Reduction of Internal Phosphorus Load in New Lakes by Pretreatment of the Former Agricultural Soil—Methods, Ecological Results and Costs
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Site
2.2. Experimental Site and Soil Treatments
2.3. Pre-Flooding Soil Sampling
2.4. Laboratory Experiments on Undisturbed Soil Cores Collected Prior to Flooding
2.4.1. Soil Core Sampling
2.4.2. Flooding Experiment
2.4.3. Sequential Extraction of Soil
2.5. Nutrients and Fe Flux from Inundated Soil
2.6. Lake Water Parameters
2.7. Statistical Analysis
3. Results
3.1. Soil Phosphorus Content
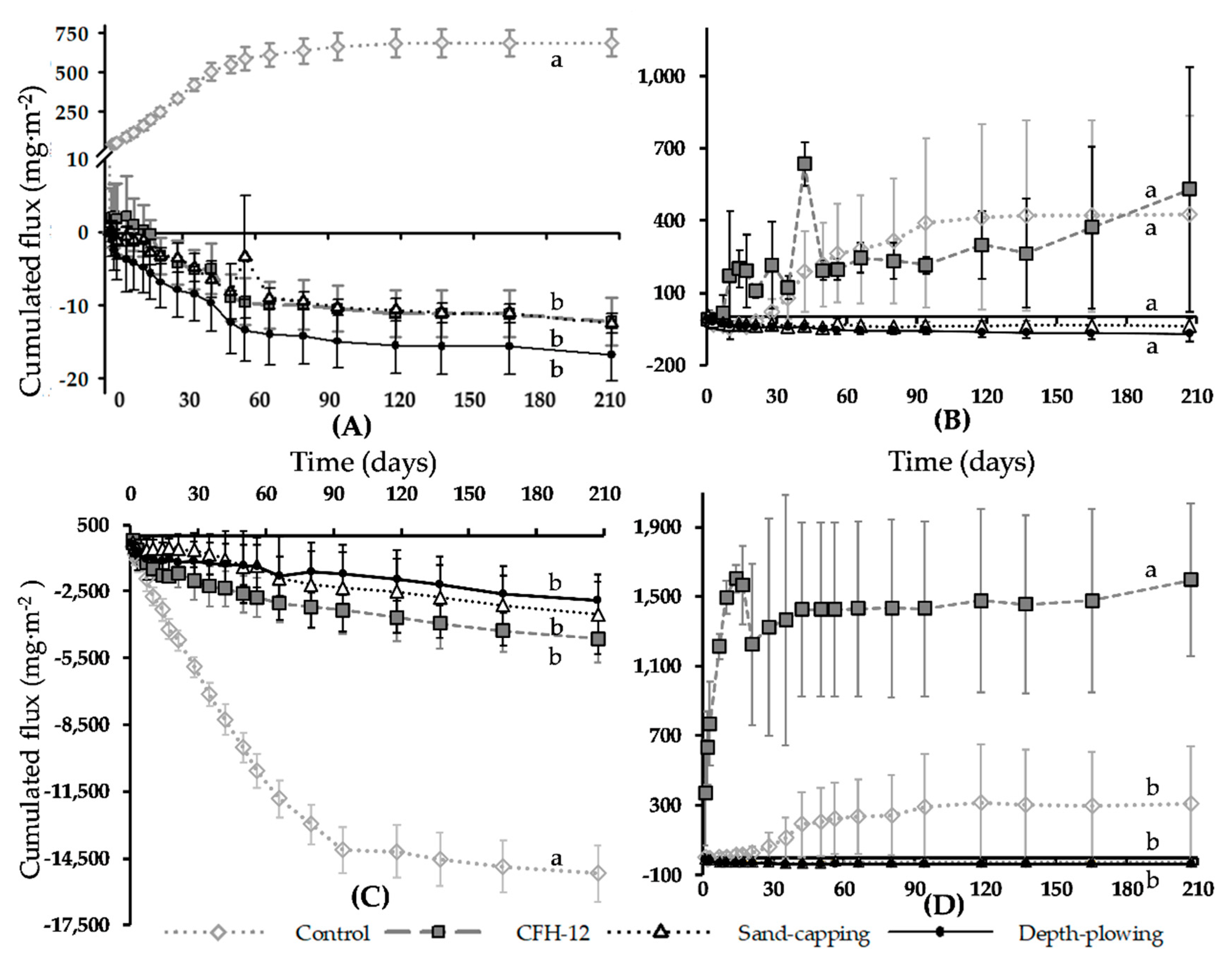
3.2. Soil–Water Flux
4. Discussion
4.1. Overview
4.2. Effect of Pretreatments
4.2.1. DIP Flux
4.2.2. DFe, NO3−, and NH4+ Flux
4.3. P Release from Agricultural Soil
4.4. Comparison of Costs of Pretreatments
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Williams, P.; Biggs, J.; Fox, G.; Nicolet, P.; Whitfield, M. History, origins and importance of temporary ponds. In Freshwater Forum; The Ponds Conservation Trust: Policy & Research; c/o Oxford Brookes University: Oxford, UK, 2001; pp. 7–14. [Google Scholar]
- Hoffmann, C.C.; Baattrup-Pedersen, A. Re-establishing freshwater wetlands in Denmark. Ecol. Eng. 2007, 30, 157–166. [Google Scholar] [CrossRef]
- Moreno-Mateos, D.; Power, M.E.; Comín, F.A.; Yockteng, R. Structural and functional loss in restored wetland ecosystems. PLoS Biol. 2012, 10, e1001247. [Google Scholar] [CrossRef] [PubMed]
- Hansen, K. Det Tabte Land—Den Store Fortælling om Magten over det Danske Landskab; Gads Forlag: København, Denmark, 2008. [Google Scholar]
- Zedler, J.B. Wetlands at your service: Reducing impacts of agriculture at the watershed scale. Front. Ecol. Environ. 2003, 1, 65–72. [Google Scholar] [CrossRef]
- Zedler, J.B.; Kercher, S. Wetland resources: Status, trends, ecosystem services, and restorability. Annu. Rev. Environ. Resour. 2005, 30, 39–74. [Google Scholar] [CrossRef]
- Mitsch, W.J.; Horne, A.J.; Nairn, R.W. Nitrogen and phosphorus retention in wetlands—Ecological approaches to solving excess nutrient problems. Ecol. Eng. 2000, 14, 1–7. [Google Scholar]
- Fisher, J.; Acreman, M.C. Wetland nutrient removal: A review of the evidence. Hydrol. Earth Syst. Sci. 2004, 8, 673–685. [Google Scholar] [CrossRef]
- Strand, J.A.; Weisner, S.E. Effects of wetland construction on nitrogen transport and species richness in the agricultural landscape experiences from Sweden. Ecol. Eng. 2013, 56, 14–25. [Google Scholar] [CrossRef]
- Ardón, M.; Montanari, S.; Morse, J.L.; Doyle, M.W.; Bernhardt, E.S. Phosphorus export from a restored wetland ecosystem in response to natural and experimental hydrologic fluctuations. J. Geophys. Res. 2010, 115, 1–12. [Google Scholar] [CrossRef]
- Steinman, A.D.; Ogdahl, M.E. Does converting agricultural fields to wetlands retain or release P? J. N. Am. Benthol. Soc. 2011, 30, 820–830. [Google Scholar] [CrossRef]
- Kragh, T.; Sand-Jensen, K.; Petersen, K.; Kristensen, E. Fast phosphorus loss by sediment resuspension in a re-established shallow lake on former agricultural fields. Ecol. Eng. 2017, 108, 2–9. [Google Scholar] [CrossRef]
- Aldous, A.R.; Craft, C.B.; Stevens, C.J.; Barry, M.J.; Bach, L.B. Soil phosphorus release from a restoration wetland, Upper Klamath Lake, Oregon. Wetlands 2007, 27, 1025–1035. [Google Scholar] [CrossRef]
- Grunth, N.L.; Askaer, L.; Elberling, B. Oxygen depletion and phosphorus release following flooding of a cultivated wetland area in Denmark. Dan. J. Geogr. 2008, 108, 17–25. [Google Scholar] [CrossRef]
- Hoffmann, C.C.; Heiberg, L.; Audet, J.; Schønfeldt, B.; Fuglsang, A.; Kronvang, B.; Ovesen, N.B.; Kjaergaard, C.; Hansen, H.C.B.; Jensen, H.S. Low phosphorus release but high nitrogen removal in two restored riparian wetlands inundated with agricultural drainage water. Ecol. Eng. 2012, 46, 75–87. [Google Scholar] [CrossRef]
- Pant, H.K.; Reddy, K.R. Potential internal loading of phosphorus in a wetland constructed in agricultural land. Water Res. 2003, 37, 965–972. [Google Scholar] [CrossRef]
- Jeke, N.N.; Zvomuja, F. Flooding depth and timing effects on phosphorus release from flooded biosolids in an end-of-life municipal lagoon. Water Air Soil Pollut. 2018, 229, 1–14. [Google Scholar] [CrossRef]
- Reddy, K.R.; DeLaune, R.D. Biogeochemistry of Wetlands: Science and Applications; CRC Press: Boca Raton, FL, USA, 2008. [Google Scholar]
- Duff, J.H.; Carpenter, K.D.; Snyder, D.T.; Lee, K.K.; Avanzino, R.J.; Triska, F.J. Phosphorus and nitrogen legacy in a restoration wetland, Upper Klamath Lake, Oregon. Wetlands 2009, 29, 735–746. [Google Scholar] [CrossRef]
- Audet, J.; Zak, D.; Bidstrup, J.; Hoffmann, C.C. Nitrogen and phosphorus retention in Danish restored wetlands. Ambio 2019. [Google Scholar] [CrossRef]
- Barberis, E.; Ajmone Marsan, F.; Scalenghe, R.; Lamers, A.; Schwertmann, U.; Edwards, A.C.; Maguire, R.; Wilson, M.J.; Delgado, A.; Torrent, J. European soils overfertilized with phosphorus. Part 1. Basic properties. Nutr. Cycl. Agroecosyst. 1995, 45, 199–207. [Google Scholar] [CrossRef]
- Reynolds, C.; Davies, P. Sources and bioavailability of phosphorus fractions in freshwaters: A British perspective. Biol. Rev. 2001, 76, 27–64. [Google Scholar] [CrossRef]
- Schärer, M.; Stamm, C.; Vollmer, T.; Frossard, E.; Oberson, A.; Flühler, H.; Sinaj, S. Reducing phosphorus losses from over-fertilized grassland soils proves difficult in the short term. Soil Use Manag. 2007, 23, 154–164. [Google Scholar] [CrossRef]
- Laboski, C.A.; Lamb, J.A. Changes in soil test phosphorus concentration after application of manure or fertilizer. Soil Sci. Soc. Am. J. 2003, 67, 544–554. [Google Scholar] [CrossRef]
- Zak, D.; Gelbrecht, J. The mobilisation of phosphorus, organic carbon and ammonium in the initial stage of fen rewetting (a case study from NE Germany). Biogeochemistry 2007, 85, 141–151. [Google Scholar] [CrossRef]
- Van Dijk, J.; Stroetenga, M.; Bos, L.; Van Bodegom, P.M.; Verhoef, H.A.; Aerts, R. Restoring natural seepage conditions on former agricultural grasslands does not lead to reduction of organic matter decomposition and soil nutrient dynamics. Biogeochemistry 2004, 71, 317–337. [Google Scholar] [CrossRef]
- Søndergaard, M.; Jensen, J.P.; Jeppesen, E. Role of sediment and internal loading of phosphorus in shallow lakes. Hydrobiologia 2003, 506, 135–145. [Google Scholar] [CrossRef]
- Jensen, H.S.; Kristensen, P.; Jeppesen, E.; Skytthe, A. Iron:phosphorus ratio in surface sediment as an indicator of phosphate release from aerobic sediments in shallow lakes. Hydrobiologica 1992, 235, 731–743. [Google Scholar] [CrossRef]
- Patrick, W.H.; Khalid, R.A. Phosphate release and sorption by soils and sediments: Effect of aerobic and anaerobic conditions. Science 1974, 186, 53–55. [Google Scholar] [CrossRef]
- Ponnamperuma, F.N. Effects of flooding on soils. In Flooding and Plantgrowth; Kozlowski, T.T., Ed.; Academic Press, Inc.: Orlando, FL, USA, 1984; pp. 9–45. [Google Scholar]
- Scalenghe, R.; Edwards, A.C.; Marsan, F.A.; Barberis, E. The effect of reducing conditions on the solubility of phosphorus in a diverse range of European agricultural soils. Eur. J. Soil Sci. 2002, 53, 439–447. [Google Scholar] [CrossRef]
- Loeb, R.; Lamers, L.P.; Roelofs, J.G.M. Prediction of phosphorus mobilization in inundated floodplain soils. Environ. Pollut. 2008, 156, 325–331. [Google Scholar] [CrossRef]
- Heiberg, L.; Pedersen, T.V.; Jensen, H.S.; Kjaergaard, C.; Hansen, H.C.B. A comparative study of phosphate sorption in lowland soils under oxic and anoxic conditions. Environ. Qual. 2010, 39, 734–743. [Google Scholar] [CrossRef]
- Smolders, A.J.P.; Lamers, L.P.M.; Lucassen, E.C.H.E.T.; Van der Velde, G.; Roelofs, J.G.M. Internal eutrophication: How it works and what to do about it—A review. Chem. Ecol. 2006, 22, 93–111. [Google Scholar] [CrossRef]
- Reitzel, K.; Andersen, F.Ø.; Egemose, S.; Jensen, H.S. Phosphate adsorption by lanthanum modified bentonite clay in fresh and brackish water. Water Res. 2013, 47, 2787–2796. [Google Scholar] [CrossRef] [PubMed]
- Reitzel, K.; Jensen, H.S.; Egemose, S. pH dependent dissolution of sediment aluminum in six Danish lakes treated with aluminum. Water Res. 2013, 47, 1409–1420. [Google Scholar] [CrossRef] [PubMed]
- Bakker, E.S.; van Donk, E.; Immers, A.K. Lake restoration by in-lake iron addition: A synopsis of iron impact on aquatic organisms and shallow lake ecosystems. Aquat. Ecol. 2016, 50, 1–15. [Google Scholar] [CrossRef]
- Palermo, M.R. Design considerations for in-situ capping of contaminated sediments. Water Sci. Technol. 1998, 37, 315–321. [Google Scholar] [CrossRef]
- Smolders, A.J.P.; Lamers, L.P.M.; Lucassen, E.C.H.E.T.; Van der Aalst, M.; Roelofs, J.G.M. Decreasing the abundance of Juncus effuses on former agricultural lands with noncalcareous soil: Possible effects of liming and soil removal. Restor. Ecol. 2008, 16, 240–248. [Google Scholar] [CrossRef]
- Geurts, J.J.M.; Van de Wouw, P.A.U.; Smolders, A.J.P.; Roelofs, J.G.M. Ecological restoration on former agricultural soils: Feasibility of in-situ phosphate fixation as an alternative to top-soil removal. Ecol. Eng. 2011, 37, 1620–1629. [Google Scholar] [CrossRef]
- Lürling, M.; Mackay, E.; Reitzel, K.; Spears, B.M. Editorial—A critical perspective on geo-engineering for eutrophication management in lakes. Water Res. 2016, 97, 1–10. [Google Scholar] [CrossRef]
- Bostic, E.M.; White, J.R. Soil phosphorus and vegetation influence on wetland phosphorus release after simulated drought. Soil Sci. Soc. Am. J. 2007, 71, 238–244. [Google Scholar] [CrossRef]
- Wang, X.Y.; Feng, J. Assessment of the effectiveness of environmental dredging in South Lake, China. Environ. Manag. 2007, 40, 314–322. [Google Scholar] [CrossRef]
- Himmelheber, D. In Situ Capping of Contaminated Sediments: Spatial and Temporal Characterization of Biogeochemical and Contaminant Biotransformation Processes. Ph.D. Thesis, Georgia Institute of Technology, Atlanta, GA, USA, April 2008. [Google Scholar]
- Rubæk, G.H.; Kristensen, K.; Olesen, S.E.; Østergaard, H.S.; Heckrath, G. Phosphorus accumulation and spatial distribution in agricultural soils in Denmark. Geoderma 2013, 209–210, 241–250. [Google Scholar] [CrossRef]
- Azcue, J.M.; Zeman, A.J.; Mudroch, A.; Rosa, F.; Patterson, T. Assessment of sediment and porewater after one year of subaqueous capping of contaminated sediments in Hamilton Harbour, Canada. Water Sci. Technol. 1998, 37, 323–329. [Google Scholar] [CrossRef]
- Jacobs, P.H.; Forstner, U. Concept of subaqueous capping of contaminated sediments with Active Barrier Systems (ABS) using natural and modified zeolites. Water Res. 1999, 33, 2083–2087. [Google Scholar] [CrossRef]
- Berg, U.; Neumann, T.; Donnert, D.; Nuesch, R.; Stuben, D. Sediment capping in eutrophic lakes-efficiency of undisturbed calcite barriers to immobilize phosphorus. Appl. Geochem. 2004, 19, 1759–1771. [Google Scholar] [CrossRef]
- Kim, G.; Jung, W. Role of sand capping in phosphorus release from sediment. KSCE J. Civ. Eng. 2010, 14, 815–821. [Google Scholar] [CrossRef]
- Noyma, N.P.; De Magalhaes, L.; Furtado, L.L.; Mucci, M.; Van Oosterhout, F.; Huszar, V.L.M.; Marinho, M.M.; Lürling, M. Controlling cyanobacterial blooms through effective flocculation and sedimentation with combined use of flocculants and phosphorus adsorbing natural soil and modified clay. Water Res. 2015, 97, 26–38. [Google Scholar] [CrossRef] [PubMed]
- Flindt, M.R.; Lange, T.; Aaskoven, N.; Wendländer, N.; Steinfurth, R.; Nielsen, B.; Kristensen, E. Sand-capping—Et nyt marint virkemiddel. Vand Jord 2019, 4, 165–168. [Google Scholar]
- Shackelford, C.; Daniel, D.E. Diffusion in saturated soil. I: Background. J. Geotech. Eng. 1991, 117, 467–484. [Google Scholar] [CrossRef]
- Arias, C.A.; Del Bubba, M.; Brix, H. Phosphorus removal by sands for use in media in subsurface flow constructed reed beds. Wat. Res. 2001, 35, 1159–1168. [Google Scholar] [CrossRef]
- Hickey, C.W.; Gibbs, M.M. Lake sediment phosphorus release management Decision support and risk assessment framework. N. Z. J. Mar. Freshw. Res. 2009, 43, 819–856. [Google Scholar] [CrossRef]
- Boers, P.C.M.; Van der Does, J.; Quaak, M.; Van der Vlugt, J. Phosphorus fixation with iron(III)chloride: A new method to combat internal phosphorus loading in shallow lakes? Arch. Hydrobiol. 1994, 129, 339–351. [Google Scholar]
- Reitzel, K.; Hansen, J.; Jensen, H.S.; Andersen, F.Ø.; Hansen, K.S. Testing aluminum addition as a tool for lake restoration in shallow, eutrophic Lake Sønderby, Denmark. Hydrobiologia 2003, 506, 781–787. [Google Scholar] [CrossRef]
- Varjo, E.; Liikanen, A.; Salonen, V.P.; Martikainen, P.J. A new gypsum-based technique to reduce methane and phosphorus release from sediments of eutrophied lakes: (Gypsum treatment to reduce internal loading). Water Res. 2003, 37, 1–10. [Google Scholar] [CrossRef]
- Dithmer, L.; Nielsen, U.G.; Lürling, M.; Spears, B.M.; Yasseri, S.; Lundberg, D.; Moore, A.; Jensen, N.D.; Reitzel, K. Responses in sediment phosphorus and lanthanum concentrations and composition across 10 lakes following applications of lanthanum modified bentonite. Water Res. 2016, 97, 101–110. [Google Scholar] [CrossRef] [PubMed]
- Novak, J.M.; Watts, D.W. Increasing the phosphorus sorption capacity of southeastern Coastal Plain soils using water treatment residuals. Soil Sci. 2004, 169, 206–214. [Google Scholar] [CrossRef]
- Agyin-Birikorang, S.; Oladeji, O.O.; O’Connor, G.A.; Obreza, T.A.; Capece, J.C. Efficacy of drinking-water treatment residual in controlling off-site phosphorus losses: A field study in Florida. J. Environ. Qual. 2009, 38, 1076–1085. [Google Scholar] [CrossRef] [PubMed]
- Jørgensen, C.A.; Jensen, H.S.; Egemose, S. Phosphate adsorption to iron sludge from waterworks, ochre precipitation basins and commercial ferrihydrite at ambient freshwater phosphate concentrations. Environ. Tech. 2016, 38, 2185–2192. [Google Scholar] [CrossRef]
- Smolders, A.J.P.; Lamers, L.P.M.; Moonen, M.; Zwaga, K.; Roelofs, J.G.M. Controlling phosphate release from phosphate-enriched sediments by adding various iron compounds. Biogeochemistry 2001, 54, 219–228. [Google Scholar] [CrossRef]
- Fuchs, E.; Funes, A.; Saar, K.; Reitzel, K.; Jensen, H.S. Evaluation of dried amorphous ferric hydroxide CFH-12® as agent for binding bioavailable phosphorous in lake sediments. Sci. Total Environ. 2018, 628–629, 990–996. [Google Scholar] [CrossRef]
- Lyngsie, G.; Borggaard, O.K.; Hansen, H.C.B. A three-step test of phosphate sorption efficiency of potential agricultural drainage filter materials. Water Res. 2014, 51, 256–265. [Google Scholar] [CrossRef]
- Ann, Y.; Reddy, K.R.; Delfino, J.J. Influence of redox potential on phosphorus solubility in chemically amended wetland organic soils. Ecol. Eng. 1999, 14, 169–180. [Google Scholar] [CrossRef]
- Østergaard, D. Afgørelse om at Hydrologi-og Skovrejsningsprojektet Rønnebæk Øst Ikke er VVM-Pligtigt; J. nr. SVANA-130-00125; Ministry of Environment and Food, Environmental Protection Agency, Nature Management: København, Denmark, 2017. [Google Scholar]
- Rasmussen, A. Udkast—Tilladelse til Restaurering af Rønnebækken, og Etablering af Søer i Tilknytning til Rønnebækken i det nye Skovrejsningsområde ved Rønnebæk By Case nr.: 06.02.10-P20-16-17; Center of Plan and Environment—The Water and Nature Team: Næstved, Denmark, 2017. [Google Scholar]
- Madsen, S. Rønnebæk Skovsø—Synergisø Ansøgning; Center of Plan and Environment—The Water and Nature Team: Næstved, Denmark, 2017. [Google Scholar]
- Greve, M.H.; Møller, A.B. Lokalitetskortlægning af Skovrejsningsområdet ved Rønnebæk; University of Aarhus: Aarhus, Denmark, 2017. [Google Scholar]
- Andersen, J.M. An ignition method for determination of total phosphorus in lake sediments. Water Res. 1976, 10, 329–331. [Google Scholar] [CrossRef]
- Rubæk, G.H.; Kristensen, K. Protocol for Bicarbonate Extraction of Inorganic Phosphate from Agricultural Soil; DCA Report No. 102; Aarhus University, Danish Centre for Food and Agriculture: Aarhus, Denmark, 2017. [Google Scholar]
- Koroleff, F. Determination of nutrients. In Methods of Seawater Analysis, 2nd ed.; Grasshof, K., Ehrhardt, M., Kremling, K., Eds.; Verlag Chemie: Weinheim, Germany, 1983; pp. 159–226. [Google Scholar]
- Reitzel, K. Separation of aluminum bound phosphate from iron bound phosphate in freshwater sediments by a sequential extraction procedure. In Phosphate in Sediments; Golterman, H.L., Serrano, L., Eds.; Backhuys Publishers: Leiden, The Netherlands, 2005; pp. 109–117. [Google Scholar]
- Gibbs, M.M. A simple method for the rapid determination of iron in natural waters. Water Res. 1979, 13, 295–297. [Google Scholar] [CrossRef]
- Psenner, R.; Pucsko, R.; Sager, M. Die fraktionierung organischer und anorganischer phosphor-verbindungen von sedimenten. Arch. Hydrobiol. Suppl. Monogr. Beitr. 1984, 70, 111–155. [Google Scholar]
- Jensen, H.S.; Thamdrup, B. Iron-bound phosphorus in marine sediments as measured by bicarbonate-dithionite extraction. Hydrobiologia 1993, 253, 47–59. [Google Scholar] [CrossRef]
- Jespersen, A.M.; Christoffersen, K. Measurements of chlorophyll—A from phytoplankton using ethanol as extraction solvent. Arch. Hydrobiol. 1987, 109, 445–454. [Google Scholar]
- Mackereth, F.J.H.; Heron, J.; Talling, J.F. Water Analysis: Some Revised Methods for Limnologists; Freshwater Biological Association Scientific Publication No. 36; Titus Wilson and Sons Ltd.: Kendal, UK, 1978. [Google Scholar]
- Sawyer, C.N.; McCarty, P.L.; Parkin, G.F. Chemistry for Environmental Engineering, 4th ed.; McGraw-Hill, Inc.: New York, NY, USA, 2000. [Google Scholar]
- De Vicente, I.; Jensen, H.; Andersen, F. Factors affecting phosphate adsorption to aluminum in lake water: Implications for lake restoration. Sci. Total Environ. 2008, 389, 29–36. [Google Scholar] [CrossRef]
- Jensen, H.S.; Reitzel, K.; Egemose, S. Evaluation of aluminum treatment efficiency on water quality and internal phosphorus cycling in six Danish lakes. Hydrobiologia 2015, 751, 189–199. [Google Scholar] [CrossRef]
- Flindt, M.R.; Jørgensen, C.; Jensen, H.S. Notat—Den Interne Fosforbelastning i Danske Søer og Indsvingningstiden Efter Reduktion af Ekstern Fosfortilførsel; The Danish Nature Agency: Copenhagen, Denmark, 2015; pp. 2–46.
- Kolath, T.; Reitzel, K.; Egemose, S.; Jensen, H.S. Variation in sediment phosphorus content and efflux between new and natural Lakes. 2020, in press. [Google Scholar]
- Reitzel, K.; Ahlgren, J.; DeBrabandere, H.; Waldebäck, M.; Gogoll, A.; Tranvik, L.; Rydin, E. Degradation rates of organic phosphorus in lake sediment. Biogeochemistry 2007, 82, 15–28. [Google Scholar] [CrossRef]
- Sjøgaard, K.S.; Treusch, A.H.; Valdemarsen, T.B. Carbon degradation in agricultural soils flooded with seawater after managed coastal realignment. Biogeosciences 2017, 14, 4375–4389. [Google Scholar] [CrossRef]
- Stumm, W.; Morgan, J.J. Aquatic Chemistry; John Wiley & Sons: New York, NY, USA, 1981. [Google Scholar]
- Canfield, D.E. The geochemistry of river particulates from the continental United States: Major elements. Geochim. Cosmochim. Acta 1997, 61, 3349–3365. [Google Scholar] [CrossRef]
- Zeman, A.J. Subaqueous capping of very soft contaminated sediments. Can. Geotech. J. 1994, 31, 570–577. [Google Scholar] [CrossRef]
- Ahlgren, J.; Tranvik, L.; Gogoll, A.; Waldebäck, M.; Markides, K.; Rydin, E. Sediment depth attenuation of biogenic phosphorus compounds measured by 31P-NMR. Environ. Sci. Technol. 2005, 39, 867–872. [Google Scholar] [CrossRef] [PubMed]
- Bleam, W.F.; Pfeffer, P.E.; Goldberg, S.; Taylor, R.W.; Dudley, R. A 31P solid-state nuclear magnetic resonance study of phosphate adsorption at the boehmite/aqueous solution interface. Langmuir 1991, 7, 1702–1712. [Google Scholar] [CrossRef]
- Weyhenmeyer, G. Resuspension in lakes and its ecological impacts: A review. Arch. Hydrobiol. Spec. Issues Advanc. Limnol. 1998, 51, 185–200. [Google Scholar]
- Windolf, J.; Wiberg-Larsen, P.; Bøgestrand, J.; Larsen, S.E.; Thodsen, H.; Bjerring, R.; Ovesen, N.B.; Kjeldgaard, A.; Kronvang, B. Vandløb 2011 NOVANA; National Center for Environment and Energy, University of Aarhus: Aarhus, Denmark, 2011. [Google Scholar]
- Hille, S.; Graeber, D.; Kronvang, B.; Rubæk, G.H.; Onnen, N.; Molina-Navarro, E.; Baattrup-Pedersen, A.; Heckrath, G.J.; Stutter, M.I. Management options to reduce phosphorus leaching from vegetated buffer strips. J. Environ. Qual. 2018, 48, 322–329. [Google Scholar] [CrossRef] [PubMed]
- Heyden, B.P.V.D.; Roychoudhury, A.N. Application, chemical interaction and fate of iron minerals in polluted sediment and soils. Curr. Pollut. Rep. 2015, 1, 265–279. [Google Scholar] [CrossRef]
- Burford, J.R.; Bremner, J.M. Relationships between denitrification capacities of soils and total, water-soluble and readily decomposable soil organic matter. Soil Biol. Biochem. 1975, 7, 389–394. [Google Scholar] [CrossRef]
- Lovley, D.R.; Phillips, E.J.P. Organic matter mineralization with reduction of ferric iron in anaerobic sediments. Appl. Environ. Microbiol. 1986, 51, 683–689. [Google Scholar] [CrossRef]
- Roden, E.E.; Zachara, J.M. Microbial reduction of crystalline iron(III) oxides: Influence of oxide surface area and potential for cell growth. Environ. Sci. Technol. 1996, 30, 1618–1628. [Google Scholar] [CrossRef]
- Sahrawat, K.L. Ammonium production in submerged soils and sediments: The role of reducible iron. Commun. Soil Sci. Plan. 2004, 35, 399–411. [Google Scholar] [CrossRef]
- Wagai, R.; Mayer, L.M. Sorptive stabilization of organic matter in soils by hydrous iron oxides. Geochim. Cosmochim. Acta 2006, 71, 25–35. [Google Scholar] [CrossRef]
- Lalonde, K.; Mucci, A.; Ouellet, A.; Gélinas, Y. Preservation of organic matter in sediments promoted by iron. Nature 2012, 483, 198–200. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Zhu-Barker, X.; Horwath, W.R.; Faeflen, S.J.; Luo, H.; Xin, X.; Jiang, X. Effect of iron oxide on nitrification in two agricultural soils with different pH. Biogeosciences 2016, 13, 5609–5617. [Google Scholar] [CrossRef]
- Zhu, X.; Silva, L.C.; Doane, T.A.; Horwath, W.R. Iron: The forgotten driver of nitrous oxide production in agricultural soil. PLoS ONE 2013, 8, e60146. [Google Scholar] [CrossRef] [PubMed]
- Audet, J.; Elsgaard, L.; Kjaergaard, C.; Larsen, S.E.; Hoffmann, C.C. Greenhouse gas emissions from a Danish riparian wetland before and after restoration. Ecol. Eng. 2013, 57, 170–182. [Google Scholar] [CrossRef]
- Wu, Y.; Wen, Y.; Zhou, J.; Wu, Y. Phosphorus release from lake sediments: Effects of pH, temperature and dissolved oxygen. KSCE J. Civ. Eng. 2013, 18, 323–329. [Google Scholar] [CrossRef]
- Andersen, F.Ø.; Jensen, H.S. The influence of chironomids on decomposition of organic matter and nutrient exchange in lake sediment. Verh. Int. Ver. Theor. Angew. Limnol. 1991, 24, 2055–3051. [Google Scholar] [CrossRef]
- Bengtsson, L.; Hellström, T. Wind-induced resuspension in a small shallow lake. Hydrobiologia 1992, 241, 163–172. [Google Scholar] [CrossRef]
- Hernandez, M.E.; Mitsch, W.J. Denitrification potential and organic matter as affected by vegetation community, wetland age, and plant introduction in created wetlands. J. Environ. Qual. 2007, 6, 333–342. [Google Scholar] [CrossRef]
- Reitzel, K.; Lotter, S.; Dubke, M.; Egemose, S.; Jensen, H.S.; Andersen, F.Ø. Effects of Phoslock® treatment and chironomids on the exchange of nutrients between sediment and water. Hydrobiologia 2012, 703, 189–202. [Google Scholar] [CrossRef]
- Søndergaard, M.; Jeppesen, E.; Jensen, J.; Bradshaw, E.; Skovgaard, H.; Grünfeld, S. Vandrammedirektivet og Danske Søer: Del 1: Søtyper, Referencetilstand og Økologiske Kvalitetsklasser. DMU nr. 475; National Center for Environment and Energy, University of Aarhus: Aarhus, Denmark, 2003. [Google Scholar]
- Ofoegbu, G.I.; Read, R.S.; Ferrante, F. Bulking Factor of Rock for Underground Openings; U.S. Nuclear Regulatory Commission: Rockville, MD, USA, 2008.
- Espensen, B.L.; Goldberg, C.; Jakobsen, E.M.; Lorentzen, C.; Thaysen, J.N.; Christensen, M.; Bojsen, T. Katalog over Omkostninger ved Etablering af Erstatningsnatur; Danish Environmental Agency, Orbicon: Copenhagen, Denmark, 2018. [Google Scholar]
- Jørgensen, B.B.; Callesen, I.; Vesterdal, L.; Riis-Nielsen, T. Reolpløjning ved Skovrejsning på Sandet Landbrugsjord: Langsigtede Effekter på Vækst, Rodudvikling og Bundflora—IGN Rapport; Institute of Geoscience and Nature management, University of Copenhagen: Copenhagen, Denmark, 2017. [Google Scholar]


| Lake Characteristics | Soil Characteristics | ||
|---|---|---|---|
| Lake area (ha)/Catchment (ha) | 7.9 1/270 | Dry weight (%) | 81.7 ± 2.6 5 |
| Volume (m3)/Residence time (month) | 118,000 1/6 1 | Loss on ignition (%) | 5.1 ± 0.7 5 |
| Max depth (m)/Secchi depth (m) | 3.5 1/1.3 ± 0.4 2 | Density (g·cm−3) | 1.97 ± 0.06 5 |
| O2 (mg·L−1)/Color (mgPt·L−1) | 8.6 ± 5.6 3/99 ± 29 2 | TP (mgP·gDW−1) | 0.62 ± 0.09 5 |
| pH/Alkalinity (meq·L−1) | 8.2 ± 0.6 3/3.7 ± 0.6 2 | P-number (mgP·100gDW−1) | 1.9 ± 0.5 5 |
| Chl-a (µg·L−1)/DOC (mg·L−1) | 12.6 ± 15.3 2/16 ± 3 2 | TFe (mgFe·gDW−1) | 11.9 ± 0.5 5 |
| SO42− (mg·L−1) | 15.4 ± 5.4 4 | TAl (mgAl·gDW−1) | 11.9 ± 0.6 5 |
| TP (µg·L−1)/DIP (µg·L−1) | 80 ± 70 2/10 ± 10 2 | Molar TFe:TP | 10.8 ± 1.5 5 |
| NO3− (µg·L−1)/NH4+ (µg·L−1) | 40 ± 80 2/50 ± 40 2 | Soil type | Clay soil 6 |
| Flux | Treatments (mg·m−2·d−1) | ||||
|---|---|---|---|---|---|
| Control | CFH-12 | Sand-Capping | Depth-Plowing | ||
| DIP | Inundated | 1.8 ± 1.9 a,x | −0.4 ± 0.6 a,x | −0.4 ± 1.2 a,x | −1.0 ± 0.4 a,x |
| Flooding | −0. 1 ± 0.2 a,x | <−0.01 a,x | <-0.01 a,x | <−0.01 a,x | |
| NO3− | Inundated | −2.6 ± 3.2 a,x | −10.9 ± 6.4 ab,x | −5.0 ± 6.8 ab,x | −10.8 ± 1.5 b,x |
| Flooding | −7.8 ± 2.1 a,x | −8.3 ± 5.7 a,x | −9.6 ± 0.3 a,x | −7.3 ± 1.9 a,x | |
| NH4+ | Inundated | 4.7 ± 14.0 a,x | 39.6 ± 42.9 ab,x | 18.7 ± 28.2 a x | 74.9 ± 3.3 b,x |
| Flooding | 0.3 ± 0.5 ab,x | 2.8 ± 2.3 ab,x | −0.1 ± 0.1 a,x | <−0.01 b,y | |
| DFe | Inundated | 3.7 ± 8.3 a,x | 18.4 ± 15.1 ab,x | 5.4 ± 13.9 a,x | 36.2 ± 8.2 b,x |
| Flooding | 0.2 ± 0.2 a,x | 3.8 ± 6.8 a,x | −0.1 ± 0.1 a,x | −0.1 ± 0.1 a,y | |
| Method | Costs (EUR·ha−1) |
|---|---|
| Depth-plowing 1,2 | 900 |
| Sand-capping 1,2 | 40,200 |
| CFH-12 3,4 | 67,000 |
| Removal of topsoil 1,2 | 502,800 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kolath, T.; Reuss, L.; Egemose, S.; Reitzel, K. Reduction of Internal Phosphorus Load in New Lakes by Pretreatment of the Former Agricultural Soil—Methods, Ecological Results and Costs. Sustainability 2020, 12, 3575. https://doi.org/10.3390/su12093575
Kolath T, Reuss L, Egemose S, Reitzel K. Reduction of Internal Phosphorus Load in New Lakes by Pretreatment of the Former Agricultural Soil—Methods, Ecological Results and Costs. Sustainability. 2020; 12(9):3575. https://doi.org/10.3390/su12093575
Chicago/Turabian StyleKolath, Thor, Lotte Reuss, Sara Egemose, and Kasper Reitzel. 2020. "Reduction of Internal Phosphorus Load in New Lakes by Pretreatment of the Former Agricultural Soil—Methods, Ecological Results and Costs" Sustainability 12, no. 9: 3575. https://doi.org/10.3390/su12093575
APA StyleKolath, T., Reuss, L., Egemose, S., & Reitzel, K. (2020). Reduction of Internal Phosphorus Load in New Lakes by Pretreatment of the Former Agricultural Soil—Methods, Ecological Results and Costs. Sustainability, 12(9), 3575. https://doi.org/10.3390/su12093575






