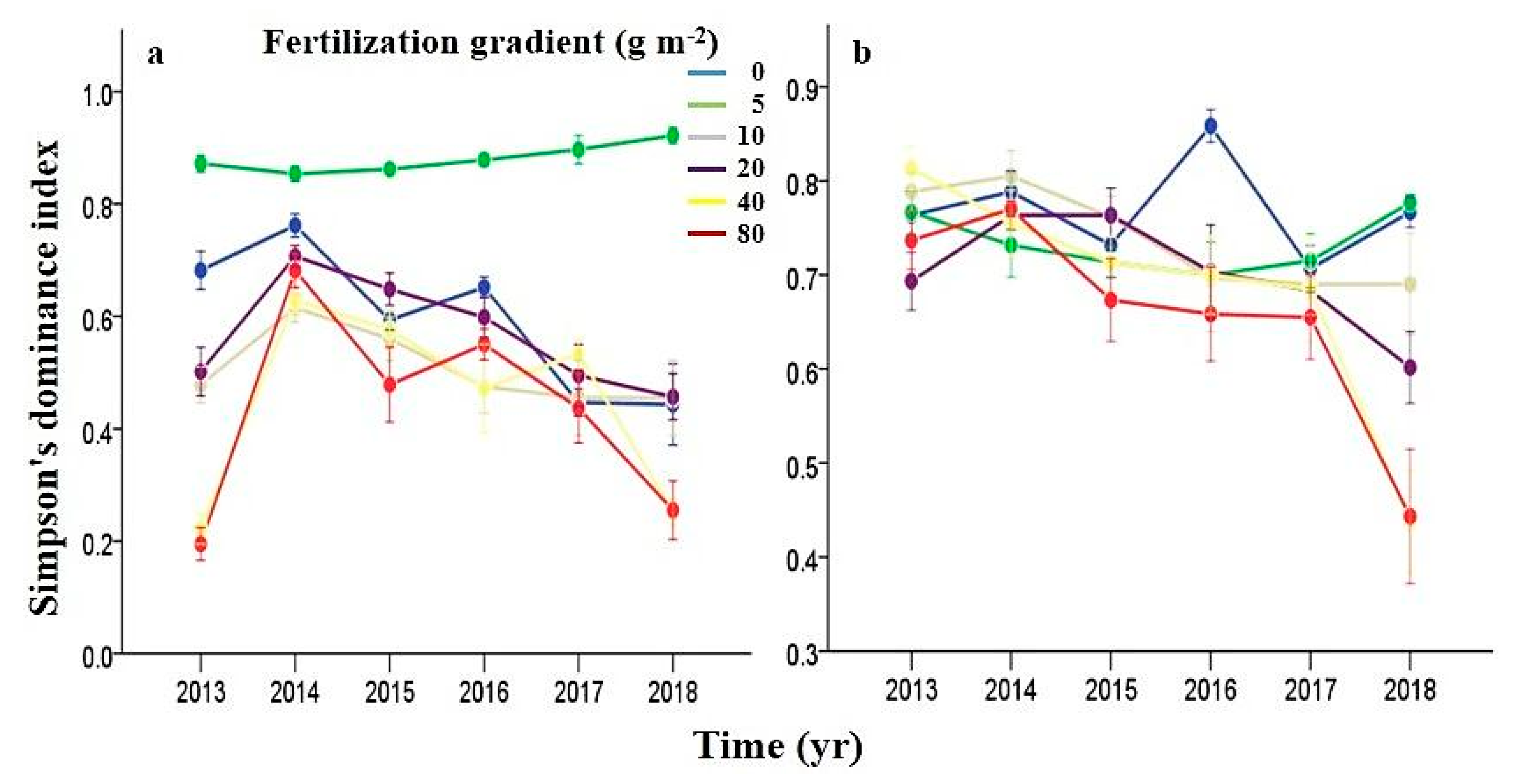
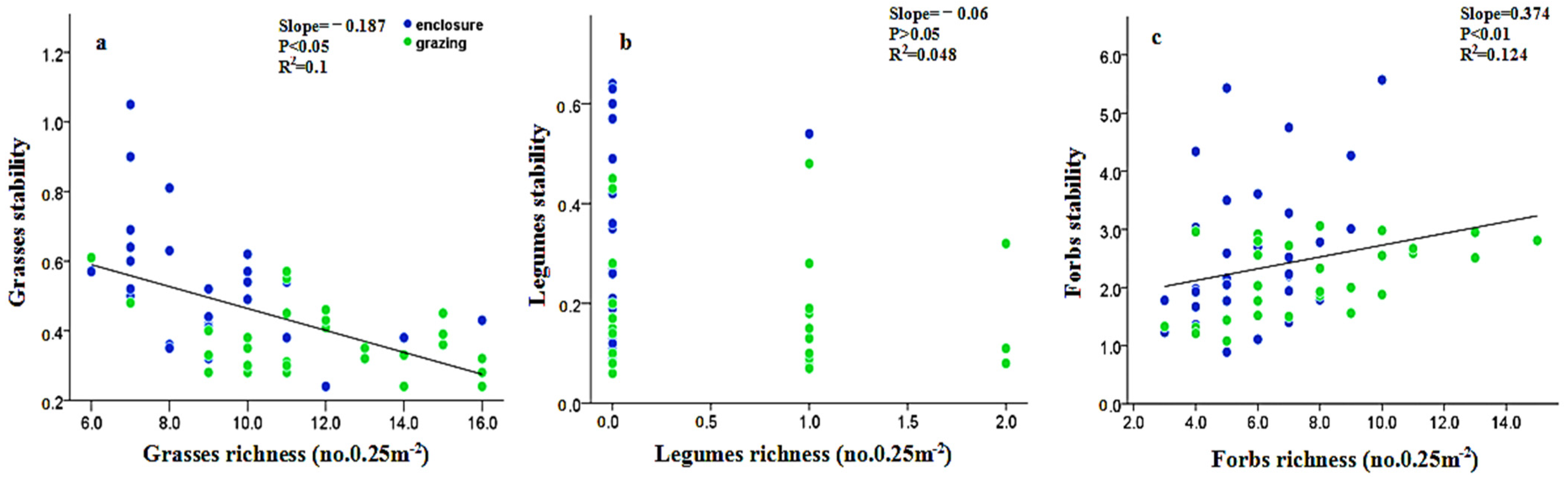
Appendix B
| N | G | N*G | |||||||
|---|---|---|---|---|---|---|---|---|---|
| df | F | p | df | F | p | df | F | p | |
| COS | 5 | 4.25 | 0.007 ** | 1 | 3.41 | 0.018 * | 5 | 2.71 | 0.031 * |
| GRS | 5 | 3.48 | 0.017 * | 1 | 0.90 | 0.495 NS | 5 | 2.22 | 0.068 NS |
| NFS | 5 | 8.44 | 0.000 ** | 1 | 8.32 | 0.000 ** | 5 | 1.04 | 0.403 NS |
| LES | 5 | 0.57 | 0.692 NS | 1 | 0.69 | 0.640 NS | 4 | 0.27 | 0.893 NS |
| Functional Groups | Species Name | Mean Population Stability | N | G | N*G | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Enclosure | Grazing | |||||||||||||||
| 0 | 5 | 10 | 20 | 40 | 80 | 0 | 5 | 10 | 20 | 40 | 80 | |||||
| Grasses | Stipa przewalskyi | 3.72 | 2.50 | 2.94 | 2.67 | 2.07 | 2.71 | 1.01 | 1.01 | 1.06 | 1.01 | 1.01 | 1.19 | 1.18 NS | 166.60 ** | 1.24 NS |
| Carex aridula | 0.96 | 0.87 | 0.96 | 0.81 | 0.63 | 0.54 | 1.37 | 2.40 | 2.41 | 1.47 | 1.01 | 1.01 | 3.63 ** | 32.06 ** | 0.49 NS | |
| Leymus secalinus | 0.56 | 0.65 | 0.65 | 0.63 | 0.45 | 0.72 | 1.01 | 1.25 | 1.11 | 1.09 | 1.05 | 1.01 | 0.45 NS | 47.72 ** | 0.35 NS | |
| Elymus dahuricus | 0.79 | 0.48 | 0.64 | 0.64 | 0.74 | 0.40 | 1.01 | 1.01 | 1.01 | 1.01 | 1.01 | 1.01 | 4.33 ** | 0.45 NS | 0.51 NS | |
| Poa annua | 0.67 | 0.58 | 0.39 | 0.36 | 0.35 | 0.31 | 1.74 | 3.06 | 1.01 | 1.01 | 1.02 | 1.02 | 4.82 ** | 57.68 ** | 0.58 NS | |
| Stipa grandis | 0.65 | 0.44 | 0.46 | 0.46 | 0.39 | 0.38 | 2.01 | 1.91 | 1.02 | 1.02 | 1.01 | 1.01 | 2.87* | 51.56 ** | 0.91 NS | |
| Agropyron cristatum | 0.37 | 0.35 | 0.30 | 0.36 | 0.30 | 0.31 | 1.60 NS | |||||||||
| Legumes | Medicago archiducis-nicolai | 0.45 | 0.35 | 0.31 | 0.36 | 0.42 | 1.01 | 1.01 | 1.02 | 1.02 | 1.37 | 0.29 NS | 25.14 ** | 0.11 NS | ||
| Astragalus tataricus | 0.41 | 0.31 | 0.43 | 1.01 | 1.05 | 1.02 | 1.13 | 1.02 | 1.01 | 1.26 NS | 113.41 ** | 4.36* | ||||
| Oxytropis ochrantha var. ochrantha | 0.28 | 0.31 | 1.01 | 1.58 | 1.01 | 1.01 | 0.38 NS | 5.54* | 4.54* | |||||||
| Forbs | Heteropappus altaicus | 0.45 | 0.45 | 0.53 | 0.62 | 0.39 | 0.31 | 1.01 | 2.36 | 1.88 | 3.31 | 1.18 | 1.13 | 2.81* | 71.41 ** | 0.40 NS |
| Artemisia sacrorum | 1.38 | 1.19 | 0.87 | 1.47 | 1.23 | 0.77 | 1.01 | 1.71 | 1.23 | 1.01 | 1.55 | 0.41 NS | 0.46 NS | 0.31 NS | ||
| Thymus mongolicus | 0.43 | 0.59 | 0.41 | 0.43 | 0.39 | 0.31 | 4.16 | 2.03 | 1.39 | 2.94 | 3.61 | 1.44 | 1.07 NS | 87.10 ** | 0.65 NS | |
| Crepis crocea | 0.37 | 0.37 | 0.45 | 0.36 | 0.31 | 0.24 NS | ||||||||||
| Trigonotis amblyosepala | 0.42 | 0.31 | 0.31 | 0.30 | 0.23 NS | |||||||||||
| Potentilla bifurca | 0.65 | 0.76 | 0.68 | 0.68 | 0.66 | 1.04 | 1.01 | 1.01 | 1.01 | 1.60 NS | 0.43 NS | 2.34 NS | ||||
| Dendranthema lavandulifolium | 1.04 | 1.09 | 0.77 | 0.59 | 0.72 | 0.65 | 1.64 | 1.09 | 1.011 | 1.01 | 1.92 | 5.21 | 1.13 NS | 14.61 ** | 1.37 NS | |
| Leontopodium leontopodioides | 0.54 | 0.64 | 0.45 | 0.63 | 0.34 | 0.43 | 1.09 | 3.97 | 1.04 | 1.13 | 1.02 | 1.01 | 11.45 ** | 145.05 ** | 5.11 ** | |
| Viola dissecta | 0.57 | 0.61 | 0.71 | 0.81 | 0.62 | 0.55 | 1.10 | 1.02 | 1.29 NS | 12.55 ** | 0.41 NS | |||||
| Galium verum | 1.43 | 1.67 | 1.39 | 1.08 | 0.95 | 0.91 | 4.51 ** | |||||||||
| Saussurea alata | 0.63 | 0.58 | 0.65 | 0.51 | 0.33 | 0.54 | 1.26 NS | |||||||||
| Dracocephalum heterophyllum | 0.31 | 0.37 | 1.01 | 5.87 | 1.01 | 1.01 | 0.63 NS | 84.10 ** | 0.02 ** | |||||||
| Scutellaria scordifolia | 0.70 | 0.70 | 0.75 | 0.52 | 0.60 | 0.50 | 1.78 NS | |||||||||
| Artemisia gansuensis | 0.31 | 0.31 | 0.42 | 0.30 | 0.31 | 1.33 | 1.64 | 1.05 | 2.85 | 1.12 | 1.08 | 0.71 NS | 63.86 ** | 1.14 NS | ||
| Viola prionantha | 0.41 | 0.35 | 0.41 | 0.31 | 0.37 | 0.18 NS | ||||||||||
| Thalictrum petaloideum | 0.41 | 0.42 | 0.41 | 0.35 | 0.30 | 0.35 | 1.01 NS | |||||||||
| Polygala tenuifolia | 0.31 | 0.46 | 0.31 | 0.37 | 1.01 | 1.01 | 1.01 | 1.32 | 1.01 | 0.73 NS | 77.79 ** | 0.51 NS | ||||
| Potentilla acaulis | 0.35 | 0.34 | 0.31 | 0.31 | 0.37 | 1.01 | 1.17 | 1.01 | 1.01 | 1.01 NS | 68.4 ** | 0.66 NS | ||||
| Adenophora stenanthina | 0.59 | 0.71 | 0.43 | 0.53 | 0.42 | 0.41 | 2.47* | |||||||||
| Androsace mariae | 0.31 | 0.31 | 0.30 | 1.01 | 1.02 | 1.01 | 1.01 | 1.01 | 0.01 NS | 1.24 NS | 0.02 NS | |||||
| Allium ramosum | 0.31 | 0.31 | 0.31 | 0.35 | 0.31 | 0.30 | 0.89 NS | |||||||||
| Salsola collina | 0.76 | 0.93 | 0.66 | 0.57 | 0.63 | 0.46 | 0.50 NS | |||||||||
| Adenophora paniculata | 0.37 | 0.34 | 0.45 | 0.51 | 0.32 | 0.33 | 1.01 | 1.01 | 1.75 NS | 49.40 NS | 0.77 NS | |||||
| Torularia humilis | 0.34 | 0.41 | 0.33 | 0.30 | 0.34 | 0.31 | 2.05 NS | |||||||||
| Euphorbia fischeriana | 1.01 | 1.02 | 1.01 | 1.01 | 1.01 | 1.01 | 0.38 NS | |||||||||
| N | G | N*G | |||||||
|---|---|---|---|---|---|---|---|---|---|
| df | F | p | df | F | p | df | F | p | |
| SSY | 5 | 0.46 | 0.807 NS | 1 | 13.81 | 0.000 ** | 5 | 0.50 | 0.774 NS |
| FSY | 5 | 1.05 | 0.410 NS | 1 | 6.94 | 0.011 * | 5 | 0.55 | 0.741 NS |
| N | G | N*G | |||||||
|---|---|---|---|---|---|---|---|---|---|
| df | F | p | df | F | p | df | F | p | |
| Z | 5 | 0.60 | 0.702 NS | 1 | 0.49 | 0.485 NS | 5 | 0.29 | 0.916 NS |
| Y | N | G | Y*N | Y*G | Y*G*N | N*G | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| df | F | p | df | F | p | df | F | p | df | F | p | df | F | p | df | F | p | df | F | p | |
| DI | 5 | 31.86 | 0.000 ** | 5 | 10.25 | 0.000 ** | 1 | 86.26 | 0.000 ** | 25 | 2.25 | 0.001 ** | 5 | 4.51 | 0.002 ** | 25 | 2.21 | 0.001 ** | 5 | 1.62 | 0.168 NS |
References
- Song, M.-H.; Yu, F.-H. Reduced compensatory effects explain the nitrogen-mediated reduction in stability of an alpine meadow on the Tibetan Plateau. New Phytol. 2015, 207, 70–77. [Google Scholar] [CrossRef]
- Wilsey, B.J.; Daneshgar, P.P.; Hofmockel, K.; Polley, H.W. Invaded grassland communities have altered stability-maintenance mechanisms but equal stability compared to native communities. Ecol. Lett. 2013, 17, 92–100. [Google Scholar] [CrossRef] [PubMed]
- Polley, H.W.; Wilsey, B.J.; Derner, J.D. Dominant species constrain effects of species diversity on temporal variability in biomass production of tallgrass prairie. Oikos 2007, 116, 2044–2052. [Google Scholar] [CrossRef]
- Fowler, M.S.; Laakso, J.; Kaitala, V.; Ruokolainen, L.; Ranta, E. Species dynamics alter community diversity-biomass stability relationships. Ecol. Lett. 2012, 15, 1387–1396. [Google Scholar] [CrossRef] [PubMed]
- Congreves, K.A.; Hooker, D.C.; Hayes, A.; Verhallen, E.A.; Van Eerd, L.L. Interaction of long-term nitrogen fertilizer appli-cation, crop rotation, and tillage system on soil carbon and nitrogen dynamics. Plant Soil 2017, 410, 113–127. [Google Scholar] [CrossRef]
- Grman, E.; Jennifer, A.L.; Donald, R.; Gross, K.L. Mechanisms contributing to stability in ecosystem function depend on the environmental context. Ecol. Lett. 2010, 13, 1400–1410. [Google Scholar] [CrossRef]
- Yang, Z.L.; Ruijven, J.V.; Du, G. The effects of long-term fertilization on the temporal stability of alpine meadow communi-ties. Plant Soil 2011, 345, 315–324. [Google Scholar] [CrossRef]
- Salgado-Luarte, C.; Escobedo, V.M.; Stotz, G.C.; Rios, R.S.; Arancio, G.; Gianoli, E. Goat grazing reduces diversity and leads to functional, taxonomic, and phylogenetic homogenization in an arid shrubland. Land Degrad. Dev. 2019, 30, 178–189. [Google Scholar] [CrossRef]
- Reinhart, K.O.; Rinella, M.J.; Waterman, R.C.; Petersen, M.K.; Vermeire, L.T. Testing rangeland health theory in the north-ern great plains. J. Appl. Ecol. 2019, 56, 319–329. [Google Scholar] [CrossRef]
- Chen, Y.; Li, Y.; Zhao, X.; Awada, T.; Shang, W.; Han, J. Effects of Grazing Exclusion on Soil Properties and on Ecosystem Carbon and Nitrogen Storage in a Sandy Rangeland of Inner Mongolia, Northern China. Environ. Manag. 2012, 50, 622–632. [Google Scholar] [CrossRef]
- Vargas, R.S.; Bataiolli, R.; Da Costa, P.B.; Lisboa, B.B.; Passaglia, L.M.P.; Beneduzi, A.; Vargas, L.K. Microbial quality of soil from the Pampa biome in response to different grazing pressures. Genet. Mol. Biol. 2015, 38, 205–212. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.-D.; Zhang, L.-L.; Zhu, Z.-H. Effects of clipping and fertilizing on the relationships between species diversity and ecosystem functioning and mechanisms of community stability in alpine meadow. Chin. J. Plant. Ecol. 2013, 37, 279–295. [Google Scholar] [CrossRef]
- Piñeiro, G.; Paruelo, J.M.; Oesterheld, M.; Jobbágy, E.G. Pathways of Grazing Effects on Soil Organic Carbon and Nitrogen. Rangel. Ecol. Manag. 2010, 63, 109–119. [Google Scholar] [CrossRef]
- Hewins, D.B.; Lyseng, M.P.; Schoderbek, D.F.; Alexander, M.; Willms, W.D.; Carlyle, C.N.; Chang, S.X.; Bork, E.W. Grazing and climate effects on soil organic carbon concentration and particle-size association in northern grasslands. Sci. Rep. 2018, 8, 1–9. [Google Scholar] [CrossRef]
- Franzluebbers, A.J.; Stuedemann, J.A. Impact of Cattle and Forage Management on Soil Surface Properties in the Southern Piedmont USA. 2003. Available online: https://www.researchgate.net/publication/251807538 (accessed on 20 March 2020).
- Hautier, Y.; Seabloom, E.W.; Borer, E.T.; Adler, P.B.; Harpole, W.S.; Hillebrand, H.; Lind, E.M.; MacDougall, A.S.; Stevens, C.J.; Bakker, J.D.; et al. Eutrophication weakens stabilizing effects of diversity in natural grasslands. Nat. Cell Biol. 2014, 508, 521–525. [Google Scholar] [CrossRef]
- Tilman, D. Biodiversity: Population Versus Ecosystem Stability. Ecology 1995, 77, 350–363. [Google Scholar] [CrossRef]
- Yang, H.; Jiang, L.; Li, L.; Li, A.; Wu, M.; Wan, S. Diversity-dependent stability under mowing and nutrient addition: Evidence from a 7-year grassland experiment. Ecol. Lett. 2012, 15, 619–626. [Google Scholar] [CrossRef]
- Loreau, M.; Mazancourt, C. Species synchrony and its drivers: Neutral and nonneutral community dynamics in fluctuating environments. Am. Nat. 2008, 172, 1455–1474. [Google Scholar] [CrossRef]
- Wildova, R.; Goldberg, D.E.; Herben, T. The contrasting roles of growth traits and architectural traits in diversity mainte-nance in clonal plant communities. Am. Nat. 2012, 180, 693–706. [Google Scholar] [CrossRef]
- Niu, D.; Yuan, X.; Cease, A.J.; Wen, H.; Zhang, C.; Fu, H.; Elser, J.J. The impact of nitrogen enrichment on grassland ecosystem stability depends on nitrogen addition level. Sci. Total. Environ. 2018, 618, 1529–1538. [Google Scholar] [CrossRef]
- Gonzalez, A.; Loreau, M. The Causes and Consequences of Compensatory Dynamics in Ecological Communities. Annu. Rev. Ecol. Evol. Syst. 2009, 40, 393–414. [Google Scholar] [CrossRef]
- Taylor, E.C.; Knopf, R.J.; Cogliano, J.A.; Barton, J.W.; Pfleiderer, W. A Facile Pyrimidine Ring Cleavage: Corrections. J. Am. Chem. Soc. 2005, 83, 2786. [Google Scholar] [CrossRef]
- Tilman, D.; Lehman, C.L.; Bristow, C.E. Diversity-stability relationships: Statistical inevitability or ecological consequence? Am. Nat. 1998, 151, 277–282. [Google Scholar] [CrossRef] [PubMed]
- Isbell, F.; Polley, H.W.; Wilsey, B.J. Biodiversity, productivity and the temporal stability of productivity: Patterns and processes. Ecol. Lett. 2009, 12, 443–451. [Google Scholar] [CrossRef] [PubMed]
- Kalyuzhny, M.; Schreiber, Y.; Chocron, R.; Flather, C.H.; Kadmon, R.; Kessler, D.A.; Shnerb, N.M. Temporal fluctuation scaling in populations and communities. Ecology 2014, 95, 1701–1709. [Google Scholar] [CrossRef] [PubMed]
- Lepš, J. Variability in population and community biomass in a grassland community affected by environmental productivity and diversity. Oikos 2004, 107, 64–71. [Google Scholar] [CrossRef]
- Steiner, C.F.; Long, Z.T.; Krumins, J.A.; Morin, P.J. Temporal stability of aquatic food webs: Partitioning the effects of species diversity, species composition and enrichment. Ecol. Lett. 2005, 8, 819–828. [Google Scholar] [CrossRef]
- Smith, M.D.; Knapp, A.K. Dominant species maintain ecosystem function with non-random species loss. Ecol. Lett. 2003, 6, 509–517. [Google Scholar] [CrossRef]
- Deng, L.; Sweeney, S.; Shangguan, Z.P. Grassland responses to grazing disturbance: Plant diversity changes with grazing intensity in a desert steppe. Grass Forage Sci. 2013, 69, 524–533. [Google Scholar] [CrossRef]
- Wang, H.X.; Wu, T.; Lu, S.H. Research progress of enclosure in China. Heilongjiang Anim. Husb. Vet. Med. 2016, 9, 89–92. [Google Scholar]
- Xu, L.; Nie, Y.; Chen, B.; Xin, X.; Yang, G.; Xu, D.; Ye, L. Effects of Fence Enclosure on Vegetation Community Characteristics and Productivity of a Degraded Temperate Meadow Steppe in Northern China. Appl. Sci. 2020, 10, 2952. [Google Scholar] [CrossRef]
- Berg, P.A. Metamorphosis for Cities: From Gray to Green. Trumpeter 1991, 8, 1–40. [Google Scholar]
- MAO. Monitoring Report of National Grassland. Ministry of Agriculture the People’s Republic of China, 2013. Available online: http://english.agri.gov.cn/ (accessed on 22 March 2020).
- Zheng, D.; Li, H.; Zhu, X. Herding behavior in institutional investors: Evidence from China’s stock market. J. Multinatl. Financ. Manag. 2015, 32, 59–76. [Google Scholar] [CrossRef]
- Zheng, Y.; Zhou, G.; Zhuang, Q.; Shimizu, H. Long-Term Elimination of Grazing Reverses the Effects of Shrub Encroachment on Soil and Vegetation on the Ordos Plateau. J. Geophys. Res. Biogeosci. 2020, 125, 125. [Google Scholar] [CrossRef]
- Hooper, D.U.; Chapin, F.S.; Ewel, J.J.; Hector, A.; Inchausti, P.; Lavorel, S.; Lawton, J.H.; Lodge, D.M.; Loreau, M.; Naeem, S.; et al. Effects of Biodiversity on Ecosystem Functioning: A Consensus of Current Knowledge. Ecol. Monogr. 2005, 75, 3–35. [Google Scholar] [CrossRef]
- Xie, Y.Z.; Becker, U.; Ute, B.; Rüdiger, W. Vegetation of the stipa loess steppe in Nigxia (northern china) in relation to grazing intensity. Grassl. Sci. 2007, 53, 143–154. [Google Scholar] [CrossRef]
- Jing, Z.; Cheng, J.; Su, J.; Bai, Y.; Jin, J. Changes in plant community composition and soil properties under 3-decade grazing exclusion in semiarid grassland. Ecol. Eng. 2014, 64, 171–178. [Google Scholar] [CrossRef]
- Qiu, L.P.; Wei, X.R.; Zhang, X.C. Ecosystem carbon and nitrogen accumulation after grazing exclusion in semiarid grassland. PLoS ONE 2013, 8, e55433. [Google Scholar] [CrossRef]
- Stöcklin, J.K.; Körner, C. Recruitment and mortality of Pinus sylvestris near the nordic treeline: The role of climatic change and herbivory. Ecol. Bull. 1999, 47, 168–177. [Google Scholar]
- Voigt, W.; Perner, J.; Jones, T.H. Using functional groups to investigate community response to environmental changes: Two grassland case studies. Glob. Chang. Biol. 2007, 13, 1710–1721. [Google Scholar] [CrossRef]
- Li, W.; Wen, S.J.; Hu, W.X.; Du, G.Z. Root-shoot competition interactions cause diversity loss after fertilization: A field ex-periment in an alpine meadow on the Tibetan Plateau. J. Plant Ecol. 2011, 4, 138–146. [Google Scholar] [CrossRef]
- Lehman, C.L.; Tilman, D. Biodiversity, stability and productivity in competitive communities. Am. Nat. 2000, 156, 534. [Google Scholar] [CrossRef] [PubMed]
- Tilman, D.; Reich, P.B.; Knops, J.; Wedin, D.; Mielke, T.; Lehman, C. Diversity and Productivity in a Long-Term Grassland. Science 2001, 294, 843–845. [Google Scholar] [CrossRef] [PubMed]
- Smith, B.; Wilson, J.B. A Consumer’s Guide to Evenness Indices. Oikos 1996, 76, 70. [Google Scholar] [CrossRef]
- Ketchen, D.J. A Primer on Partial Least Squares Structural Equation Modeling (PLS-SEM). Long Range Plann. 2013, 46, 184–185. [Google Scholar] [CrossRef]
- Eviner, V.T.; Chapin, F.S. The influence of plant species, fertilization and elevated CO2 on soil aggregate stability. Plant Soil 2002, 246, 211–219. [Google Scholar] [CrossRef]
- Micheli, F.; Cottingham, K.L.; Bascompte, J.; Bjørnstad, O.N.; Eckert, G.L.; Fischer, J.M.; Keitt, T.H.; Kendall, B.E.; Klug, J.L.; Rusak, J.A.; et al. The Dual Nature of Community Variability. Oikos 1999, 85, 161. [Google Scholar] [CrossRef]
- Zhang, Y.; Feng, J.; Loreau, M.; He, N.; Han, X.G.; Jiang, L. Nitrogen addition does not reduce the role of spatial asynchrony in stabilising grassland communities. Ecol. Lett. 2019, 22, 563–571. [Google Scholar] [CrossRef]
- Ren, H.; Taube, F.; Stein, C.; Zhang, Y.; Bai, Y.; Hu, S. Grazing weakens temporal stabilizing effects of diversity in the Eurasian steppe. Ecol. Evol. 2017, 8, 231–241. [Google Scholar] [CrossRef]
- Beck, J.; Hernández, D.L.; Pasari, J.R.; Zavaleta, E.S. Grazing maintains native plant diversity and promotes community stability in an annual grassland. Ecol. Appl. 2015, 25, 1259–1270. [Google Scholar] [CrossRef]
- Li, Y.; Dong, S.; Gao, Q.; Zhang, Y.; Liu, S.; Ganjurjav, H.; Hu, G.; Wang, X.; Yan, Y.; Wu, H.; et al. Rotational grazing promotes grassland aboveground plant biomass and its temporal stability under changing weather conditions on the Qinghai-Tibetan plateau. Land Degrad. Dev. 2020, 31, 2662–2671. [Google Scholar] [CrossRef]
- Pellkofer, S.; Van Der Heijden, M.G.A.; Schmid, B.; Wagg, C. Soil Communities Promote Temporal Stability and Species Asynchrony in Experimental Grassland Communities. PLoS ONE 2016, 11, e0148015. [Google Scholar] [CrossRef] [PubMed]
- McGrady-Steed, J.; Morin, P.J. Biodiversity, density compensation, and the dynamics of populations and functional groups. Ecology 2000, 81, 361–373. [Google Scholar] [CrossRef]
- Jiang, L.; Pu, Z. Different Effects of Species Diversity on Temporal Stability in Single-Trophic and Multitrophic Communities. Am. Nat. 2009, 174, 651–659. [Google Scholar] [CrossRef]
- Amy, L.; Bryan, L.; Mathew, A. Multiple diversity—Stability mechanisms enhance population and community stability in aquatic food webs. Ecology 2014, 95, 173–184. [Google Scholar]
- Lee, Y.-Y.; Hong, S.; Cho, K.-S. Design and shelf stability assessment of bacterial agents for simultaneous removal of methane and odors. J. Environ. Sci. Heal. Part. A 2019, 54, 906–913. [Google Scholar] [CrossRef]
- Niu, K.; He, J.-S.; Zhang, S.; Lechowicz, M.J. Grazing increases functional richness but not functional divergence in Tibetan alpine meadow plant communities. Biodivers. Conserv. 2015, 25, 2441–2452. [Google Scholar] [CrossRef]
- Taddese, G.; Saleem, M.A.M.; Abyie, A.; Wagnew, A. Impact of grazing on plant species richness, plant biomass, plant attribute, and soil physical and hydrological properties of vertisol in East African highlands. Environ. Manag. 2002, 29, 279–289. [Google Scholar] [CrossRef]
- Anna, O.; Panu, H. Grazers increaseβ-diversity of vascular plants and bryophytes in wood-pastures. J. Veg. Sci. 2016, 27, 1084–1093. [Google Scholar]
- Pérez-Ramos, I.M.; Díaz-Delgado, R.; De La Riva, E.G.; Villar, R.; Lloret, F.; Marañón, T. Climate variability and community stability in Mediterranean shrublands: The role of functional diversity and soil environment. J. Ecol. 2017, 105, 1335–1346. [Google Scholar] [CrossRef]
- Xue, Y.F.; Zong, N.; He, N.P.; Tian, J.; Zhang, Y.Q. Influence of long-term enclosure and free grazing on soil microbial community structure and carbon metabolic diversity of alpine meadow. Ying Yong Sheng Tai Xue Bao J. Appl. Ecol. 2018, 29, 2705–2712. [Google Scholar]
- Tian, Q.; Liu, N.; Bai, W.; Li, L.; Chen, J.; Reich, P.B.; Yu, Q.; Guo, D.; Smith, M.D.; Knapp, A.K.; et al. A novel soil manganese mechanism drives plant species loss with increased nitrogen deposition in a temperate steppe. Ecolology 2016, 97, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Midolo, G.; Alkemade, R.; Schipper, A.M.; Benítez-López, A.; Perring, M.P.; De Vries, W. Impacts of nitrogen addition on plant species richness and abundance: A global meta-analysis. Glob. Ecol. Biogeogr. 2019, 28, 398–413. [Google Scholar] [CrossRef]
- Haddad, N.M.; Crutsinger, G.M.; Gross, K.; Haarstad, J.; Tilman, D. Plant diversity and the stability of foodwebs. Ecol. Lett. 2010, 14, 42–46. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.F.; Han, X.G.; Wu, J.G.; Chen, Z.Z.; Li, L.H. Ecosystem stability and compensatory effects in the Inner Mongolia grass-land. Nature 2004, 431, 4–181. [Google Scholar] [CrossRef] [PubMed]

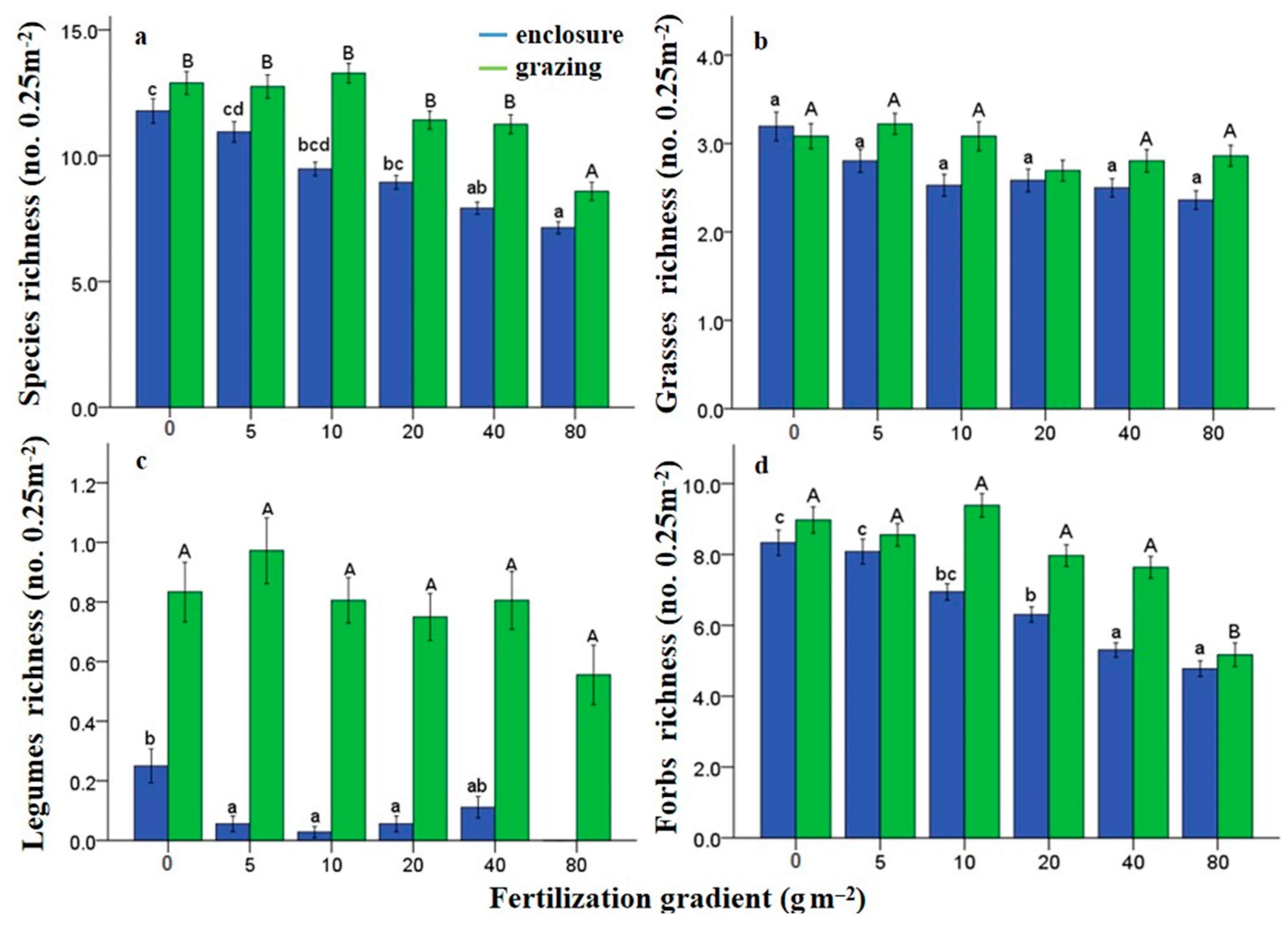

| Y | N | G | Y*N | Y*G | Y*G*N | N*G | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| df | F | p | df | F | p | df | F | p | df | F | p | df | F | p | df | F | p | df | F | p | |
| SR | 5 | 69.46 | 0.000 ** | 5 | 21.96 | 0.000 ** | 1 | 57.94 | 0.000 ** | 25 | 1.97 | 0.005 ** | 5 | 6.67 | 0.000 ** | 25 | 2.30 | 0.001 ** | 5 | 1.83 | 0.121 NS |
| GR | 5 | 20.82 | 0.000 ** | 5 | 1.72 | 0.000 ** | 1 | 8.45 | 0.005 ** | 25 | 1.24 | 0.206 NS | 5 | 8.47 | 0.000 ** | 25 | 1.24 | 0.201 NS | 5 | 0.74 | 0.601 NS |
| LR | 5 | 23.49 | 0.000 ** | 5 | 1.72 | 0.160 NS | 1 | 138.52 | 0.000 ** | 25 | 1.69 | 0.023 * | 5 | 15.93 | 0.000 ** | 25 | 1.64 | 0.031 * | 5 | 0.85 | 0.521 NS |
| NR | 5 | 29.23 | 0.000 ** | 5 | 24.21 | 0.000 ** | 1 | 19.24 | 0.000 ** | 25 | 1.77 | 0.015 * | 5 | 3.37 | 0.010 ** | 25 | 2.32 | 0.001 ** | 5 | 2.32 | 0.054 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, J.; Yang, X.; Ghanizadeh, H.; Guo, Q.; Fan, Y.; Zhang, B.; Yan, X.; Wen, Z.; Li, W. Long-Term Enclosure Can Benefit Grassland Community Stability on the Loess Plateau of China. Sustainability 2021, 13, 213. https://doi.org/10.3390/su13010213
Liu J, Yang X, Ghanizadeh H, Guo Q, Fan Y, Zhang B, Yan X, Wen Z, Li W. Long-Term Enclosure Can Benefit Grassland Community Stability on the Loess Plateau of China. Sustainability. 2021; 13(1):213. https://doi.org/10.3390/su13010213
Chicago/Turabian StyleLiu, Jing, Xue Yang, Hossein Ghanizadeh, Qian Guo, Yongming Fan, Bo Zhang, Xinhui Yan, Zhongming Wen, and Wei Li. 2021. "Long-Term Enclosure Can Benefit Grassland Community Stability on the Loess Plateau of China" Sustainability 13, no. 1: 213. https://doi.org/10.3390/su13010213
APA StyleLiu, J., Yang, X., Ghanizadeh, H., Guo, Q., Fan, Y., Zhang, B., Yan, X., Wen, Z., & Li, W. (2021). Long-Term Enclosure Can Benefit Grassland Community Stability on the Loess Plateau of China. Sustainability, 13(1), 213. https://doi.org/10.3390/su13010213






