Economic Gain vs. Ecological Pain—Environmental Sustainability in Economies Based on Renewable Biological Resources
Abstract
1. Introduction
2. Biomass Production in Different Ecosystems
2.1. Global Biomass
2.2. Terrestrial Systems
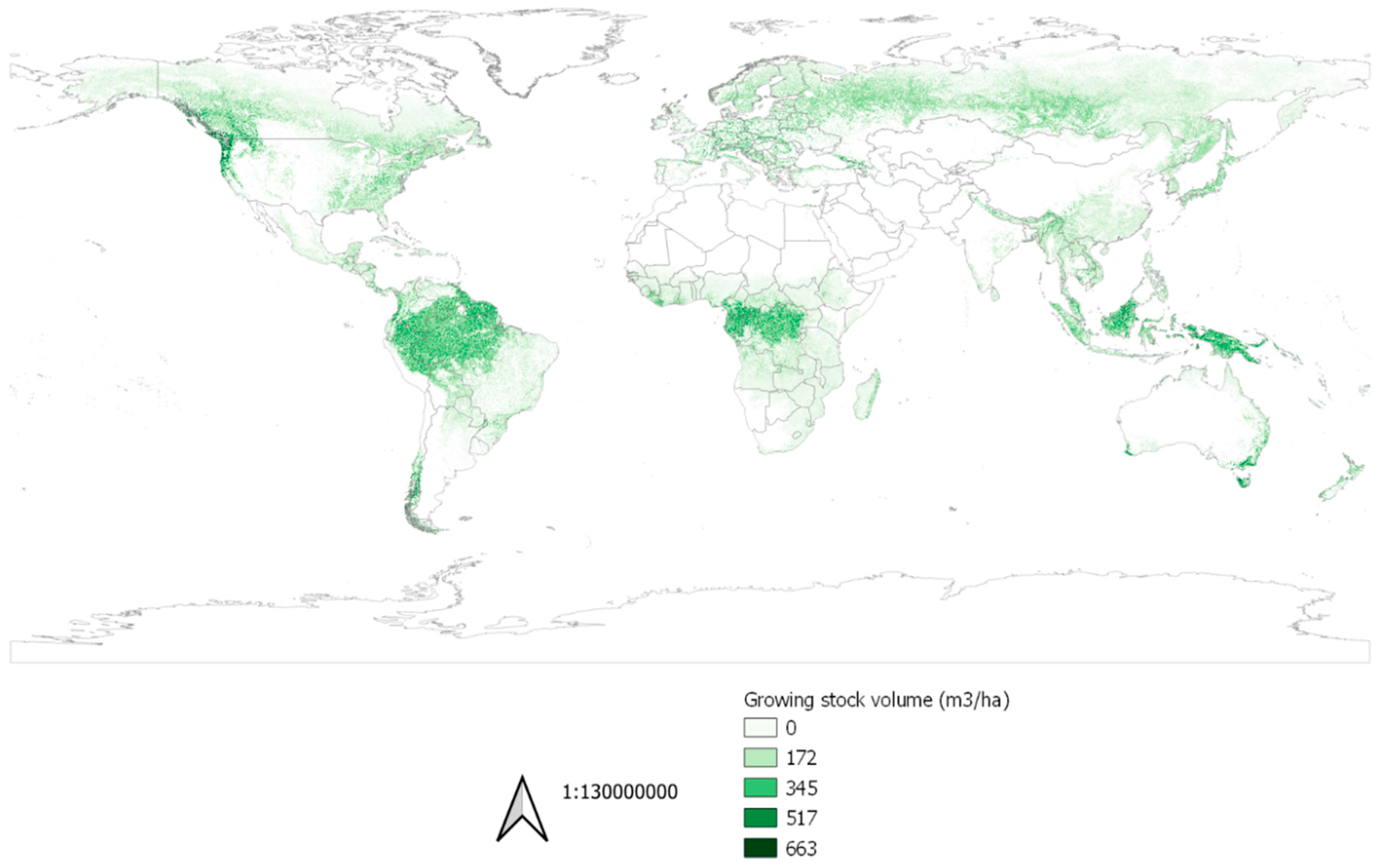
2.2.1. Forest Production
2.2.2. Non-timber Forest Products
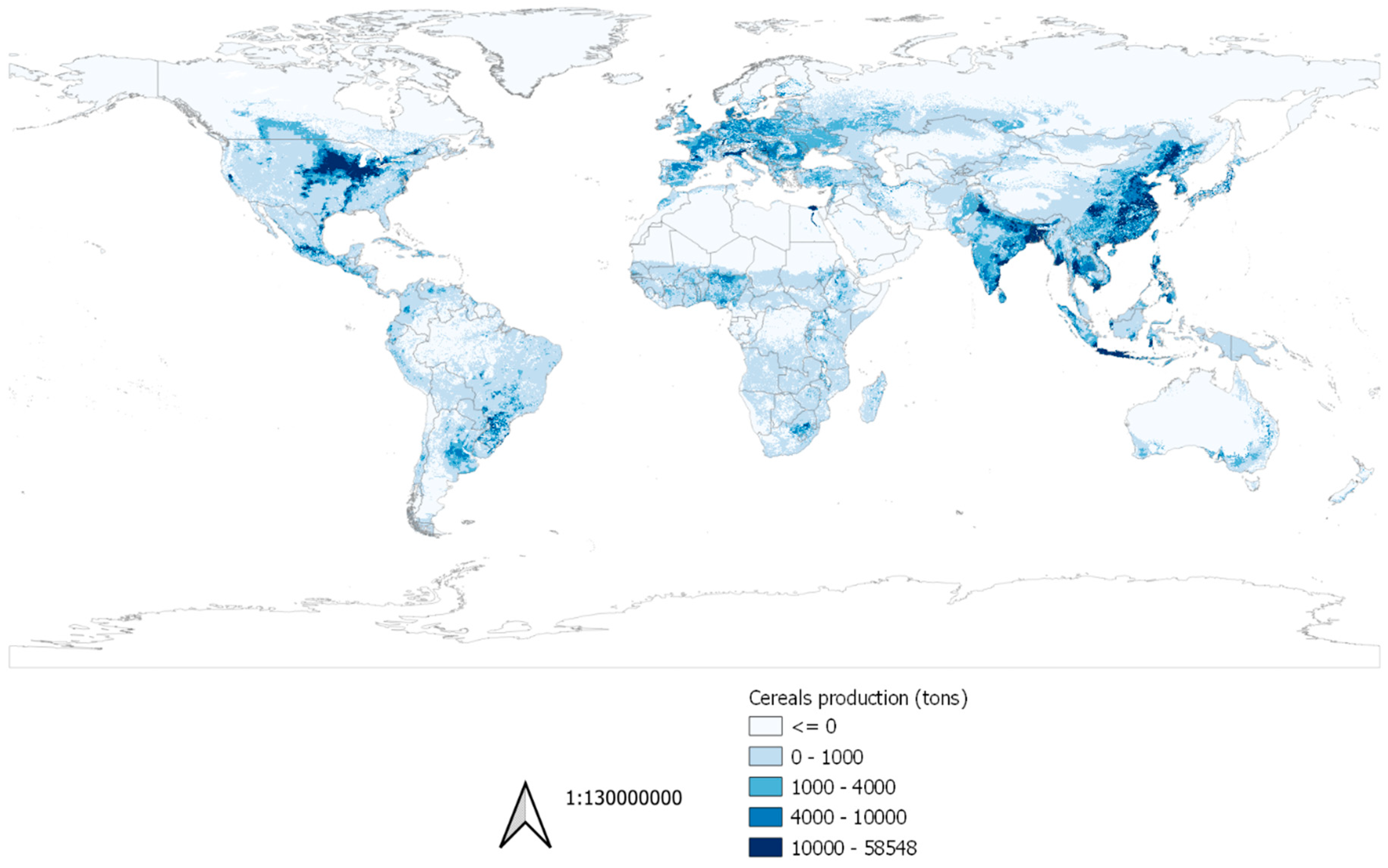
2.2.3. Crops Production
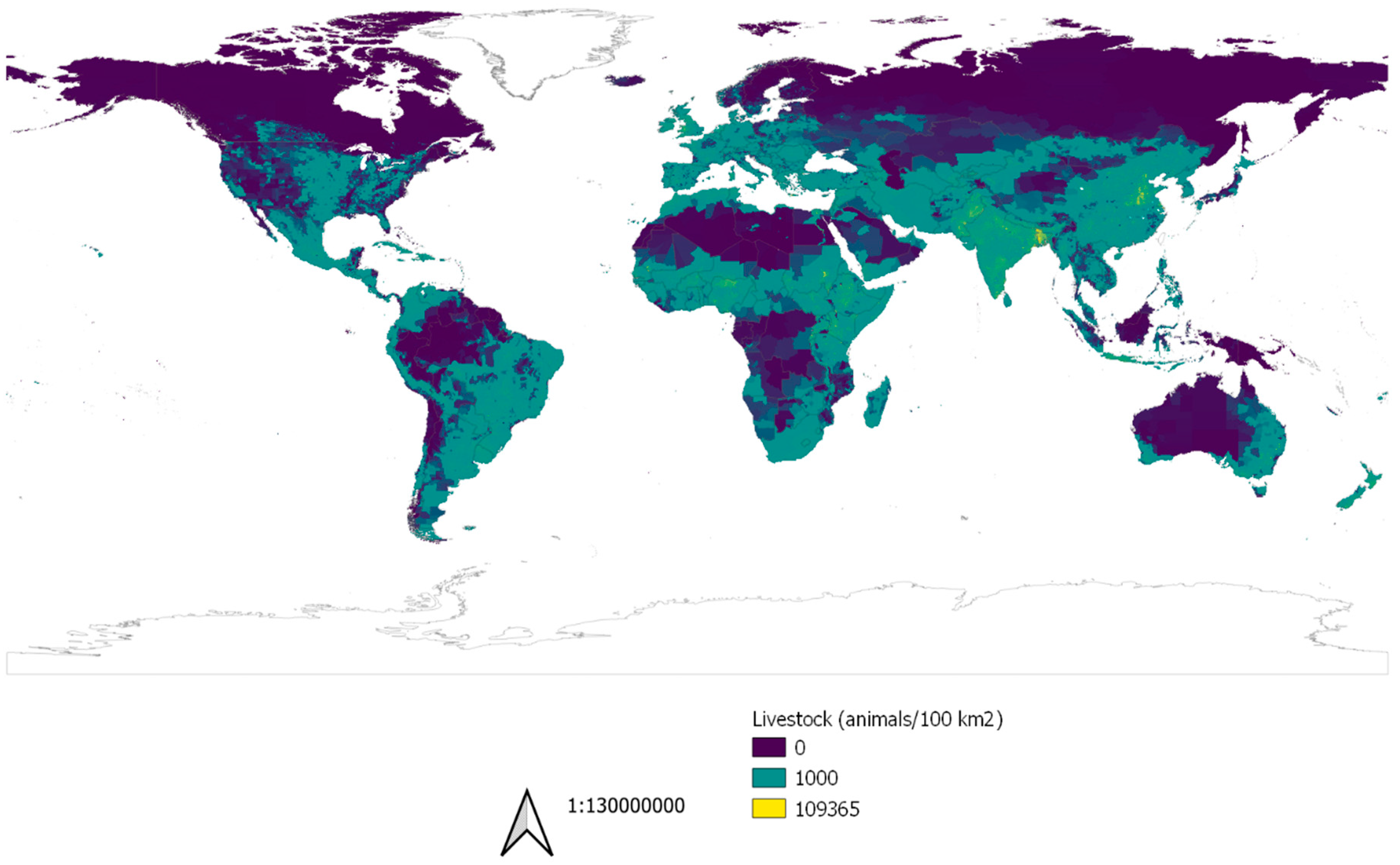
2.2.4. Livestock Production
2.3. Marine and Freshwater Systems
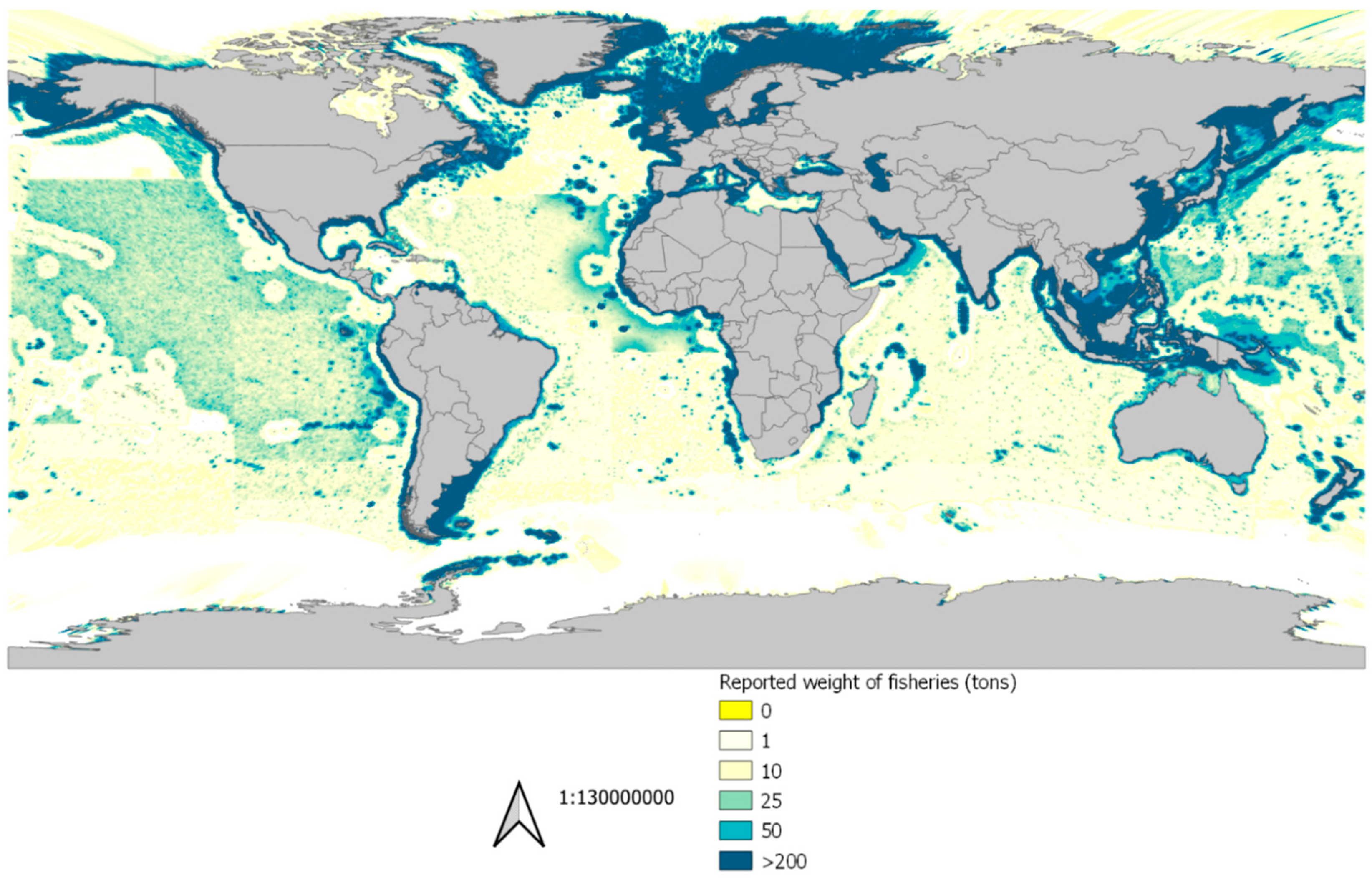
2.3.1. Marine Production
2.3.2. Freshwater Production
2.3.3. Aquaculture Production
3. Bioeconomy Strategies and the Economic Gain and Ecological Pain Model
3.1. Bioeconomy Strategies
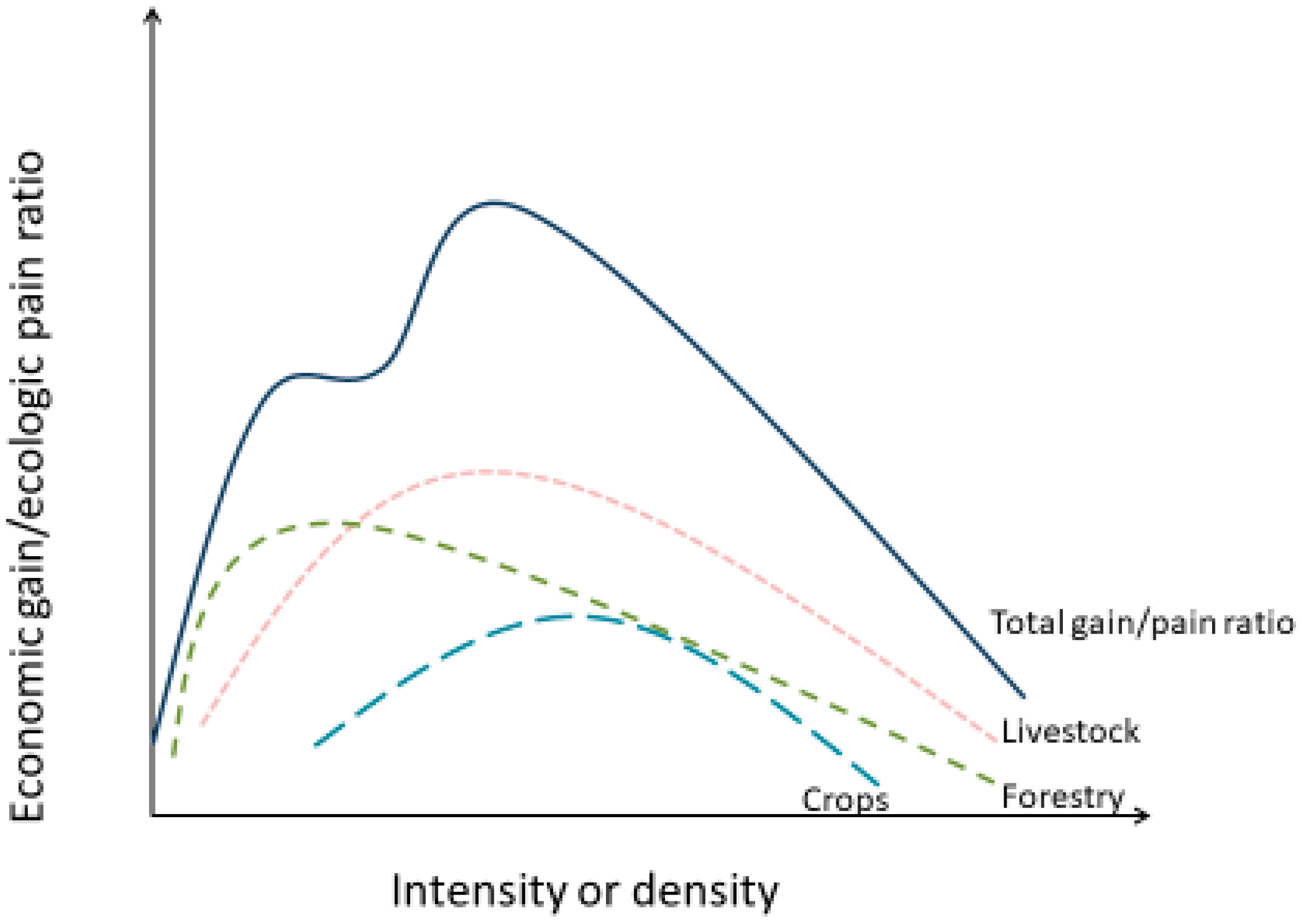
3.2. The Economic Gain and Ecological Pain Model
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- European Commission. Innovating for Sustainable Growth: A Bioeconomy for Europe. Communication from the Commission to the European Parliament, the Council, the European Economic and Social Committee and the Committee of the Regions. 2012. Available online: https://op.europa.eu/en/publication-detail/-/publication/1f0d8515-8dc0-4435-ba53-9570e47dbd51/language-en (accessed on 10 February 2020).
- Ripple, W.J.; Wolf, C.; Newsome, T.M.; Galetti, M.; Alamgir, M.; Crist, E.; Mahmoud, M.I.; Laurance, W.F.; 15,364 scientist signatories from 184 countries. World scientists’ warning to humanity: A second notice. BioScience 2017, 67, 1026–1028. [Google Scholar] [CrossRef]
- de Schutter, L.; Giljum, S.; Häyhä, T.; Bruckner, M.; Naqvi, A.; Omann, I.; Stagl, S. Bioeconomy Transitions through the Lens of Coupled Social-Ecological Systems: A Framework for Place-Based Responsibility in the Global Resource System. Sustainability 2019, 11, 5705. [Google Scholar] [CrossRef]
- Costanza, R.; d’Arge, R.; De Groot, R.; Farber, S.; Grasso, M.; Hannon, B.; Limburg, K.; Naeem, S.; O’neill, R.V.; Paruelo, J. The value of the world’s ecosystem services and natural capital. Nature 1997, 387, 253–260. [Google Scholar] [CrossRef]
- Martínez, A.C.; García-Llorente, M.; Martín-López, B.; Palomo, I.; Iniesta-Arandia, I. Multidimensional approaches in ecosystem services assessment. Earth Obs. Ecosyst. Serv. 2013, 441, 441–468. [Google Scholar]
- WTTC. World Travel and Tourism Council. Economic Impact of Travel and Tourism 2014 Annual Update: Summary. 2014. Available online: http://www.ontit.it/opencms/export/sites/default/ont/it/documenti/files/ONT_2014-03-31_03008.pdf (accessed on 15 April 2020).
- SWD. Biodiversity Strategy. European Commision, 2011. 2012. Available online: http://ec.europa.eu/environment/nature/biodiversity/strategy/index_en.htm (accessed on 10 February 2020).
- European Commission. A Sustainable Bioeconomy for Europe: Strengthening the Connection between Economy, Society and the Environment; European Commission: Brussels, Belgium, 2018. [Google Scholar] [CrossRef]
- Piotrowski, S.; Carus, M.; Essel, R. Global bioeconomy in the conflict between biomass supply and demand. Ind. Biotechnol. 2015, 11, 308–315. [Google Scholar] [CrossRef]
- UNEP. The United Nations Convension on Biological Diversity; UNEP: Ginebra, Switzerland, 1992. [Google Scholar]
- Laurila-Pant, M.; Lehikoinen, A.; Uusitalo, L.; Venesjärvi, R. How to value biodiversity in environmental management? Ecol. Indic. 2015, 55, 1–11. [Google Scholar] [CrossRef]
- Bastille-Rousseau, G.; Schaefer, J.A.; Peers, M.J.L.; Ellington, E.H.; Mumma, M.A.; Rayl, N.D.; Mahoney, S.P.; Murray, D.L. Climate change can alter predator-prey dynamics and population viability of prey. Oecologia 2018, 186, 141–150. [Google Scholar] [CrossRef]
- Bar-On, Y.M.; Phillips, R.; Milo, R. The biomass distribution on Earth. Proc. Natl. Acad. Sci. USA 2018, 115, 6506–6511. [Google Scholar] [CrossRef]
- Groombridge, B.; Jenkins, M.D. Global Biodiversity: Earth’s Living Resources in the 21st Century; World Conservation Monitoring Centre: Cambridge, UK, 2000. [Google Scholar]
- Wilson, R.; Millero, F.J.; Taylor, J.; Walsh, P.; Christensen, V.; Jennings, S.; Grosell, M. Contribution of fish to the marine inorganic carbon cycle. Science 2009, 323, 359–362. [Google Scholar] [CrossRef]
- Whitman, W.B.; Coleman, D.C.; Wiebe, W.J. Prokaryotes: The unseen majority. Proc. Natl. Acad. Sci. USA 1998, 95, 6578–6583. [Google Scholar] [CrossRef]
- The World Bank. Biodiversity Conservation in Forest Ecosystems—World Bank Assistance, 1992–2002; The World Bank Group: Washington, DC, USA, 2002. [Google Scholar]
- FAO. Global Forest Resources Assessment 2010: Main Report; Food and Agriculture Organization: Rome, Italy, 2010; Available online: http://www.fao.org/3/a-i1757e.pdf (accessed on 15 April 2020).
- TEEB. The Economics of Ecosystems and Biodiversity. TEEB for Business. 2009. Available online: http://www.teebweb.org/wp-content/uploads/Study%20and%20Reports/Reports/Business%20and%20Enterprise/TEEB%20for%20Business%20Report/TEEB%20for%20Business.pdf (accessed on 15 April 2020).
- Santoro, M. GlobBiomass—Global datasets of forest biomass. PANGAEA10 2018, 1594. [Google Scholar] [CrossRef]
- Ranius, T.; Roberge, J.-M. Effects of intensified forestry on the landscape-scale extinction risk of dead wood dependent species. Biodivers. Conserv. 2011, 20, 2867–2882. [Google Scholar] [CrossRef]
- Naumov, V.; Manton, M.; Elbakidze, M.; Rendenieks, Z.; Priednieks, J.; Uhlianets, S.; Yamelynets, T.; Zhivotov, A.; Angelstam, P. How to reconcile wood production and biodiversity conservation? The Pan-European boreal forest history gradient as an “experiment”. J. Environ. Manag. 2018, 218, 1–13. [Google Scholar] [CrossRef]
- Food and Agriculture Organization of the United Nations. State of the World’s Forests 2016: Forests and Agriculture: Land-Use Challenges and Opportunities; FAO Report; FAO: Rome, Italy, 2016. [Google Scholar]
- Dijkman, W.; Boot, R.G.; Zuidema, P.A. Dynamics and Sustainability of Non-Timber Forest Products Extraction; Seminar Proceedings NTFP Research in the Tropenbos Programme: Results and Perspectives; Tropenbos: Wageningen, The Netherlands, 1999; pp. 169–173. [Google Scholar]
- Ros, T.; Dijkman, W.; van Bueren, E.L. Commercial and Sustainable Extraction of Non-Timber Forest Products: Towards a Policy and Management Oriented Research Strategy; Tropenbos Foundation: Wageningen, The Netherlands, 1995. [Google Scholar]
- FAO. State of the World’s Forests; Food and Agriculture Organization: Rome, Italy, 1997; Available online: http://www.fao.org/3/w4345e/w4345e00.htm (accessed on 15 March 2020).
- Reid, W.V.; Mooney, H.A.; Cropper, A.; Capistrano, D.; Carpenter, S.R.; Chopra, K.; Dasgupta, P.; Dietz, T.; Duraiappah, A.K.; Hassan, R. Ecosystems and Human Well-Being-Synthesis: A Report of the Millennium Ecosystem Assessment; Island Press: Washington, DC, USA, 2005. [Google Scholar]
- Ros-Tonen, M.A. The role of non-timber forest products in sustainable tropical forest management. Holz Als Roh-Und Werkst. 2000, 58, 196–201. [Google Scholar] [CrossRef]
- Chamberlain, J.L.; Bush, R.J.; Hammett, A.; Araman, P.A. Managing national forests of the eastern United States for non-timber forest products. In Proceedings of the XXI IUFRO World Congress 2000, Forests and Society: The Role of Research 1, Kuala Lumpur, Malaysia, 7–12 August 2000; pp. 407–420. [Google Scholar]
- Arnold, J.M.; Pérez, M.R. Can non-timber forest products match tropical forest conservation and development objectives? Ecol. Econ. 2001, 39, 437–447. [Google Scholar] [CrossRef]
- Ros-Tonen, M. Forest products—Non-timber. Berkshire Encyclopedia of Sustainability. Vol. 4: Natural Resources and Sustainability; Berkshire Publishing: Great Barrington, MA, USA, 2011; pp. 163–167. [Google Scholar]
- Chediack, S.E. The effect of forest exploitation on structure, diversity, and floristic composition of palmito-dominated Atlantic forests at Misiones, Argentina. Rev. Biol. Trop. 2008, 56, 721–738. [Google Scholar]
- Wollenberg, E.; Ingles, A. Incomes from the Forest: Methods for the Development and Conservation of Forest Products for Local Communities; Cifor: Bogor, Indonesia, 1998. [Google Scholar]
- Wickens, G.E. Management issues for development of non-timber forest products. Unasylva 1991, 42, 3–8. [Google Scholar]
- Apollonio, M.; Andersen, R.; Putman, R. European Ungulates and Their Management in the 21st Century; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2010. [Google Scholar]
- Milner, J.M.; Bonenfant, C.; Mysterud, A.; GAILLARD, J.M.; Csányi, S.; Stenseth, N.C. Temporal and spatial development of red deer harvesting in Europe: Biological and cultural factors. J. Appl. Ecol. 2006, 43, 721–734. [Google Scholar] [CrossRef]
- Bennett, E.L.; Robinson, J.G. Hunting of Wildlife in Tropical Forests: Implications for Biodiversity and Forest Peoples. FAO, Open Knowledge Repository, OKR. 2000. Available online: http://agris.fao.org/agris-search/search.do?recordID=US2014603031 (accessed on 10 February 2020).
- Bleier, N.; Biró, Z.; Galló, J.; Szemethy, L.; Csányi, S. Trend in game meat production and consumption in Hungary over the period 1970–2012. In Proceedings of the 2nd International Symposium on Hunting, Novi Sad, Serbia, 17–20 October 2013; pp. 17–20. [Google Scholar]
- Gangås, K.E.; Kaltenborn, B.P.; Andreassen, H.P. Global differences in the motivation for illegal hunting. Nat. Conserv. 2020. in review. [Google Scholar]
- Milner-Gulland, E.J.; Bennett, E.L. Wild meat: The bigger picture. Trends Ecol. Evol. 2003, 18, 351–357. [Google Scholar] [CrossRef]
- FAO. World Agriculture: Towards 2015/30—An FAO Perspective; Food and Agriculture Organization: Rome, Italy, 2010. [Google Scholar]
- Clay, D.E.; Clay, S.A.; Reitsma, K.D.; Dunn, B.H.; Smart, A.J.; Carlson, G.G.; Horvath, D.; Stone, J.J. Does the conversion of grasslands to row crop production in semi-arid areas threaten global food supplies? Glob. Food Secur. 2014, 3, 22–30. [Google Scholar] [CrossRef]
- Monfreda, C.; Ramankutty, N.; Foley, J.A. Farming the planet: 2. Geographic distribution of crop areas, yields, physiological types, and net primary production in the year 2000. Glob. Biogeochem. CY 2008, 22. [Google Scholar] [CrossRef]
- Jones, P.G.; Thornton, P.K. The potential impacts of climate change on maize production in Africa and Latin America in 2055. Glob. Environ. Chang. 2003, 13, 51–59. [Google Scholar] [CrossRef]
- Fischer, G.; Shah, M.; van Velthuizen, H.; Nachtergaele, F.O. Global Agro-Ecological Assessment for Agriculture in the 21st Century; IIASA Research Repoort 02-02; International Institute for Applied Systems Analysis: Laxenburg, Austria, 2001; p. 119. [Google Scholar]
- Parry, M.E. Assessment of Potential Effects and Adaptations for Climate Change in Europe—The Europe Acacia Project; Jackson Environment Institute, University of East Anglia: Norwich, UK, 2000. [Google Scholar]
- Parry, M.; Rosenzweig, C.; Iglesias, A.; Fischer, G.; Livermore, M. Climate change and world food security: A new assessment. Glob. Environ. Chang. 1999, 9, S51–S67. [Google Scholar] [CrossRef]
- Easterling, D.R.; Meehl, G.A.; Parmesan, C.; Changnon, S.A.; Karl, T.R.; Mearns, L.O. Climate extremes: Observations, modeling, and impacts. Science 2000, 289, 2068–2074. [Google Scholar] [CrossRef]
- IPCC. Climate Change 2001: The Scientific Basis; Contribution of Working Group I to the Third Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2001. [Google Scholar]
- IPCC. Climate Change 2001: Impacts, Adaptation and Vulnerability; Contribution of Working Group II to the Third Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2001. [Google Scholar]
- OECD. OECD Environmental Outlook to 2020; OECD: Paris, France, 2001. [Google Scholar] [CrossRef]
- IPCC. Climate Change 2001: Synthesis Report; A Contribution of Working Groups I, II, and III to the Third Assessment Report of the Integovernmental Panel on Climate Change; Watson, R.T., the Core Writing Team, Eds.; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2001; p. 398. [Google Scholar]
- FAO. Food and Agriculture Organization of the United Nations. Livestock and the Environment. 2020. Available online: http://www.fao.org/livestock-environment/en/ (accessed on 15 April 2020).
- Steinfeld, H.; Gerber, P.; Wassenaar, T.; Castel, V.; Rosales, M.; Rosales, M.; de Haan, C. Livestock’s Long Shadow: Environmental Issues and Options; Food & Agriculture Org.: Rome, Italy, 2006. [Google Scholar]
- Sukhdev, P.; Wittmer, H.; Schröter-Schlaack, C.; Nesshöver, C.; Bishop, J.; Brink, P.t.; Gundimeda, H.; Kumar, P.; Simmons, B. The Economics of Ecosystems and Biodiversity: Mainstreaming the Economics of Nature: A Synthesis of the Approach, Conclusions and Recommendations of TEEB; UNEP: Ginebra, Switzerland, 2010. [Google Scholar]
- McDonald, P.; Edwards, R.A.; Greenhalgh, J.F.D.; Sinclair, L.A.; Wilkinson, R.G. Animal Nutrition; Pearson Education Unlimited: Essex, UK, 2011; p. 672. ISBN 9781408204238. [Google Scholar]
- Gilbert, M.; Nicolas, G.; Cinardi, G.; van Boeckel, T.P.; Vanwambeke, S.; Wint, W.G.R.; Robinson, T.P. Global Cattle Distribution in 2010 (5 Minutes of arc); Harvard Dataverse, V3; 2018. [Google Scholar] [CrossRef]
- Gilbert, M.; Nicolas, G.; Cinardi, G.; van Boeckel, T.P.; Vanwambeke, S.; Wint, W.G.R.; Robinson, T.P. Global Goats Distribution in 2010 (5 Minutes of arc); Harvard Dataverse, V3; 2018. [Google Scholar] [CrossRef]
- Gilbert, M.; Nicolas, G.; Cinardi, G.; van Boeckel, T.P.; Vanwambeke, S.; Wint, W.G.R.; Robinson, T.P. Global Horses Distribution in 2010 (5 Minutes of arc); Harvard Dataverse, V3; 2018. [Google Scholar] [CrossRef]
- Gilbert, M.; Nicolas, G.; Cinardi, G.; van Boeckel, T.P.; Vanwambeke, S.; Wint, W.G.R.; Robinson, T.P. Global Sheep Distribution in 2010 (5 Minutes of arc); Harvard Dataverse, V3; 2018. [Google Scholar] [CrossRef]
- Kaldy, M. Protein yield of various crops as related to protein value. Econ. Bot. 1972, 26, 142–144. [Google Scholar] [CrossRef]
- Hoekstra, A.Y. Virtual Water Trade: Proceedings of the International Expert Meeting on Virtual Water Trade, Delft, The Netherlands, 12–13 December 2002; Value of Water Research Report Series No. 12; UNESCO-IHE: Delft, The Netherlands, 2003. [Google Scholar]
- Toutain, B.; Ickowicz, A.; Dutilly-Diane, C.; Reid, R.; Diop, A.T.; Taneja, V.K.; Gibon, A.; Genin, D.; Ibrahim, M.; Behnke, R. Impacts of livestock systems on terrestrial ecosystems. In Livestock in a Changing Landscape, Volume 1: Drivers, Consequences, and Responses; Bibliovault OAI Repository; The University of Chicago Press: Chicago, IL, USA, 2010; p. 1165. [Google Scholar]
- Steinfeld, H.; Gerber, P. Livestock production and the global environment: Consume less or produce better? Proc. Natl. Acad. Sci. USA 2010, 107, 18237–18238. [Google Scholar] [CrossRef]
- Thornton, P.K. Livestock production: Recent trends, future prospects. Philos. Trans. R. Soc. B Biol. Sci. 2010, 365, 2853–2867. [Google Scholar] [CrossRef]
- Reid, R.; Bedelian, C.; Said, M.; Kruska, R.; Mauricio, R.; Castel, V.; Olson, J.; Thornton, P. Global Livestock Impacts on Biodiversity. In Livestock in a Changing Landscape. Drivers, Consequences, and Responses; Steinfeld, H., Mooney, H.A., Schneider, F., Neville, L.E., Eds.; Island Press: Washington, DC, USA, 2010; Volume 1, pp. 111–138. [Google Scholar]
- Hampicke, U.; Plachter, H. Livestock grazing and nature conservation objectives in Europe. In Large-Scale Livestock Grazing: A Management Tool for Nature Conservation; Plachter, H., Hampicke, U., Eds.; Springer-Verlag: Berlin, Germany, 2010; pp. 2–25. [Google Scholar]
- Beaumont, N.; Austen, M.; Atkins, J.; Burdon, D.; Degraer, S.; Dentinho, T.; Derous, S.; Holm, P.; Horton, T.; Van Ierland, E. Identification, definition and quantification of goods and services provided by marine biodiversity: Implications for the ecosystem approach. Mar. Pollut. Bull. 2007, 54, 253–265. [Google Scholar] [CrossRef]
- Waycott, M.; Duarte, C.M.; Carruthers, T.J.; Orth, R.J.; Dennison, W.C.; Olyarnik, S.; Calladine, A.; Fourqurean, J.W.; Heck, K.L.; Hughes, A.R. Accelerating loss of seagrasses across the globe threatens coastal ecosystems. Proc. Natl. Acad. Sci. USA 2009, 106, 12377–12381. [Google Scholar] [CrossRef]
- FAO. The State of World Fisheries and Aquaculture-2012; Food and agriculture organization of the United Nations: Rome, Italy, 2012. [Google Scholar]
- Watson, R.A. A database of global marine commercial, small-scale, illegal and unreported fisheries catch 1950–2014. Sci. Data 2017, 4, 170039. [Google Scholar] [CrossRef]
- Godfray, H.C.J.; Beddington, J.R.; Crute, I.R.; Haddad, L.; Lawrence, D.; Muir, J.F.; Pretty, J.; Robinson, S.; Thomas, S.M.; Toulmin, C. Food security: The challenge of feeding 9 billion people. Science 2010, 327, 812–818. [Google Scholar] [CrossRef]
- Merino, G.; Barange, M.; Blanchard, J.L.; Harle, J.; Holmes, R.; Allen, I.; Allison, E.H.; Badjeck, M.C.; Dulvy, N.K.; Holt, J. Can marine fisheries and aquaculture meet fish demand from a growing human population in a changing climate? Glob. Environ. Chang. 2012, 22, 795–806. [Google Scholar] [CrossRef]
- Barbier, E.B. Progress and challenges in valuing coastal and marine ecosystem services. Rev. Environ. Econ. Policy 2012, 6, 1–19. [Google Scholar] [CrossRef]
- Lotze, H.K.; Lenihan, H.S.; Bourque, B.J.; Bradbury, R.H.; Cooke, R.G.; Kay, M.C.; Kidwell, S.M.; Kirby, M.X.; Peterson, C.H.; Jackson, J.B. Depletion, degradation, and recovery potential of estuaries and coastal seas. Science 2006, 312, 1806–1809. [Google Scholar] [CrossRef]
- Worm, B.; Hilborn, R.; Baum, J.K.; Branch, T.A.; Collie, J.S.; Costello, C.; Fogarty, M.J.; Fulton, E.A.; Hutchings, J.A.; Jennings, S. Rebuilding global fisheries. Science 2009, 325, 578–585. [Google Scholar] [CrossRef]
- WWF. Americas Regional Process Event. Water for our Future. 2014. Available online: https://c402277.ssl.cf1.rackcdn.com/publications/740/files/original/10-20_World_Water_Forum_WP_FIN_15_122.pdf?1416335200 (accessed on 15 March 2020).
- Aylward, B.; Bandyopadhyay, J.; Belausteguigotia, J.-C.; Borkey, P.; Cassar, A.; Meadors, L.; Saade, L.; Siebentritt, M.; Stein, R.; Tognetti, S. Freshwater ecosystem services. Ecosyst. Hum. Well-Being Policy Responses 2005, 3, 213–256. [Google Scholar]
- FAO. The State of World Aquaculture and Fisheries 2006; Food and Agriculture Organization of the United Nations: Rome, Italy, 2007. [Google Scholar]
- IPCC. Climate Change 2007: Impacts, Adaptation and Vulnerability; Contribution of Working Group II to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change; Parry, M.L., Canziani, O.F., Palutikof, J.P., van der Linden, P.J., Hanson, C.E., Eds.; Cambridge University Press: Cambridge, UK, 2007; p. 976. [Google Scholar]
- UNESCO-WWAP. Water: A Shared Responsibility; the United Nations World Water Development Report 2. 2006. Available online: https://unesdoc.unesco.org/ark:/48223/pf0000145405 (accessed on 15 April 2020).
- Barbier, E.B. Sustainable use of wetlands valuing tropical wetland benefits: Economic methodologies and applications. Geogr. J. 1993, 159, 22–32. [Google Scholar] [CrossRef]
- UN-Water. Coping with Water Scarcity: A Strategic Issue and Priority for System-Wide Action. 2006. Available online: https://www.un.org/waterforlifedecade/pdf/2006_unwater_coping_with_water_scarcity_eng.pdf (accessed on 15 February 2020).
- Cada, G.; Sale, M.; Dauble, D. Environmental impact of hydropower. In Encyclopedia of Energy; Cleveland, J.C., Ed.; Elsevier: New York, NY, USA, 2004; Volume 3, pp. 291–300. [Google Scholar]
- Deutsch, L.; Gräslund, S.; Folke, C.; Troell, M.; Huitric, M.; Kautsky, N.; Lebel, L. Feeding aquaculture growth through globalization: Exploitation of marine ecosystems for fishmeal. Glob. Environ. Chang. 2007, 17, 238–249. [Google Scholar] [CrossRef]
- Naylor, R.L.; Eagle, J.; Smith, W.L. Salmon aquaculture in the Pacific Northwest a global industry with local impacts. Environ. Sci. Policy Sustain. Dev. 2003, 45, 18–39. [Google Scholar] [CrossRef]
- Focardi, S.; Corsi, I.; Franchi, E. Safety issues and sustainable development of European aquaculture: New tools for environmentally sound aquaculture. Aquac. Int. 2005, 13, 3. [Google Scholar] [CrossRef]
- Radziemska, M.; Mazur, Z. Effect of compost from by-product of the fishing industry on crop yield and microelement content in maize. J. Ecol. Eng. 2015, 16, 168–175. [Google Scholar] [CrossRef]
- Palm, H.W.; Knaus, U.; Appelbaum, S.; Goddek, S.; Strauch, S.M.; Vermeulen, T.; Jijakli, M.H.; Kotzen, B. Towards commercial aquaponics: A review of systems, designs, scales and nomenclature. Aquac. Int. 2018, 26, 813–842. [Google Scholar] [CrossRef]
- Alriksson, B.; Hörnberg, A.; Gudnason, A.E.; Knobloch, S.; Arnason, J.; Johannsson, R. Fish feed from wood. Cellul. Chem. Technol. 2014, 48, 843–848. [Google Scholar]
- Barnosky, A.D.; Matzke, N.; Tomiya, S.; Wogan, G.O.; Swartz, B.; Quental, T.B.; Marshall, C.; McGuire, J.L.; Lindsey, E.L.; Maguire, K.C. Has the Earth’s sixth mass extinction already arrived? Nature 2011, 471, 51–57. [Google Scholar] [CrossRef]
- Dirzo, R.; Raven, P.H. Global state of biodiversity and loss. Ann. Rev. Environ. Resour. 2003, 28, 137–167. [Google Scholar] [CrossRef]
- Wake, D.B.; Vredenburg, V.T. Are we in the midst of the sixth mass extinction? A view from the world of amphibians. Proc. Natl. Acad. Sci. USA 2008, 105, 11466–11473. [Google Scholar] [CrossRef]
- IPBES. Global Assessment Report on Biodiversity and Ecosystem Services; IPBES Secretariat: Bonn, Germany, 2019. [Google Scholar]
- Bugge, M.M.; Hansen, T.; Klitkou, A. What Is the Bioeconomy? A Review of the Literature. Sustainability 2016, 8, 691. [Google Scholar] [CrossRef]
- Bracco, S.; Calicioglu, O.; Gomez San Juan, M.; Flammini, A. Assessing the Contribution of Bioeconomy to the Total Economy: A Review of National Frameworks. Sustainability 2018, 10, 1698. [Google Scholar] [CrossRef]
- Egenolf, V.; Bringezu, S. Conceptualization of an Indicator System for Assessing the Sustainability of the Bioeconomy. Sustainability 2019, 11, 443. [Google Scholar] [CrossRef]
- Ramcilovic-Suominen, S.; Pülzl, H. Sustainable development—A ‘selling point’of the emerging EU bioeconomy policy framework? J. Clean. Prod. 2018, 172, 4170–4180. [Google Scholar] [CrossRef]
- UNECSO. Global Ocean Science Report—The Current Status of Ocean Science around the World; UNECSO: Paris, France, 2007. [Google Scholar]
- Hansen, M.C.; Potapov, P.V.; Moore, R.; Hancher, M.; Turubanova, S.A.; Tyukavina, A.; Thau, D.; Stehman, S.V.; Goetz, S.J.; Loveland, T.R.; et al. High-Resolution Global Maps of 21st-Century Forest Cover Change. Science 2013, 342, 850–853. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, A.; Verones, F.; de Baan, L.; Hellweg, S. Quantifying Land Use Impacts on Biodiversity: Combining Species–Area Models and Vulnerability Indicators. Environ. Sci. Technol. 2015, 49, 9987–9995. [Google Scholar] [CrossRef] [PubMed]
- Pauly, D. Anecdotes and the shifting baseline syndrome of fisheries. Trends Ecol. Evol. 1995, 10, 430. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pedersen, S.; Gangås, K.E.; Chetri, M.; Andreassen, H.P. Economic Gain vs. Ecological Pain—Environmental Sustainability in Economies Based on Renewable Biological Resources. Sustainability 2020, 12, 3557. https://doi.org/10.3390/su12093557
Pedersen S, Gangås KE, Chetri M, Andreassen HP. Economic Gain vs. Ecological Pain—Environmental Sustainability in Economies Based on Renewable Biological Resources. Sustainability. 2020; 12(9):3557. https://doi.org/10.3390/su12093557
Chicago/Turabian StylePedersen, Simen, Kristin E. Gangås, Madhu Chetri, and Harry P. Andreassen. 2020. "Economic Gain vs. Ecological Pain—Environmental Sustainability in Economies Based on Renewable Biological Resources" Sustainability 12, no. 9: 3557. https://doi.org/10.3390/su12093557
APA StylePedersen, S., Gangås, K. E., Chetri, M., & Andreassen, H. P. (2020). Economic Gain vs. Ecological Pain—Environmental Sustainability in Economies Based on Renewable Biological Resources. Sustainability, 12(9), 3557. https://doi.org/10.3390/su12093557





