Bacterial Augmented Floating Treatment Wetlands for Efficient Treatment of Synthetic Textile Dye Wastewater
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of Textile Effluent
2.2. Macrophytes
2.3. Endophytic Bacterial Strains
2.4. Fabrication of FTWs and Experimental Setup
2.5. Persistence of Inoculated Bacteria in Treated Water and Plants
2.6. Plant Biomass
2.7. Statistical Analysis
3. Results
3.1. Changes in Physicochemical Parameters of Treated Textile Effluent
3.2. Removal of Heavy Metals from Water
3.3. Bacterial Persistence in Roots, Shoots and Water
3.4. Plant Growth in Response to Bacterial Inoculation
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Evans, A.E.; Mateo-Sagasta, J.; Qadir, M.; Boelee, E.; Ippolito, A. Agricultural water pollution: Key knowledge gaps and research needs. Curr. Opin. Environ. Sustain. 2019, 36, 20–27. [Google Scholar] [CrossRef]
- Madhav, S.; Ahamad, A.; Singh, P.; Mishra, P.K. A review of textile industry: Wet processing, environmental impacts, and effluent treatment methods. Environ. Qual. Manag. 2018, 27, 31–41. [Google Scholar] [CrossRef]
- Khan, S.; Malik, A. Environmental and health effects of textile industry wastewater. In Environmental Deterioration and Human Health; Springer: Berlin/Heidelberg, Germany, 2014; pp. 55–71. [Google Scholar]
- Carmen, Z.; Daniela, S. Textile Organic Dyes—Characteristics, Polluting Effects and Separation/Elimination Procedures from Industrial Effluents—A Critical Overview, Organic Pollutants Ten Years after the Stockholm Convention-Environmental and Analytical Update; InTech: Rijeka, Croatia, 2012; p. 31. [Google Scholar]
- Chandanshive, V.V.; Kadam, S.K.; Khandare, R.V.; Kurade, M.B.; Jeon, B.-H.; Jadhav, J.P.; Govindwar, S.P. In situ phytoremediation of dyes from textile wastewater using garden ornamental plants, effect on soil quality and plant growth. Chemosphere 2018, 210, 968–976. [Google Scholar] [CrossRef] [PubMed]
- Bello, A.O.; Tawabini, B.S.; Khalil, A.B.; Boland, C.R.; Saleh, T.A. Phytoremediation of cadmium-, lead-and nickel-contaminated water by Phragmites australis in hydroponic systems. Ecol. Eng. 2018, 120, 126–133. [Google Scholar] [CrossRef]
- Khandare, R.V.; Kabra, A.N.; Kurade, M.B.; Govindwar, S.P. Phytoremediation potential of Portulaca grandiflora Hook. (Moss-Rose) in degrading a sulfonated diazo reactive dye Navy Blue HE2R (Reactive Blue 172). Bioresour. Technol. 2011, 102, 6774–6777. [Google Scholar] [CrossRef]
- Patil, A.V.; Lokhande, V.H.; Suprasanna, P.; Bapat, V.A.; Jadhav, J.P. Sesuvium portulacastrum (L.) L.: A potential halophyte for the degradation of toxic textile dye, Green HE4B. Planta 2012, 235, 1051–1063. [Google Scholar] [CrossRef]
- Telke, A.A.; Kagalkar, A.N.; Jagtap, U.B.; Desai, N.S.; Bapat, V.A.; Govindwar, S.P. Biochemical characterization of laccase from hairy root culture of Brassica juncea L. and role of redox mediators to enhance its potential for the decolorization of textile dyes. Planta 2011, 234, 1137–1149. [Google Scholar] [CrossRef]
- Khan, M.U.; Sessitsch, A.; Harris, M.; Fatima, K.; Imran, A.; Arslan, M.; Shabir, G.; Khan, Q.M.; Afzal, M. Cr-resistant rhizo-and endophytic bacteria associated with Prosopis juliflora and their potential as phytoremediation enhancing agents in metal-degraded soils. Front. Plant Sci. 2015, 5, 755. [Google Scholar] [CrossRef] [Green Version]
- Tara, N.; Arslan, M.; Hussain, Z.; Iqbal, M.; Khan, Q.M.; Afzal, M. On-site performance of floating treatment wetland macrocosms augmented with dye-degrading bacteria for the remediation of textile industry wastewater. J. Clean. Prod. 2019, 217, 541–548. [Google Scholar] [CrossRef]
- Roy, D.C.; Biswas, S.K.; Saha, A.K.; Sikdar, B.; Rahman, M.; Roy, A.K.; Prodhan, Z.H.; Tang, S.-S. Biodegradation of Crystal Violet dye by bacteria isolated from textile industry effluents. PeerJ 2018, 6, e5015. [Google Scholar] [CrossRef]
- Shahid, M.J.; Arslan, M.; Ali, S.; Siddique, M.; Afzal, M. Floating Wetlands: A Sustainable Tool for Wastewater Treatment. CLEAN-Soil Air Water 2018, 46, 1800120. [Google Scholar] [CrossRef]
- Wu, S.; Vymazal, J.; Brix, H. Critical Review: Biogeochemical Networking of Iron, Is It Important in Constructed Wetlands for Wastewater Treatment? Environ. Sci. Technol. 2019. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Nurul, S.; Schmitt, H.; Sutton, N.B.; Murk, T.A.; Blokland, M.H.; Rijnaarts, H.H.; Langenhoff, A.A. Evaluation of attenuation of pharmaceuticals, toxic potency, and antibiotic resistance genes in constructed wetlands treating wastewater effluents. Sci. Total Environ. 2018, 631, 1572–1581. [Google Scholar] [CrossRef] [PubMed]
- Tanner, C.C.; Headley, T.R. Components of floating emergent macrophyte treatment wetlands influencing removal of stormwater pollutants. Ecol. Eng. 2011, 37, 474–486. [Google Scholar] [CrossRef]
- White, S.A.; Cousins, M.M. Floating treatment wetland aided remediation of nitrogen and phosphorus from simulated stormwater runoff. Ecol. Eng. 2013, 61, 207–215. [Google Scholar] [CrossRef]
- Yeh, N.; Yeh, P.; Chang, Y.-H. Artificial floating islands for environmental improvement. Renew. Sustain. Energy Rev. 2015, 47, 616–622. [Google Scholar] [CrossRef]
- Karstens, S.; Nazzari, C.; Bâlon, C.; Bielecka, M.; Grigaitis, Ž.; Schumacher, J.; Stybel, N.; Razinkovas-Baziukas, A. Floating wetlands for nutrient removal in eutrophicated coastal lagoons: Decision support for site selection and permit process. Mar. Policy 2018, 97, 51–60. [Google Scholar] [CrossRef]
- Keizer-Vlek, H.E.; Verdonschot, P.F.; Verdonschot, R.C.; Dekkers, D. The contribution of plant uptake to nutrient removal by floating treatment wetlands. Ecol. Eng. 2014, 73, 684–690. [Google Scholar] [CrossRef]
- Ladislas, S.; Gerente, C.; Chazarenc, F.; Brisson, J.; Andres, Y. Floating treatment wetlands for heavy metal removal in highway stormwater ponds. Ecol. Eng. 2015, 80, 85–91. [Google Scholar] [CrossRef]
- Ijaz, A.; Iqbal, Z.; Afzal, M. Remediation of sewage and industrial effluent using bacterially assisted floating treatment wetlands vegetated with Typha domingensis. Water Sci. Technol. 2016, 74, 2192–2201. [Google Scholar] [CrossRef] [Green Version]
- Emenike, C.U.; Jayanthi, B.; Agamuthu, P.; Fauziah, S. Biotransformation and removal of heavy metals: A review of phytoremediation and microbial remediation assessment on contaminated soil. Environ. Rev. 2018, 26, 156–168. [Google Scholar] [CrossRef] [Green Version]
- Ijaz, A.; Shabir, G.; Khan, Q.M.; Afzal, M. Enhanced remediation of sewage effluent by endophyte-assisted floating treatment wetlands. Ecol. Eng. 2015, 84, 58–66. [Google Scholar] [CrossRef]
- Shahid, M.J.; Arslan, M.; Siddique, M.; Ali, S.; Tahseen, R.; Afzal, M. Potentialities of floating wetlands for the treatment of polluted water of river Ravi, Pakistan. Ecol. Eng. 2019, 133, 167–176. [Google Scholar] [CrossRef]
- Compant, S.; Clément, C.; Sessitsch, A. Plant growth-promoting bacteria in the rhizo-and endosphere of plants: Their role, colonization, mechanisms involved and prospects for utilization. Soil Biol. Biochem. 2010, 42, 669–678. [Google Scholar] [CrossRef] [Green Version]
- Afzal, M.; Khan, Q.M.; Sessitsch, A. Endophytic bacteria: Prospects and applications for the phytoremediation of organic pollutants. Chemosphere 2014, 117, 232–242. [Google Scholar] [CrossRef] [PubMed]
- Tara, N.; Iqbal, M.; Mahmood Khan, Q.; Afzal, M. Bioaugmentation of floating treatment wetlands for the remediation of textile effluent. Water Environ. J. 2019, 33, 124–134. [Google Scholar] [CrossRef]
- Wang, C.-Y.; Sample, D.J.; Day, S.D.; Grizzard, T.J. Floating treatment wetland nutrient removal through vegetation harvest and observations from a field study. Ecol. Eng. 2015, 78, 15–26. [Google Scholar] [CrossRef]
- Shahid, A.; Singh, J.; Bisht, S.; Teotia, P.; Kumar, V. Biodegradation of textile dyes by fungi isolated from north Indian field soil. EnvironmentAsia 2013, 6, 51–57. [Google Scholar]
- Arslan, M.; Imran, A.; Khan, Q.M.; Afzal, M. Plant–bacteria partnerships for the remediation of persistent organic pollutants. Environ. Sci. Pollut. Res. 2017, 24, 4322–4336. [Google Scholar] [CrossRef]
- Saleem, H.; Arslan, M.; Rehman, K.; Tahseen, R.; Afzal, M. Phragmites australis—A helophytic grass—Can establish successful partnership with phenol-degrading bacteria in a floating treatment wetland. Saudi J. Biol. Sci. 2019, 26, 1179–1186. [Google Scholar] [CrossRef]
- Sutton, S. Measurement of microbial cells by optical density. J. Valid. Technol. 2011, 17, 46–49. [Google Scholar]
- Rehman, K.; Imran, A.; Amin, I.; Afzal, M. Inoculation with bacteria in floating treatment wetlands positively modulates the phytoremediation of oil field wastewater. J. Hazard. Mater. 2018, 349, 242–251. [Google Scholar] [CrossRef] [PubMed]
- Saleem, H.; Rehman, K.; Arslan, M.; Afzal, M. Enhanced degradation of phenol in floating treatment wetlands by plant-bacterial synergism. Int. J. Phytorem. 2018, 20, 692–698. [Google Scholar] [CrossRef] [PubMed]
- Shahid, M.J.; Tahseen, R.; Siddique, M.; Ali, S.; Iqbal, S.; Afzal, M. Remediation of polluted river water by floating treatment wetlands. Water Supply 2019, 19, 967–977. [Google Scholar] [CrossRef]
- Chen, W.; Hao, H.; Hughes, D.; Shi, Y.; Cui, J.; Li, Z.-X. Static and dynamic mechanical properties of expanded polystyrene. Mater. Des. 2015, 69, 170–180. [Google Scholar] [CrossRef]
- Apha, A. Standard Methods for the Examination of Water and Wastewater; WEF: Cologny, Switzerland, 2012; Volume 22. [Google Scholar]
- Kämpfer, P.; Erhart, R.; Beimfohr, C.; Böhringer, J.; Wagner, M.; Amann, R. Characterization of bacterial communities from activated sludge: Culture-dependent numerical identification versus in situ identification using group-and genus-specific rRNA-targeted oligonucleotide probes. Microb. Ecol. 1996, 32, 101–121. [Google Scholar] [CrossRef]
- Chen, T.; Xu, M.; Tu, J.; Wang, H.; Niu, X. Relationship between Omnibus and Post-hoc Tests: An Investigation of performance of the F test in ANOVA. Shanghai Arch. Psychiatry 2018, 30, 60–64. [Google Scholar]
- Rehman, K.; Imran, A.; Amin, I.; Afzal, M. Enhancement of oil field-produced wastewater remediation by bacterially-augmented floating treatment wetlands. Chemosphere 2019, 217, 576–583. [Google Scholar] [CrossRef]
- Borne, K.E.; Fassman, E.A.; Tanner, C.C. Floating treatment wetland retrofit to improve stormwater pond performance for suspended solids, copper and zinc. Ecol. Eng. 2013, 54, 173–182. [Google Scholar] [CrossRef]
- Borne, K.E. Floating treatment wetland influences on the fate and removal performance of phosphorus in stormwater retention ponds. Ecol. Eng. 2014, 69, 76–82. [Google Scholar] [CrossRef]
- Ma, Y.; Rajkumar, M.; Oliveira, R.S.; Zhang, C.; Freitas, H. Potential of plant beneficial bacteria and arbuscular mycorrhizal fungi in phytoremediation of metal-contaminated saline soils. J. Hazard. Mater. 2019, 379, 120813. [Google Scholar] [CrossRef] [PubMed]
- Billore, S.; Sharma, J. Treatment performance of artificial floating reed beds in an experimental mesocosm to improve the water quality of river Kshipra. Water Sci. Technol. 2009, 60, 2851–2859. [Google Scholar] [CrossRef] [PubMed]
- Shehzadi, M.; Fatima, K.; Imran, A.; Mirza, M.; Khan, Q.; Afzal, M. Ecology of bacterial endophytes associated with wetland plants growing in textile effluent for pollutant-degradation and plant growth-promotion potentials. Plant. Biosyst. 2016, 150, 1261–1270. [Google Scholar] [CrossRef]
- Shehzadi, M.; Afzal, M.; Khan, M.U.; Islam, E.; Mobin, A.; Anwar, S.; Khan, Q.M. Enhanced degradation of textile effluent in constructed wetland system using Typha domingensis and textile effluent-degrading endophytic bacteria. Water Res. 2014, 58, 152–159. [Google Scholar] [CrossRef] [PubMed]
- Winston, R.J.; Hunt, W.F.; Kennedy, S.G.; Merriman, L.S.; Chandler, J.; Brown, D. Evaluation of floating treatment wetlands as retrofits to existing stormwater retention ponds. Ecol. Eng. 2013, 54, 254–265. [Google Scholar] [CrossRef]
- Joshi, P.; Jaybhaye, S.; Mhatre, K. Biodegradation of dyes using consortium of bacterial strains isolated from textile effluent. Eur. J. Exp. Biol. 2015, 5, 36–40. [Google Scholar]
- Manjate, E.; Ramos, S.; Almeida, C.M.R. Potential interferences of microplastics in the phytoremediation of Cd and Cu by the salt marsh plant Phragmites australis. J. Environ. Chem. Eng. 2020, 8, 103658. [Google Scholar] [CrossRef]
- Maxwell, B.; Winter, D.; Birgand, F. Floating treatment wetland retrofit in a stormwater wet pond provides limited water quality improvements. Ecol. Eng. 2020, 149, 105784. [Google Scholar] [CrossRef]
- Kong, Z.; Wu, Z.; Glick, B.R.; He, S.; Huang, C.; Wu, L. Co-occurrence patterns of microbial communities affected by inoculants of plant growth-promoting bacteria during phytoremediation of heavy metal-contaminated soils. Ecotoxicol. Environ. Saf. 2019, 183, 109504. [Google Scholar] [CrossRef]
- Afzal, M.; Shabir, G.; Tahseen, R.; Islam, E.; Iqbal, S.; Khan, Q.M.; Khalid, Z.M. Endophytic Burkholderia sp. strain PsJN improves plant growth and phytoremediation of soil irrigated with textile effluent. CLEAN–Soil Air Water 2014, 42, 1304–1310. [Google Scholar] [CrossRef]
- Kong, Z.; Glick, B.R. The role of plant growth-promoting bacteria in metal phytoremediation. Adv. Microb. Physiol. 2017, 71, 97–132. [Google Scholar]
- Zhang, N.; Wang, D.; Liu, Y.; Li, S.; Shen, Q.; Zhang, R. Effects of different plant root exudates and their organic acid components on chemotaxis, biofilm formation and colonization by beneficial rhizosphere-associated bacterial strains. Plant Soil 2014, 374, 689–700. [Google Scholar] [CrossRef]
- Ojuederie, O.; Babalola, O. Microbial and plant-assisted bioremediation of heavy metal polluted environments: A review. Int. J. Environ. Res. Public Health 2017, 14, 1504. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Afzal, M.; Rehman, K.; Shabir, G.; Tahseen, R.; Ijaz, A.; Hashmat, A.J.; Brix, H. Large-scale remediation of oil-contaminated water using floating treatment wetlands. NPJ Clean Water 2019, 2, 3. [Google Scholar] [CrossRef]
- Ali, H.; Khan, E.; Sajad, M.A. Phytoremediation of heavy metals—Concepts and applications. Chemosphere 2013, 91, 869–881. [Google Scholar] [CrossRef]
- Ashraf, S.; Afzal, M.; Naveed, M.; Shahid, M.; Ahmad Zahir, Z. Endophytic bacteria enhance remediation of tannery effluent in constructed wetlands vegetated with Leptochloa fusca. Int. J. Phytorem. 2018, 20, 121–128. [Google Scholar] [CrossRef]
- Afridi, M.S.; Mahmood, T.; Salam, A.; Mukhtar, T.; Mehmood, S.; Ali, J.; Khatoon, Z.; Bibi, M.; Javed, M.T.; Sultan, T. Induction of tolerance to salinity in wheat genotypes by plant growth promoting endophytes: Involvement of ACC deaminase and antioxidant enzymes. Plant Physiol. Biochem. 2019, 139, 569–577. [Google Scholar] [CrossRef] [PubMed]
- Glick, B.R. Plant growth-promoting bacteria: Mechanisms and applications. Scientifica 2012, 2012, 963401. [Google Scholar] [CrossRef] [Green Version]
- Fahid, M.; Arslan, M.; Shabir, G.; Younus, S.; Yasmeen, T.; Rizwan, M.; Siddique, K.; Ahmad, S.R.; Tahseen, R.; Iqbal, S. Phragmites australis in combination with hydrocarbons degrading bacteria is a suitable option for remediation of diesel-contaminated water in floating wetlands. Chemosphere 2020, 240, 124890. [Google Scholar] [CrossRef]
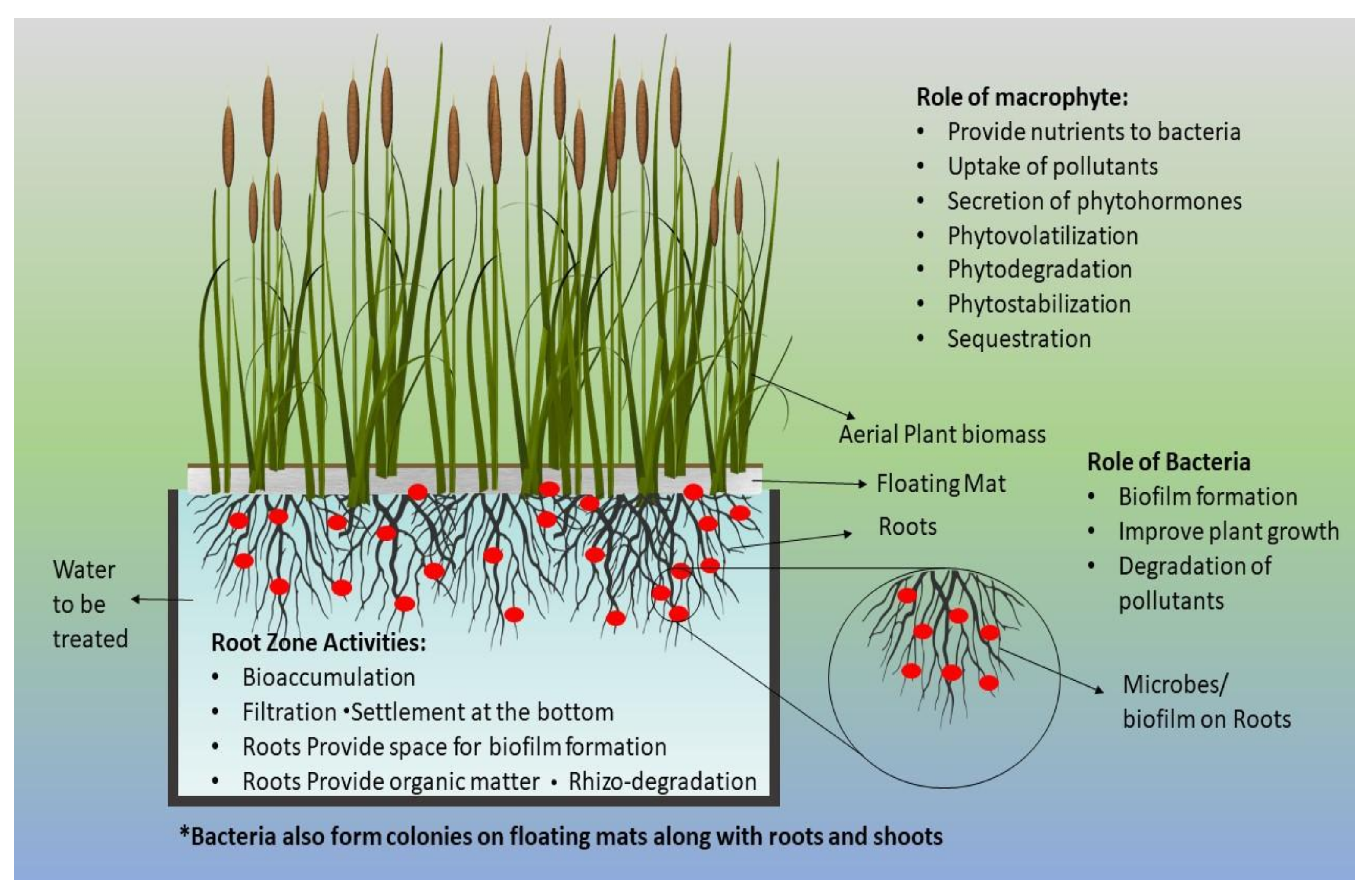
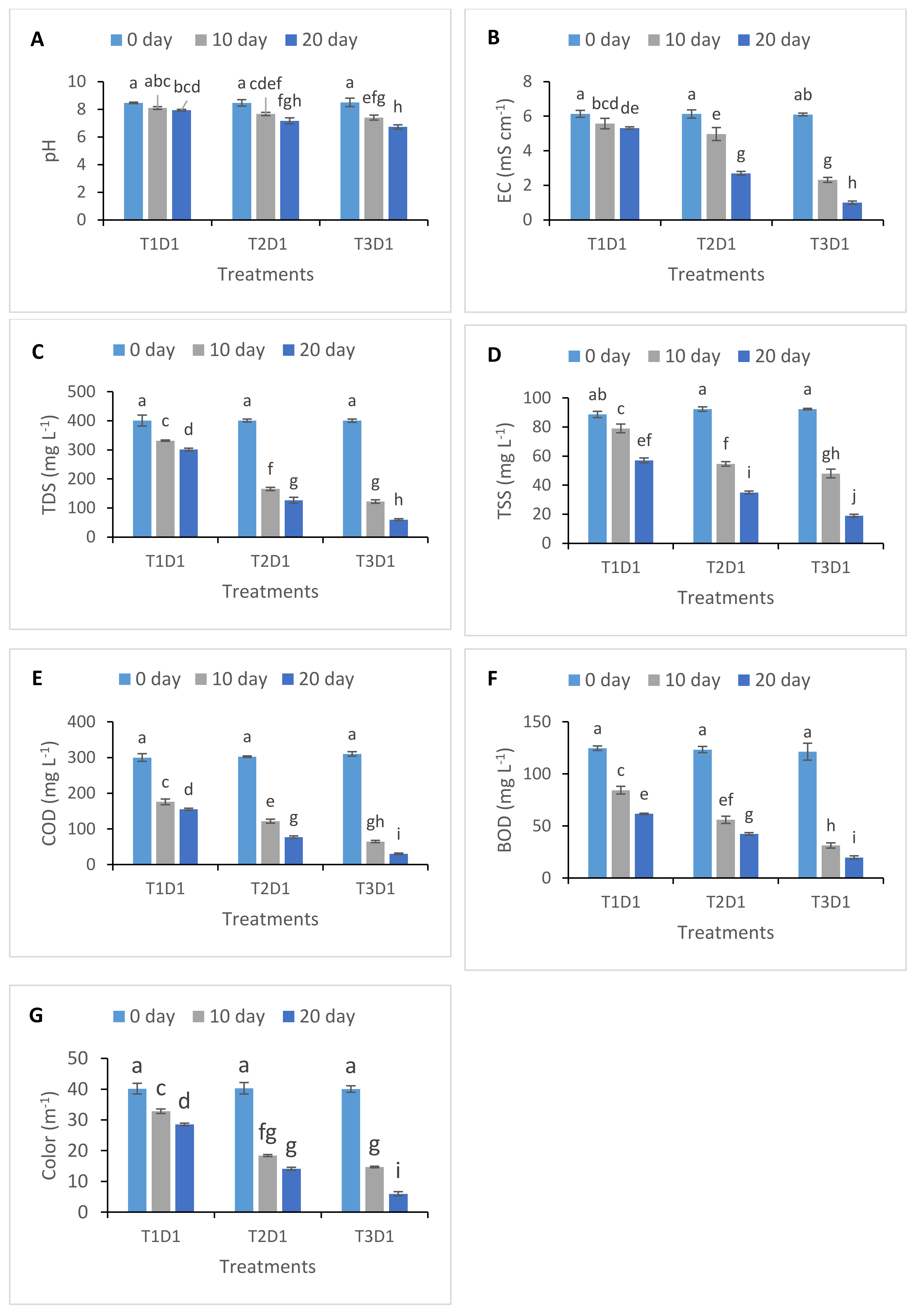
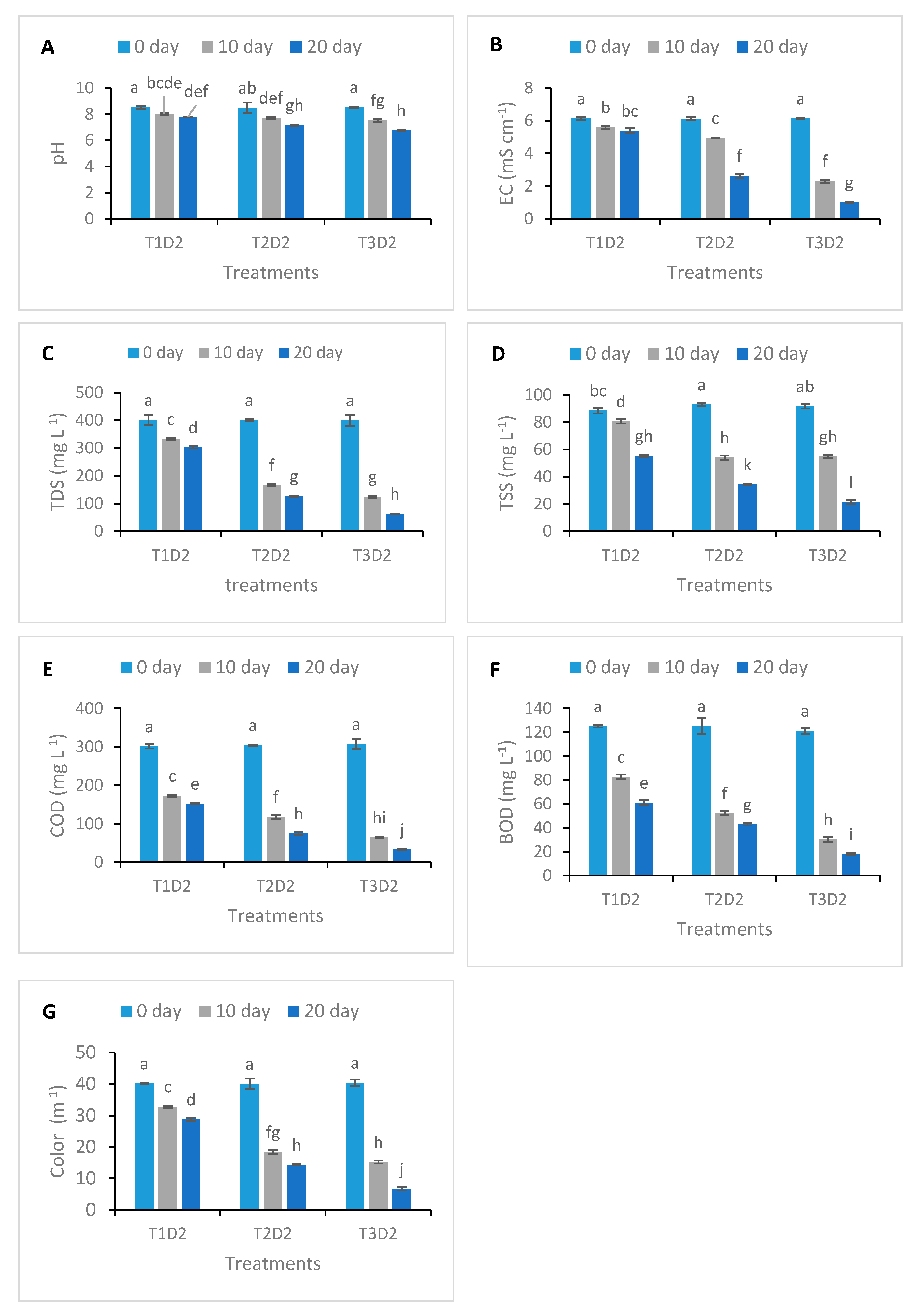
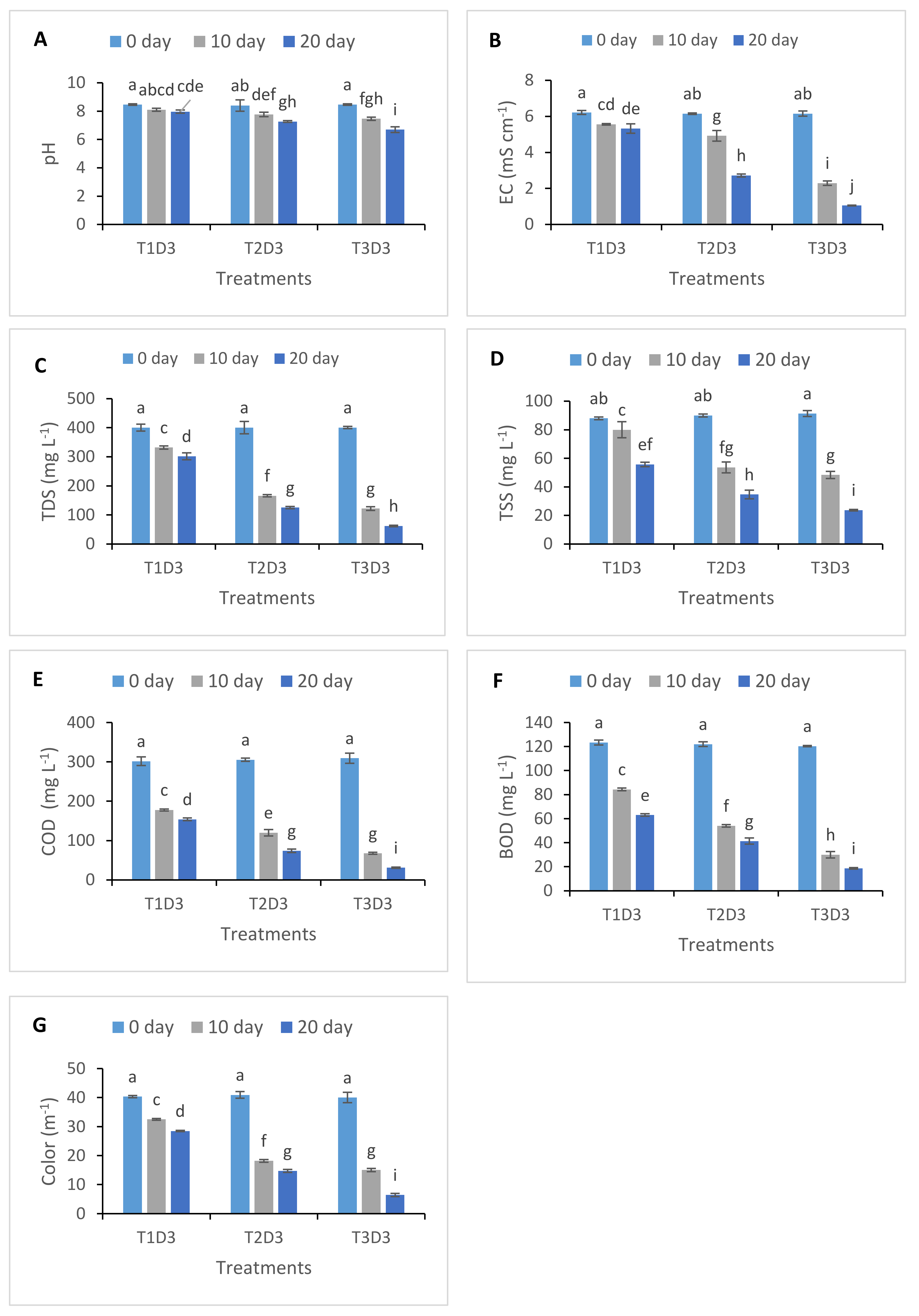
| Treatment | T1 | T2 | T3 | ||||
|---|---|---|---|---|---|---|---|
| Only Dye | Dye + Plant | Dye + Plant + Bacteria | |||||
| Dye | Metals | 10 Days | 20 Days | 10 Days | 20 Days | 10 Days | 20 Days |
| D1 | Cu | 20.0 b,c | 30.0 c,d | 58.5 e | 65.9 e,f,g | 67.5 e,f,g | 75.0 g |
| (0.0) | (0.0) | (0.0) | (0.0) | (0.0) | (0.0) | ||
| Ni | 19.4 b,c | 32.3 d,e | 40.0 e,f | 60.0 g,h | 60.0 g,h | 73.3 h | |
| (0.0) | (0.0) | (0.0) | (0.0) | (0.0) | (0.0) | ||
| Zn | 8.8 c | 21.1 d | 60.0 e,f | 66.7 f | 75.4 g | 86.9 h | |
| (0.0) | (0.0) | (0.0) | (0.0) | (0.0) | (0.0) | ||
| Fe | 7.5 b,c | 12.5 c | 48.8 e | 65.9 g | 62.5 f,g | 75.0 h | |
| (0.0) | (0.0) | (0.0) | (0.0) | (0.0) | (0.0) | ||
| Mn | 13.3 b,c | 23.3 c,d | 34.5 d,e | 48.3 e,f | 56.7 f,g | 70.0 h | |
| (0.0) | (0.0) | (0.0) | (0.0) | (0.0) | (0.0) | ||
| Pb | 20.0 b,c | 26.7 c,d | 40.0 d,e,f | 60.0 g | 46.7 f | 76.7 h | |
| (0.0) | (0.0) | (0.0) | (0.0) | (0.0) | (0.0) | ||
| D2 | Cu | 20.0 c | 27.5 c | 55.0 d,e,f | 65.0 f,g,h | 70.0 g,h,i | 77.5 i |
| (0.0) | (0.0) | (0.0) | (0.0) | (0.0) | (0.0) | ||
| Ni | 16.7 b,c | 30.0 c | 46.7 d | 60.0 d,e,f | 60.0 d,e,f | 73.3 f | |
| (0.0) | (0.0) | (0.0) | (0.0) | (0.0) | (0.0) | ||
| Zn | 13.3 b,c | 33.3 c | 56.9 d,e | 65.5 e,f | 75.0 f,g | 83.3 g | |
| (0.0) | (0.0) | (0.0) | (0.0) | (0.0) | (0.0) | ||
| Fe | 5.1 a,b | 12.8 b,c | 48.8 c,d | 65.9 e,f | 62.5 e | 77.5 f | |
| (0.0) | (0.0) | (0.0) | (0.0) | (0.0) | (0.0) | ||
| Mn | 22.6 b | 25.8 b,c | 40.0 c,d,e | 50.0 e,f,g | 60.0 f,g | 66.7 g | |
| (0.0) | (0.0) | (0.0) | (0.0) | (0.0) | (0.0) | ||
| Pb | 22.6 b | 29.0 b,c | 43.3 c,d,e | 56.7 e,f,g | 50.0 e,f | 73.3 g | |
| (0.0) | (0.0) | (0.0) | (0.0) | (0.0) | (0.0) | ||
| D3 | Cu | 20.0 a,b,c | 30.0 a,b,c,d,e | 55.0 d,e,f,h | 65.0 f,g | 67.5 e,f,g | 77.5 g |
| (0.0) | (0.0) | (0.0) | (0.0) | (0.0) | (0.0) | ||
| Ni | 20.0 c,d | 30.0 d,e | 43.8 e,f | 59.4 g,h | 60.0 g,h,i | 73.3 i | |
| (0.0) | (0.0) | (0.0) | (0.0) | (0.0) | (0.0) | ||
| Zn | 10.5 c | 24.6 d | 58.3 e | 70.0 f,g,h | 75.9 g,h | 89.7 i | |
| (0.0) | (0.0) | (0.0) | (0.0) | (0.0) | (0.0) | ||
| Fe | 14.6 b,c | 19.5 c | 52.4 d,e | 69.0 f,g | 66.7 e,f | 81.0 g | |
| (0.0) | (0.0) | (0.0) | (0.0) | (0.0) | (0.0) | ||
| Mn | 10.0 a,b,c | 26.7 c,d,e | 35.7 d,e,f | 46.4 f,g,h | 63.3 g,h | 70.0 h | |
| (0.0) | (0.0) | (0.0) | (0.0) | (0.0) | (0.0) | ||
| Pb | 23.3 bc | 30.0 cd | 43.3 ef | 56.7 hi | 44.8 fg | 65.5 i | |
| (0.0) | (0.0) | (0.0) | (0.0) | (0.0) | (0.0) | ||
| Treatment | Days | Dye 1 | Dye 2 | Dye 3 |
|---|---|---|---|---|
| Only Dye (T1) | 5 | 1.5 × 103 a | 1.6 × 103 a | 1.5 × 103 a |
| (0.3) | (0.2) | (0.4) | ||
| 10 | 1.6 × 103 a | 1.6 × 103 a | 1.7 × 103 a | |
| (0.4) | (0.5) | (0.5) | ||
| 15 | 1.9 × 103 a | 1.8 × 103 a | 1.8 × 103 a | |
| (0.6) | (0.7) | (0.6) | ||
| 20 | 1.8 × 103 a | 1.8 × 103 a | 2.0 × 103 a | |
| (0.6) | (0.8) | (0.9) | ||
| Dye + Plant (T2) | 5 | 2.1 × 105 b | 2.3 × 105 b | 2.2 × 105 b |
| (1.0) | (1.0) | (0.9) | ||
| 10 | 2.7 × 105 b | 2.9 × 105 b | 2.5 × 105 b | |
| (1.1) | (1.2) | (1.1) | ||
| 15 | 3 × 105 b,c | 3.3 × 105 b,c | 3.4 × 105 b,c | |
| (0.9) | (0.8) | (0.9) | ||
| 20 | 3.7 × 105 c | 3.5 × 105 c | 3.6 × 105 c | |
| (1.1) | (1.1) | (1.1) | ||
| Dye + Plant + Bacteria (T3) | 5 | 9.6 × 108 d | 9.8 × 108 d | 9.9 × 108 d |
| (0.5) | (0.6) | (0.5) | ||
| 10 | 7.1 × 109 e | 7.2 × 109 e | 7.1 × 109 e | |
| (0.7) | (0.5) | (0.4) | ||
| 15 | 6.4 × 109 f | 6.6 × 109 f | 6.6 × 109 f | |
| (0.2) | (0.6) | (0.6) | ||
| 20 | 5.0 × 108 g | 5.1 × 108 g | 4.9 × 108 g | |
| (0.6) | (0.6) | (0.7) |
| Root/Shoot | Treatment | Days | Dye 1 | Dye 2 | Dye 3 |
|---|---|---|---|---|---|
| Root | Dye + Plant (T2) | 5 | 2 × 102 a | 2.2 × 102 a | 2.2 × 102 a |
| (0.8) | (0.9) | (0.8) | |||
| 10 | 3.2 × 102 b | 3.3 × 102 b | 3.3 × 102 b | ||
| (1.0) | (1.0) | (1.0) | |||
| 15 | 3.7 × 102 b,c | 3.7 × 102 b,c | 3.7 × 102 b,c | ||
| (0.8) | (0.8) | (0.8) | |||
| 20 | 4.1 × 102 c | 4.0 × 102 c | 4.1 × 102 c | ||
| (0.9) | (0.9) | (0.9) | |||
| Dye + Plant + Bacteria (T3) | 5 | 4 × 103 d | 4.4 × 103 d | 4.3 × 103 d | |
| (1.2) | (1.3) | (0.9) | |||
| 10 | 11.9 × 103 e | 12.0 × 103 e | 11.6 × 103 e | ||
| (1.1) | (1.3) | (1.1) | |||
| 15 | 17.2 × 103 f | 17.6 × 103 f | 18.5 × 103 f | ||
| (1.1) | (1.3) | (0.9) | |||
| 20 | 22.8 × 103 g | 23.1 × 103 g | 23.4 × 103 g | ||
| (1.1) | (1.1) | (1.1) | |||
| Shoot | Dye + Plant (T2) | 5 | 1.1 × 102 a | 1.2 × 102 a | 1.2 × 102 a |
| (0.2) | (0.2) | (0.3) | |||
| 10 | 1.2 × 102 a | 1.0 × 102 a | 1.2 × 102 a | ||
| (0.4) | (0.5) | (0.3) | |||
| 15 | 1.3 × 102 a | 1.3 × 102 a | 1.2 × 102 a | ||
| (0.2) | (0.2) | (0.7) | |||
| 20 | 1.3 × 102 a | 1.2 × 102 a | 1.3 × 102 a | ||
| (0.2) | (0.3) | (0.5) | |||
| Dye + Plant + Bacteria (T3) | 5 | 1.4 × 103 b | 1.6 × 103 b | 1.5 × 103 b | |
| (0.3) | (0.2) | (0.4) | |||
| 10 | 6.2 × 103 c | 6.0 × 103 c | 6.2 × 103 c | ||
| (0.7) | (0.7) | (0.8) | |||
| 15 | 10.5 × 103 d | 10.1 × 103 d | 11.2 × 103 d | ||
| (0.4) | (0.9) | (1.3) | |||
| 20 | 14.3 × 103 e | 13.9 × 103 e | 14.0 × 103 e | ||
| (2.1) | (2.3) | (2.5) |
| Treatments | Shoot Length (cm) | Root Length (cm) | ||||
|---|---|---|---|---|---|---|
| Dye 1 | Dye 2 | Dye 3 | Dye 1 | Dye 2 | Dye 3 | |
| Dye + Plants | 187.7 d | 197.7 c,d | 202.7 b,c,d | 29.7 c | 30.7 c | 31.0 c |
| (T2) | (24.9) | (7.8) | (3.8) | (0.58) | (1.2) | (0.0) |
| Dye + Plants+ Bacteria | 222.0 a,b,c | 228.0 a,b | 224.3 a,b,c | 38.0 b | 39.0 b | 38.3 b |
| (T3) | (10.8) | (2.6) | (9.3) | (1.0) | (1.0) | (1.2) |
| Fresh water + Plants | 233.3 a | 232.3 a | 230.0 a,b | 43.0 a | 44.3 a | 43.7 a |
| (T4) | (3.1) | (2.5) | (2.6) | (1.0) | (0.58) | (2.1) |
| Treatments | Shoot Dry Weight (g) | Root Dry Weight (g) | ||||
|---|---|---|---|---|---|---|
| Dye 1 | Dye 2 | Dye 3 | Dye 1 | Dye 2 | Dye 3 | |
| Dye + Plants | 492.0 c | 517.7 b,c | 533.0 a,b,c | 62.3 c | 64.3 c | 64.7 c |
| (T2) | (65.3) | (20.3) | (9.6) | (1.2) | (3.2) | (0.6) |
| Dye + Plants+ Bacteria | 572.7 a,b | 591.3 a,b | 581.3 a,b | 79.3 a,b,c | 81.0 b,c | 80.3 a,b,c |
| (T3) | (29.3) | (7.1) | (21.4) | (2.1) | (1.7) | (2.1) |
| Fresh water + Plants | 605.0 a | 609.0 a | 603.7 a | 89.3 a,b | 93.0 a | 90.0 a,b |
| (T4) | (8.2) | (9.5) | (8.3) | (3.1) | (1.7) | (1.7) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nawaz, N.; Ali, S.; Shabir, G.; Rizwan, M.; Shakoor, M.B.; Shahid, M.J.; Afzal, M.; Arslan, M.; Hashem, A.; Abd_Allah, E.F.; et al. Bacterial Augmented Floating Treatment Wetlands for Efficient Treatment of Synthetic Textile Dye Wastewater. Sustainability 2020, 12, 3731. https://doi.org/10.3390/su12093731
Nawaz N, Ali S, Shabir G, Rizwan M, Shakoor MB, Shahid MJ, Afzal M, Arslan M, Hashem A, Abd_Allah EF, et al. Bacterial Augmented Floating Treatment Wetlands for Efficient Treatment of Synthetic Textile Dye Wastewater. Sustainability. 2020; 12(9):3731. https://doi.org/10.3390/su12093731
Chicago/Turabian StyleNawaz, Neeha, Shafaqat Ali, Ghulam Shabir, Muhammad Rizwan, Muhammad Bilal Shakoor, Munazzam Jawad Shahid, Muhammad Afzal, Muhammad Arslan, Abeer Hashem, Elsayed Fathi Abd_Allah, and et al. 2020. "Bacterial Augmented Floating Treatment Wetlands for Efficient Treatment of Synthetic Textile Dye Wastewater" Sustainability 12, no. 9: 3731. https://doi.org/10.3390/su12093731
APA StyleNawaz, N., Ali, S., Shabir, G., Rizwan, M., Shakoor, M. B., Shahid, M. J., Afzal, M., Arslan, M., Hashem, A., Abd_Allah, E. F., Alyemeni, M. N., & Ahmad, P. (2020). Bacterial Augmented Floating Treatment Wetlands for Efficient Treatment of Synthetic Textile Dye Wastewater. Sustainability, 12(9), 3731. https://doi.org/10.3390/su12093731








