Sustainable Processes and Chemical Characterization of Natural Food Additives: Palmyra Palm (Borassus Flabellifer Linn.) Granulated Sugar
Abstract
1. Introduction
2. Materials and Methods
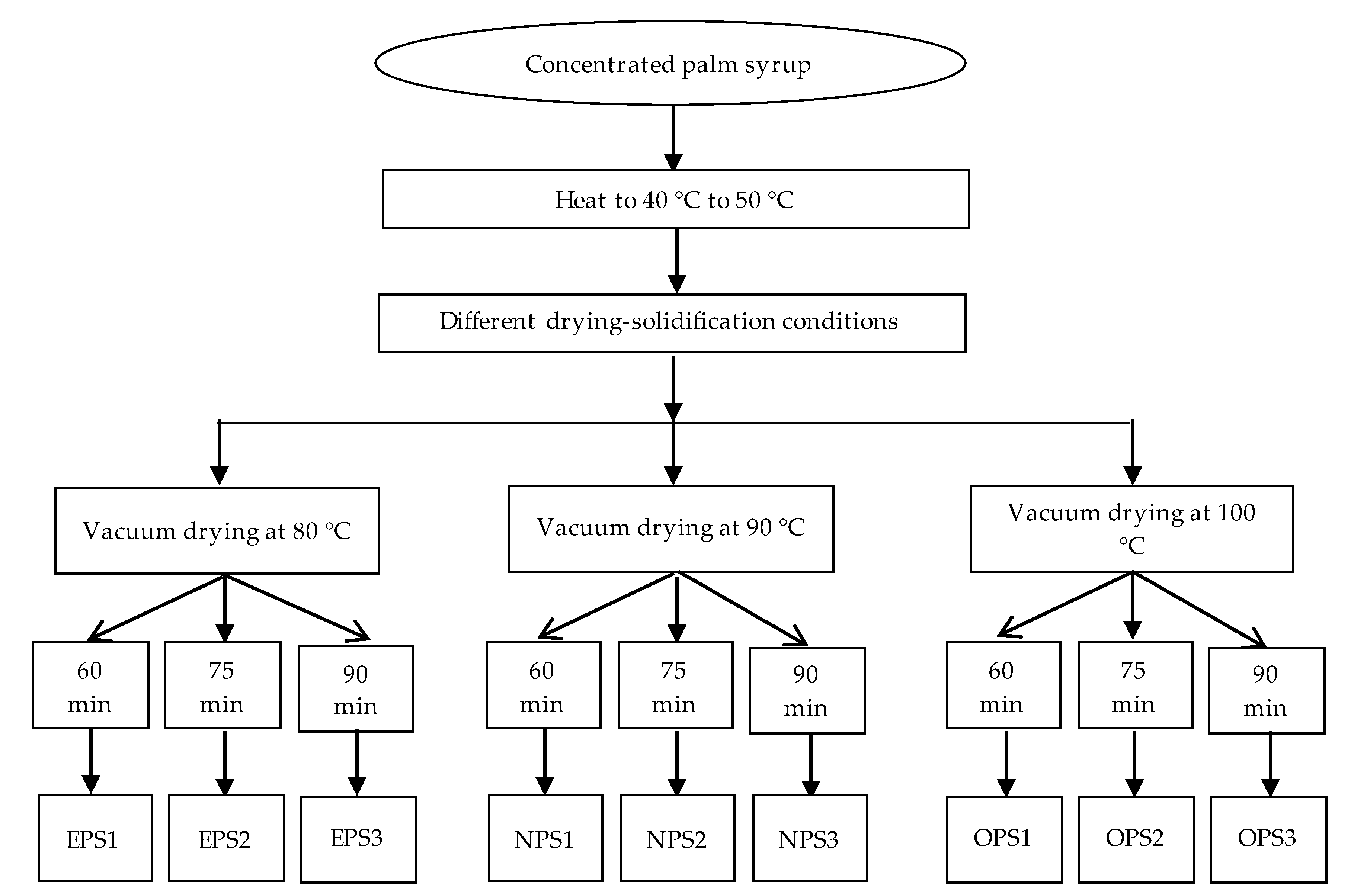
2.1. Preparation of Palmyra Palm Granulated Sugar
2.2. Measurement of Color, pH, Moisture Content, and Water Activity
2.3. Determination of Total Sugar and Reducing Sugar
2.4. Determination of Mineral Content
2.5. Determination of Vitamin Content
2.6. Determination of Total Phenolic Content
2.7. Determination of DPPH Radical Scavenging Activity
2.8. Determination of Ferric Reducing Antioxidant Power (FRAP)
2.9. Determination of 5-Hydroxymethylfurfural (HMF) Content
2.10. Determination of Volatile Compositions
2.11. Odor Description and Detection Analyses
2.12. Cytoprotective Effect of Palm Granulated Sugar against tert-Butyl Hydroperoxide (tBuOOH)
2.13. Data Analysis
3. Results
3.1. Physicochemical Characteristics
3.2. Chemical Composition
3.3. Vitamin Content
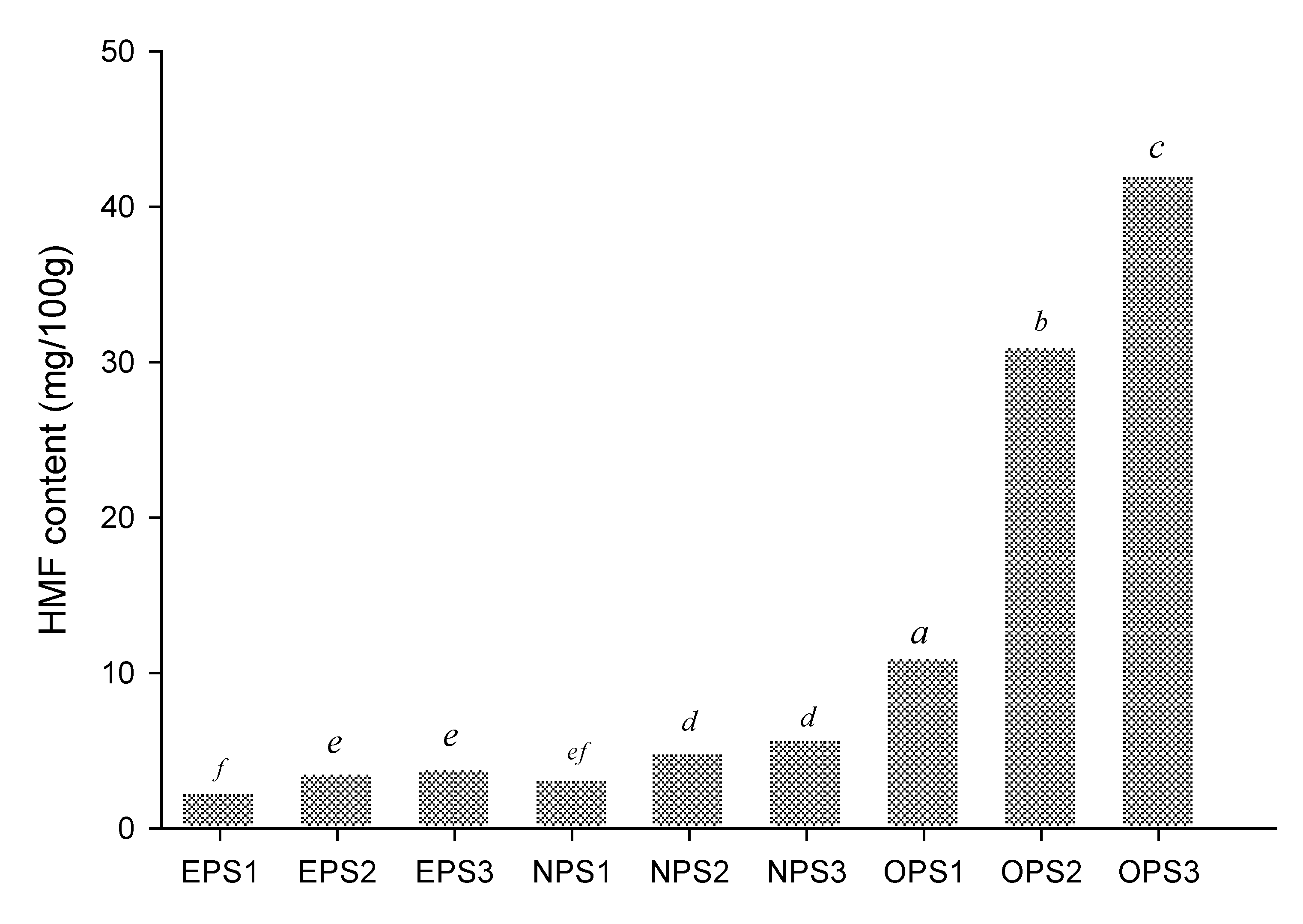
3.4. HMF content
3.5. Composition of Volatiles
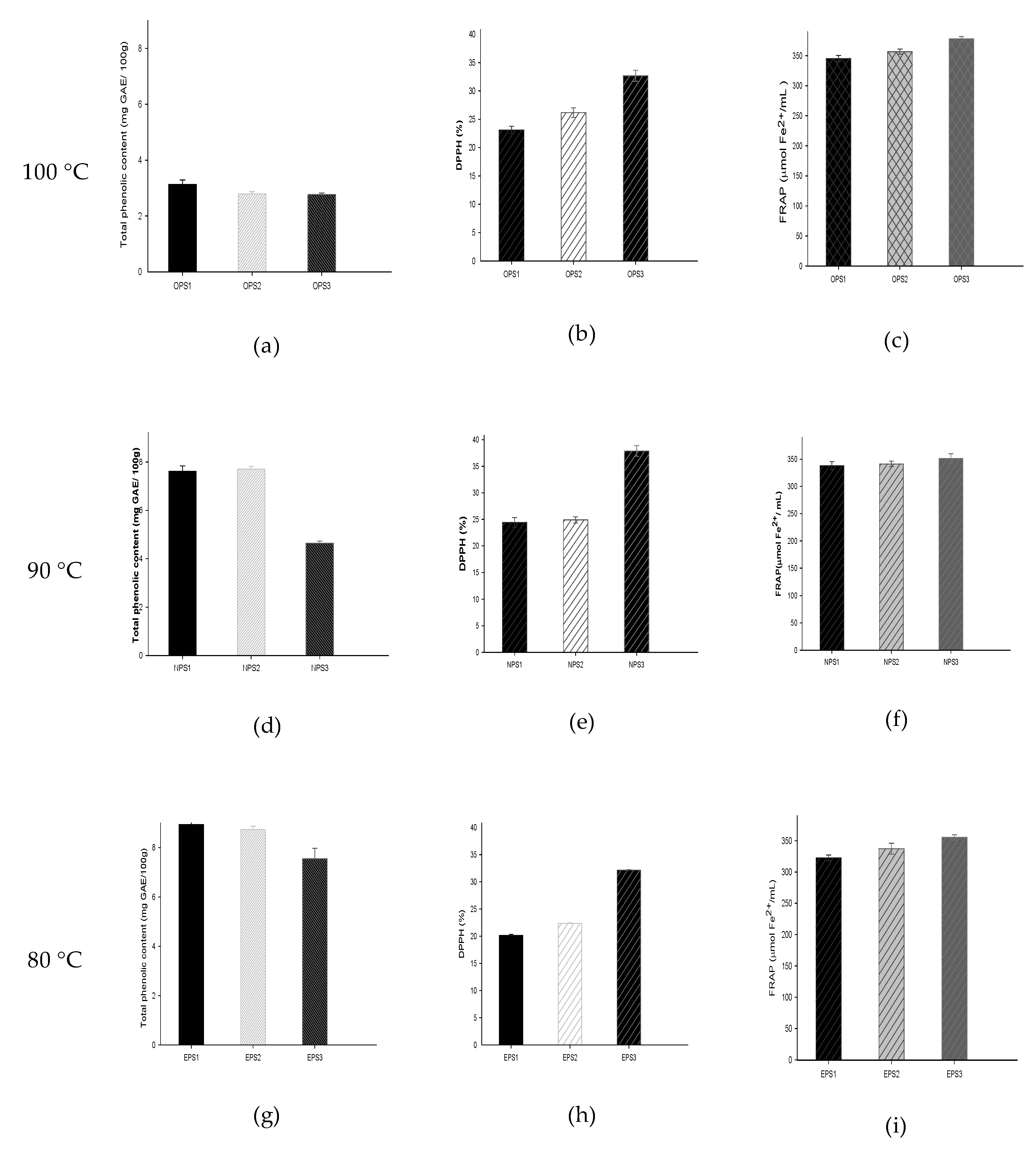
3.6. Total Phenolic Content and Antioxidant Properties
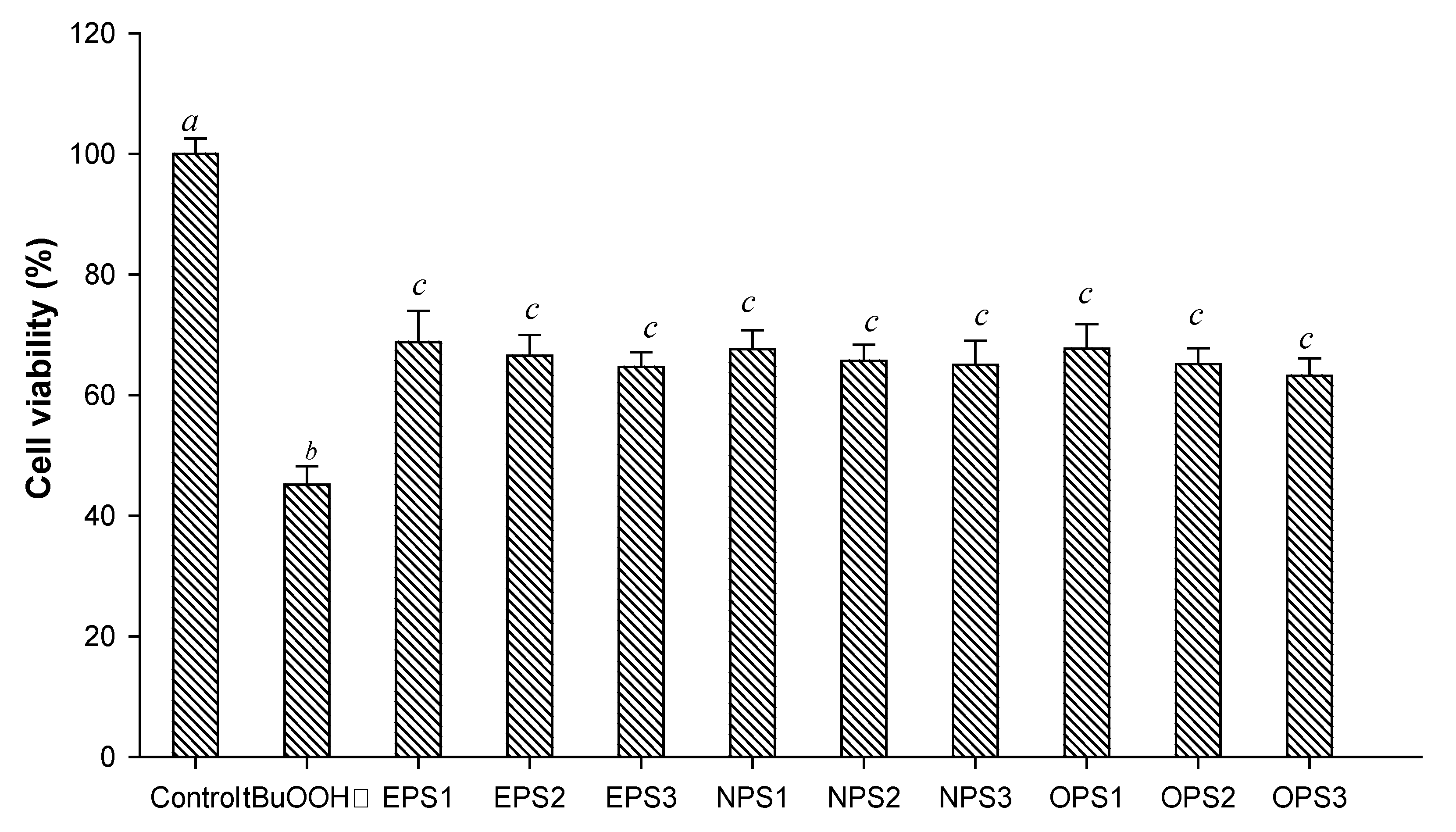
3.7. Cytoprotective Effect
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A

References
- Lim, T.K. Edible Medicinal and Medicinal Plants, 1st ed.; Springer: Dordrecht, The Netherlands, 2012; pp. 293–300. [Google Scholar] [CrossRef]
- Vengaiah, P.C.; Murthy, G.N.; Sattiraju, M.; Maheswarappa, H.P. Value Added Food Products from Palmyrah Palm (Borassus Flabellifer L.). JNHS 2017, 4, 1–3. [Google Scholar] [CrossRef][Green Version]
- Mohite, M.; Pramod, H.J.; Yadav, A.V.; Raje, V.N.; Wadkar, G.H. Evaluation of antiulcer activity of aqueous extract of Borassus flabellifer (Linn.) Fruits. J. Pharm. Res. 2012, 5, 3782–3786. Available online: http://jprsolutions.info/newfiles/journal-file-56bc119b914ed0.51098376.pdf (accessed on 27 June 2012).
- Singh, T.; Ravi Kumar, V.; Kumar, R.; Yashaswini, Y.; Pravalika, D.; Pravalika, V. Comparative study of in vitro anthelmintic activity of sap Borassus Flabellifer. World J. Pharm. Pharm. Sci. 2015, 5, 701–706. Available online: https://www.researchgate.net/publication/317715107_COMPARATIVE_STUDY_OF_IN_VITRO_ANTHELMINTIC_ACTIVITY_OF_SAP_OF_BORASSUS_FLABELLIFER (accessed on 1 December 2015).
- Yoshikawa, M.; Xu, F.; Morikawa, T.; Pongpiriyadacha, Y.; Nakamura, S.; Asao, Y.; Kumahara, A.; Matsuda, H. Medicinal flowers. XII. (1) New spirostane-type steroid saponins with antidiabetogenic activity from Borassus flabellifer. Chem. Pharm. Bull. 2007, 55, 308–316. [Google Scholar] [CrossRef] [PubMed]
- Paschapur, M.S.; Patil, M.B.; Kumar, R.; Patil, S.R. Evaluation of anti-inflammatory activity of ethanolic extract of Borassus flabellifer L. male flowers (inflorescences) in experimental animals. J. Med. Plant Res. 2009, 3, 49–54. Available online: http://citeseerx.ist.psu.edu/viewdoc/download?doi=10.1.1.875.7726&rep=rep1&type=pdf (accessed on 19 December 2008).
- Paschapur, M.S.; Patil, S.; Patil, S.R.; Kumar, R.; Patil, M.B. Evaluation of the anagesic and antipyretic activities of ethanolic extract of male folwers (inflorescences) of Borassus Flabellifer L. (Arecaceae). Int. J. Pharm. Pharm. Sci. 2009, 2, 98–106. Available online: https://pdfs.semanticscholar.org/8423/5cac7f7bd64de1b7a54a53159a7310f649e5.pdf (accessed on 24 July 2009).
- Reshma, M.V.; Jacob, J.; Syamnath, V.L.; Habeeba, V.P.; Kumar, B.S.; Lankalapalli, R.S. First report on isolation of 2,3,4-trihydroxy-5-methylacetophenone from palmyra palm (Borassus flabellifer Linn.) syrup, its antioxidant and antimicrobial properties. Food Chem. 2017, 228, 491–496. [Google Scholar] [CrossRef]
- Mahilrajan, S.; Balakumar, S.; Arasaratnam, V.; Kumanan, T.; Kailayalinkam, R. Glycemic Index and Insulin Index of Palmyrah Based Edible Products Commonly Consumed in Jaffna. IOSR-JBB 2017, 3, 37–42. [Google Scholar] [CrossRef]
- Radam, R.R.; Sari, H.N.; Lusyani, H.L. Chemical Compounds Of Granulated Palm Sugar Made From Sap Of Nipa Palm (Nypa Fruticans Wurmb) Growing In Three Different Places. J. Wetlands Environ. Manag. 2014, 2, 108–114. Available online: https://ijwem.ulm.ac.id/index.php/ijwem/article/view/37/23 (accessed on 1 April 2014).
- Khongsak, S.; Janya, S.; Wirot, L. Productions and Functional Properties of Palm Sugars. WJST 2018, 16, 897–907. Available online: http://wjst.wu.ac.th/index.php/wjst/article/view/5323 (accessed on 28 August 2018).
- Jagannadha Rao, P.V.K.; Das, M.; Das, S.K. Changes in physical and thermo-physical properties of sugarcane, palmyra-palm and date-palm juices at different concentration of sugar. J. Food Eng. 2009, 90, 559–566. [Google Scholar] [CrossRef]
- Sanyang, M.L.; Sapuan, S.M.; Jawaid, M.; Ishak, M.R.; Sahari, J. Recent developments in sugar palm (Arenga pinnata) based biocomposites and their potential industrial applications: A review. Renew. Sust. Energy Rev. 2016, 54, 533–549. [Google Scholar] [CrossRef]
- Madhava, M.; Ravindra Babu, D.; Vengaiah, P.C.; Hari Babu, B. Optimization of Process Parameters for Production of Palmyrah Palm Jaggery. JAE 2015, 52, 14–19. Available online: https://www.indianjournals.com/ijor.aspx?target=ijor:joae&volume=52&issue=1&article=002 (accessed on 26 March 2020).
- Inyang, U.; Oboh, I.; Etuk, B. Drying and the Different Techniques. Int. J. Food Nutr. Saf. 2017, 8, 45–72. Available online: http://modernscientificpress.com/Journals/ViewArticle.aspx?6ZIT7oAL6Lqarm6Ljqm1ABiuFTINYiNR0EKTnHl+ifbzl+1BOQ8iCs1yNd6FcqQb (accessed on 20 November 2017).
- Naknean, P.; Meenune, M.; Roudaut, G. Changes in properties of palm sugar syrup produced by an open pan and a vacuum evaporator during storage. IFRJ 2013, 20, 2323–2334. Available online: https://pdfs.semanticscholar.org/7673/13f5204742cbe963f10a5ffa5ecb9318a745.pdf (accessed on 26 March 2020).
- Aeimsard, R.; Thumthanaruk, B.; Jumnongpon, R.; Lekhavat, S. Effect of drying on total phenolic compounds, antioxidant activities and physical properties of palm sugar. JFAT 2015, 1, 126–130. Available online: http://rs.mfu.ac.th/ojs/index.php/jfat/article/view/39/36 (accessed on 28 January 2015).
- Valder, R.; Nooralabettu, K.P. Physico-chemical changes in Palmyra Palm (Borassus flabellifer) sap at different temperature. IJSER 2018, 9, 761–766. Available online: https://www.ijser.org/onlineResearchPaperViewer.aspx?Physico-chemical-Changes-in-Palmyra-Palm-Borassus-flabellifer-sap-at-different-temperature.pdf (accessed on 1 January 2018).
- Apriyantono, A.; Aristyani, A.; Nurhayati; Lidya, Y.; Budiyanto, S.; Soekarto, S.T. Rate of browning reaction during preparation of coconut and palm sugar. Int. Congr. Ser. 2002, 1245, 275–278. [Google Scholar] [CrossRef]
- Nielsen, S.S. Total Carbohydrate by Phenol-Sulfuric Acid Method. In Food Analysis Laboratory Manual, 3rd ed.; Nielsen, S.S., Ed.; Springer: Cham, Switzerland, 2017; pp. 137–141. [Google Scholar] [CrossRef]
- Miller, G.L. Use of Dinitrosalicylic Acid Reagent for Determination of Reducing Sugar. Anal. Chem. 1959, 31, 426–428. [Google Scholar] [CrossRef]
- Guerra, M.; Mujica, M. Physical and chemical properties of granulated cane sugar “panelas”. Ciênc. Tecnol. Aliment. 2010, 30, 250–257. [Google Scholar] [CrossRef]
- Rizzolo, A.; Polesello, S. Chromatographic determination of vitamins in foods. J. Chromatogr. A 1992, 103–152. [Google Scholar] [CrossRef]
- Antakli, S.; Sarkees, N.; Sarraf, T. Determination of water-soluble Vitamins B1, B2, B3, B6, B9, B12 and C on C18 column with particle size 3 µM in some manufactured food products b HPLC with UV-DAD/FLD detection. Int. J. Pharm. Pharm. Sci. 2015, 7, 219–224. [Google Scholar]
- Zhang, Q.; Zhang, J.; Shen, J.; Silva, A.; Dennis, D.; Barrow, C. A Simple 96-Well Microplate Method for Estimation of Total Polyphenol Content in Seaweeds. J. Appl. Phycol. 2006, 18, 445–450. [Google Scholar] [CrossRef]
- Lee, J.S.; Ramalingam, S.; Jo, I.G.; Kwon, Y.S.; Bahuguna, A.; Oh, Y.S.; Kwon, O.J.; Kim, M. Comparative study of the physicochemical, nutritional, and antioxidant properties of some commercial refined and non-centrifugal sugars. Food Res. Int. 2018, 109, 614–625. [Google Scholar] [CrossRef] [PubMed]
- Payet, B.; Sing, A.S.; Smadja, J. Assessment of antioxidant activity of cane brown sugars by ABTS and DPPH radical scavenging assays: Determination of their polyphenolic and volatile constituents. J. Agric. Food Chem. 2005, 53, 10074–10079. [Google Scholar] [CrossRef]
- Asikin, Y.; Hirose, N.; Tamaki, H.; Ito, S.; Oku, H.; Wada, K. Effects of different drying–solidification processes on physical properties, volatile fraction, and antioxidant activity of non-centrifugal cane brown sugar. LWT Food Sci. Technol. 2016, 66, 340–347. [Google Scholar] [CrossRef]
- Phillips, K.M.; Carlsen, M.H.; Blomhoff, R. Total Antioxidant Content of Alternatives to Refined Sugar. J. Am. Diet. Assoc. 2009, 109, 64–71. [Google Scholar] [CrossRef]
- Rattanathanalerk, M.; Naphaporn, C.; Walaiporn, S. Effect of thermal processing on the quality loss of pineapple juice. J. Food Eng. 2005, 66, 259–265. [Google Scholar] [CrossRef]
- Nardini, M.; Pisu, P.; Gentili, V.; Natella, F.; Felice, M.D.; Piccolella, E.; Scaccini, C. Effect of caffeic acid on tert-butyl hydroperoxide-induced oxidative stress in U937. Free Radic. Biol. Med. 1998, 25, 1098–1105. [Google Scholar] [CrossRef]
- Nayaka, M.A.; Sathisha, U.V.; Manohar, M.P.; Chandrashekar, K.B.; Dharmesh, S.M. Cytoprotective and antioxidant activity studies of jaggery sugar. Food Chem. 2009, 115, 113–118. [Google Scholar] [CrossRef]
- Hansen, M.J.; Nielsen, S.E.; Berg, K. Re-examination and further development of a precise and rapid dye method for measuring cell growth/cell kill. J. Immunol. Methods 1989, 119, 203–210. [Google Scholar] [CrossRef]
- Jaffé, W.R. Nutritional and functional components of non-centrifugal cane sugar: A compilation of the data from the analytical literature. J. Food Compos. Anal. 2015, 43, 194–202. [Google Scholar] [CrossRef]
- Seguí, L.; Calabuig-Jiménez, L.; Betoret, N.; Fito, P. Physicochemical and antioxidant properties of non-refined sugarcane alternatives to white sugar. IJFST 2015, 50, 2579–2588. [Google Scholar] [CrossRef]
- Unde, P.A.; Adagale, P.V.; Hashmi, S.I.; Raheem, A.K.; Gandhi, R. Effect of Different Particle Sizes of Jaggery Powder on Storability. WJAS 2010, 7, 157–160. Available online: http://www.panelamonitor.org/media/docrepo/document/files/effect-of-different-particle-sizes-of-jaggery-powder-on-storability.pdf (accessed on 26 March 2020).
- Akochi-K, E.; Alli, I.; Kermasha, S. Characterization of the Pyrazines Formed during the Processing of Maple Syrup. J. Agric. Food Chem. 1997, 45, 3368–3373. [Google Scholar] [CrossRef]
- Ho, C.W.; Aida, W.M.W.; Maskat, M.Y.; Osman, H. Changes in volatile compounds of palm sap (Arenga pinnata) during the heating process for production of palm sugar. Food Chem. 2007, 102, 1156–1162. [Google Scholar] [CrossRef]
- Siddiqui, A.; Nazzal, S. Measurement of surface color as an expedient QC method for the detection of deviations in tablet hardness. Int. J. Pharm. 2007, 341, 173–180. [Google Scholar] [CrossRef]
- Choong, C.C.; Anzian, A.; Sapawi, C.W.; Hussin, A.S. Characterization of sugar from Arenga pinnata and Saccharum officinarum sugars. IFRJ 2016, 23, 1642–1652. Available online: http://www.asean-cites.org/index.php?r=articles%2Fpublic-view&id=187096 (accessed on 29 December 2015).
- Slade, L.; Levine, H. Beyond water activity: Recent advances based on an alternative approach to the assessment of food quality and safety. Crit. Rev. Food Sci. Nutr. 1991, 30, 115–360. [Google Scholar] [CrossRef]
- Martins, S.I.F.S.; Jongen, W.M.F.; Van Boekel, M.A.J.S. A Review of Maillard Reaction in Food and Implications to Kinetic Modelling. Trends Food Sci. Technol. 2000, 11, 364–373. [Google Scholar] [CrossRef]
- Hebbar, K.; Manivannan, A.; Ramarathinam, M.; Mathew, A.; Thamban, C.; Thomas, G.; Chowdappa, P. Coconut Inflorescence Sap and its Value Addition as Sugar—Collection Techniques, Yield, Properties and Market Perspective. Curr. Sci. 2015, 109, 1411–1417. [Google Scholar] [CrossRef]
- Abdullah, W.G.; Rianse, U.; Iswandi, R.M.; Taridala, S.A.; Widayati, W.; Rianse, I.S.; Zulfikar Baka, L.R.; Abdi, M.; Baka, W.K.; Mudihin, S. Potency of Natural Sweetener: Brown Sugar. Adv. Environ. Biol. 2014, 8, 374–385. Available online: https://fdocuments.in/document/potency-of-natural-sweetener-brown-sugar.html (accessed on 26 March 2020).
- Aburto, N.J.; Hanson, S.; Gutierrez, H.R.; Hooper, L.; Elliott, P.; Cappuccio, F.P. Effect of increased potassium intake on cardiovascular risk factors and disease: Systematic review and meta-analyses. BMJ 2013, 346, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Weerawatanakorn, M.; Asikin, Y.; Takahashi, M.; Tamaki, H.; Wada, K.; Chi-Tang, H.; Chuekittisak, R. Physico-chemical properties, wax composition, aroma profiles, and antioxidant activity of granulated non-centrifugal sugars from sugarcane cultivars of Thailand. J. Food Sci. Technol. 2016, 53, 4084–4092. [Google Scholar] [CrossRef]
- Chawla, J.; Kvarnberg, D. Hydrosoluble vitamins. In Handbook of Clinical Neurology, 3rd ed.; Jose, B., Jose, M.F., Eds.; Elsevier B.V: Amsterdam, The Netherlands, 2014; Volume 120, pp. 891–914. [Google Scholar] [CrossRef]
- Srivastava, A.; Bishnoi, S.K.; Sarkar, P.K.; Anuradha Srivastava, S.B. Value addition in palmyra palm (Borassus flabellifer L.): A potential strategy for livelihood security and poverty alleviation. Rashtriya Krishi 2017, 12, 110–112. Available online: https://www.researchgate.net/publication/320908186_Value_addition_in_palmyra_palm_Borassus_flabellifer_L_A_potential_strategy_for_livelihood_security_and_poverty_alleviation (accessed on 1 June 2017).
- Barh, D.; Mazumdar, B. Comparative Nutritive Values of Palm Saps Before and after Their Partial Fermentation and Effective Use of Wild Date (Phoenix sylvestris Roxb.) Sap in Treatment of Anemia. RJMMS 2008, 3, 173–176. Available online: http://arnmsmb.com/old/rjmms/rjmms/2008/173-176.pdf (accessed on 26 March 2020).
- Morton, J. Notes on distribution, propagation, and products of Borassus Palms (Arecaceae). Econ. Bot. 1988, 42, 420–441. [Google Scholar] [CrossRef]
- Zhang, L.-L.; Sun, Y.; Zhang, Y.-Y.; Sun, B.-G.; Chen, H.-T. Determination and Quantification of 5-Hydroxymethylfurfural in Vinegars and Soy Sauces. J. Food Qual. 2017, 2017, 1–8. [Google Scholar] [CrossRef]
- Anese, M.; Suman, M. Mitigation strategies of furan and 5-hydroxymethylfurfural in food. Food Res. Int. 2013, 51, 257–264. [Google Scholar] [CrossRef]
- Fallico, B.; Zappalà, M.; Arena, E.; Verzera, A. Effects of conditioning on HMF content in unifloral honeys. Food Chem. 2004, 85, 305–313. [Google Scholar] [CrossRef]
- Shapla, U.M.; Solayman, M.; Alam, N.; Khalil, M.I.; Gan, S.H. 5-Hydroxymethylfurfural (HMF) levels in honey and other food products: Effects on bees and human health. Chem. Cent. J. 2018, 12, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Billaud, C.; Brun-Merimee, S.; Louarme, L.; Nicolas, J. Effect of glutathione and Maillard reaction products prepared from glucose or fructose with glutathione on polyphenoloxidase from apple—I: Enzymatic browning and enzyme activity inhibition. Food Chem. 2004, 84, 223–233. [Google Scholar] [CrossRef]
- Naknean, P.; Meenune, M. Characteristics and antioxidant activity of palm sugar syrup produced in Songkhla Province, Southern Thailand. AJOFAI 2012, 4, 204–212. Available online: https://pdfs.semanticscholar.org/4fcb/c836187f2da33e3c9d017f38a31d8e587476.pdf (accessed on 26 March 2020).
- Jousse, F.; Jongen, T.; Agterof, W.; Russell, S.; Braat, P. Simplified Kinetic Scheme of Flavor Formation by the Maillard Reaction. J. Food Sci. 2002, 67, 2534–2542. [Google Scholar] [CrossRef]
- Yu, A.; Zhang, A. The effect of pH on the formation of aroma compounds produced by heating a model system containing L-ascorbic acid with L-threonine/L-serine. Food Chem. 2010, 119, 214–219. [Google Scholar] [CrossRef]
- Chuyen, N.V. Maillard Reaction and Food Processing. In Advances in Experimental Medicine and Biology; Ho, C.T., Chuyen, N.V., Eds.; Springer: Boston, MA, USA, 1998; Volume 434, pp. 213–235. [Google Scholar] [CrossRef]
- van Boekel, M.A.J.S. Formation of flavour compounds in the Maillard reaction. Biotechnol. Adv. 2006, 24, 230–233. [Google Scholar] [CrossRef]
- Wong, K.H.; Abdul Aziz, S.; Mohamed, S. Sensory aroma from Maillard reaction of individual and combinations of amino acids with glucose in acidic conditions. IJFST 2008, 43, 1512–1519. [Google Scholar] [CrossRef]
- Asikin, Y.; Kamiya, A.; Mizu, M.; Takara, K.; Tamaki, H.; Wada, K. Changes in the physicochemical characteristics, including flavour components and Maillard reaction products, of non-centrifugal cane brown sugar during storage. Food Chem. 2014, 149, 170–177. [Google Scholar] [CrossRef]
- Qureshi, M.S.; Bhongale, S.S.; Thorave, A.K. Determination of organic acid impurities in lactic acid obtained by fermentation of sugarcane juice. J. Chromatogr. A 2011, 1218, 7147–7157. [Google Scholar] [CrossRef]
- Cerny, C. The aroma side of the Maillard reaction. Ann. N. Y. Acad. Sci. 2008, 1126, 66–71. [Google Scholar] [CrossRef]
- Mustafa, R.A.; Abdul Hamid, A.; Mohamed, S. Total Phenolic Compounds, Flavonoids, and Radical Scavenging Activity of 21 Selected Tropical Plants. J. Food Sci. 2010, 75, C28–C35. [Google Scholar] [CrossRef] [PubMed]
- Chung, Y.; Chang, C.; Chao, W.; Lin, C.; Chou, S. Antioxidative Activity and Safety of the 50% Ethanolic Extract from Red Bean Fermented by Bacillus subtilis IMR-NK1. J. Agric. Food Chem. 2002, 50, 2454–2458. [Google Scholar] [CrossRef] [PubMed]
- Vijaya Kumar Reddy, C.; Sreeramulu, D.; Raghunath, M. Antioxidant activity of fresh and dry fruits commonly consumed in India. Food Res. Int. 2010, 43, 285–288. [Google Scholar] [CrossRef]
- Mahattanatawee, K.; Manthey, J.A.; Luzio, G.A.; Talcott, S.T.; Goodner, K.L.; Baldwin, E.A. Total antioxidant activity and fiber content of select Florida-grown tropical fruits. J. Agric. Food Chem. 2006, 54, 7355–7363. [Google Scholar] [CrossRef] [PubMed]
- Benjakul, S.; Lertittikul, W.; Bauer, F. Antioxidant activity of Maillard reaction products from a porcine plasma protein–sugar model system. Food Chem. 2005, 93, 189–196. [Google Scholar] [CrossRef]
- Siddhuraju, P.; Becker, K. The antioxidant and free radical scavenging activities of processed cowpea (Vigna unguiculata (L.) Walp.) seed extracts. Food Chem. 2007, 101, 10–19. [Google Scholar] [CrossRef]
- Samydurai, P.; Thangapandian, T. Nutritional assessment, polyphenols evaluation and antioxidant activity of food resource plant Decalepis hamiltonii Wight & Arn. J. Appl. Pharm. Sci. 2012, 2, 106–110. [Google Scholar] [CrossRef][Green Version]
- Ozgen, M.; Reese, R.; Tulio, A.Z.; Scheerens, J.C.; Miller, A.R. Modified 2,2-azino-bis-3-ethylbenzothiazoline-6-sulfonic acid (abts) method to measure antioxidant capacity of Selected small fruits and comparison to ferric reducing antioxidant power (FRAP) and 2,2′-diphenyl-1-picrylhydrazyl (DPPH) methods. J. Agric. Food Chem. 2006, 54, 1151–1157. [Google Scholar] [CrossRef]
- Magalhaes, L.M.; Segundo, M.A.; Reis, S.; Lima, J.L. Methodological aspects about in vitro evaluation of antioxidant properties. Anal. Chim. Acta 2008, 613, 1–19. [Google Scholar] [CrossRef]
- Farrukh, A.; Iqbal, A.; Zafar, M. Antioxidant and free radical scavenging properties of twelve traditionally used Indian medicinal plants. Turk. J. Biol. 2006, 30, 177–183. [Google Scholar] [CrossRef]
| Properties | EPS1 | EPS2 | EPS3 | NPS1 | NPS2 | NPS3 | OPS1 | OPS2 | OPS3 |
|---|---|---|---|---|---|---|---|---|---|
| Moisture content (%) | 5.12 ± 0.18 a | 4.24 ± 0.25 b | 3.88 ± 0.09 c | 5.08 ± 0.06 a | 3.31 ± 0.18 d | 3.26 ± 0.08 d | 4.97 ± 0.07 a | 3.00 ± 0.06 de | 2.91 ± 0.02 e |
| Water activity (Aw) | 0.48 ± 0.01 a | 0.45 ± 0.02 b | 0.35 ± 0.03 de | 0.40 ± 0.01 c | 0.33 ± 0.01 def | 0.31 ± 0.01 f | 0.35 ± 0.00 d | 0.32 ± 0.01 ef | 0.30 ± 0.02 f |
| pH | 6.90 ± 0.04 a | 6.96 ± 0.03 a | 6.99 ± 0.01 a | 6.92 ± 0.04 a | 6.95 ± 0.18 a | 6.99 ± 0.08 a | 6.86 ± 0.11 a | 6.98 ± 0.05 a | 6.99 ± 0.03 a |
| L* value | 116.24 ± 0.28 cd | 117.13 ± 0.84 cd | 119.14 ± 0.72 b | 115.69 ± 0.39 d | 116.25 ± 2.02 cd | 121.04 ± 0.22 a | 117.83 ± 0.39 bc | 122.57 ± 0.62 a | 121.54 ± 1.05 a |
| a* value | −0.21 ± 0.02 e | −0.17 ± 0.03 e | −0.21 ± 0.02 e | −0.11 ± 0.02 e | 0.05 ± 0.02 d | 0.15 ± 0.02 cd | 0.26 ± 0.04 bc | 0.31 ± 0.02 ab | 0.39 ± 0.09 a |
| b* value | 3.18 ± 0.24 ab | 2.85 ± 0.21 abc | 2.38 ± 0.20 cd | 2.86 ± 0.35 abc | 1.84 ± 0.42 de | 1.83 ± 0.31 de | 3.27 ± 0.18 a | 2.39 ± 0.24 bcd | 1.57 ± 0.27 e |
| Compositions | EPS1 | EPS2 | EPS3 | NPS1 | NPS2 | NPS3 | OPS1 | OPS2 | OPS3 |
|---|---|---|---|---|---|---|---|---|---|
| Total sugar (%) | 91.04 ± 0.75 d | 91.65 ± 0.72 abcd | 92.01 ± 0.16 abcd | 91.23 ± 0.78 cd | 92.87 ± 0.64 abcd | 92.93 ± 0.93 abc | 91.36 ± 0.44 bcd | 93.11 ± 0.13 ab | 93.28 ± 0.71 a |
| Reducing sugars (%) | 6.61 ± 0.30 a | 5.87 ± 0.22 bcd | 5.81 ± 0.21 bcd | 6.48 ± 0.22 ab | 5.73 ± 0.36 cd | 5.63 ± 0.16 d | 6.34 ± 0.17 abc | 5.62 ± 0.27 d | 5.55 ± 0.13 d |
| Sodium (mg) | 24.12 ± 4.85 a | 24.39 ± 1.62 a | 23.52 ± 3.93 a | 24.16 ± 3.84 a | 23.73 ±.59 a | 24.50 ±.21 a | 23.32 ±.87 a | 23.07 ±.89 a | 22.72 ±.13 a |
| Potassium (mg) | 688.45 ± 8.43 a | 689.63 ± 5.07 a | 690.76 ± 3.14 a | 702.13 ± 10.47 a | 699.93 ± 7.48 a | 698.45 ± 4.67 a | 701.49 ± 3.65 a | 705.27 ± 5.85 a | 699.73 ± 6.95 a |
| Iron (mg) | 1.99 ± 0.14 a | 2.02 ± 0.07 a | 2.03 ± 0.08 a | 1.88 ± 0.08 a | 1.92 ± 0.04 a | 1.98 ± 0.03 a | 2.05 ± 0.07 a | 2.04 ± 0.05 a | 2.01 ± 0.04 a |
| Vitamin (Per 100 g) | EPS1 | EPS2 | EPS3 | NPS1 | NPS2 | NPS3 | OPS1 | OPS2 | OPS3 |
|---|---|---|---|---|---|---|---|---|---|
| Vit A (mg) | 1.86 ± 0.09 a | 1.84 ± 0.06 a | 1.74 ± 0.06 ab | 1.84 ± 1.11 a | 1.76 ± 0.04 ab | 1.65 ± 0.05 b | 1.76 ± 0.04 ab | 1.72 ± 0.04 ab | 1.60 ± 0.04 ab |
| B1 (mg) | 0.97 ± 0.09 a | 0.92 ± 0.05 ab | 0.91 ± 0.09 ab | 0.90 ± 0.02 abc | 0.83 ± 0.05 abcd | 0.72 ± 0.04 cd | 0.83 ± 0.10 abcd | 0.77 ± 0.05 bcd | 0.70 ± 0.04 d |
| B2 (mg) | 0.07 ± 0.01 a | 0.06 ± 0.01 abc | 0.06 ± 0.01 ab | 0.06 ± 0.01 ab | 0.05 ± 0.01 abc | 0.05 ± 0.00 abc | 0.05 ± 0.01 bc | 0.05 ± 0.01 abc | 0.04 ± 0.01 c |
| B3 (mg) | 2.15 ± 0.04 a | 2.15 ± 0.02 a | 2.14 ± 0.07 a | 2.1 ± 0.12 a | 2.09 ± 0.11 a | 2.08 ± 0.07 a | 2.01 ± 0.04 a | 1.98 ± 0.12 a | 1.95 ± 0.07 a |
| B5 (mg) | 0.66 ± 0.08 ab | 0.58 ± 0.05 abc | 0.50 ± 0.02 cd | 0.67 ± 0.02 a | 0.56 ± 0.03 bc | 0.5 ± 0.02 cd | 0.61 ± 0.04 abc | 0.56 ± 0.02 bc | 0.44 ± 0.04 d |
| B6 (mg) | 0.19 ± 0.02 a | 0.14 ± 0.02 bc | 0.11 ± 0.02 cd | 0.16 ± 0.01 ab | 0.13 ± 0.02 bc | 0.12 ± 0.01 cd | 0.15 ± 0.02 abc | 0.12 ± 0.03 bcd | 0.09 ± 0.01 d |
| Folic acid (μg) | 3.12 ± 0.15 a | 3.08 ± 0.04 ab | 2.95 ± 0.11 abc | 3.01 ± 0.06 abc | 2.85 ± 0.10 abc | 2.72 ± 0.11 bc | 2.89 ± 0.09 abc | 2.72 ± 0.17 bc | 2.64 ± 0.24 c |
| Vit C (mg) | 4.01 ± 0.14 a | 3.45 ± 0.17 bcd | 3.15 ± 0.16 de | 3.85 ± 0.07 ab | 3.41 ± 0.21 bcd | 3.07 ± 0.14 de | 3.66 ± 0.23 abc | 3.21 ± 0.16 cde | 2.78 ± 0.09 e |
| Vit D2 (mg) | 2.15 ± 0.04 abc | 2.23 ± 0.02 a | 2.11 ± 0.05 c | 2.17 ± 0.03 abc | 2.21 ± 0.03 ab | 2.14 ± 0.02 bc | 2.17 ± 0.02 abc | 2.11 ± 0.05 c | 2.15 ± 0.02 abc |
| Vit E (mg) | 55.12 ± 0.88 a | 54.68 ± 1.11 a | 54.34 ± 0.69 ab | 55.01 ± 0.18 a | 54.98 ± 1.07 a | 54.23 ± 1.06 ab | 54.23 ± 0.75 ab | 54.12 ± 1.10 ab | 52.15 ± 0.60 b |
| No. | RI | Compound | Content (mg/100 g) | Odor Description | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| EPS1 | EPS2 | EPS3 | NPS1 | NPS2 | NPS3 | OPS1 | OPS2 | OPS3 | ||||
| Total alcohols | 0.560 | 0.518 | 0.498 | 0.545 | 0.513 | 0.504 | 0.544 | 0.498 | 0.477 | |||
| 1 | 931 | Ethanol | 0.211 ± 0.010 a | 0.208 ± 0.009 ab | 0.201 ± 0.004 ab | 0.213 ± 0.014 a | 0.206 ± 0.013 ab | 0.198 ± 0.010 ab | 0.208 ± 0.009 ab | 0.200 ± 0.008 ab | 0.184 ± 0.007 b | Alcoholic, solvent |
| 2 | 1540 | R-(R’,R’)-2,3-butanediol | 0.088 ± 0.003 a | 0.078 ± 0.004 abc | 0.072 ± 0.004 cd | 0.084 ± 0.005 ab | 0.077 ± 0.005 bc | 0.070 ± 0.003 cd | 0.085 ± 0.005 ab | 0.068 ± 0.002 cd | 0.066 ± 0.003 d | Sweet, grassy, fruity |
| 3 | 1579 | S-(R’,R’)-2,3-butanediol | 0.239 ± 0.011 a | 0.213 ± 0.006 cd | 0.207 ± 0.005 d | 0.227 ± 0.006 abc | 0.21 ± 0.005 cd | 0.219 ± 0.006 bcd | 0.232 ± 0.005 ab | 0.211 ± 0.006 cd | 0.210 ± 0.004 cd | Sweet, flowery, rancid |
| 4 | 1656 | 2-Furanmethanol | 0.019 ± 0.001 a | 0.015 ± 0.002 b | 0.015 ± 0.001 b | 0.018 ± 0.02 ab | 0.017 ± 0.001 a | 0.014 ± 0.001 b | 0.016 ± 0.001 b | 0.016 ± 0.002 ab | 0.014 ± 0.001 ab | Roasted, nutty, fruity |
| 5 | 1720 | 5-Methyl-2-furanmethanol | 0.002 ± 0.000 a | 0.002 ± 0.000 a | 0.002 ± 0.000 a | 0.002 ± 0.000 a | 0.002 ± 0.000 a | 0.002 ± 0.000 a | 0.002 ± 0.000 a | 0.002 ± 0.000 a | 0.002 ± 0.000 a | Sweet, fruity, minty |
| 6 | 2069 | 5-Methyl-2-pyrazinylmethanol | 0.001 ± 0.000 a | 0.001 ± 0.000 a | 0.001 ± 0.000 a | 0.001 ± 0.000 a | 0.001 ± 0.000 a | 0.001 ± 0.000 a | 0.001 ± 0.000 a | 0.001 ± 0.000 a | 0.001 ± 0.000 a | Acidic, sweat-like, sweet |
| Total ketones | 0.351 | 0.341 | 0.338 | 0.348 | 0.385 | 0.383 | 0.368 | 0.378 | 0.388 | |||
| 7 | 1256 | 4,5-Dihydro-2-methyl-3(2H)-furanone | 0.003 ± 0.000 a | 0.002 ± 0.000 b | 0.002 ± 0.000 b | 0.002 ± 0.000 b | 0.003 ± 0.000 b | 0.002 ± 0.000 a | 0.002 ± 0.000 b | 0.003 ± 0.000 a | 0.001 ± 0.000 c | Toasted, buttery |
| 8 | 1278 | 3-Hydroxy-2-butanone | 0.004 ± 0.000 a | 0.004 ± 0.000 a | 0.004 ± 0.000 a | 0.004 ± 0.000 a | 0.004 ± 0.000 a | 0.004 ± 0.000 a | 0.004 ± 0.000 a | 0.004 ± 0.000 a | 0.004 ± 0.000 a | Sweet, nutty, dairy-like |
| 9 | 1292 | 1-Hydroxy-2-propanone | 0.048 ± 0.003 a | 0.044 ± 0.003 ab | 0.042 ± 0.003 abc | 0.04 ± 0.002 bc | 0.036 ± 0.003 cd | 0.032 ± 0.003 d | 0.041 ± 0.002 bc | 0.033 ± 0.002 d | 0.032 ± 0.002 d | Sweet, grassy, coffee-like |
| 10 | 1614 | Butyrolactone | 0.009 ± 0.000 a | 0.006 ± 0.000 d | 0.005 ± 0.000 e | 0.007 ± 0.000 c | 0.006 ± 0.000 d | 0.007 ±00.000 c | 0.008 ±0.000 b | 0.006 ±0.001 d | 0.007 ±0.000 c | Cooked, sweet |
| 11 | 1746 | 2(5H)-Furanone | 0.008 ± 0.000 ab | 0.006 ± 0.001 c | 0.006 ± 0.001 c | 0.009 ± 0.001 a | 0.006 ± 0.001 c | 0.006 ± 0.000 c | 0.007 ± 0.000 bc | 0.004 ± 0.000 d | 0.004 ± 0.000 d | Pungent, cheesy |
| 12 | 1826 | 3-Methyl-1,2-cyclopentanedione | 0.001 ± 0.000 a | 0.001 ± 0.000 a | 0.001 ± 0.000 a | 0.001 ± 0.000 a | 0.001 ± 0.000 a | 0.001 ± 0.000 a | 0.001 ± 0.000 a | 0.001 ± 0.000 a | 0.001 ± 0.000 a | Sweet, maple-like, medicinal |
| 13 | 1966 | 2-Acetyl pyrrole | 0.014 ± 0.004 a | 0.012 ± 0.003 a | 0.008 ± 0.001 ab | 0.012 ± 0.003 a | 0.008 ± 0.001 ab | 0.006 ± 0.001 b | 0.010 ± 0.002 ab | 0.008 ± 0.001 ab | 0.006 ± 0.001 b | Herbaceous, metallic, sweet |
| 14 | 2027 | Pantolactone | 0.029 ± 0.002 ab | 0.026 ± 0.003 bc | 0.022 ± 0.002 c | 0.031 ± 0.002 ab | 0.030 ± 0.003 ab | 0.028 ± 0.002 a | 0.033 ± 0.003 a | 0.029 ± 0.002 ab | 0.026 ± 0.003 bc | Sweet, caramel |
| 15 | 2035 | 2,5-Dimethyl-4-hydroxy-3(2H)-furanone | 0.001 ±0.000 c | 0.003 ±0.003 a | 0.002 ±0.000 b | 0.001 ±0.000 c | 0.002 ±0.000 b | 0.001 ±0.000 c | 0.002 ±0.000 b | 0.002 ±0.000 b | 0.002 ±0.000 b | Sweet, cotton candy-like, caramel |
| 16 | 2044 | 2-Pyrrolidinone | 0.002 ± 0.00 a | 0.002 ± 0.00 a | 0.002 ± 0.00 a | 0.002 ± 0.00 a | 0.002 ± 0.00 a | 0.002 ± 0.00 a | 0.002 ± 0.00 a | 0.002 ± 0.00 a | 0.002 ± 0.00 a | Sweet, cotton candy-like, caramel |
| 17 | 2268 | 2,3-Dihydro-3,5-dihydroxy-6-methyl-4H-pyran-4-one | 0.188 ± 0.012 c | 0.192 ± 0.009 c | 0.202 ± 0.009 c | 0.198 ± 0.009 c | 0.242 ± 0.010 ab | 0.247 ± 0.013 a | 0.217 ± 0.006 bc | 0.243 ± 0.013 ab | 0.254 ± 0.011 a | Sweet, maple-like, caramel |
| 18 | 2467 | 2,5-Pyrrolidinedione | 0.044 ±0.002 ab | 0.043 ±0.002 ab | 0.042 ±0.002 b | 0.041 ±0.003 b | 0.045 ±0.003 ab | 0.047 ±0.002 ab | 0.041 ±0.002 b | 0.043 ±0.003 ab | 0.049 ±0.002 a | Sweet, cotton candy-like, caramel |
| Total pyrazines | 0.081 | 0.069 | 0.064 | 0.078 | 0.07 | 0.064 | 0.073 | 0.065 | 0.065 | |||
| 19 | 1262 | 2-Methyl-pyrazine | 0.006 ± 0.000 a | 0.005 ± 0.000 a | 0.005 ± 0.000 a | 0.005 ± 0.000 a | 0.006 ± 0.000 a | 0.006 ± 0.000 a | 0.005 ± 0.000 a | 0.006 ± 0.000 a | 0.005 ± 0.000 a | Sweet, grassy, acidic |
| 20 | 1321 | 2,5-Dimethyl-pyrazine | 0.047 ± 0.003 ab | 0.041 ± 0.002 bc | 0.043 ± 0.002 abc | 0.049 ± 0.003 a | 0.046 ± 0.001 abc | 0.042 ± 0.002 bc | 0.041 ± 0.001 bc | 0.040 ± 0.003 c | 0.046 ± 0.002 abc | Nutty, earthy, roasted |
| 21 | 1327 | 2,6-Dimethyl-pyrazine | 0.016 ± 0.002 ab | 0.013 ± 0.001 bc | 0.007 ± 0.007 e | 0.017 ± 0.002 a | 0.008 ± 0.001 de | 0.006 ± 0.001 e | 0.015 ± 0.015 ab | 0.011 ± 0.011 cd | 0.005 ± 0.001 e | Nutty, sweet |
| 22 | 1345 | 2,3-Dimethyl-pyrazine | 0.002 ± 0.000 a | 0.001 ± 0.000 b | 0.002 ± 0.000 a | 0.002 ± 0.000 a | 0.002 ± 0.000 a | 0.001 ± 0.000 b | 0.001 ± 0.000 b | 0.002 ± 0.000 a | 0.001 ± 0.000 b | Nutty, roasted, coffee-like |
| 23 | 1407 | 2,3,5-Trimethyl-pyrazine | 0.009 ± 0.001 a | 0.006 ± 0.001 bc | 0.005 ± 0.001 cd | 0.004 ± 0.000 d | 0.006 ± 0.001 bc | 0.007 ± 0.000 ab | 0.008 ± 0.001 a | 0.005 ± 0.000 cd | 0.006 ± 0.001 bc | Nutty, earthy, roasted |
| 24 | 1458 | 2-Ethyl-3,6-dimethyl-pyrazine | 0.001 ± 0.000 a | 0.003 ± 0.000 c | 0.002 ± 0.000 ab | 0.001 ± 0.000 a | 0.002 ± 0.000 ab | 0.002 ± 0.000 ab | 0.003 ± 0.00 c | 0.001 ± 0.000 a | 0.002 ± 0.000 ab | Nutty, earthy, coffee-like |
| Total acids | 0.637 | 0.545 | 0.500 | 0.633 | 0.581 | 0.522 | 0.624 | 0.554 | 0.495 | |||
| 25 | 1528 | Propanoic acid | 0.047 ± 0.002 a | 0.042 ± 0.001 ab | 0.037 ± 0.003 bc | 0.042 ± 0.002 ab | 0.038 ±0.002 bc | 0.036 ±0.002 cd | 0.039 ±0.003 bc | 0.031 ±0.002 de | 0.029 ±0.002 e | Rancid, acidic |
| 26 | 1560 | 2-Methyl-propanoic acid | 0.013 ± 0.002 ab | 0.007 ± 0.001 cd | 0.01 ± 0.000 bc | 0.012 ± 0.003 ab | 0.011 ± 0.000 e | 0.007 ± 0.001 cd | 0.014 ± 0.002 a | 0.006 ± 0.001 d | 0.004 ± 0.001 de | ---- |
| 27 | 1618 | Butanoic acid | 0.015 ± 0.002 bc | 0.012 ± 0.002 c | 0.011 ± 0.002 c | 0.019 ± 0.002 ab | 0.016 ± 0.001 abc | 0.012 ± 0.002 c | 0.021 ± 0.002 a | 0.015 ± 0.002 bc | 0.012 ± 0.003 c | Cheesy, yogurt-like, acidic |
| 28 | 1622 | 2-Propenoic acid | 0.241 ± 0.005 abc | 0.227 ± 0.010 bc | 0.213 ± 0.017 c | 0.266 ± 0.012 a | 0.245 ± 0.009 abc | 0.223 ± 0.012 bc | 0.275 ± 0.018 a | 0.256 ± 0.012 ab | 0.227 ± 0.014 bc | Baked, vinegar-like |
| 29 | 1664 | 3-Methyl-butanoic acid | 0.029 ± 0.003 a | 0.021 ± 0.003 bc | 0.019 ± 0.003 bc | 0.018 ± 0.002 c | 0.026 + 0.003 ab | 0.024 ± 0.002 abc | 0.023 ± 0.003 abc | 0.021 ± 0.002 bc | 0.019 ± 0003 bc | Cheesy, foul smell, acidic |
| 30 | 1735 | Pentanoic acid | 0.002 ± 0.000 a | 0.002 ± 0.000 a | 0.002 ± 0.000 a | 0.002 ± 0.000 a | 0.002 ± 0.000 a | 0.002 ± 0.000 a | 0.002 ± 0.000 a | 0.002 ± 0.000 a | 0.002 ± 0.000 a | Rancid, buttery |
| 31 | 2176 | 2-Hydroxy-propanoic acid | 0.088 ± 0.005 a | 0.073 ± 0.004 bc | 0.062 ± 0.004 d | 0.089 ± 0.004 a | 0.082 ± 0.003 ab | 0.071 ± 0.003 bcd | 0.084 ± 0.005 a | 0.080 ± 0.004 ab | 0.068 ± 0.003 cd | Grassy, sweat-like |
| 32 | 2417 | Benzoic acid | 0.158 ± 0.030 a | 0.121 ± 0.002 bc | 0.108 ± 0.006 cde | 0.143 ± 0.004 a | 0.117 ± 0.009 bcd | 0.109 ± 0.001 cde | 0.127 ± 0.003 b | 0.103 ± 0.004 de | 0.094 ± 0.003 e | Sweet, caramel |
| 33 | 2482 | Dodecanoic acid | 0.044 ± 0.002 a | 0.040 ± 0.002 ab | 0.038 ± 0.001 b | 0.042 ± 0.002 ab | 0.044 ± 0.002 a | 0.038 ± 0.002 b | 0.039 ± 0.002 b | 0.040 ± 0002 ab | 0.041 ± 0.002 ab | Dairy-like, caramel |
| Total sulfurs | 0.163 | 0.137 | 0.133 | 0.169 | 0.148 | 0.12 | 0.163 | 0.139 | 0.114 | |||
| 34 | 1581 | Dimethyl sulfoxide | 0.144 ± 0.003 ab | 0.121 ± 0.004 c | 0.117 ± 0.006 cd | 0.154 ± 0.005 a | 0.132 ± 0.010 bc | 0.105 ± 0.004 de | 0.149 ± 0.003 a | 0.125 ± 0.008 c | 0.101 ± 0.004 e | Rancid, pungent, metallic |
| 35 | 1895 | Dimethyl sulfone | 0.019 ± 0.002 a | 0.016 ± 0.001 ab | 0.016 ± 0.001 ab | 0.015 ± 0.001 b | 0.016 ± 0.001 ab | 0.015 ± 0.002 b | 0.014 ± 0.001 b | 0.014 ± 0.001 b | 0.013 ± 0.001 b | Sweet, waxy, sulfuric |
| Total phenols and aldehyde | 0.026 | 0.023 | 0.024 | 0.023 | 0.019 | 0.017 | 0.026 | 0.021 | 0.016 | |||
| 36 | 1852 | 2-Methoxy-phenol | 0.015 ± 0.001 ab | 0.014 ± 0.001 ab | 0.013 ± 0.001 abc | 0.012 ± 0.001 bc | 0.011 ± 0.002 cd | 0.009 ± 0.001 d | 0.016 ± 0.001 a | 0.012 ± 0.002 bc | 0.009 ± 0.001 d | Sweet, medicinal, herbaceous |
| 37 | 2263 | 2,6-Dimethoxy-phenol | 0.009 ± 0.000 a | 0.006 ± 0.000 d | 0.008 ± 0.000 b | 0.007 ± 0.000 c | 0.006 ± 0.000 d | 0.005 ± 0.000 e | 0.007 ± 0.000 c | 0.006 ± 0.000 d | 0.005 ± 0.000 e | Sweet, maple-like, caramel |
| 38 | 2549 | Vanillin | 0.002 ± 0.000 c | 0.003 ± 0.000 b | 0.003 ± 0.000 b | 0.004 ± 0.000 a | 0.002 ± 0.000 c | 0.003 ± 0.000 b | 0.003 ± 0.000 b | 0.003 ± 0.000 b | 0.002 ± 0.000 c | Sweet, cotton candy-like |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huynh Thi Le, D.; Lu, W.-C.; Li, P.-H. Sustainable Processes and Chemical Characterization of Natural Food Additives: Palmyra Palm (Borassus Flabellifer Linn.) Granulated Sugar. Sustainability 2020, 12, 2650. https://doi.org/10.3390/su12072650
Huynh Thi Le D, Lu W-C, Li P-H. Sustainable Processes and Chemical Characterization of Natural Food Additives: Palmyra Palm (Borassus Flabellifer Linn.) Granulated Sugar. Sustainability. 2020; 12(7):2650. https://doi.org/10.3390/su12072650
Chicago/Turabian StyleHuynh Thi Le, Dung, Wen-Chien Lu, and Po-Hsien Li. 2020. "Sustainable Processes and Chemical Characterization of Natural Food Additives: Palmyra Palm (Borassus Flabellifer Linn.) Granulated Sugar" Sustainability 12, no. 7: 2650. https://doi.org/10.3390/su12072650
APA StyleHuynh Thi Le, D., Lu, W.-C., & Li, P.-H. (2020). Sustainable Processes and Chemical Characterization of Natural Food Additives: Palmyra Palm (Borassus Flabellifer Linn.) Granulated Sugar. Sustainability, 12(7), 2650. https://doi.org/10.3390/su12072650






