Abundance and Phenotypic Diversity of the Medicinal Sideritis Scardica Griseb. in Relation to Floristic Composition of Its Habitat in Northern Greece
Abstract
1. Introduction
2. Materials and Methods
2.1. The Studied Species
2.2. Study Area
2.3. Analysis of Soil Characteristics
2.4. Diversity Data
2.5. Evaluation of Phenotypic Characteristics
2.6. Statistical Analysis
3. Results
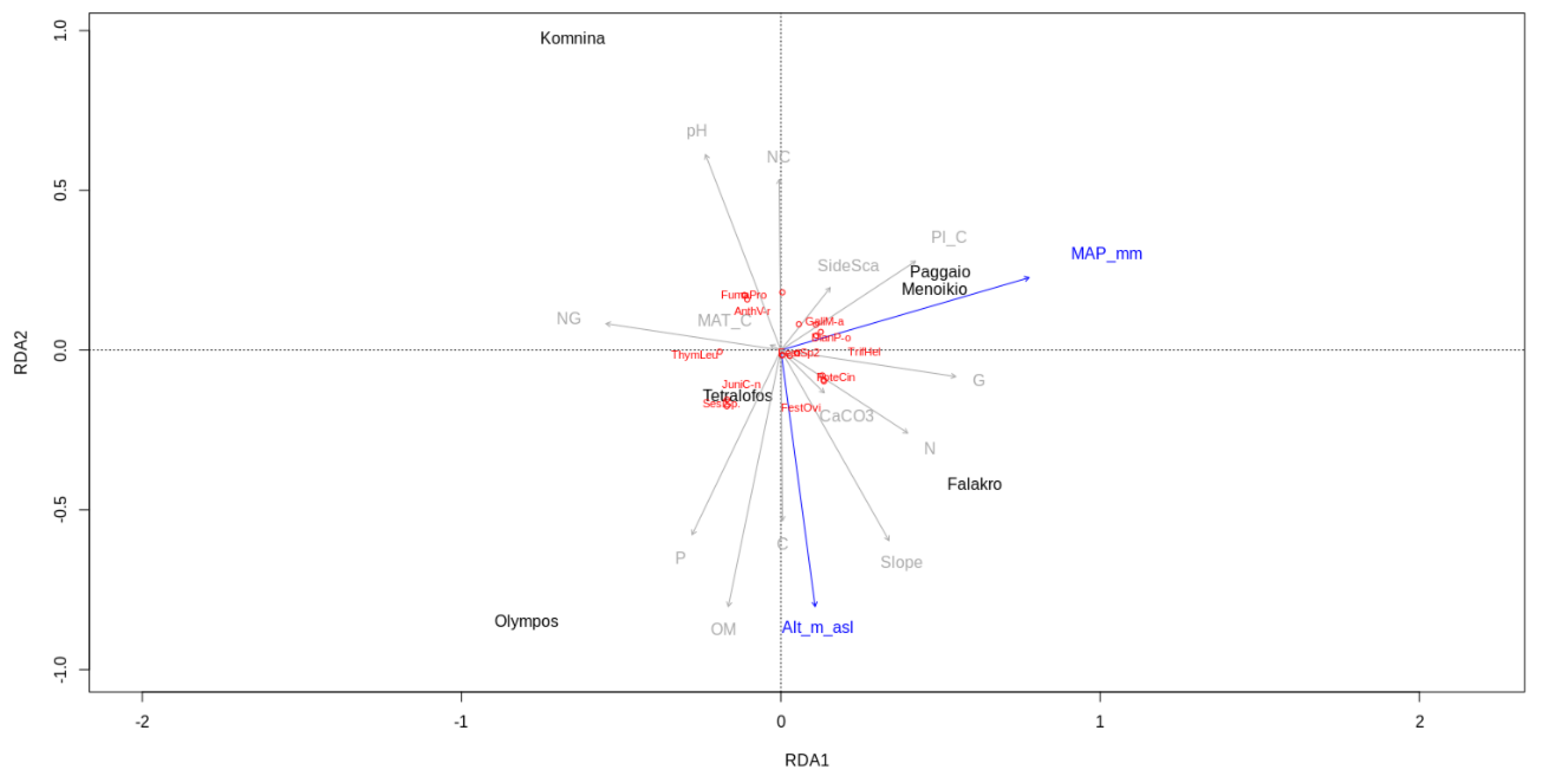
3.1. Floristic Composition and Diversity of the Studied Areas
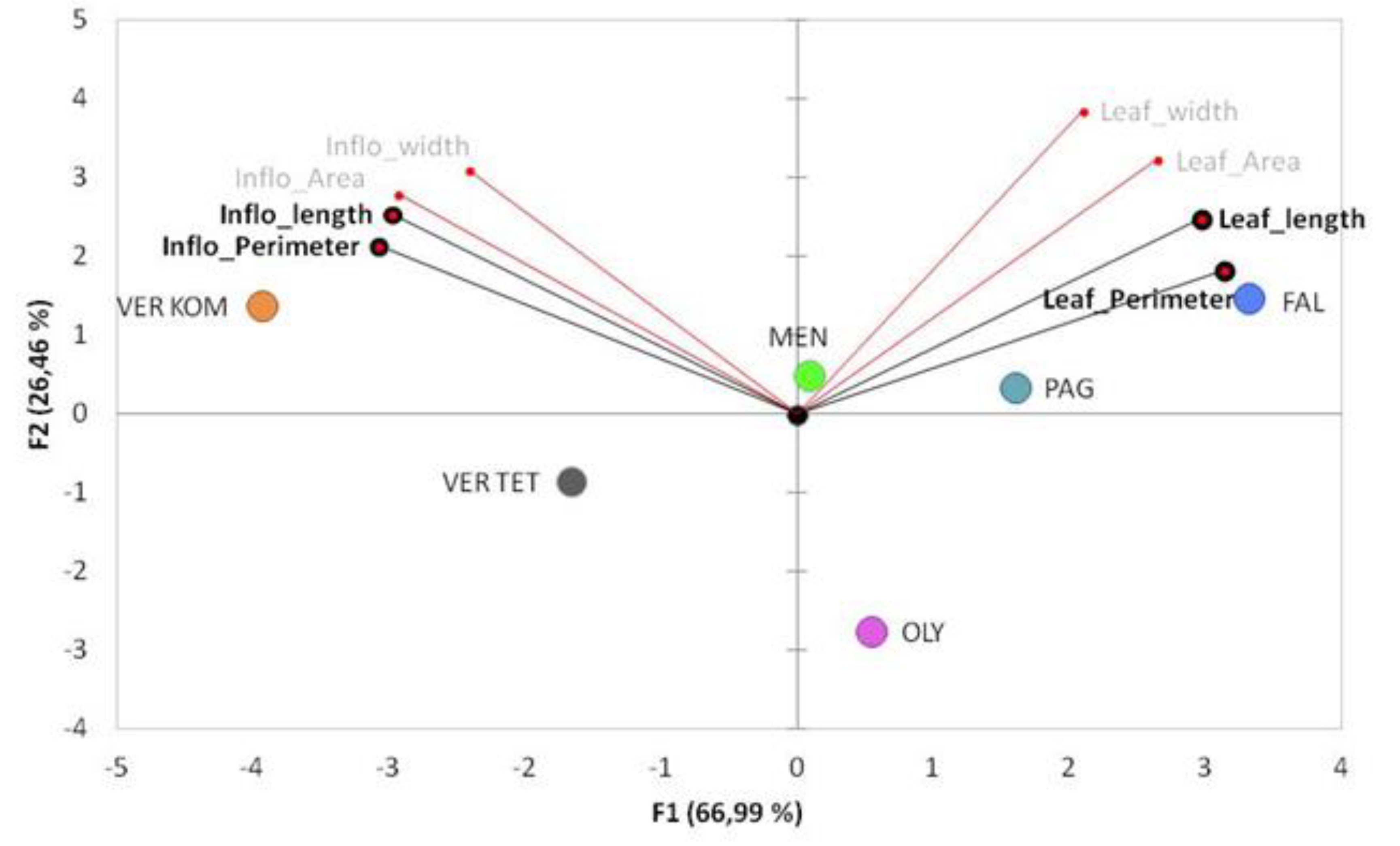
3.2. Evaluation of the Phenotypic Diversity among the Populations in the Studied Areas
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Duman, H.; Sideritis, L. Sideritis L. In Flora of Turkey and East Aegean Islands (Supplement 2); Edinburgh University Press: Edinburgh, UK, 2000; Volume 11, pp. 201–205. [Google Scholar]
- Todorova, M.; Trendafilova, A. Sideritis scardica Griseb, an endemic species of Balkan peninsula: Traditional uses, cultivation, chemical composition, biological activity. J. Ethnopharmacol. 2014, 152, 256–265. [Google Scholar] [CrossRef] [PubMed]
- González-Burgos, E.; Carretero, M.E.; Gómez-Serranillos, M.P. Sideritis spp.: Uses, chemical composition and pharmacological activities—A review. J. Ethnopharmacol. 2011, 135, 209–225. [Google Scholar]
- Tadić, V.M.; Jeremic, I.; Dobric, S.; Isakovic, A.; Markovic, I.; Trajkovic, V.; Arsic, I. Anti-inflammatory, gastroprotective, and cytotoxic effects of Sideritis scardica extracts. Planta Med. 2012, 78, 415–427. [Google Scholar]
- Petreska Stanoeva, J.; Stefova, M. Assay of urinary excretion of polyphenols after ingestion of a cup of mountain tea (Sideritis scardica) measured by HPLC-DAD-ESI-MS/MS. J. Agric. Food Chem. 2013, 61, 10488–10497. [Google Scholar] [CrossRef]
- Hofrichte, R.J.; Krohn, M.; Schumacher, T.; Lange, C.; Feistel, B.; Walbroel, B.; Pahnke, J. Sideritis spp. extracts enhance memory and learning in Alzheimer’s β-amyloidosis mouse models and aged C57Bl/6 mice. J. Alzheimers Dis. 2016, 53, 967–980. [Google Scholar] [CrossRef]
- Heiner, F.; Feistel, B.; Wink, M. Sideritis scardica extracts inhibit aggregation and toxicity of amyloid-β in Caenorhabditis elegans used as a model for Alzheimer’s disease. PeerJ 2018, 6, e4683. [Google Scholar] [CrossRef]
- Irakli, M.; Tsifodimou, K.; Sarrou, E.; Chatzopoulou, P. Optimization infusions conditions for improving phenolic content and antioxidant activity in Sideritis scardica tea using response surface methodology. JARMAP 2018, 8, 67–74. [Google Scholar] [CrossRef]
- Khela, S. Sideritis scardica. The IUCN Red List of Threatened Species. 2013: E.T203271A2762714.
- Dumont, B.; Farruggia, A.; Garel, J.P.; Bachelard, P.; Boitier, E.; Frain, M. How does grazing intensity influence the diversity of plants and insects in a species-rich upland grassland on basalt soils? Grass Forage Sci. 2009, 64, 92–105. [Google Scholar] [CrossRef]
- De Bello, F.; Lepš, J.; Sebastià, M.-T. Predictive value of plant traits to grazing along a climatic gradient in the Mediterranean. J. Appl. Ecol. 2005, 42, 824–833. [Google Scholar] [CrossRef]
- Gusmeroli, S.; Piccione, S.; Rotondi, D. A capability-based security approach to manage access control in the internet of things. Math. Comput Model. 2013, 58, 1189–1205. [Google Scholar] [CrossRef]
- Yankova-Tsvetkova, E.; Yurukova-Grancharova, P.; Vitkova, A. Reproductive biology of the Balkan endemic Sideritis scardica (Lamiaceae). Botanica Serbica 2013, 37, 83–87. [Google Scholar]
- Yang, W.Z.; Jin, H.; Li, W.Y.; Zhang, Z.H.; Zhao, Z.L.; Zhang, J.Y. A study on phenotypic diversity in different populations of endangered Coptis teeta wall of Yunnan. J. Yunnan Univ. 2013, 35, 719–726. [Google Scholar]
- Jump, S.; Peñuelas, J. Running to stand still: Adaptation and the response of plants to rapid climate change. Ecol. Lett. 2005, 8, 1010–1020. [Google Scholar] [CrossRef]
- Sultan, S.E. Promising directions in plant phenotypic plasticity. Perspect. Plant Ecol. Evol. Syst. 2004, 6, 227–233. [Google Scholar] [CrossRef]
- Zunzunegui, M.; Barradas, M.C.D.; Ain-Lhout, F.; Alvarez-Cansino, L.; Esquivias, M.P.; Novo, F.G. Seasonal physiological plasticity and recovery capacity after summer stress in Mediterranean scrub communities. Plant Ecol. 2011, 212, 127–142. [Google Scholar] [CrossRef]
- Pintado, A.; Valladares, F.; Sancho, L.G. Exploring phenotypic plasticity in the lichen Ramalina capitata: Morphology, water relations and chlorophyll content in North- and South-facing populations. Ann. Bot. 1997, 80, 345–353. [Google Scholar] [CrossRef]
- Scheiner, S.M. The genetics of phenotypic plasticity. VII. Evolution in a spatially-structured environment. J. Evol. Biol. 1998, 11, 303–320. [Google Scholar] [CrossRef]
- Grdiša, M.; Radosavljević, I.; Liber, Z.; Stefkov, G.; Ralli, P.; Chatzopoulou, P.S.; Carović-Stanko, K.; Šatović, Z. Divergent selection and genetic structure of Sideritis scardica populations from southern Balkan Peninsula as revealed by AFLP fingerprinting. Sci. Rep. 2019, 9, 1–14. [Google Scholar]
- Yordanova, M.; Apostolova, I. Estimation of the status of representative populations of Sideritis scardica Griseb. in the Rhodopi Mts. Phytol. Balc. 2000, 6, 43–57. [Google Scholar]
- Lipman, E. Report of a Working Group on Medicinal and Aromatic Plants; Second Meeting, Strumica, Macedonia, 16–18 December 2004; FYR/Third Meeting, Olomouc, Czech Republic, 26–28 June 2007; Biodiversity International: Rome, Italy, 2010. [Google Scholar]
- Von Halácsy, E. Conspectus Florae Graecae; Sumptipus Guilelmi Engelmann: Lipsiae, Germany, 1902; Volume 3. [Google Scholar]
- Papanikolaou, K.; Kokkini, S. A Taxonomic Revision of Sideritis L. Section Empedoclia (Rafin.) Bentham (Labiatae) in Greece. In Aromatic Plants; Springer: Dordrecht, The Netherlands, 1982; pp. 101–128. [Google Scholar]
- Abu-Asab, M.S.; Cantino, P.D. Systematic implications of pollen morphology in subfamilies Lamioideae and Pogostemonoideae (Labiatae). Ann. Missouri Bot. Gard. 1994, 81, 653–686. [Google Scholar] [CrossRef]
- Evstatieva, L. Sideritis scardica Griseb. Red Data Book of the Republic of Bulgaria; Joint Edition of the Bulgarian Academy of Sciences & Ministry of Environment and Water of Bulgaria: Sofia, Bulgaria, 2011. [Google Scholar]
- Kožuharov, S.; Kuzmanov, B. A Contribution to the Karyological Knowledge of the Bulgarian Plants. Caryologia 1965, 18, 349–351. [Google Scholar]
- Strid, A.; Tan, K. Mountain Flora of Greece; Edinburgh University Press: Edinburgh, UK, 1991; Volume 2. [Google Scholar]
- Strid, A. Mountain Flora of Greece; Cambridge University Press: Cambridge, UK, 1986; Volume 1. [Google Scholar]
- Ministry of Environment and Energy. 2018. Available online: http://www.ypeka.gr/Default.aspx?tabid=432&language=el-GR (accessed on 17 December 2018).
- European Commission DG Environment. Interpretation Manual of European Union Habitats—EUR28; Nature ENV B.3.: Brussels, Belgium, 2013. [Google Scholar]
- Ben Salem, H.; Papachristou, T.G. Methodology for Studying Vegetation of Grazing Lands and Determination of Grazing Animal Responses. Sustainable Grazing, Nutritional Utilization and Quality of Sheep and Goat Products; Molina Alcaide, E., Ben Salem, H., Biala, K., Morand-Fehr, P., Eds.; CIHEAM Publisher: Zaragoza, Spain, 2005; Volume 67, pp. 291–305. [Google Scholar]
- Karger, D.N.; Conrad, O.; Böhner, J.; Kawohl, T.; Kreft, H.; Soria-Auza, R.W.; Zimmermann, N.E.; Linder, H.P.; Kessler, M. Climatologies at high resolution for the earth’s land surface areas. Sci. Data 2017, 4, 170122. [Google Scholar] [CrossRef] [PubMed]
- Bingman, E. Experimental observations: Introduction. Ann. N. Y. Acad. Sci. 1982, 1, 215. [Google Scholar] [CrossRef]
- Kent, M.; Coker, P. Vegetation Description and Analysis: A Practical Approach; John Wiley & Sons: Chichester, UK, 1992. [Google Scholar]
- Barbour, M.G.; Burk, J.H.; Pitts, W.D.; Gilliam, F.S.; Schwartz, M.W. Terrestrial Plant Ecology, 3rd ed.; Addison Wesley Longman, Inc.: Menlo Park, CA, USA, 1999. [Google Scholar]
- Tutin, T.G.; Heywood, V.H.; Burges, N.A.; Moore, D.M.; Valentine, D.H.; Walters, S.M.; Webb, D.A. Flora Europaea; Cambridge University Press: Cambridge, UK, 2010; Volumes 2–5. [Google Scholar]
- Tutin, T.G.; Burges, N.A.; Chater, A.O.; Edmondson, J.R.; Heywood, V.H.; Moore, D.M.; Valentine, D.H.; Walters, S.M.; Webb, D.A. Flora Europaea, 2nd ed.; Cambridge University Press: Cambridge, UK, 1993; Volume 1. [Google Scholar]
- Pignatti, S. Flora d’ Italia; Edagricole—Edizioni Agricole della Calderini s.r.l.: Bologna, Italy, 1997; Volumes 1–3. [Google Scholar]
- Euro+Med (2006-): Euro+Med PlantBase—The Information Resource for Euro-Mediterranean Plant Diversity. Available online: http://ww2.bgbm.org/EuroPlusMed (accessed on 27 March 2019).
- Magurran, A.E. Ecological Diversity and Its Measurement; Chapman and Hall: London, UK, 1991. [Google Scholar]
- Henderson, J. Inducing History Representations for Broad Coverage Statistical Parsing. In Proceedings of the Joint Meeting of North American Chapter of the Association for Computational Linguistics and the Human Language Technology Conference, Edmonton, AB, Canada, 27 May–1 June 2003; pp. 103–110. [Google Scholar]
- IBM Corp. IBM SPSS Statistics for Windows, v.25.0.; Released; IBM Corp.: Armonk, NY, USA, 2017. [Google Scholar]
- Lavorel, S. Global change effects on landscape and regional patterns of plant diversity. Divers. Distrib. 1999, 5, 239–240. [Google Scholar] [CrossRef]
- Pearson, G.R.; Dawson, P.T. Predicting the impacts of climate change on the distribution of species: Are bioclimate envelope models models useful? Glob. Ecol. Biogeogr. 2003, 12, 361–371. [Google Scholar] [CrossRef]
- Dainese, M.; Bragazza, L. Plant traits across different habitats of the Italian Alps: A comparative analysis between native and alien species. Alp. Bot. 2012, 122, 11–21. [Google Scholar] [CrossRef]
- Dybzinski, R.; Tilman, D. Resource use patterns predict long-term outcomes of plant competition for nutrients and light. Am. Nat. 2007, 170, 305–318. [Google Scholar] [CrossRef]
- Hautier, Y.; Niklaus, P.A.; Hector, A. Competition for light causes plant biodiversity loss after eutrophication. Science 2009, 324, 636–638. [Google Scholar] [CrossRef]
- Borer, E.T.; Seabloom, E.W.; Gruner, D.S.; Harpole, W.S.; Hillebrand, H.; Lind, E.M.; Biederman, L. Herbivores and nutrients control grassland plant diversity via light limitation. Nature 2014, 508, 517–520. [Google Scholar] [CrossRef]
- Stephenson, A.G. Flower and fruit abortion: Proximate causes and ultimate functions. Annu. Rev. Ecol. Syst. 1981, 12, 253–279. [Google Scholar] [CrossRef]
- Harper, J.L. Population Biology of Plants; The Blackburn Press: Caldwell, NJ, USA, 1977. [Google Scholar]
- Huntly, N. Herbivores and the dynamics of communities and ecosystems. Annu. Rev. Ecol. Syst. 1991, 22, 477–503. [Google Scholar] [CrossRef]
- Holt, R.D.; Grover, J.; Tilman, D. Simple rules for interspecific dominance in systems with exploitative and apparent competition. Am. Nat. 1994, 144, 741–771. [Google Scholar] [CrossRef]
- Harpole, W.S.; Tilman, D. Grassland species loss resulting from reduced niche dimension. Nature 2007, 446, 791–793. [Google Scholar] [CrossRef] [PubMed]
- House, S.M. Pollination success in a population of dioecious rain forest trees. Oecologia 1993, 96, 555–561. [Google Scholar] [CrossRef]
- Kunin, W.E. Population Biology and Rarity: On the Complexity of Density Dependence in Insect—Plant Interactions. In The Biology of Aarity; Springer: Dordrecht, The Netherlands, 1997; pp. 150–173. [Google Scholar]
- Bosch, M.; Waser, N.M. Experimental manipulation of plant density and its effect on pollination and reproduction of two confamilial montane herbs. Oecologia 2001, 126, 76–83. [Google Scholar] [CrossRef]
- Sanjuán, Y.; Arnáez, J.; Beguería, S.; Lana-Renault, N.; Lasanta, T.; Gómez-Villar, A.; Álvarez-Martínez, J.; Coba-Pérez, P.; García-Ruiz, J.M. Woody plant encroachment following grazing abandonment in the subalpine belt: A case study in northern Spain. Reg. Environ. Chang. 2018, 18, 1103–1115. [Google Scholar]
- Korzeniak, J. Mountain Nardus stricta grasslands as a relic of past farming—The effects of grazing abandonment in relation to elevation and spatial scale. Folia Geob. 2016, 51, 93–113. [Google Scholar] [CrossRef]
- Lindburg, R.; Eriksson, O. Historical landscape connectivity affects present plant species diversity. Ecology 2004, 85, 1840–1845. [Google Scholar] [CrossRef]
- Helm, A.; Hanski, I.; Partel, M. Slow response of plant species richness to habitat loss and fragmentation. Ecol. Lett. 2006, 9, 72–77. [Google Scholar] [CrossRef]
- Aneva, I.; Zhelev, P. Morphometric studies of Sideritis scardica Grsb. and S. syriaca L. in their natural populations in Bulgaria. Boletin Latinoam. Caribe Plantas 2019, 18, 71–80. [Google Scholar] [CrossRef]
- Kremer, D.; Bolarić, S.; Ballian, D.; Bogunić, F.; Stešević, D.; Karlović, K.; Bezić, N. Morphological, genetic and phytochemical variation of the endemic Teucrium arduini L. (Lamiaceae). Phytochemistry 2015, 116, 111–119. [Google Scholar] [CrossRef] [PubMed]
- Talebi, S.M. Infra-specific morphological diversity in Phlomis olivieri (Labiatae). J. Biosci. 2014, 22, 59–67. [Google Scholar] [CrossRef]
- Koohdar, F.; Sheidai, M.; Talebi, S.M.; Noormohammadi, Z.; Ghasemzadeh-Baraki, S. Genetic diversity, population structure and morphological variability in the Lallemantia royleana (Lamiaceae) from Iran. Phytol. Balcan. 2016, 22, 29–38. [Google Scholar]
- Patelou, E.; Chatzopoulou, P.; Polidoros, A.N.; Mylona, P.V. Genetic diversity and structure of Sideritis raeseri Boiss. & Heldr. (Lamiaceae) wild populations from Balkan Peninsula. J. Appl. Res. Med. Aromatic 2020, 100241. [Google Scholar] [CrossRef]
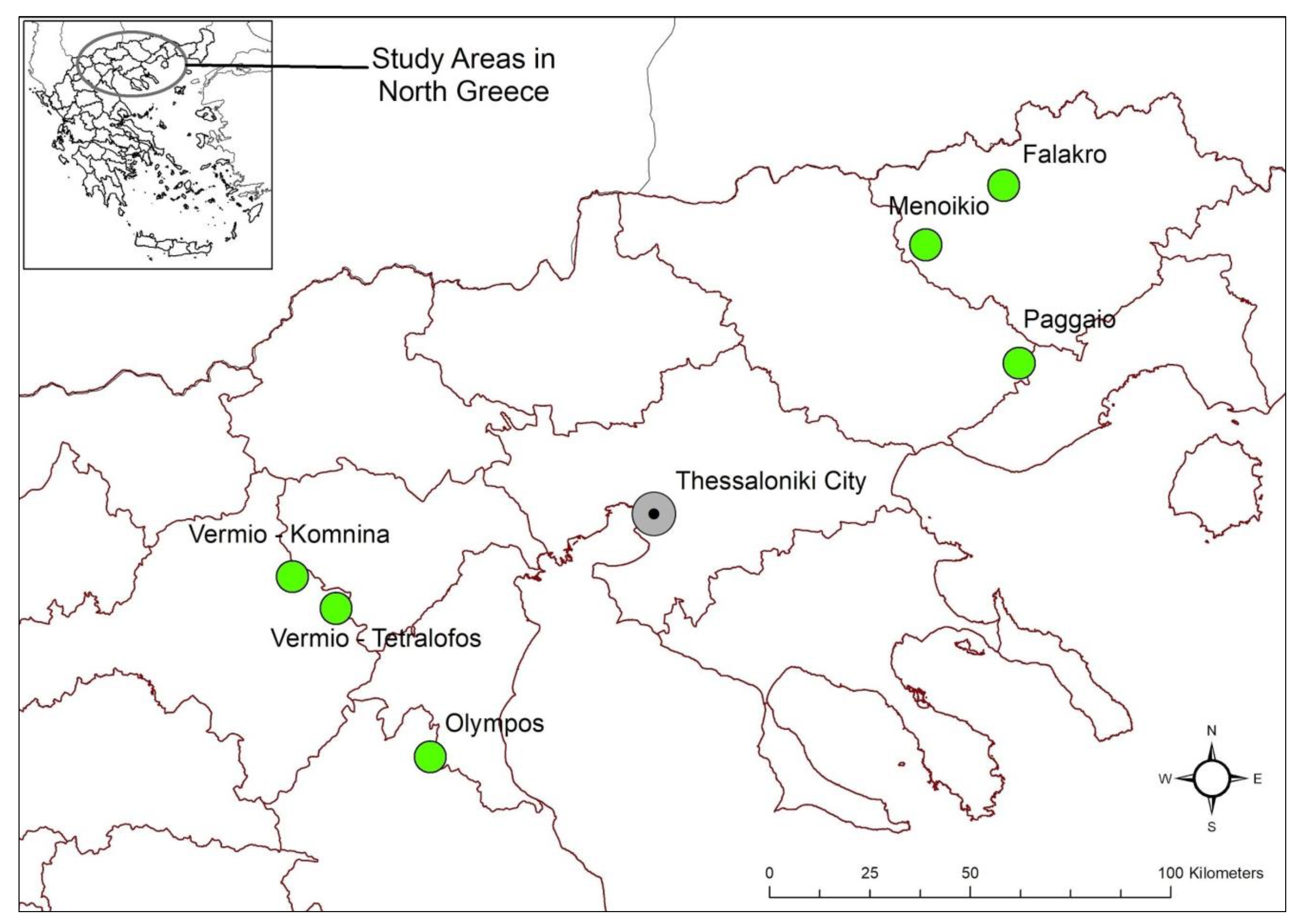

| Study Areas | Habitats | Habitat Types | Code | Natura 2000-Area Category | Dominant Species |
|---|---|---|---|---|---|
| Komnina (VerKom) | Shrubland | - | GR1210001 | SAC 1 | Helianthemum canum ssp. canum, Fumana procumbens, Sesleria caerulea |
| Tetralofos (VerTet) | Pseudo-alpine grassland | - | GR1210001 | SAC | Pilosella hoppeana ssp. testimonialis, Thymus longicaulis ssp. longicaulis, Anthyllis vulneraria ssp. rubriflora |
| Olympos (Oly) | Pseudo-alpine grassland | 6170 | GR1250001 | SAC and SPA 2 | Sesleria sp., Festuca ovina, Lotus corniculatus |
| Falakro (Fal) | Pseudo-alpine grassland | 62A0 | GR1140004 | SAC | Festuca ovina, Trifolium heldreichianum, Helianthemum canum ssp. canum |
| Paggaio (Pag) | Pseudo-alpine grassland | 6170 | GR1150005 and GR1150011 | SAC and SPA | Galium mollugo aggr., Festuca ovina, Trifolium heldreichianum |
| Menoikio (Men) | Pseudo-alpine grassland | 6170 | GR1260004 and GR1260009 | SAC and SPA | Festuca sp. 2, Fragaria vesca, Potentilla cinerea |
| Study Areas | VerKom | VerTet | Oly | Fal | Pag | Men |
|---|---|---|---|---|---|---|
| Altitude (m asl) | 1047 | 1703 | 2042 | 1958 | 1381 | 1696 |
| Latitude | 40°35′ 51.33″ | 40°27′ 57.55″ | 40°2′ 54.36″ | 41°18′ 3.42″ | 40°56′ 13.68″ | 41°11′ 33.11″ |
| Longitude | 21°48′ 58.04″ | 21°59′ 51.07″ | 22°20′ 57.13″ | 24°4′ 45.82″ | 24°4′ 49.72″ | 23°45′ 46.28″ |
| Explanatory Variables | ||||||
| Mean annual precipitation (mm) | 662 | 720 | 532 | 822 | 816 | 769 |
| Mean annual temperature (°C) | 10 | 6.3 | 4 | 3.7 | 7.4 | 5.7 |
| Management | ||||||
| Plant Cover % | 45.5 | 65.0 | 45.3 | 48.3 | 63.0 | 72.3 |
| Grazing 1 | 0 | 0 | 1 | 1 | 1 | 1 |
| Collection 2 | 1 | 1 | 1 | 0 | 1 | 0 |
| Soil features | ||||||
| MAP (mm) 3 | 662 | 720 | 532 | 822 | 816 | 769 |
| MAT (°C) 4 | 10 | 6.3 | 4 | 3.7 | 7.4 | 5.7 |
| Pl C (%) 5 | 45.5 | 65 | 45.33 | 48.33 | 63 | 72.33 |
| pH | 8.1 | 7.9 | 7.7 | 7.15 | 6.8 | 7.9 |
| OM (%) 6 | 7 | 7 | 8 | 7 | 7.7 | 4.8 |
| CaCO3 (%) | 4 | 4.4 | 25.5 | 9.8 | 4 | 6.3 |
| N | 43.17 | 40 | 199 | 16 | 73.13 | 48.6 |
| P | 33.73 | 30 | 10.6 | 22 | 38.59 | 4.16 |
| S. scardica (individuals/m2) | 0.83 | 1 | 0.83 | 3.67 | 1.17 | 4.33 |
| Study Areas | G 1 % | G 1 No 6 | L 2 % | L 2 No 6 | F 3 % | F 3 No 6 | W 4 % | W 4 No 6 | S 5 % | S 5 No 7 |
|---|---|---|---|---|---|---|---|---|---|---|
| VerKom | 29.2ab | 2.3a | 5.7b | 1.2a | 58.2a | 7.5bc | 5.8ab | 1.2ab | 1.1b | 0.8b |
| VerTet | 13.2c | 3.3a | 20.1ab | 3.2a | 52.1ab | 11.8a | 13.8ab | 1.8ab | 0.8b | 1.0b |
| Oly | 40.5a | 2.7a | 12.3ab | 1.2a | 29.3b | 5.8c | 16.4a | 2.5a | 1.4b | 0.8b |
| Fal | 30.9ab | 1.7a | 27.3a | 2.8a | 31.9b | 6.8bc | 6.3ab | 0.8b | 3.7ab | 3.7ab |
| Pag | 23.4bc | 2.5a | 15.3ab | 2.0a | 56.3a | 9.0abc | 3.6ab | 1.7ab | 1.4b | 1.2b |
| Men | 23.2bc | 1.7a | 6.1b | 1.3a | 61.8a | 10.2ab | 3.2b | 1.2ab | 5.8a | 4.3a |
| Study Areas | VerKom | VerTet | Oly | Fal | Pag | Men |
|---|---|---|---|---|---|---|
| VerKom | 1 | |||||
| VerTet | 0.115385 | 1 | ||||
| Oly | 0.061225 | 0.140845 | 1 | |||
| Fal | 0.102041 | 0.136986 | 0.066667 | 1 | ||
| Pag | 0.044776 | 0.125 | 0.066667 | 0.1 | 1 | |
| Men | 0.096154 | 0.075 | 0.0625 | 0.152174 | 0.189655 | 1 |
| Study Areas | Shannon-Wiener | Simpson | Species Number | Margalef | Equitability | Berger-Parker | McIntosh |
|---|---|---|---|---|---|---|---|
| VerKom | 2.06b | 6.87a | 12.17b | 2.60b | 0.61a | 0.28a | 0.67a |
| VerTet | 2.55a | 10.73a | 20.17a | 3.89a | 0.63a | 0.22a | 0.73a |
| Oly | 2.16ab | 8.00a | 12.17b | 2.74b | 0.69a | 0.24a | 0.71a |
| Fal | 2.04b | 6.65a | 12.17b | 2.46b | 0.63a | 0.32a | 0.64a |
| Pag | 2.40ab | 10.70a | 15.17b | 3.25ab | 0.65a | 0.20a | 0.75a |
| Men | 2.29ab | 8.62a | 14.33b | 2.91b | 0.69a | 0.22a | 0.72a |
| Inflorescence | ||||||||||
| Population | Individuals Number | Max. Stem length | Area (cm2) | CV% | Perimeter (cm) | CV% | Length (cm) | CV% | Width (cm) | CV% |
| VerKom | 24 | 38.2 ± 1.7 a | 16.2 ± 0.7 a | 20.2 | 28.0 ± 1.3 a | 22.2 | 8.7 ± 0.4 a | 19.5 | 3 ± 0.1 | 13.2 |
| VerTet | 24 | 27.3 ± 1.7 b | 10.7 ± 0.6 b | 28.8 | 21.2 ± 0.7 b | 15.3 | 6.2 ± 0.2 b | 18.3 | 3 ± 0.1 | 19.1 |
| Oly | 16 | 11.4 ± 0.7 c | 6.1 ± 0.4 d | 29.2 | 13.4 ± 0.7 c | 20.8 | 3.5 ± 0.2 d | 20.5 | 2.8 ± 0.1 | 12.7 |
| Pag | 24 | 26.8 ± 1.3 b | 9.0 ± 0.5 bc | 27.9 | 15.7 ± 0.8 c | 25.2 | 5.0± 0.3 bc | 27.8 | 2.8 ± 0.1 | 13.5 |
| Men | 24 | 27.9 ± 0.9 b | 10.3 ± 0.6 b | 27.6 | 16.5 ± 0.6 c | 18.3 | 5.2 ± 0.2 bc | 20.8 | 3 ± 0.1 | 12.2 |
| Fal | 24 | 14.4 ± 0.7 c | 7.2 ± 0.2 cd | 16.9 | 14.5 ± 0.4 c | 12.4 | 4.1 ± 0.1 cd | 12.3 | 2.9 ± 0.1 | 13.5 |
| Leaves | ||||||||||
| Population | Individuals number | No. of stems/plant | Area (cm2) | CV% | Perimeter (cm) | CV% | Length (cm) | CV% | Width (cm) | CV% |
| VerKom | 24 | 4.2 ± 0.7 ab | 2.6 ± 0.2 b | 43.1 | 10.3 ± 0.5 b | 24.5 | 4.7 ± 0.3 b | 29.6 | 0.9 ± 0 | 25.8 |
| VerTet | 24 | 3.6 ± 0.6 b | 2.7 ± 0.2 b | 42.2 | 10.2 ± 0.3 b | 16.3 | 4.8 ± 0.2 b | 16.6 | 0.9 ± 0 | 18.1 |
| Oly | 16 | 2.9 ± 0.7 b | 2.5 ± 0.2 b | 36.1 | 11.2 ± 0.7 ab | 24.6 | 4.9 ± 0.3 b | 23.7 | 0.9 ± 0.1 | 22.7 |
| Pag | 24 | 3.6 ± 0.7 b | 3.1 ± 0.2 ab | 35.5 | 11.8 ± 0.5 ab | 18.5 | 5.6 ± 0.2 ab | 19.4 | 1 ± 0 | 19.2 |
| Men | 24 | 3.7 ±0.4 b | 2.9 ± 0.3 ab | 43.2 | 11.7 ± 0.6 ab | 23.2 | 5.1 ± 0.3 b | 24.2 | 1 ± 0 | 20.3 |
| Fal | 24 | 6.5 ± 0.6 a | 3.6 ± 0.2 a | 25.4 | 13.1 ± 0.4 a | 13.2 | 6.3 ± 0.2 a | 13.3 | 1 ± 0 | 16.9 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Papaporfyriou, P.K.; Sarrou, E.; Avramidou, E.; Abraham, E.M. Abundance and Phenotypic Diversity of the Medicinal Sideritis Scardica Griseb. in Relation to Floristic Composition of Its Habitat in Northern Greece. Sustainability 2020, 12, 2542. https://doi.org/10.3390/su12062542
Papaporfyriou PK, Sarrou E, Avramidou E, Abraham EM. Abundance and Phenotypic Diversity of the Medicinal Sideritis Scardica Griseb. in Relation to Floristic Composition of Its Habitat in Northern Greece. Sustainability. 2020; 12(6):2542. https://doi.org/10.3390/su12062542
Chicago/Turabian StylePapaporfyriou, Pinelopi K., Eirini Sarrou, Eleni Avramidou, and Eleni M. Abraham. 2020. "Abundance and Phenotypic Diversity of the Medicinal Sideritis Scardica Griseb. in Relation to Floristic Composition of Its Habitat in Northern Greece" Sustainability 12, no. 6: 2542. https://doi.org/10.3390/su12062542
APA StylePapaporfyriou, P. K., Sarrou, E., Avramidou, E., & Abraham, E. M. (2020). Abundance and Phenotypic Diversity of the Medicinal Sideritis Scardica Griseb. in Relation to Floristic Composition of Its Habitat in Northern Greece. Sustainability, 12(6), 2542. https://doi.org/10.3390/su12062542






