Toxicity and Hazards of Biodegradable and Non-Biodegradable Sunscreens to Aquatic Life of Quintana Roo, Mexico
Abstract
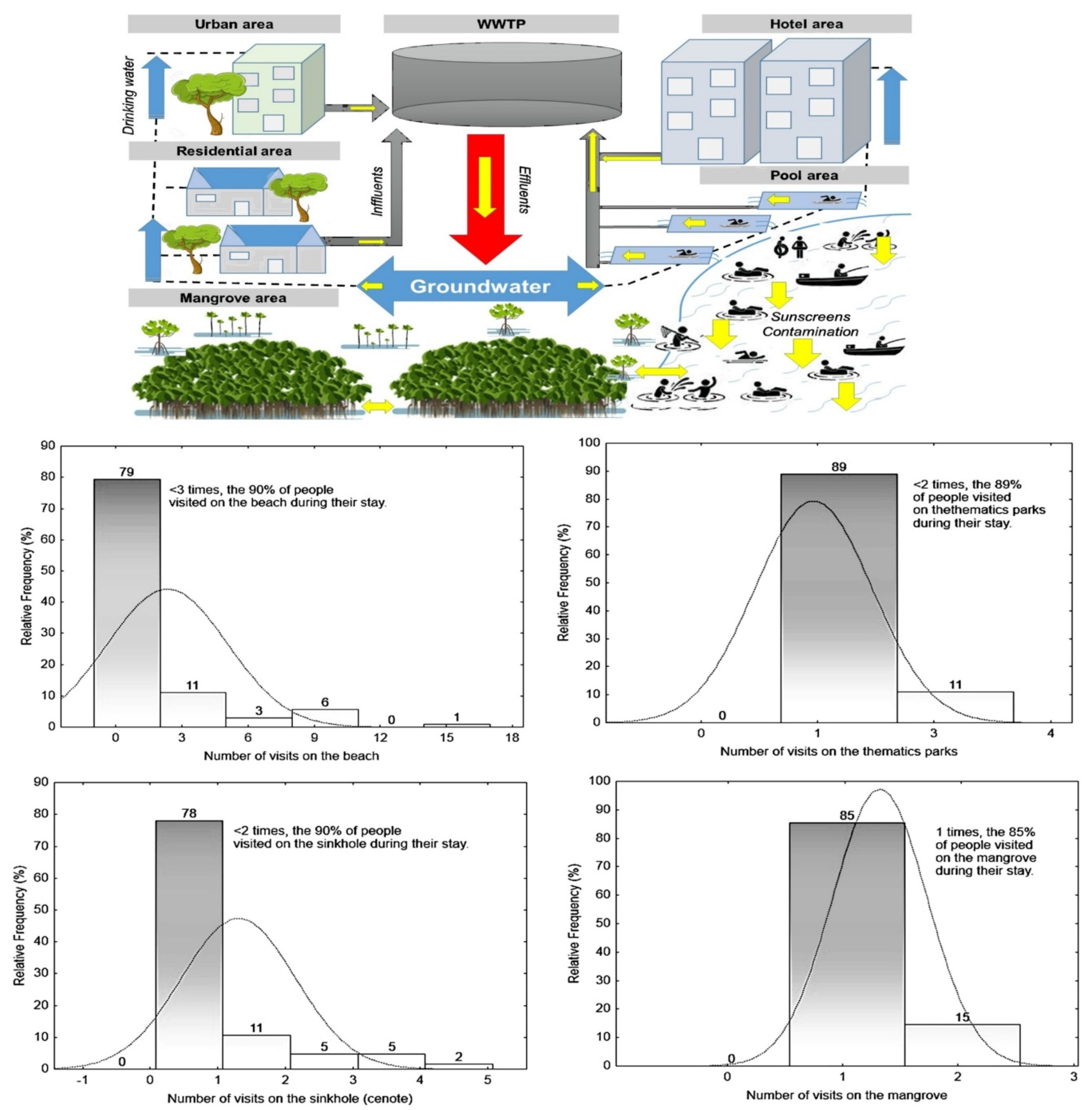
1. Introduction
2. Materials and Methods
2.1. Zooplankton Sampling and Identification
2.2. Isolation and Creation of Monoclonal Cultures of the Zooplankton Species
2.3. Sunscreen Selection and Preparation of the Sunscreen Stock
2.4. Acute Toxicity Test in Zooplankton
2.5. Risk Assessment and Statistical Analyses
3. Results
3.1. Statistics on Sunscreens, Quintana Roo, Mexico
3.2. Bioindicator Species
3.3. Toxicity of Sunscreens in Zooplankton Species
3.4. Risk Assessment
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- McCoshum, S.; Schlarb, M.A.; Baum, A.K. Direct and indirect effects of sunscreen exposure for reef biota. Hydrobiologia 2016, 776, 139–146. [Google Scholar] [CrossRef]
- Giokas, D.L.; Salvador, A.; Chisvert, A. UV filters: From sunscreens to human body and the environment. TrAC Trends Anal. Chem. 2007, 26, 360–374. [Google Scholar] [CrossRef]
- Brausch, J.M.; Rand, G.M. A review of personal care products in the aquatic environment: Environmental concentrations and toxicity. Chemosphere 2011, 82, 1518–1532. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Quiles, D.; Tovar-Sánchez, A. Are sunscreens a new environmental risk associated with coastal tourism? Environ. Int. 2015, 83, 158–170. [Google Scholar] [CrossRef]
- Poiger, T.; Buser, H.-R.; Balmer, E.M.; Bergqvist, P.-A.; Müller, M.D. Occurrence of UV filter compounds from sunscreens in surface waters: Regional mass balance in two Swiss lakes. Chemosphere 2004, 55, 951–963. [Google Scholar] [CrossRef] [PubMed]
- Danovaro, R.; Bongiorni, L.; Corinaldesi, C.; Giovannelli, D.; Damiani, E.; Astolfi, P.; Greci, L.; Pusceddu, A. Sunscreens Cause Coral Bleaching by Promoting Viral Infections. Environ. Health Perspect. 2008, 116, 441–447. [Google Scholar] [CrossRef] [PubMed]
- Casas-Beltrán, D.; Hernández-Pedraza, M.; Alvarado-Flores, J. Estimation of the Discharge of Sunscreens in Aquatic Environments of the Mexican Caribbean. Environments 2020, 7, 15. [Google Scholar] [CrossRef]
- SECTUR. Resultados de la Actividad Turística. 2018. Available online: https://www.datatur.sectur.gob.mx/RAT/RAT-2018-12(ES).pdf (accessed on 10 December 2019).
- Rodríguez-Fuentes, G.; Luna-Ramírez, K.; Soto, M. Sunscreen use behavior and most frequently used active ingredients among beachgoers on Cancun, Mexico. WebmedCentral Dermatol. 2010, 1, WMC001364. [Google Scholar] [CrossRef]
- Bozec, Y.M.; Acosta-González, G.; Núñez-Lara, E.; Arias-González, J.E. Impacts of coastal development on ecosystem structure and function of Yucatan coral reefs, Mexico. In Proceedings of the 11th International Coral Reef Symposium, Fort Lauderdale, FL, USA, 7–11 July 2008; Volume 2, pp. 691–695. [Google Scholar]
- Sieratowicz, A.; Kaiser, D.; Behr, M.; Oetken, M.; Oehlmann, J. Acute and chronic toxicity of four frequently used UV filter substances for Desmodesmus subspicatus and Daphnia magna. J. Environ. Health Sci. Part A 2011, 46, 1311–1319. [Google Scholar] [CrossRef]
- Clément, L.; Hurel, C.; Marmier, N. Toxicity of TiO2 nanoparticles to cladocerans, algae, rotifers and plants—Effects of size and crystalline structure. Chemosphere 2013, 90, 1083–1090. [Google Scholar] [CrossRef]
- Mills, S.; Alcántara-Rodríguez, J.A.; Ciros-Pérez, J.; Gómez, A.; Hagiwara, A.; Galindo, K.H.; Jersabek, C.D.; Malekzadeh-Viayeh, R.; Leasi, F.; Lee, J.S.; et al. Fifteen species in one: Deciphering the Brachionus plicatilis species complex (Rotifera, Monogononta) through DNA taxonomy. Hydrobiologia 2017, 796, 39–58. [Google Scholar] [CrossRef]
- Elías-Gutiérrez, M.; Suárez-Morales, E.; Gutiérrez Aguirre, M.A.; Silva-Briano, M.; Granados Ramírez, J.G.; Garfias Espejo, T. Cladócera y Copépoda de las Aguas Continentales de Mexico. Guía Ilustrada; Ávila Valdivieso, J.M., Ed.; Universidad Nacional Autónoma de Mexico: Ciudad de Mexico, Mexico, 2008. [Google Scholar]
- Nagler, C.; Geist, J.; Matzke-Karasz, R. Revision of genus Tanycypris Ostrácoda, Cypricercinae) con la description de Tanycypris alfonsin. sp., and an identification key to the genus. Zootaxa 2014, 4, 401–424. [Google Scholar] [CrossRef]
- López-Gutiérrez, L.F.; Rubio-Franchini, I.; Rico-Martínez, R.; Mesquita-Joanes, F.; Ramírez-López, E.M.; Arredondo-Figueroa, J.L.; Silva-Briano, M. Inter- and Intraspecific Variability in Invertebrate Acute Toxicity Response to Arsenic and Fluoride Exposure. J. Environ. Health Sci. 2018, 4, 1–10. [Google Scholar]
- Pérez-Legaspi, I.A.; Garatachia-Vargas, M.; García-Villar, A.M.; Rubio-Franchini, I. Evaluación de la sensibilidad del cladócero tropical Ceriodaphnia cornuta a metales pesados. Rev. Int. Contam. Ambient. 2017, 33, 49–56. [Google Scholar] [CrossRef]
- Garza-León, C.V.; Arzate-Cárdenas, M.A.; Rico-Martínez, R. Toxicity evaluation of cypermethrin, glyphosate, and malathion, on two indigenous zooplanktonic species. Environ. Sci. Pollut. Res. 2017, 24, 18123–18134. [Google Scholar] [CrossRef] [PubMed]
- EPA. Methods for Measuring the Acute Toxicity of Effluents and Receiving Waters to Freshwater and Marine Organisms; Office of Water (4303T): Washington, DC, USA, 2002. [Google Scholar]
- Nichols, H.W. Growth media-freshwater. In Handbook of Physiological Methods; Stein, R., Ed.; Cambridge University Press: Cambridge, UK, 1973. [Google Scholar]
- Casas-Beltrán, D.A.; Gallaher, C.; Maloof, Y.; Hernandez, E.; Moreno, K.F.; Voglesonger, K.; Leal-Bautista, R.M.; Lenczewski, M. Seaweed Invasion! Temporal Changes in Beach Conditions Lead to Increasing Cenote Usage and Contamination in the Riviera Maya. Sustainability 2020, 12, 2474. [Google Scholar] [CrossRef]
- Pérez-Legaspi, I.A.; Rico-Martínez, R. Acute toxicity test on three species of the genus Lecane (Rotifera:Monogononta). Hydrobiologia 1998, 446, 375–381. [Google Scholar]
- Santos-Medrano, G.; Rico-Martínez, R. Acute Sensitivity Comparison among Daphnia magna Straus, 1820 Daphnia pulex Leydig, 1860 and Simocephalus vetulus Müller, 1776, Exposed to Nine Toxicants. Turk. J. Fish. Aquat. Sci. 2018, 19, 615–623. [Google Scholar]
- Karlsson, C. Risk Assessment of Compounds that Could Impair the Aquatic Environment; Swedish Environmental Protection Agency: Stockholm, Sweden, 2007; pp. 1–56. [Google Scholar]
- Zhao, J.; Chen, B. Species sensitivity distribution for chlorpyrifos to aquatic organisms: Model choice and sample size. Ecotoxicol. Environ. Saf. 2016, 125, 161–169. [Google Scholar] [CrossRef]
- Cruzeiro, C.; Amaral, S.; Rocha, E.; Rocha, M.J. Determination of 54 pesticides in waters of the Iberian Douro River estuary and risk assessment of environmentally relevant mixtures using theoretical approaches and Artemia salina and Daphnia magna bioassays. Ecotoxicol. Environ. Saf. 2017, 14, 126–134. [Google Scholar] [CrossRef]
- Beyer, J.; Petersen, K.; Song, Y.; Russ, A.; Grung, M.; Bakke, T.; Tollefsen, K.E. Environmental risk assessment of combined effects in aquatic ecotoxicology: A discussion paper. Mar. Environ. Res. 2014, 96, 81–91. [Google Scholar] [CrossRef] [PubMed]
- Van Wijngaarden, R.P.A.; Arts, G.H.P.; Belgers, J.D.M.; Boonstra, H.; Rossink, I.; Schroer, A.F.W.; Brock, T.C.M. The species sensitivity distribution approach compared to a microcosm study: A case study with the fungicide fluazinam. Ecotoxicol. Environ. Saf. 2010, 73, 109–122. [Google Scholar] [CrossRef] [PubMed]
- Backhaus, T.; Faust, M. Predictive environmental risk assessment of chemical mixtures: A conceptual framework. Environ. Sci. Technol. 2012, 46, 2564–3573. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.-W.; Won, Y.L.; Park, D.J.; Kim, Y.S.; Jin, E.S.; Lee, S.K. Combined toxic effects of polar and nonpolar chemicals on human hepatocytes (HepG2) cell by quantitative property-activity relationships modeling. Toxicol. Res. 2016, 32, 337–343. [Google Scholar] [CrossRef]
- PubChem. Available online: https://pubchem.ncbi.nlm.nih.gov/ (accessed on 10 December 2019).
- Balmer, M.E.; Buser, H.R.; Muller, M.D.; Poiger, T. Occurrence of the organic UV-filter compounds BP-3, 4-MBC, EHMC, and OC in wastewater, surface waters, and in fish from Swiss lakes. Environ. Sci. Technol. 2004, 39, 953–962. [Google Scholar] [CrossRef]
- Kapler, R.; Crago, J.; Barr, J.; Arndt, J.; Setyowatu, K.; Chen, J. Toxicity biomarker expression in daphnids exposed to manufactured nanoparticles: Changes in toxicity with functionalization. Environ. Pollut. 2009, 157, 1152–1156. [Google Scholar]
- Heinlaan, M.; Ivask, A.; Blinova, I.; Dubourguier, H.C.; Kahru, A. Toxicity of nanosized and bulk ZnO, CuO, and TiO2 to bacteria Vibrio fischeri and crustaceans Daphnia magna and Thamnocephalus platyrus. Chemosphere 2008, 71, 1308–1316. [Google Scholar] [CrossRef]
- Fent, K.; Kunz, P.Y.; Zneker, A.; Rapp, M. A tentative environmental risk assessment of the UV-filters 3-(4-methylbenzylidene-camphor), 2-ethyl-hexyl-4-trimethoxycinnamate, benzophenone-3, benzophenone-4 and 3-benzylidene camphor. Mar. Environ. Res. 2010, 69, 54–56. [Google Scholar] [CrossRef]
- Gago-Ferrero, P.; Díaz-Cruz, M.S.; Barceló, D. Occurrence of multiclass UV filters in treated sewage sludge from wastewater treatment plants. Chemosphere 2011, 84, 1158–1165. [Google Scholar] [CrossRef]
- Mirabelle, M.P.T.; Leung, H.W.; Tak-Cheung, W.; Nobuyoshi, Y.; Sachi, T.; Wenhua, L.; Lam, P.K.S.; Murphy, M.B. Occurrence, distribution and ecological risk assessment of multiple classes of UV filters in surface water from different countries. Water Res. 2014, 67, 55–65. [Google Scholar]
| Species | Sunscreens | Keys | r2 | LC50 g/L | LC10 g/L |
|---|---|---|---|---|---|
| Brachionus cf ibericus SianKan C7 | Kinsun | B1 | 0.888 | 1.68E+00 | 7.00E-01 |
| Cypridopsis vidua | Kinsun | B1 | 0.921 | 1.53E+00 | 4.51E-01 |
| Diaphanocypris meridana | Kinsun | B1 | 0.999 | 6.77E+00 | 7.00E-01 |
| D. meridana | Maya Solar | B2 | 0.978 | 3.90E-01 | 1.00E-02 |
| Cypridopsis vidua | Protectyl Vegetal | B3 | 0.891 | 3.40E-01 | 8.00E-02 |
| Brachionus cf ibericus Cancun C16 | Hawaiian TropicTM BO | B4 | 0.932 | 5.00E-01 | 1.60E-01 |
| B. cf ibericus Cancun C20 | Hawaiian TropicTM BO | B4 | 0.736 | 6.50E-01 | 1.90E-01 |
| B. cf ibericus SianKan C6 | Hawaiian TropicTM BO | B4 | 0.898 | 6.30E-01 | 2.20E-01 |
| B. cf ibericus SianKan C7 | Hawaiian TropicTM BO | B4 | 0.907 | 5.40E-01 | 1.70E-01 |
| Cypridopsis vidua | Hawaiian TropicTM BO | B4 | 0.903 | 3.30E-01 | 1.80E-01 |
| Diaphanocypris meridana | Hawaiian TropicTM BO | B4 | 0.872 | 1.80E-01 | 4.00E-02 |
| Cypridopsis vidua | Banana Boat | NB1 | 0.893 | 2.10E-01 | 1.00E-01 |
| Diaphanocypris meridana | Banana Boat | NB1 | 0.824 | 1.00E-01 | 1.00E-02 |
| Macrothrix triserialis | Banana Boat | NB1 | 0.917 | 1.00E+00 | 4.60E-01 |
| Cypridopsis vidua | Coopertone Babe | NB2 | 0.839 | 2.30E-01 | 1.10E-01 |
| Diaphanocypris meridana | Coopertone Babe | NB2 | 0.947 | 2.30E-01 | 7.00E-02 |
| D. meridana | Hawaiian TropicTM ST | NB3 | 0.869 | 1.80E-01 | 5.00E-02 |
| Macrothrix triserialis | Hawaiian TropicTM ST | NB3 | 0.804 | 2.70E-01 | 7.00E-02 |
| Cypridopsis vidua | Hawaiian TropicTM O | NB4 | 0.937 | 2.30E-01 | 4.00E-02 |
| Diaphanocypris meridana | Hawaiian TropicTM O | NB4 | 0.946 | 4.80E-01 | 2.00E-02 |
| D. meridana | Nivea Sun | NB5 | 0.934 | 2.46E+00 | 6.60E-01 |
| Sunscreens | Keys | MATC of LC10 in g/L | PNEC in g/L | RQ = MEC (0.001 g/L)/PNEC | Interpretation |
|---|---|---|---|---|---|
| Kinsun | B1 | 6.05E-01 | 6.05E-04 | 1.65E+00 | Moderate |
| Maya Solar | B2 | 1.00E-02 | 1.00E-05 | 1.00E+02 | High |
| Hawaiian TropicTM BO | B3 | 1.42E-01 | 1.42E-04 | 7.04E+00 | Moderate |
| Protectyl Vegetal | B4 | 8.00E-02 | 8.00E-05 | 1.25E+01 | High |
| Banana Boat | NB1 | 7.72E-02 | 7.72E-05 | 1.30E+01 | High |
| Coopertone® Babe | NB2 | 8.77E-02 | 8.77E-05 | 1.14E+01 | High |
| Hawaiian TropicTM ST | NB3 | 5.92E-02 | 5.92E-05 | 1.69E+01 | High |
| Hawaiian TropicTM O | NB4 | 2.83E-02 | 2.83E-05 | 3.54E+01 | High |
| Nivea Sun | NB5 | 6.60E-01 | 6.60E-04 | 1.52E+00 | Moderate |
| Biodegradable | B | 1.57E-01 | 1.57E-04 | 6.36E+00 | Moderate |
| Non-Biodegradable | NB | 7.61E-02 | 7.61E-05 | 1.31E+01 | High |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hernández-Pedraza, M.; Caballero-Vázquez, J.A.; Peniche-Pérez, J.C.; Pérez-Legaspi, I.A.; Casas-Beltran, D.A.; Alvarado-Flores, J. Toxicity and Hazards of Biodegradable and Non-Biodegradable Sunscreens to Aquatic Life of Quintana Roo, Mexico. Sustainability 2020, 12, 3270. https://doi.org/10.3390/su12083270
Hernández-Pedraza M, Caballero-Vázquez JA, Peniche-Pérez JC, Pérez-Legaspi IA, Casas-Beltran DA, Alvarado-Flores J. Toxicity and Hazards of Biodegradable and Non-Biodegradable Sunscreens to Aquatic Life of Quintana Roo, Mexico. Sustainability. 2020; 12(8):3270. https://doi.org/10.3390/su12083270
Chicago/Turabian StyleHernández-Pedraza, Miguel, José Adán Caballero-Vázquez, Jorge Carlos Peniche-Pérez, Ignacio Alejandro Pérez-Legaspi, Diego Armando Casas-Beltran, and Jesús Alvarado-Flores. 2020. "Toxicity and Hazards of Biodegradable and Non-Biodegradable Sunscreens to Aquatic Life of Quintana Roo, Mexico" Sustainability 12, no. 8: 3270. https://doi.org/10.3390/su12083270
APA StyleHernández-Pedraza, M., Caballero-Vázquez, J. A., Peniche-Pérez, J. C., Pérez-Legaspi, I. A., Casas-Beltran, D. A., & Alvarado-Flores, J. (2020). Toxicity and Hazards of Biodegradable and Non-Biodegradable Sunscreens to Aquatic Life of Quintana Roo, Mexico. Sustainability, 12(8), 3270. https://doi.org/10.3390/su12083270





