Effects of Different Weeding Methods on the Biomass of Vegetation and Soil Evaporation in Eucalyptus Plantations
Abstract
1. Introduction
2. Materials and Methods
2.1. Site Description
2.2. Field Investigation and Experimental Plot Design
2.3. Measurement Indexes and Methods
2.3.1. Investigation of Shrub Species, Coverage, and Litter
2.3.2. Evaluation of the Weeding Effect
2.3.3. Evaluation of Tree Growth
2.3.4. Soil Evaporation Observation Method
2.3.5. Experimental Design of Soil Evaporation under Different Moisture Contents
2.3.6. Statistical Analysis
3. Results
3.1. Control Effect of Herbicide and Mechanical Weeding on Undergrowth Plants
3.2. Characteristics of Soil Evaporation under Different Weeding Measures
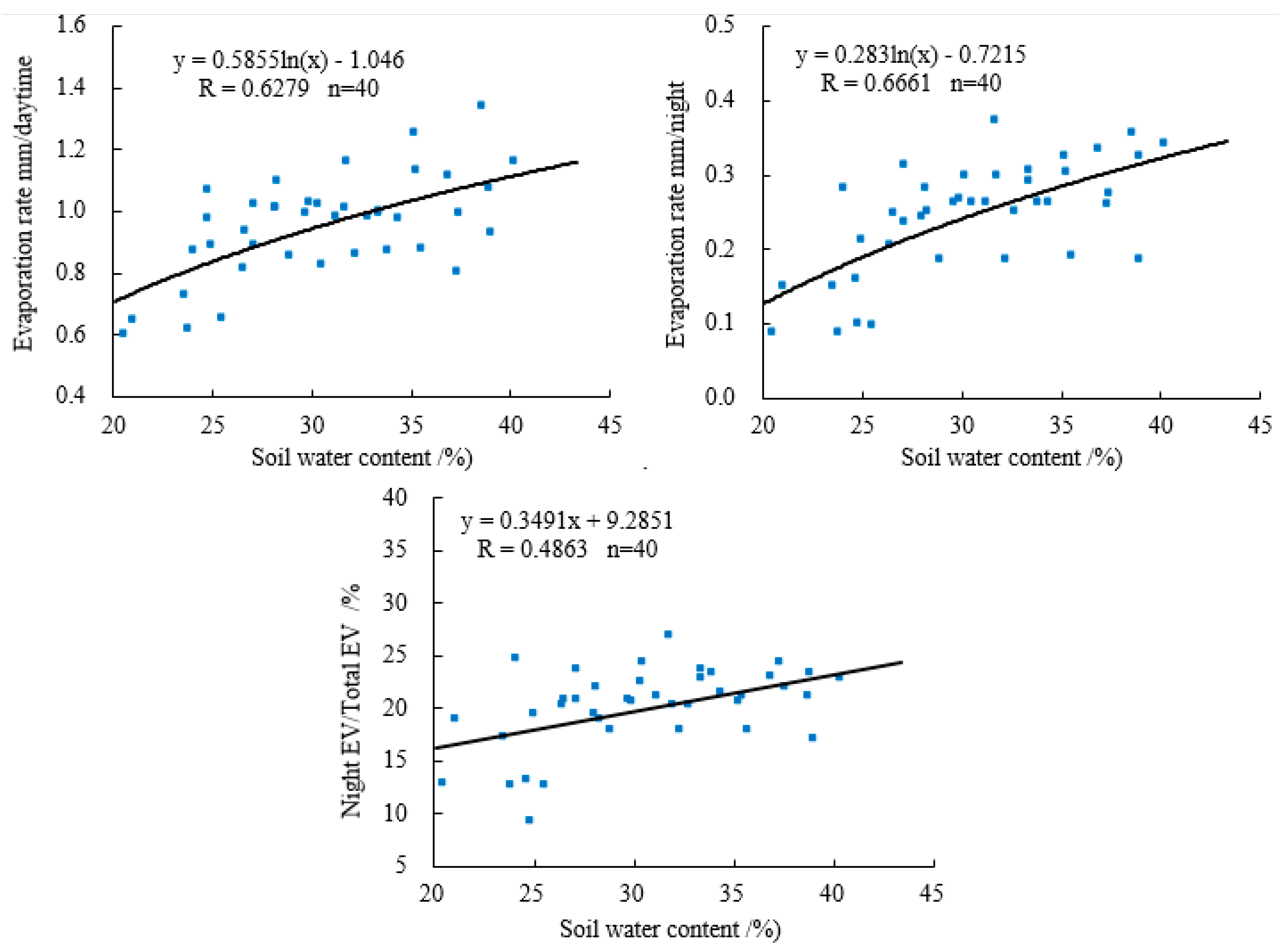
3.3. Effect of Different Water Contents on the Soil Evaporation of Eucalyptus Plantations
3.4. Effect of Different Weeding Measures on Eucalyptus Growth
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Cossalter, C.; Pye-Smith, C. Fast-Wood Forestry: Myths and Realities; Center for International Forestry Research: Bogor, Indonesia, 2003. [Google Scholar]
- Hubbard, R.M.; Stape, J.; Ryan, M.G.; Almeida, A.C.; Rojas, J. Effects of irrigation on water use and water use efficiency in two fast growing Eucalyptus plantations. For. Ecol. Manag. 2010, 259, 1714–1721. [Google Scholar] [CrossRef]
- Wen, Y.G.; Zhou, X.G.; Yu, S.F.; Zhu, H.G. The predicament and countermeasures of development of global Eucalyptus plantations. Guangxi Sci. 2018, 25, 107–116. (In Chinese) [Google Scholar]
- Yang, G.R.; Shi, X.H.; Cai, D.S.; Huang, C.B.; Lei, S.M.; Zhang, H. Water quality of throughfall and stemflow in planted forest in Guangxi, China. J. Food Agric. Environ. 2011, 9, 947–953. [Google Scholar]
- O’ Connell, A.M.; Grove, T.S.; Mendham, D.S.; Rance, S.J. Impact of harvest residue management on soil nitrogen dynamics in Eucalyptus globulus plantations in south western Australia. Soil Biol. Biochem. 2004, 36, 39–48. [Google Scholar] [CrossRef]
- Fernández, C.; Vega, J.A.; Gras, J.M.; Fonturbel, T.; Cuinas, P.; Dambrine, E.; Alonso, M. Soil erosion after Eucalyptus globulus clearcutting: Differences between logging slash disposal treatments. For. Ecol. Manag. 2004, 195, 85–95. [Google Scholar] [CrossRef]
- Wilson, G.W.; Fredlund, D.G.; Barbour, S.L. Coupled soil-atmosphere modelling for soil evaporation. Can. Geotech. J. 1993, 31, 151–161. [Google Scholar] [CrossRef]
- Li, T.C.; Shao, M.A.; Jia, Y.H. Characteristics of soil evaporation and temperature under aggregate mulches created by burrowing ants. Soil Sci. Soc. Am. J. 2017, 81, 114–123. [Google Scholar] [CrossRef]
- Lauenroth, W.K.; Bradford, J.B. Ecohydrology and the partitioning AET between transpiration and evaporation in a semiarid steppe. Ecosystems 2006, 9, 756–767. [Google Scholar] [CrossRef]
- Lawrence, D.M.; Thornton, P.E.; Oleson, K.W.; Bonan, G.B. The partitioning of evapotranspiration into transpiration, soil evaporation, and canopy evaporation in a GCM: Impacts on land-atmosphere interaction. J. Hydrometeorol. 2007, 8, 862–880. [Google Scholar] [CrossRef]
- Chattopadhyay, N.; Hulme, M. Evaporation and potential evapotranspiration in India under conditions of recent and future climate change. Agric. For. Meteorol. 1997, 87, 55–73. [Google Scholar] [CrossRef]
- Donohue, R.J.; McVicar, T.R.; Roderick, M.L. Assessing the ability of potential evaporation formulations to capture the dynamics in evaporative demand within a changing climate. J. Hydrol. 2010, 386, 186–197. [Google Scholar] [CrossRef]
- Biederman, J.A.; Harpold, A.A.; Gochis, D.J.; Ewers, B.E.; Reed, D.E.; Papuga, S.A.; Brooks, P.D. Increased evaporation following widespread tree mortality limits streamflow response. Water Resour. Res. 2014, 50, 5395–5409. [Google Scholar] [CrossRef]
- McNaughton, K.G.; Jarvis, P.G. Predicting effects of vegetation changes on transpiration and evaporation. Water Deficits Plant Growth 1983, 7, 1–47. [Google Scholar]
- Davidson, E.A.; Savage, K.V.L.V.; Verchot, L.V.; Navarro, R. Minimizing artifacts and biases in chamber-based measurements of soil respiration. Agric. For. Meteorol. 2002, 113, 21–37. [Google Scholar] [CrossRef]
- Stannard, D.I.; Weltz, M.A. Partitioning evapotranspiration in sparsely vegetated rangeland using a portable chamber. Water Resour. Res. 2006, 42, 18–27. [Google Scholar] [CrossRef]
- Rousseaux, M.C.; Figuerola, P.I.; Correa-Tedesco, G.; Searles, P.S. Seasonal variations in sap flow and soil evaporation in an olive (Olea europaea L.) grove under two irrigation regimes in an arid region of Argentina. Agric. Water Manag. 2009, 96, 1037–1044. [Google Scholar] [CrossRef]
- Bosch, J.M.; Hewlett, J.D. A review of catchment experiments to determine the effect of vegetation changes on water yield and evapotranspiration. J. Hydrol. 1982, 55, 3–23. [Google Scholar] [CrossRef]
- Almeida, A.C.; Soares, J.V.; Landsberg, J.J.; Rezende, G.D. Growth and water balance of Eucalyptus grandis hybrid plantations in Brazil during a rotation for pulp production. For. Ecol. Manag. 2007, 251, 10–21. [Google Scholar] [CrossRef]
- Rodríguez-Suárez, J.A.; Soto, B.; Perez, R.; Diaz-Fierros, F. Influence of Eucalyptus globulus plantation growth on water table levels and low flows in a small catchment. J. Hydrol. 2011, 396, 321–326. [Google Scholar] [CrossRef]
- Ward, S.M.; Cousens, R.D.; Bagavathiannan, M.V.; Barney, J.N.; Beckie, H.J.; Busi, R.; Gallandt, E.R. Agricultural weed research: A critique and two proposals. Weed Sci. 2014, 62, 672–678. [Google Scholar] [CrossRef]
- Wiltshire, J.J.J.; Tillett, N.D.; Hague, T. Agronomic evaluation of precise mechanical hoeing and chemical weed control in sugar beet. Weed Res. 2003, 43, 236–244. [Google Scholar] [CrossRef]
- Song, B.K.; Chuah, T.S.; Tam, S.M.; Olsen, K.M. Malaysian weedy rice shows its true stripes: Wild Oryza and elite rice cultivars shape agricultural weed evolution in Southeast Asia. Mol. Ecol. 2014, 23, 5003–5017. [Google Scholar] [CrossRef] [PubMed]
- Gourley, M.; Vomocil, M.; Newton, M. Forest weeding reduces the effect of deer-browsing on douglas fir. For. Ecol. Manag. 1990, 36, 177–185. [Google Scholar] [CrossRef]
- Garau, A.M.; Ghersa, C.M.; Lemcoff, J.H.; Barañao, J.J. Weeds in Eucalyptus globulus subsp. Maidenii (F. Muell) establishment: Effects of competition on sapling growth and survivorship. New For. 2009, 37, 251–264. [Google Scholar] [CrossRef]
- Van der Weide, R.Y.; Bleeker, P.O.; Achten, V.T.J.M.; Lotz, L.A.P.; Fogelberg, F.; Melander, B. Innovation in mechanical weed control in crop rows. Weed Res. 2008, 48, 215–224. [Google Scholar] [CrossRef]
- Ma, X.; Qi, L.; Liang, B.; Tan, Z.; Zuo, Y. Present status and prospects of mechanical weeding equipment and technology in paddy field. Trans. Chin. Soc. Agric. Eng. 2011, 27, 162–168. [Google Scholar]
- Owen, M.D.; Zelaya, I.A. Herbicide-resistant crops and weed resistance to herbicides. Pest Manag. Sci. 2005, 61, 301–311. [Google Scholar] [CrossRef]
- Beckie, H.J. Herbicide-resistant weeds: Management tactics and practices. Weed Technol. 2006, 20, 793–814. [Google Scholar] [CrossRef]
- Green, J.M.; Owen, M.D. Herbicide-resistant crops: Utilities and limitations for herbicide-resistant weed management. J. Agric. Food Chem. 2010, 59, 5819–5829. [Google Scholar] [CrossRef]
- Chen, L.; Huang, Z.; Gong, J.; Fu, B.; Huang, Y. The effect of land cover/vegetation on soil water dynamic in the hilly area of the Loess Plateau, China. Catena 2007, 70, 200–208. [Google Scholar] [CrossRef]
- Chen, C.L.; Luo, X.L.; Zhang, J.Q.; Zhu, G.L.; Jin, G.R. Effect and economic benefits of seeding amount and weeding methods on kenaf growth and soil available nutrients. Acta Agric. Zhejiangensis 2015, 27, 1692–1697. (In Chinese) [Google Scholar]
- Lei, L. Effects of Different Weed Control Methods on Ecological Environment, the Growth and Development and Yield of Cotton. Master’s Thesis, Henan Agriculture University, Zhengzhou, China, June 2017. (In Chinese). [Google Scholar]
- Tan, C.L.; He, B. Effects of Different Tending Methods on Growth of Young Cunning hamia lanceolata Plantation. J. Anhui Agric. 2009, 37, 17166–17167. (In Chinese) [Google Scholar]
- Benayas, J.M.R.; Navarro, J.; Espigares, T.; Nicolau, J.M.; Zavala, M.A. Effects of artificial shading and weed mowing in reforestation of Mediterranean abandoned cropland with contrasting Quercus species. For. Ecol. Manag. 2005, 212, 302–314. [Google Scholar] [CrossRef]
- Cen, J.Y. Study on two-way tree volume dynamic model of eucalyptus plantations in Guangxi. J. South China Agric. Univ. 2007, 28, 91–95. [Google Scholar]
- Figuerola, P.I.; Rousseaux, M.C.; Searles, P.S. Soil evaporation beneath and between olive trees in a non-Mediterranean climate under two contrasting irrigation regimes. J. Arid Environ. 2013, 97, 182–189. [Google Scholar] [CrossRef]
- Duke, S.O.; Powles, S.B. Glyphosate: A once in a century herbicide. Pest Manag. Sci. 2008, 64, 319–325. [Google Scholar] [CrossRef]
- Doĝan, M.N.; Öĝüt, D.; Mülleder, N.; Boz, Ö.; Brants, I.; Voegler, W. Effect of water volume and water quality on the efficacy of glyphosate on some important weed species in turkey. Julius-Kühn-Archiv 2012, 434, 229–234. [Google Scholar]
- Buhler, D.D.; Doll, J.D.; Proost, R.T.; Visocky, M.R. Integrating mechanical weeding with reduced herbicide use in conservation tillage corn production systems. Agron. J. 1995, 87, 507–512. [Google Scholar] [CrossRef]
- Wu, J.; Cui, N.X.; Cheng, S.P. Effects of sediment anoxia on growth and root respiratory metabolism of iris pseudacorus: Implications for vegetation restoration of eutrophic waters in China. Ecol. Eng. 2013, 53, 194–199. [Google Scholar] [CrossRef]
- Huang, G.Q.; Zhao, Q.G. The history, status quo, ecological problems and countermeasures of Eucalyptus plantations in Guangxi. Acta Ecol. Sin. 2014, 34, 5142–5152. (In Chinese) [Google Scholar]
- Rajcan, I.; Swanton, C.J. Understanding maize-weed competition: Resource competition, light quality and the whole plant. Field Crop. Res. 2001, 71, 139–150. [Google Scholar] [CrossRef]
- Villegas, J.C.; Breshears, D.D.; Zou, C.B. Law, D.J. Ecohydrological controls of soil evaporation in deciduous drylands: How the hierarchical effects of litter, patch and vegetation mosaic cover interact with phenology and season. J. Arid Environ. 2010, 74, 595–602. [Google Scholar] [CrossRef]
- Deal, L.M.; Hess, F.D. An analysis of the growth inhibitory characteristics of alachlor and metolachlor. Weed Sci. 1980, 28, 168–175. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, Y. Soil disturbance and cutting forces of four different sweeps for mechanical weeding. Soil Till. Res. 2017, 168, 167–175. [Google Scholar] [CrossRef]
- Schwartz, R.C.; Baumhardt, R.L.; Evett, S.R. Tillage effects on soil water redistribution and bare soil evaporation throughout a season. Soil Till. Res. 2010, 110, 221–229. [Google Scholar] [CrossRef]
- Yamanaka, T.; Takeda, A.; Sugita, F. A modified surface-resistance approach for representing bare-soil evaporation: Wind tunnel experiments under various atmospheric conditions. Water Resour. Res. 1997, 33, 2117–2128. [Google Scholar] [CrossRef]
- Cook, H.F.; Valdes, G.S.; Lee, H.C. Mulch effects on rainfall interception, soil physical characteristics and temperature under Zea mays L. Soil Till. Res. 2006, 91, 227–235. [Google Scholar] [CrossRef]
- Neave, M.; Abrahams, A.D. Vegetation influences on water yields from grassland and shrubland ecosystems in the Chihuahuan Desert. Earth Surf. Proc. Land. 2002, 27, 1011–1020. [Google Scholar] [CrossRef]
- Sakaguchi, K.; Zeng, X. Effects of soil wetness, plant litter, and under-canopy atmospheric stability on ground evaporation in the Community Land Model (CLM3.5). J. Geophys. Res-Atmos. 2009, 114, 1–14. [Google Scholar] [CrossRef]
- Peters, D.P.; Herrick, J.E.; Monger, H.C.; Huang, H. Soil-vegetation-climate interactions in arid landscapes: Effects of the North American monsoon on grass recruitment. J. Arid Environ. 2010, 74, 618–623. [Google Scholar] [CrossRef]
- Gong, W.; Hu, T.X.; Wang, J.Y.; Gong, Y.B.; Ran, H. Water holding characteristics of litter layer after natural evergreen broadleaved forest artificial regeneration in southern Sichuan Province. J. Soil Water Conserv. 2006, 3, 51–55. [Google Scholar]
- Deng, Y.S.; Duan, X.Q.; Ding, S.W.; Cai, C.F.; Chen, J.Z. Suction stress characteristics in granite red soils and their relationship with the collapsing gully in south China. Catena 2018, 171, 505–522. [Google Scholar] [CrossRef]
- Scholl, P.; Leitner, D.; Kammerer, G.; Loiskandl, W.; Kaul, H.P.; Bodner, G. Root induced changes of effective 1D hydraulic properties in a soil column. Plant Soil 2014, 381, 193–213. [Google Scholar] [CrossRef] [PubMed]
- Duan, X.Q.; Deng, Y.S.; Tao, Y.; He, Y.B.; Lin, L.R.; Chen, J.Z. Variation in soil saturated hydraulic conductivity along the hillslope of collapsing granite gullies. Hydrolog. Sci. J. 2018, 63, 803–817. [Google Scholar] [CrossRef]
- Tani, M. Runoff generation processes estimated from hydrological observations on a steep forested hillslope with a thin soil layer. J. Hydrol. 1997, 200, 84–109. [Google Scholar] [CrossRef]
- Ciais, P.; Reichstein, M.; Viovy, N.; Granier, A.; Ogée, J.; Allard, V.; Chevallier, F. Europe-wide reduction in primary productivity caused by the heat and drought in 2003. Nature 2005, 437, 529–533. [Google Scholar] [CrossRef]
- Huxman, T.E.; Wilcox, B.P.; Breshears, D.D.; Scott, R.L.; Snyder, K.A.; Small, E.E.; Jackson, R.B. Ecohydrological implications of woody plant encroachment. Ecology 2005, 86, 308–319. [Google Scholar] [CrossRef]
- Williams, R.A. Mitigating biodiversity concerns in Eucalyptus plantations located in South China. J. Biosci. Med. 2015, 3, 1–8. [Google Scholar] [CrossRef]
- Hartnett, D.C.; Potgieter, A.F.; Wilson, G.W.T. Fire effects on mycorrhizal symbiosis and root system architecture in southern African savanna grasses. Afr. J. Ecol. 2005, 42, 328–337. [Google Scholar] [CrossRef]
- Chiatante, D.; Di Iorio, A.; Sciandra, S.; Scippa, G.S.; Mazzoleni, S. Effect of drought and fire on root development in Quercus pubescens Willd. and Fraxinus ornus L. seedlings. Environ. Exp. Bot. 2006, 56, 190–197. [Google Scholar] [CrossRef]
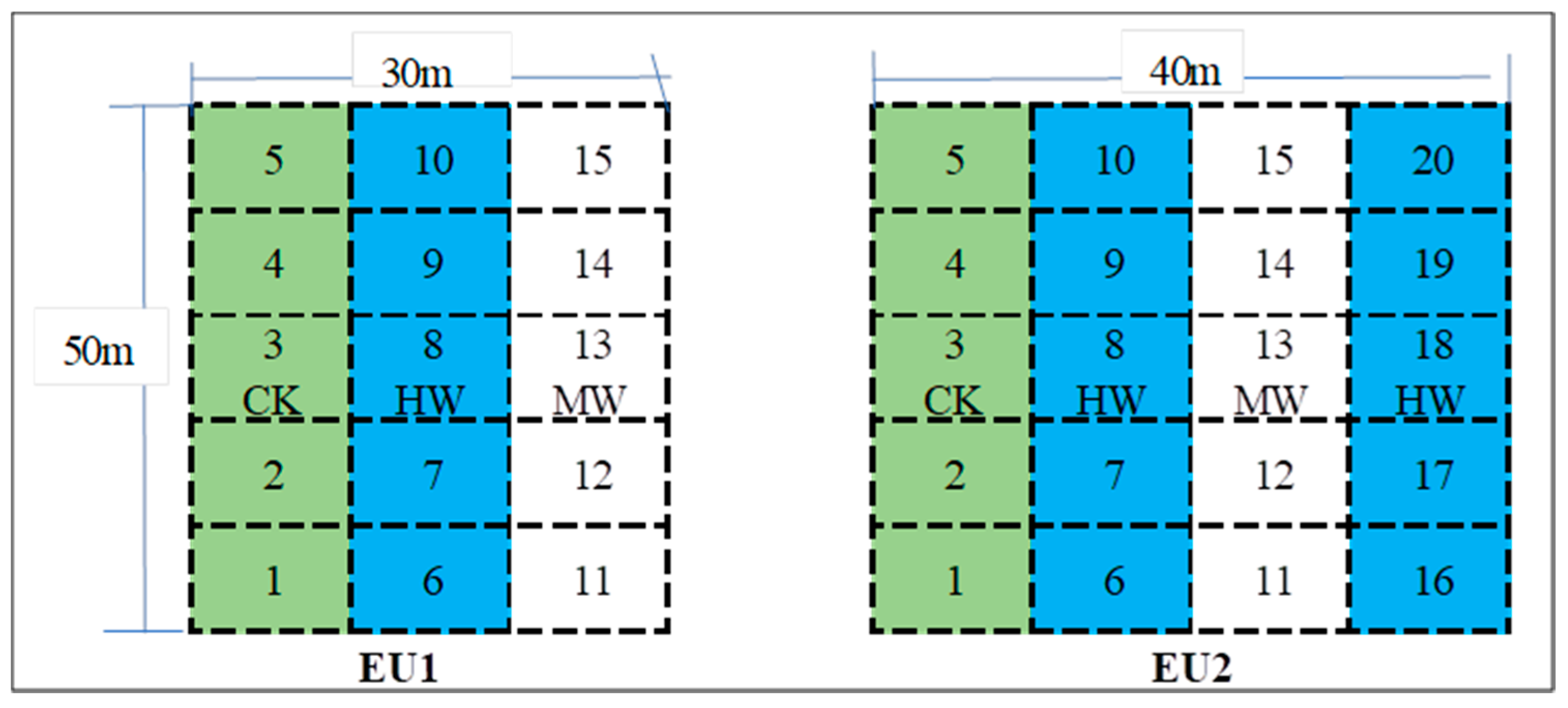
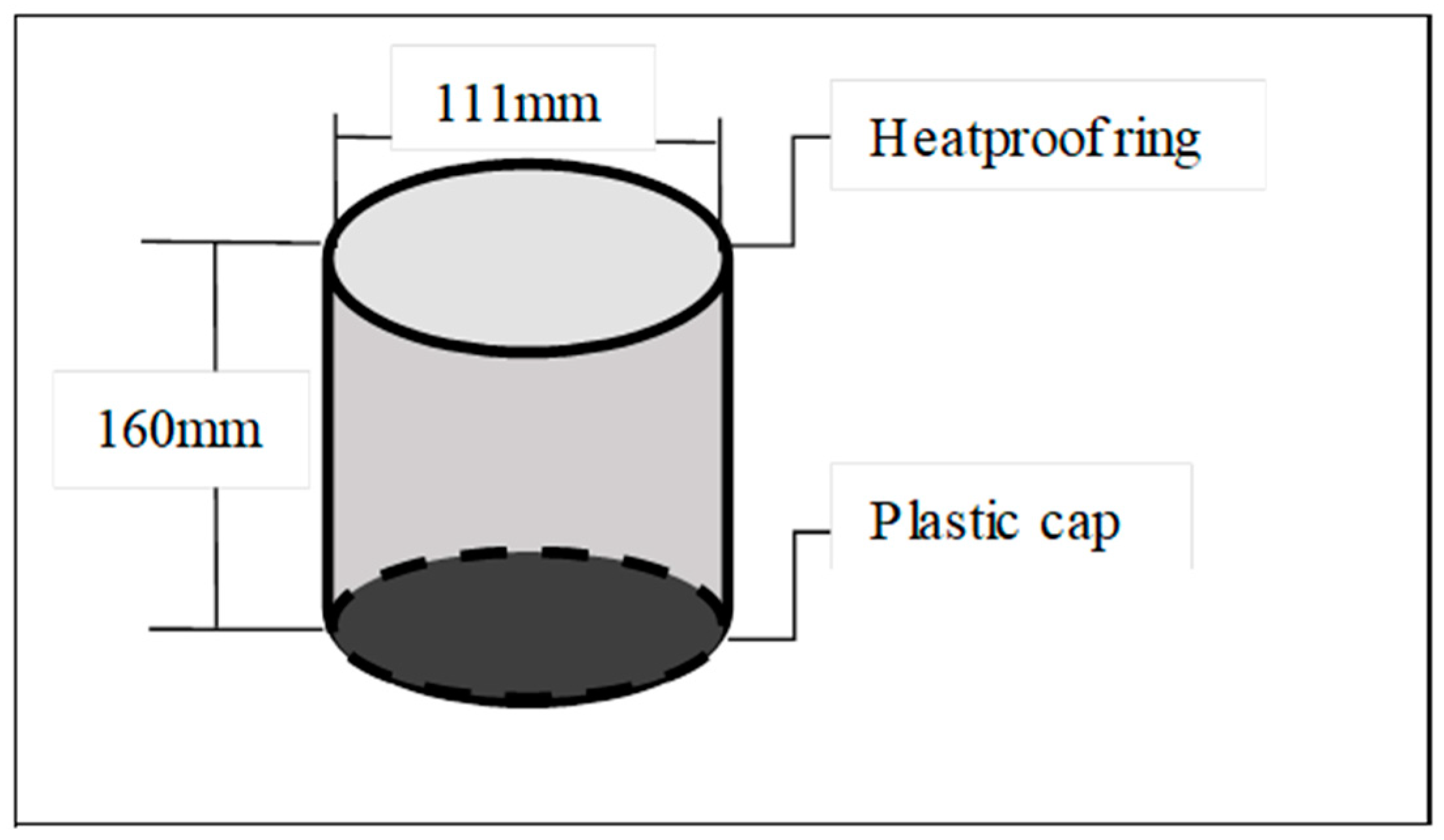
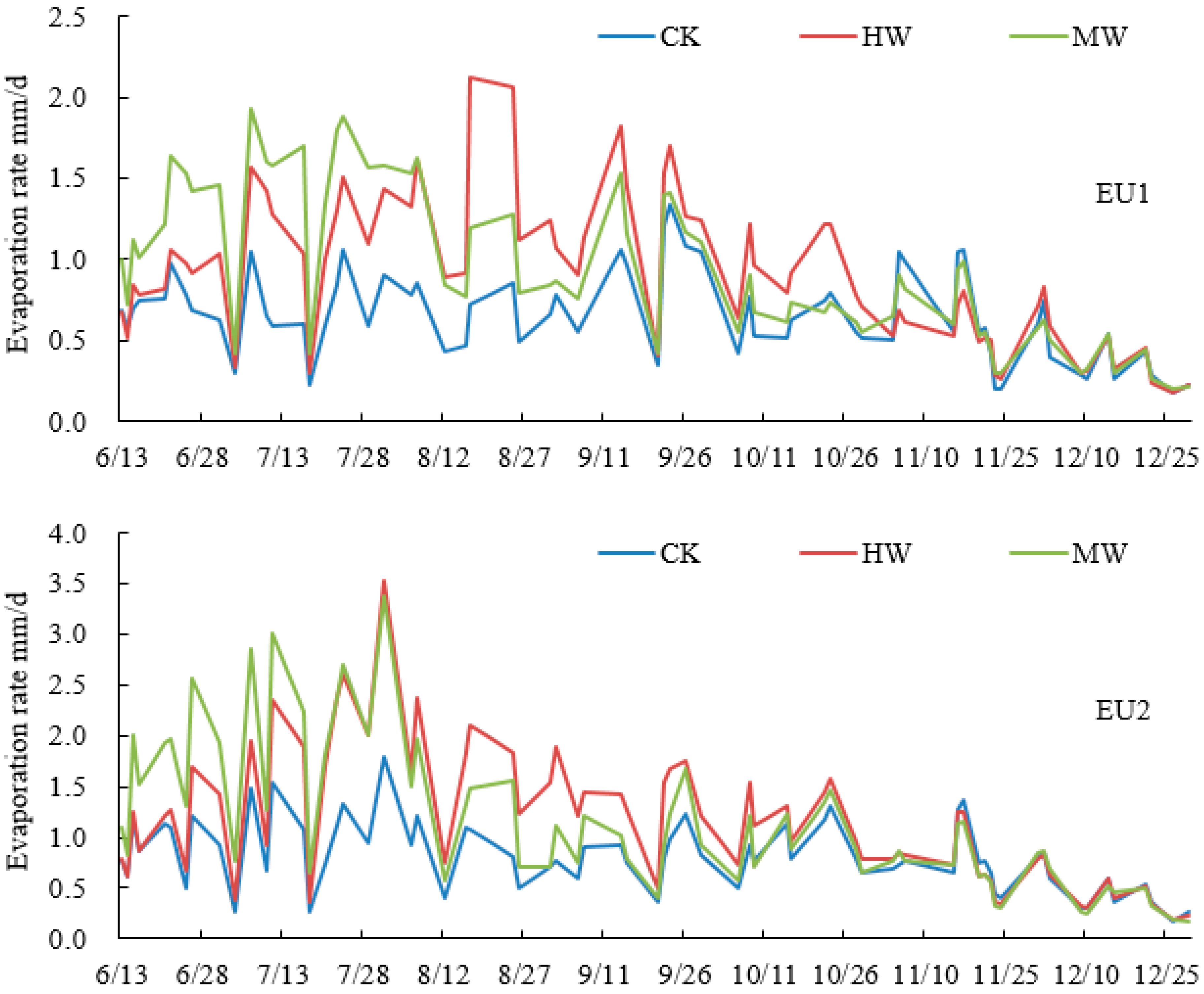
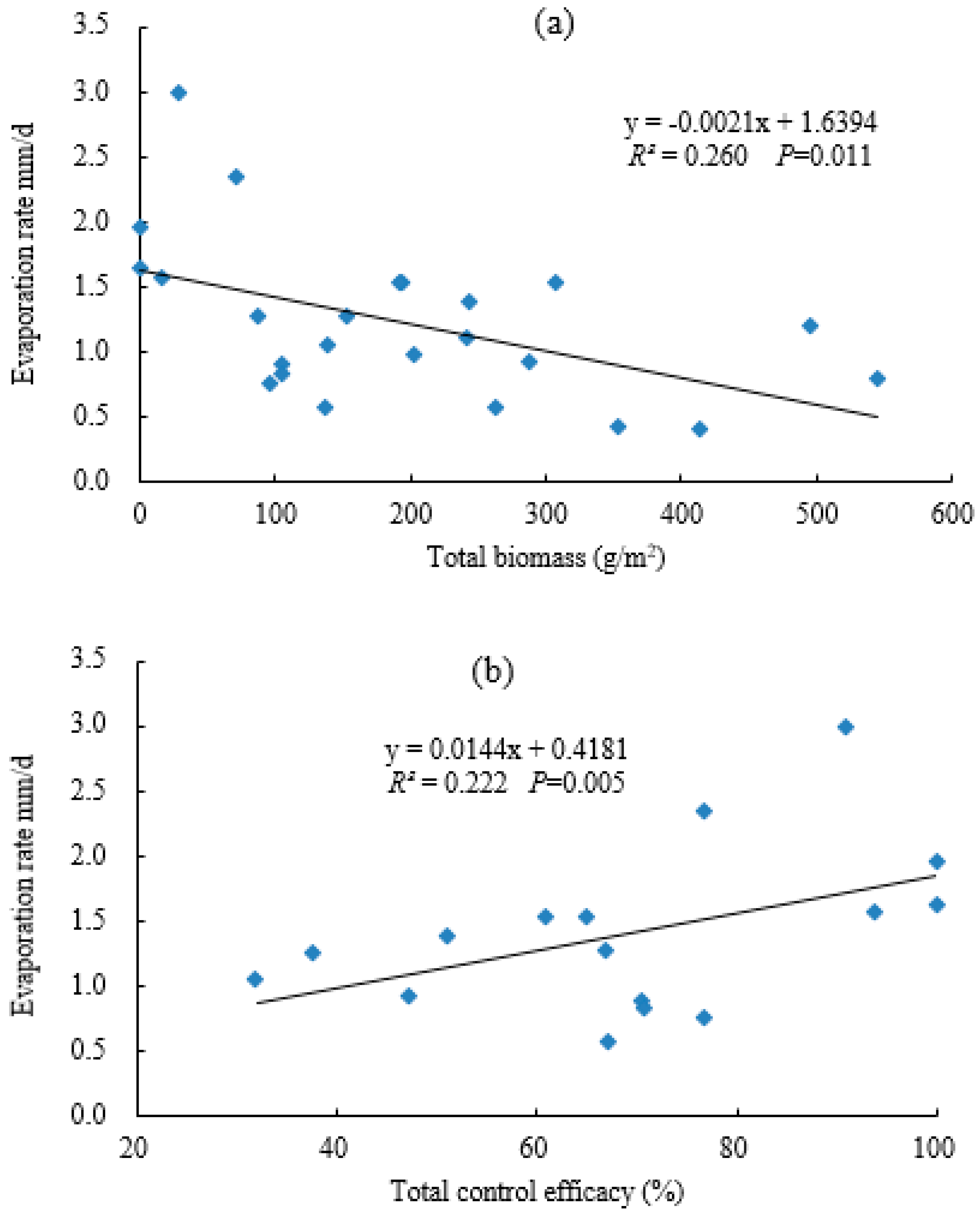



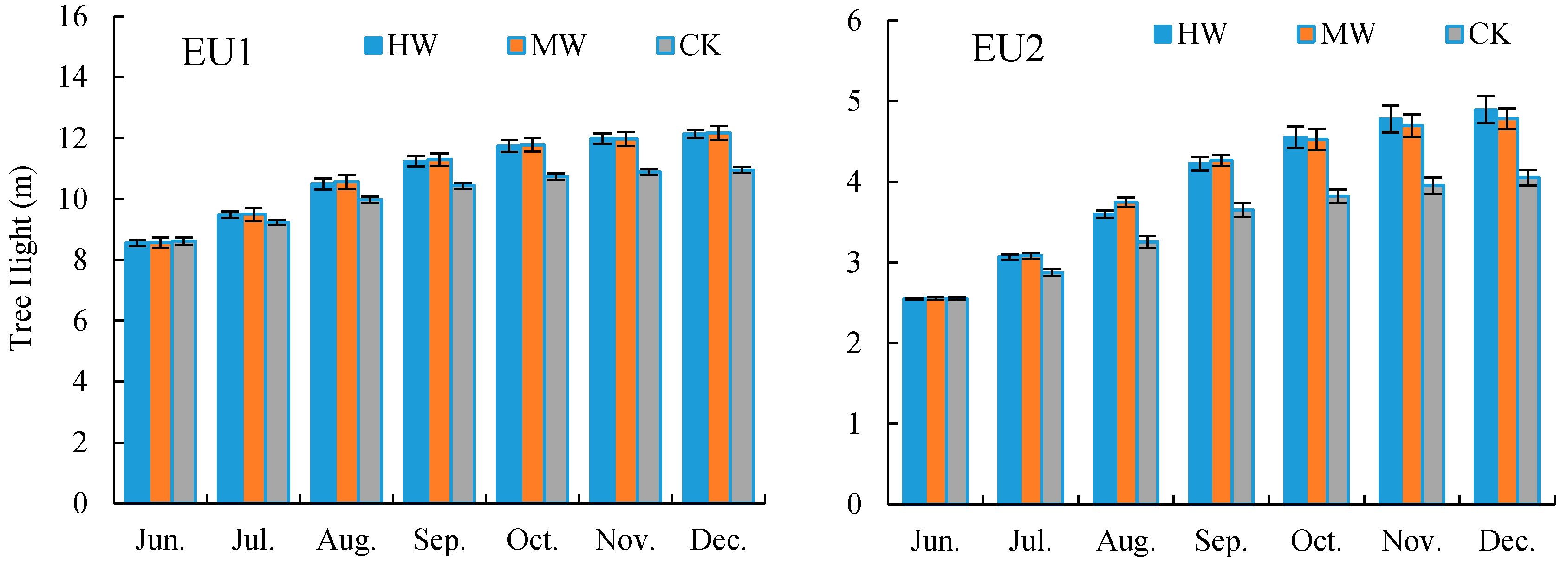


| Sample Plot | pH | BD (g/cm3) | CP (%) | NCP (%) | Sand (%) | Silt (%) | Clay (%) | OC (g/kg) | TN (g/kg) | TP (g/kg) | AN (mg/kg) | AP (mg/kg) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| EU1 | 4.8 | 1.18 | 33.5 | 21.3 | 16.1 | 74.2 | 9.7 | 19.3 | 1.52 | 0.39 | 124.3 | 4.20 |
| EU2 | 4.7 | 1.20 | 32.7 | 22.6 | 18.3 | 73.2 | 8.5 | 17.5 | 1.50 | 0.40 | 117.7 | 4.25 |
| Sample Plot | CH (%) | CS (%) | CC (%) | CE | TL (kg/hm2) |
|---|---|---|---|---|---|
| EU1 | 15.6 | 7.5 | 17.4 | 0.40 | 1890 |
| EU2 | 20.3 | 9.1 | 23.1 | 0.21 | 105 |
| Vegetation Types | 10d | 30d | 60d | 100d | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HW | MW | CK | HW | MW | CK | HW | MW | CK | HW | MW | CK | |
| Microstegium vagans (Nees ex Steud.) A. Camus | 0.0 | 0.0 | 30.2 | 0.0 | 4.6 | 45.6 | 0.0 * | 31.6 | 70.2 | 39.8 * | 80.9 | 120.7 |
| Miscanthus floridulus (Lab.) Warb. ex Schum. et Laut. | 15.2 * | 0.0 | 25.3 | 14.1 * | 2.0 | 40.4 | 13.9 * | 25.3 | 63.2 | 21.5 * | 46.4 | 85.9 |
| Dianella ensifolia | 22.5 * | 0.0 | 21.4 | 27.3 * | 1.5 | 25.8 | 31.4 * | 10.5 | 30.1 | 38.4 * | 18.4 | 35.4 |
| Dicranopteris dichotoma (Thunb.) Bernh | 27.4 * | 0.0 | 39.1 | 0.0 | 2.3 | 46.1 | 0.6 * | 10.6 | 55.9 | 3.8 * | 30.2 | 75.5 |
| Indocalamus tessellatus (Munro) Keng f. | 22.6 * | 0.0 | 25.1 | 11.8 * | 2.0 | 33.6 | 15.6 | 12.4 | 45.8 | 24.1 | 22.5 | 53.6 |
| Melastoma candidum D. Don | 17.2 * | 0.0 | 15.8 | 13.7 * | 0 | 17.4 | 20.0 * | 1.5 | 25 | 27.2 * | 7.3 | 33.2 |
| Rhodomyrtus tomentosa | 20.3 * | 0.0 | 24.1 | 17.1 * | 1.5 | 25.5 | 17.7 * | 2.6 | 28.6 | 23.6 * | 12.9 | 35.7 |
| Blechnum orientale | 12.5 * | 0.0 | 21.2 | 3.2 | 2.6 | 28.7 | 5.4 | 9.6 | 35.7 | 15.5 * | 24.9 | 55.4 |
| Total biomass (g/m2) | 137.5 * | 0.0 | 202.2 | 87.2 * | 16.6 | 263.1 | 104.5 | 104.1 | 354.5 | 193.9 * | 243.5 | 495.4 |
| Total control efficacy (%) | 32.0 * | 100.0 | 66.9 * | 93.7 | 70.5 | 70.6 | - | 60.9 * | 50.9 | |||
| Vegetation Types | 10d | 30d | 60d | 100d | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HW | MW | CK | HW | MW | CK | HW | MW | CK | HW | MW | CK | |
| Microstegium vagans (Nees ex Steud.) A. Camus | 0.0 | 0.0 | 32.7 | 0.0 * | 6.7 | 47.9 | 0.0 * | 36.5 | 74.5 | 44.6 * | 78.9 | 114.3 |
| Miscanthus floridulus (Lab.) Warb. ex Schum. et Laut. | 18.5 * | 0.0 | 35.6 | 12.6 * | 4.2 | 52.6 | 15.9 * | 34.9 | 79.4 | 23.3 * | 63.9 | 101.4 |
| Dianella ensifolia | 13.4 * | 0.0 | 15.2 | 15.1 * | 1.1 | 17.8 | 17.9 * | 8.9 | 22.8 | 22.3 * | 16.1 | 28.7 |
| Dicranopteris dichotoma (Thunb.) Bernh | 30.5 * | 0.0 | 46.9 | 0.0 * | 4.3 | 53.2 | 1.4 * | 14.4 | 68.4 | 4.5 * | 38.1 | 90.7 |
| Indocalamus tessellatus (Munro) Keng f. | 27.5 * | 0.0 | 32.4 | 13.4 * | 3.4 | 41.9 | 18.2 | 16.5 | 55.1 | 28.1 | 28.1 | 62.5 |
| Melastoma candidum D. Don | 14.2 * | 0.0 | 12.8 | 11.0 * | 0.0 | 14.3 | 17.9 * | 1.4 | 22.7 | 25.5 * | 7.2 | 31.1 |
| Rhodomyrtus tomentosa | 22.6 * | 0.0 | 19.4 | 14.8 * | 1.3 | 21.7 | 16.0* | 2.8 | 25.4 | 21.2 * | 14.9 | 33.2 |
| Blechnum orientale | 24.7 * | 0.0 | 47.5 | 4.7 | 7.0 | 58.3 | 9.1 * | 20.9 | 65.3 | 21.7 * | 41.0 | 83.6 |
| Total biomass (g/m2) | 151.4 * | 0.0 | 242.5 | 71.6 * | 27.9 | 307.7 | 96.4 * | 136.3 | 413.6 | 191.3 * | 288.1 | 545.5 |
| Total control efficacy (%) | 37.6 * | 100.0 | 76.7 * | 90.9 | 76.7 * | 67.1 | 64.9 * | 47.2 | ||||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deng, Y.; Yang, G.; Xie, Z.; Yu, J.; Jiang, D.; Huang, Z. Effects of Different Weeding Methods on the Biomass of Vegetation and Soil Evaporation in Eucalyptus Plantations. Sustainability 2020, 12, 3669. https://doi.org/10.3390/su12093669
Deng Y, Yang G, Xie Z, Yu J, Jiang D, Huang Z. Effects of Different Weeding Methods on the Biomass of Vegetation and Soil Evaporation in Eucalyptus Plantations. Sustainability. 2020; 12(9):3669. https://doi.org/10.3390/su12093669
Chicago/Turabian StyleDeng, Yusong, Gairen Yang, Zhifeng Xie, Jingrui Yu, Daihua Jiang, and Zhigang Huang. 2020. "Effects of Different Weeding Methods on the Biomass of Vegetation and Soil Evaporation in Eucalyptus Plantations" Sustainability 12, no. 9: 3669. https://doi.org/10.3390/su12093669
APA StyleDeng, Y., Yang, G., Xie, Z., Yu, J., Jiang, D., & Huang, Z. (2020). Effects of Different Weeding Methods on the Biomass of Vegetation and Soil Evaporation in Eucalyptus Plantations. Sustainability, 12(9), 3669. https://doi.org/10.3390/su12093669





