Adsorption of Mercury from a Cyanide Leaching Solution Using Various Activation Rates of Granular Activated Carbon: A Laboratory- and Industrial-Scale Study
Abstract
1. Introduction
2. Experimental Procedures
2.1. Ore and Adsorbent
2.2. Laboratory Tests
2.3. Modeling
2.4. Industrial Scale Test
3. Results and Discussion
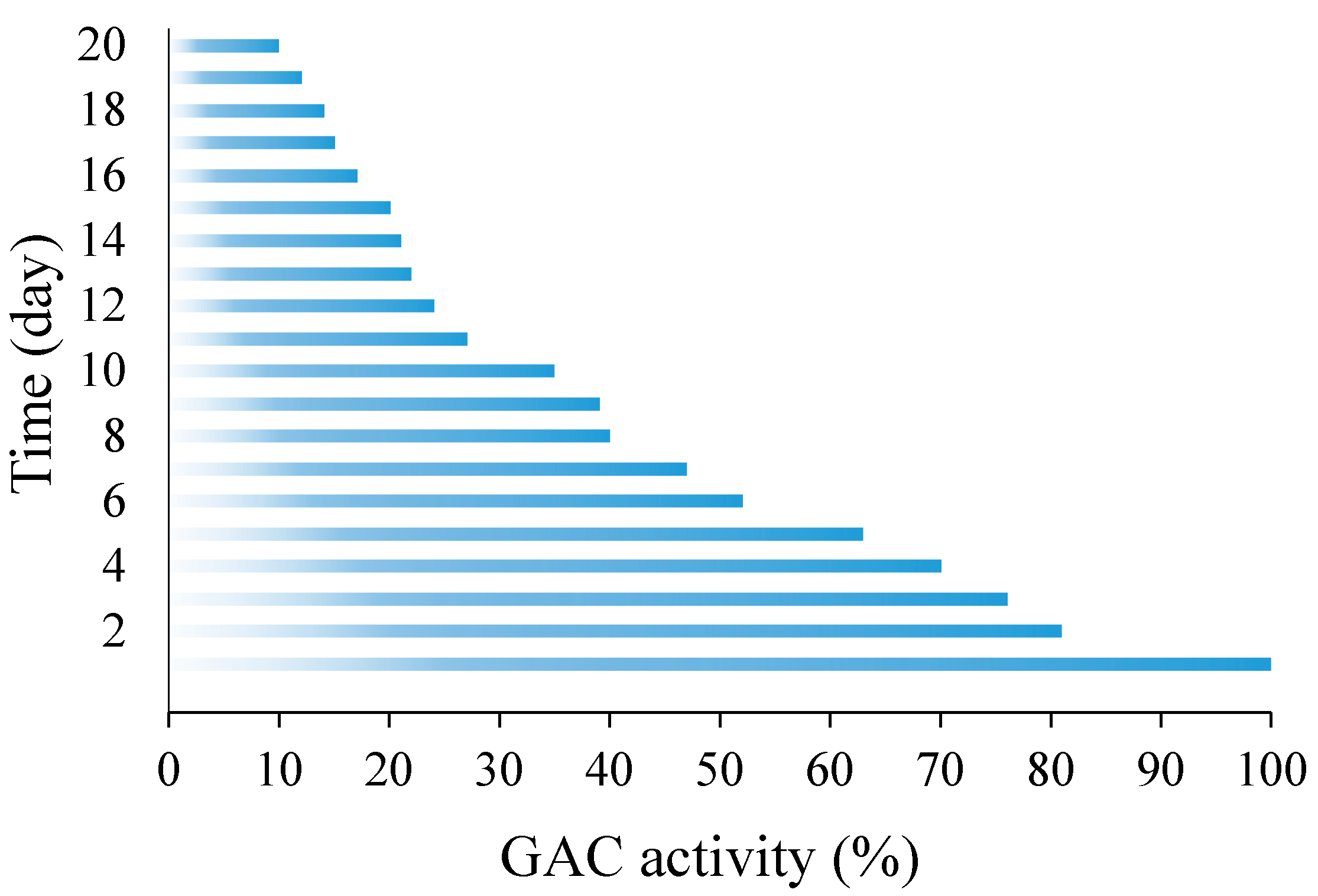
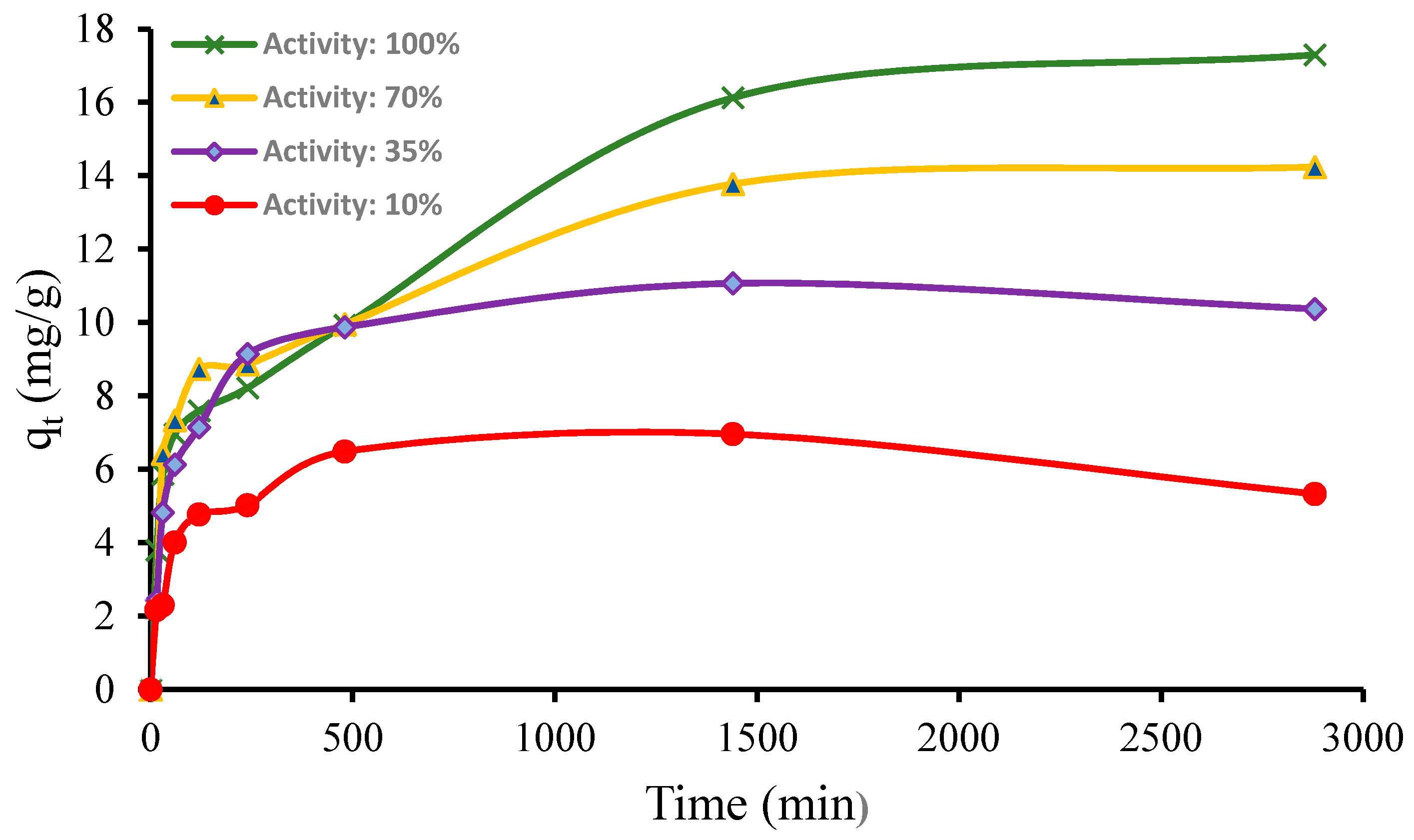
3.1. Laboratory-Scale Studies
3.2. Modeling
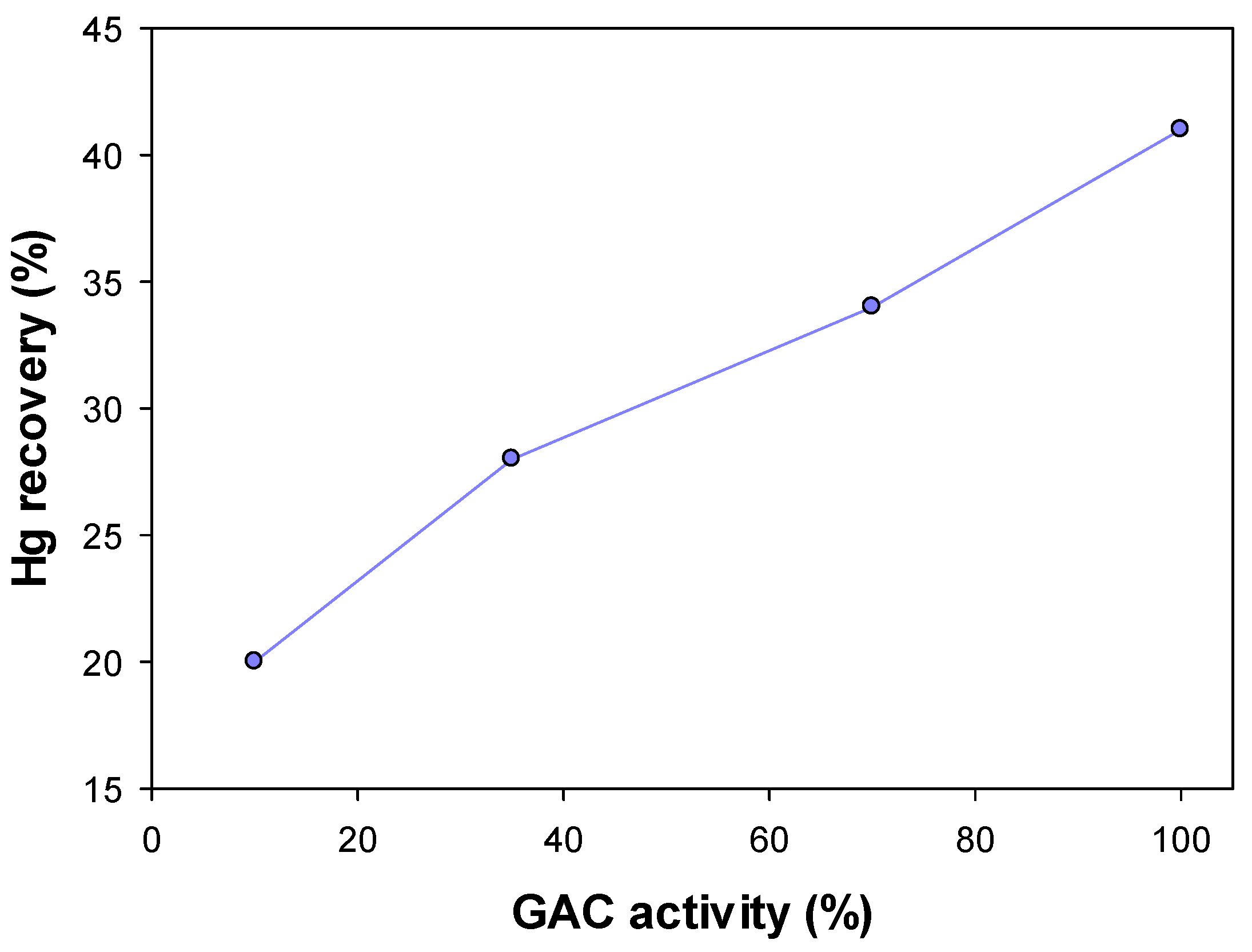
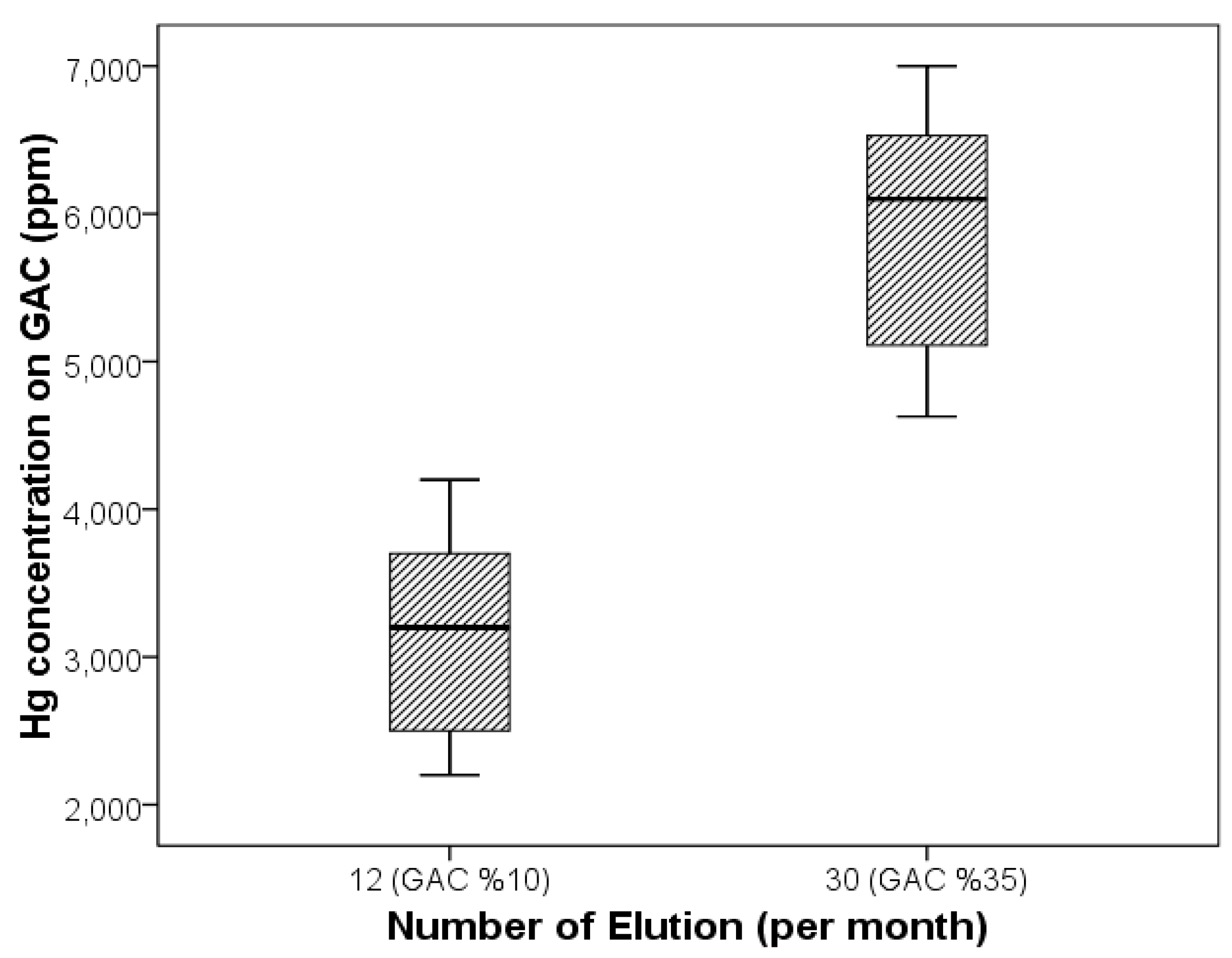
3.3. Industrial-Scale Studies
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Yanuar, E.; Suprapto, B. Leaching and adsorption of gold from lape-sumbawa rocks (Indonesia) by hypochlorite-chloride. Procedia Chem. 2015, 17, 59–65. [Google Scholar] [CrossRef]
- Wadnerkar, D.; Tade, M.O.; Pareek, V.K.; Utikar, R.P. Modeling and optimization of Carbon in leach (CIL) circuit for gold recovery. Miner. Eng. 2015, 83, 136–148. [Google Scholar] [CrossRef]
- Gupta, H.; Singh, S. Kinetics and thermodynamics of phenanthrene adsorption from water on orange rind activated carbon. Environ. Technol. Innov. 2018, 10, 208–214. [Google Scholar] [CrossRef]
- Souza, C.; Majuste, D.; Dantas, M.S.S.; Ciminelli, V.S.T. Effect of zinc ion on copper speciation and adsorption on activated carbon. Hydrometallurgy 2018, 176, 78–86. [Google Scholar] [CrossRef]
- Qu, W.; Yuan, T.; Yin, G.; Xu, S.; Zhang, Q.; Su, H. Effect of properties of activated carbon on malachite green adsorption. Fuel 2019, 249, 45–53. [Google Scholar] [CrossRef]
- Chandra, M.Z.; Mubarok, S. On the use of lignin-based biopolymer in improving gold and silver recoveries during cyanidation leaching. Miner. Eng. 2016, 89, 1–9. [Google Scholar] [CrossRef]
- Yang, X.; Yi, H.; Tang, X.; Zhao, S.; Yang, Z.; Ma, Y.; Feng, T.; Cui, X. Behaviors and kinetics of toluene adsorption-desorption on activated carbons with varying pore structure. J. Environ. Sci. 2018, 67, 104–114. [Google Scholar] [CrossRef]
- Saleh, T.A.; Adio, S.O.; Asif, M.; Dafalla, H. Statistical analysis of phenols adsorption on diethylenetriamine-modified activated carbon. J. Clean. Prod. 2018, 182, 960–968. [Google Scholar] [CrossRef]
- Zhou, L.; Yu, Q.; Cui, Y.; Xie, F.; Li, W.; Li, Y.; Chen, M. Adsorption properties of activated carbon from reed with a high adsorption capacity. Ecol. Eng. 2017, 102, 443–450. [Google Scholar] [CrossRef]
- Jiang, L.; Roskilly, A.P.; Wang, R.Z. Performance exploration of temperature swing adsorption technology for carbon dioxide capture. Energy Convers. Manag. 2018, 165, 396–404. [Google Scholar] [CrossRef]
- Radhik, R.; Jayalath, T.; Rekh Krishnan, G.; Jacob, S.; Rajeev, R.; George, B.K. Adsorption performance of packed bed column for the removal of perchlorate using modified activated carbon. Process Saf. Environ. Prot. 2018, 117, 350–362. [Google Scholar] [CrossRef]
- Di Natale, F.; Orefice, M.; La Motta, F.; Erto, A.; Lancia, A. Unveiling the potentialities of activated carbon in recovering palladium from model leaching solutions. Sep. Purif. Technol. 2017, 174, 183–193. [Google Scholar] [CrossRef]
- AWWA Standard B605-07. Reactivation of Granular Activated Carbon. Foreword Ixexi., n.d.; American Water Works Association: Denver, CO, USA, 2007. [Google Scholar]
- Effry, K.; Pleysier, M.; Bunney, R. Elution behaviour of metals from carbon. In Proceedings of the Hydrometallurgy Conference, Muldersdrift, South Africa, 24–26 February 2009; The Southern African Institute of Mining and Metallurgy: Johannesburg, South Africa, 2009. [Google Scholar]
- Snyders, C.A.; Bradshaw, S.M.; Akdogan, G.; Eksteen, J.J. Determination of the equilibrium and film diffusion constants of the platinum cyanide anions during the elution from activated carbon. Miner. Eng. 2015, 80, 57–68. [Google Scholar] [CrossRef]
- Dong, L.; Liu, W.; Jiang, R.; Wang, Z. Study on reactivation cycle of biological activated carbon (BAC) in water treatment. Int. Biodeterior. Biodegrad. 2015, 102, 209–213. [Google Scholar] [CrossRef]
- Seyedhakimi, A.; Bastami, S.A.; Ghassa, S.; Razavi, H.; Chehreh Chelgani, S. Exploring relationships between various activations of granular activated carbon on silver and gold adsorption: A kinetic and equilibrium study. Sep. Sci. Technol. 2019, 54, 1710–1721. [Google Scholar] [CrossRef]
- Xu, B.; Li, K.; Li, Q.; Yang, Y.; Liu, X.; Jiang, T. Kinetic studies of gold leaching from a gold concentrate calcine by thiosulfate with cobalt-ammonia catalysis and gold recovery by resin adsorption from its pregnant solution. Sep. Purif. Technol. 2019, 213, 368–377. [Google Scholar] [CrossRef]
- Ibrahim, M.N.M.; Ngah, W.S.W.; Norliyana, M.S.; Daud, W.R.W.; Rafatullah, M.; Sulaiman, O.; Hashim, R. A novel agricultural waste adsorbent for the removal of lead (II) ions from aqueous solutions. J. Hazard. Mater. 2010, 182, 377–385. [Google Scholar] [CrossRef]
- Syafiuddin, A.; Salmiati, S.; Jonbi, J.; Fulazzaky, M.A. Application of the kinetic and isotherm models for better understanding of the behaviors of silver nanoparticles adsorption onto different adsorbents. J. Environ. Manag. 2018, 218, 59–70. [Google Scholar] [CrossRef]
- Moosakazemi, F.; Ghassa, S.; Mohammadi, M.R.T. Environmentally friendly hydrometallurgical recovery of tin and lead from waste printed circuit boards: Thermodynamic and kinetics studies. J. Clean. Prod. 2019, 228, 185–196. [Google Scholar] [CrossRef]
- Lagergren, S. About the theory of so-called adsorption of soluble substances. K. Sven. Vetensk. Handl. Band 1898, 241, 1–39. [Google Scholar]
- Ho, Y.; McKay, G. Pseudo-second order model for sorption processes. Process Biochem. 1999, 34, 451–465. [Google Scholar] [CrossRef]
- Low, M. Kinetics of chemisorption of gases on solids. Chem. Rev. 1960, 60, 267–312. [Google Scholar] [CrossRef]
- Weber, W.M.J. Kinetics of adsorption on carbon from solutions. J. Sanit. Eng. Div. 1963, 86, 31–60. [Google Scholar]
- Asuquo, E.; Martin, A.; Nzerem, P.; Siperstein, F.; Fan, X. Adsorption of Cd(II) and Pb(II) ions from aqueous solutions using mesoporous activated carbon adsorbent: Equilibrium, kinetics and characterisation studies. J. Environ. Chem. Eng. 2017, 5, 679–698. [Google Scholar] [CrossRef]
- Konicki, W.; Hełminiak, A.; Arabczyk, W.; Mijowska, E. Adsorption of cationic dyes onto Fe@graphite core–shell magnetic nanocomposite: Equilibrium, kinetics and thermodynamics. Chem. Eng. Res. Des. 2018, 129, 259–270. [Google Scholar] [CrossRef]

| Au | Ag | Hg | Fe | Cu | Zn | Sb | Pb | CN |
|---|---|---|---|---|---|---|---|---|
| 0.8 | 1.1 | 18 | 1.1 | 3.3 | 37.5 | 1.2 | 0.06 | 120 |
| Activity (%) | Model | Constants | Correlation Coefficients | |
|---|---|---|---|---|
| 10 | Pseudo-first-order | kf (1/min) | 0.0117 | 0.9192 |
| qe (mg/g) | 5.9258 | |||
| Pseudo-second-order | ks (g/mg. min) | 241.5423 | 0.9393 | |
| qe (mg/g) | 0.1622 | |||
| h0 (mg/g.min) | 6.3547 | |||
| chemisorption | α (mg/g.min) | 1.1994 | 0.8712 | |
| β (g/mg) | 1.2118 | |||
| Intra-particle diffusion | kid (mg/g.min0.5) | 0.0903 | 0.5084 | |
| 35 | Pseudo-first-order | kf (1/min) | 0.0152 | 0.9593 |
| qe (mg/g) | 10.0568 | |||
| Pseudo-second-order | ks (g/mg. min) | 202.0494 | 0.9893 | |
| qe (mg/g) | 0.2318 | |||
| h0 (mg/g.min) | 10.8564 | |||
| chemisorption | α (mg/g.min) | 1.1004 | 0.9485 | |
| β (g/mg) | 0.6407 | |||
| Intra-particle diffusion | kid (mg/g.min0.5) | 0.1710 | 0.6382 | |
| 70 | Pseudo-first-order | kf (1/min) | 0.0163 | 0.8522 |
| qe (mg/g) | 11.6868 | |||
| Pseudo-second-order | ks (g/mg. min) | 0.0014 | 0.9237 | |
| qe (mg/g) | 0.1303 | |||
| h0 (mg/g.min) | 12.04 | |||
| chemisorption | α (mg/g.min) | 0.9029 | 0.9658 | |
| β (g/mg) | 0.4935 | |||
| Intra-particle diffusion | kid (mg/g.min0.5) | 0.2326 | 0.7792 | |
| 100 | Pseudo-first-order | kf (1/min) | 0.0047 | 0.7641 |
| qe (mg/g) | 15.2671 | |||
| Pseudo-second-order | ks (g/mg. min) | 894.3341 | 0.8604 | |
| qe (mg/g) | 0.1341 | |||
| h0 (mg/g.min) | 16.0826 | |||
| chemisorption | α (mg/g.min) | 0.5830 | 0.9482 | |
| β (g/mg) | 0.3986 | |||
| Intra-particle diffusion | kid (mg/g.min0.5) | 0.2957 | 0.9115 | |
| Activity | No. of Elutions (per Month) | No. of Activations (per Month) | Hg Recovery (%) | Hg (mg/L) | |
|---|---|---|---|---|---|
| Initial | Tenor | ||||
| 10 | 12 | 6 | 40 | 18 | 10.8 |
| 35 | 30 | 15 | 90 | 18 | 1.8 |
| Activity | No. of Elutions | No. of Activations | Gold (ppm) | Silver (ppm) | ||
|---|---|---|---|---|---|---|
| Feed | Residual | Feed | Residual | |||
| 10 | 12 | 6 | 0.7 | 0.012 | 1.2 | 0.25 |
| 30 | 30 | 15 | 0.6 | 0.003 | 1.12 | 0.03 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bastami, S.; Ghassa, S.; Seyedhakimi, A.; Chehreh Chelgani, S. Adsorption of Mercury from a Cyanide Leaching Solution Using Various Activation Rates of Granular Activated Carbon: A Laboratory- and Industrial-Scale Study. Sustainability 2020, 12, 3287. https://doi.org/10.3390/su12083287
Bastami S, Ghassa S, Seyedhakimi A, Chehreh Chelgani S. Adsorption of Mercury from a Cyanide Leaching Solution Using Various Activation Rates of Granular Activated Carbon: A Laboratory- and Industrial-Scale Study. Sustainability. 2020; 12(8):3287. https://doi.org/10.3390/su12083287
Chicago/Turabian StyleBastami, Sina, Sina Ghassa, Amin Seyedhakimi, and Saeed Chehreh Chelgani. 2020. "Adsorption of Mercury from a Cyanide Leaching Solution Using Various Activation Rates of Granular Activated Carbon: A Laboratory- and Industrial-Scale Study" Sustainability 12, no. 8: 3287. https://doi.org/10.3390/su12083287
APA StyleBastami, S., Ghassa, S., Seyedhakimi, A., & Chehreh Chelgani, S. (2020). Adsorption of Mercury from a Cyanide Leaching Solution Using Various Activation Rates of Granular Activated Carbon: A Laboratory- and Industrial-Scale Study. Sustainability, 12(8), 3287. https://doi.org/10.3390/su12083287






