Quantifying Changes in Plant Species Diversity in a Savanna Ecosystem Through Observed and Remotely Sensed Data
Abstract
1. Introduction
2. Materials and Methods
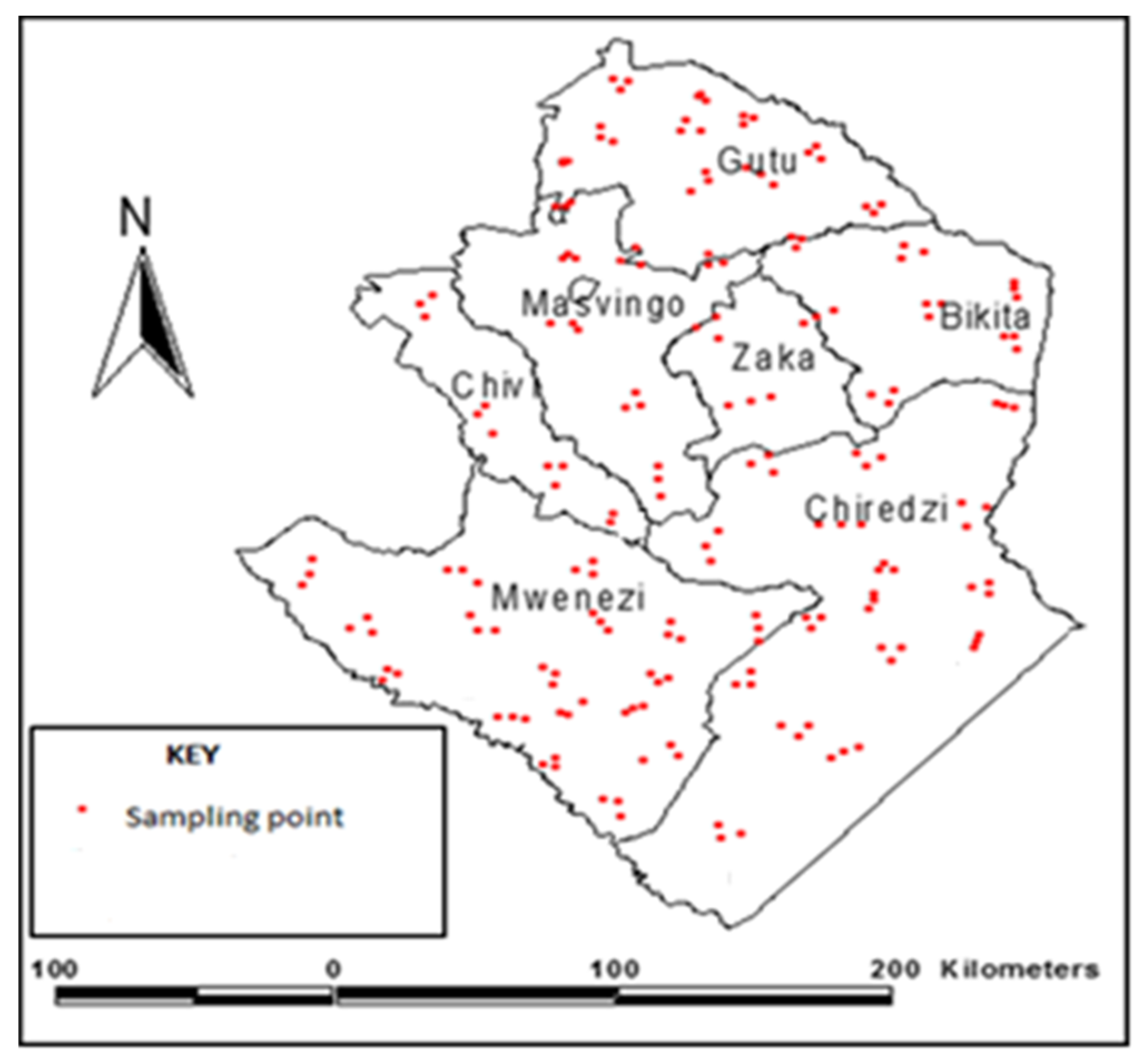
2.1. Description of the Study Area
2.2. Sampling and Data Collection
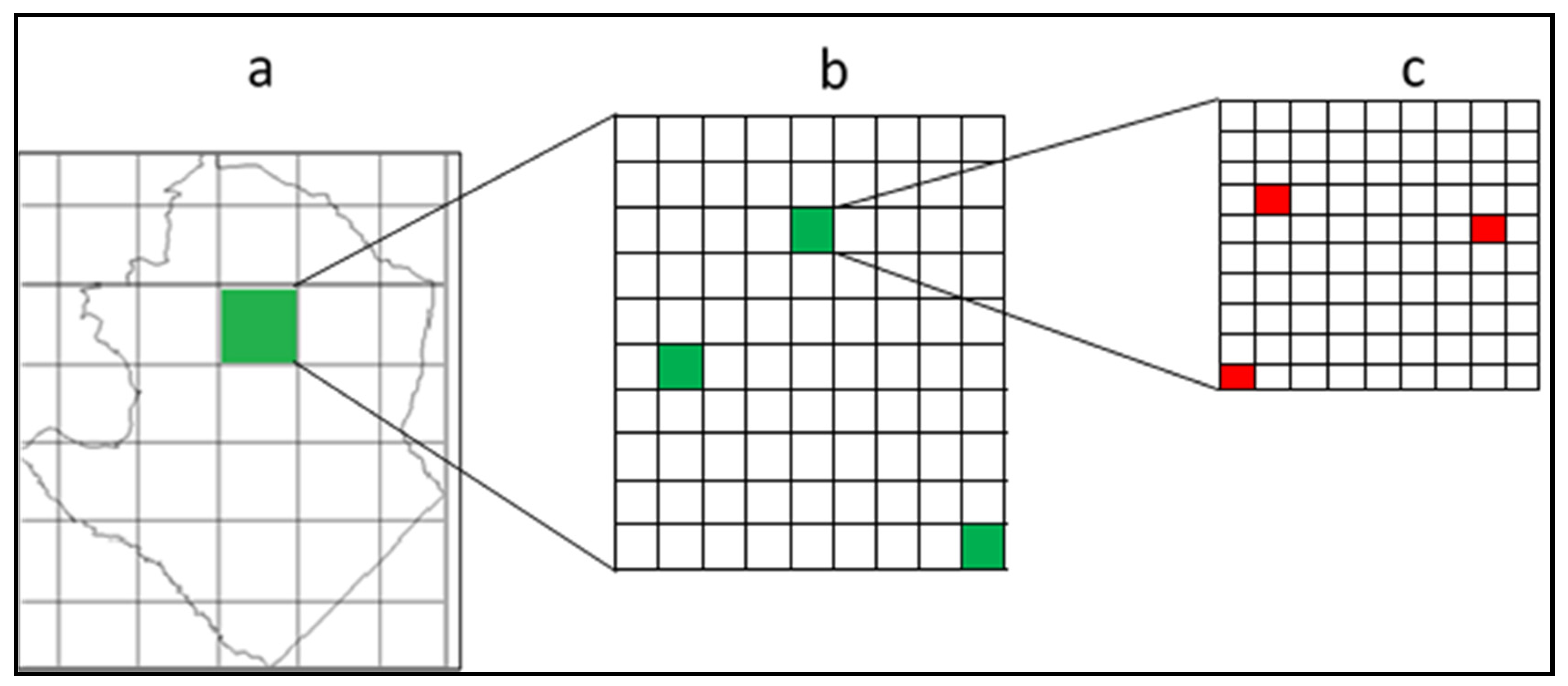
- Step 1: Grids of the same size were overlaid on the map of Masvingo province (Figure 2a). These grids were meant to divide the study area in a way that would allow samples to be obtained from all areas of geographic differences. All grids covering more than 40% of the province were selected. Each grid represents an area from which samples were taken. This makes the samples more representative as they cover all geographic areas throughout the study area. A total of 22 grids were selected.
- Step 2: Grids selected in step 1 were further subdivided into smaller grids of the same size (Figure 2b), from which 3 were randomly selected using the random point generator in ArcGIS. Thus, the number of selected grids increased to 66.
- Step 3: Grids in step 2 were further subdivided to come up with smaller grids of the same size (Figure 2c). Three smaller grids or sub-cells were randomly selected from each larger grid established in step 2. Thus, the number of selected grids increased to 198. These were the sampling points from which data were collected. From these selected grids, plots of 30 m x 30 m were established.
2.3. Satellite Image Processing
2.4. Rainfall and Temperature Data
2.5. Data Analysis
2.6. Trend Testing
2.7. Interpolation
2.8. Shannon Wiener Index (H)
2.9. Questionnaire Surveys
2.10. Key Informant Interviews
3. Results and Discussion
3.1. Climate Change Trends in Masvingo Province
3.1.1. Temperature
3.1.2. Precipitation
3.2. Climate Change and Plant Species Diversity
3.2.1. Plant Species Diversity and Precipitation-Related Variables
3.2.2. Vegetative Species Diversity and Temperature-Related Variables
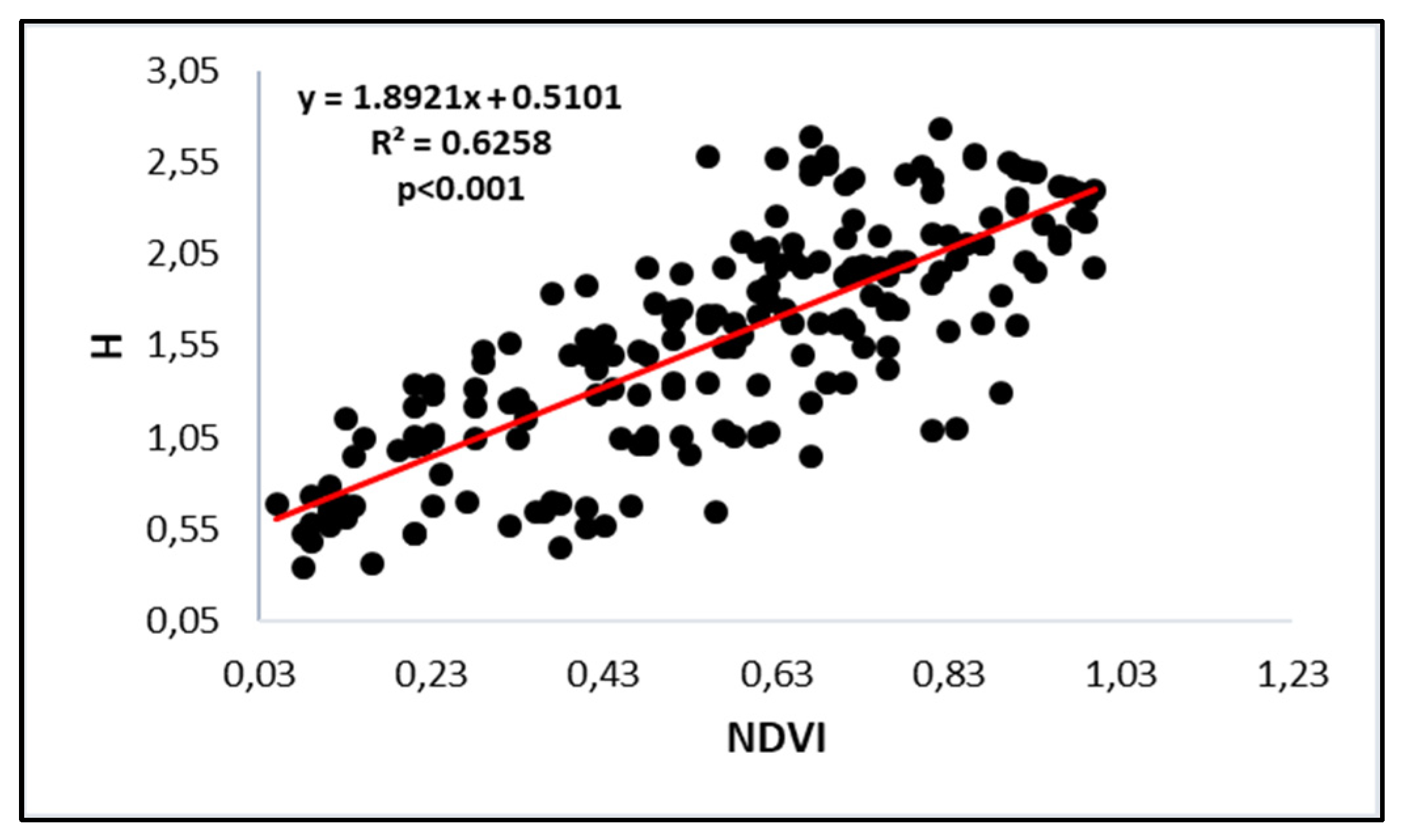
3.3. Diversity Dynamics Depicted Through Remote Sensing
3.4. Changes in Species Diversity Between 1974 and 2014
3.5. Evidence of Changes in Vegetative Species Diversity Through Interviews
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bellard, C.; Bertelsmeier, C.; Leadley, P.; Thuiller, W.; Courchamp, F. Impacts of climate change on the future of biodiversity. Ecol. Lett. 2012, 15, 365–377. [Google Scholar] [CrossRef] [PubMed]
- Henson, S.A.; Beaulieu, C.; Ilyina, T.; John, J.G.; Long, M.; Séférian, R.; Tjiputra, J.; Sarmiento, J.L. Rapid emergence of climate change in environmental drivers of marine ecosystems. Nat. Commun. 2017, 8, 14682. [Google Scholar] [CrossRef]
- Liu, H.; Mi, Z.; Lin, L.; Wang, Y.; Zhang, Z.; Zhang, F.; Wang, H.; Liu, L.; Zhu, B.; Cao, G. Shifting plant species composition in response to climate change stabilizes grassland primary production. Proc. Natl. Acad. Sci. USA 2018, 115, 4051–4056. [Google Scholar] [CrossRef] [PubMed]
- Jewitt, D.; Erasmus, B.F.; Goodman, P.S.; O’Connor, T.G.; Hargrove, W.W.; Maddalena, D.M.; Witkowski, E.T. Climate-induced change of environmentally defined floristic domains: A conservation based vulnerability framework. Appl. Geogr. 2015, 63, 33–42. [Google Scholar] [CrossRef]
- Ummenhofer, C.C.; Meehl, G.A. Extreme weather and climate events with ecological relevance: A review. Philos. Trans. R. Soc. B Biol. Sci. 2017, 372, 20160135. [Google Scholar] [CrossRef] [PubMed]
- Handmer, J.; Honda, Y.; Kundzewicz, Z.W.; Arnell, N.; Benito, G.; Hatfield, J.; Mohamed, I.F.; Peduzzi, P.; Wu, S.; Sherstyukov, B. Changes in impacts of climate extremes: Human systems and ecosystems. In Managing the Risks of Extreme Events and Disasters to Advance Climate Change Adaptation, Special Report of the Intergovernmental Panel on Climate Change (IPCC); IPCC: Cambridge, UK, 2012; pp. 231–290. [Google Scholar]
- Banerjee, K.; Gatti, R.C.; Mitra, A. Climate change-induced salinity variation impacts on a stenoecious mangrove species in the Indian Sundarbans. Ambio 2017, 46, 492–499. [Google Scholar] [CrossRef] [PubMed]
- Abreu, R.C.; Hoffmann, W.A.; Vasconcelos, H.L.; Pilon, N.A.; Rossatto, D.R.; Durigan, G. The biodiversity cost of carbon sequestration in tropical savanna. Sci. Adv. 2017, 3, e1701284. [Google Scholar] [CrossRef]
- Tsalyuk, M.; Kelly, M.; Getz, W.M. Improving the prediction of African savanna vegetation variables using time series of MODIS products. ISPRS J. Photogramm. Remote Sens. 2017, 131, 77–91. [Google Scholar] [CrossRef]
- Nhamo, L.; Magidi, J.; Dickens, C. Determining wetland spatial extent and seasonal variations of the inundated area using multispectral remote sensing. Water SA 2017, 43, 543–552. [Google Scholar] [CrossRef]
- FAO. The State of Food and Agriculture. Climate Change, Agriculture and Food Security; Food and Agriculture Organization of the United Nations (FAO): Rome, Italy, 2016; p. 194. [Google Scholar]
- Devine, A.P.; McDonald, R.A.; Quaife, T.; Maclean, I.M. Determinants of woody encroachment and cover in African savannas. Oecologia 2017, 183, 939–951. [Google Scholar] [CrossRef]
- Timpane-Padgham, B.L.; Beechie, T.; Klinger, T. A systematic review of ecological attributes that confer resilience to climate change in environmental restoration. PLoS ONE 2017, 12, e0173812. [Google Scholar] [CrossRef] [PubMed]
- Parmesan, C.; Yohe, G. A globally coherent fingerprint of climate change impacts across natural systems. Nature 2003, 421, 37. [Google Scholar] [CrossRef] [PubMed]
- Gatti, R.C.; Di Paola, A.; Bombelli, A.; Noce, S.; Valentini, R. Exploring the relationship between canopy height and terrestrial plant diversity. Plant Ecol. 2017, 218, 899–908. [Google Scholar] [CrossRef]
- Walther, G.-R.; Post, E.; Convey, P.; Menzel, A.; Parmesan, C.; Beebee, T.J.; Fromentin, J.-M.; Hoegh-Guldberg, O.; Bairlein, F. Ecological responses to recent climate change. Nature 2002, 416, 389. [Google Scholar] [CrossRef] [PubMed]
- Hillebrand, H.; Blasius, B.; Borer, E.T.; Chase, J.M.; Downing, J.A.; Eriksson, B.K.; Filstrup, C.T.; Harpole, W.S.; Hodapp, D.; Larsen, S. Biodiversity change is uncoupled from species richness trends: Consequences for conservation and monitoring. J. Appl. Ecol. 2018, 55, 169–184. [Google Scholar] [CrossRef]
- Dobson, A. Monitoring global rates of biodiversity change: Challenges that arise in meeting the Convention on Biological Diversity (CBD) 2010 goals. Philos. Trans. R. Soc. B Biol. Sci. 2005, 360, 229–241. [Google Scholar] [CrossRef]
- Tittensor, D.P.; Walpole, M.; Hill, S.L.; Boyce, D.G.; Britten, G.L.; Burgess, N.D.; Butchart, S.H.; Leadley, P.W.; Regan, E.C.; Alkemade, R. A mid-term analysis of progress toward international biodiversity targets. Science 2014, 346, 241–244. [Google Scholar] [CrossRef] [PubMed]
- Hoehndorf, R.; Alshahrani, M.; Gkoutos, G.V.; Gosline, G.; Groom, Q.; Hamann, T.; Kattge, J.; de Oliveira, S.M.; Schmidt, M.; Sierra, S. The flora phenotype ontology (FLOPO): Tool for integrating morphological traits and phenotypes of vascular plants. J. Biomed. Semant. 2016, 7, 65. [Google Scholar] [CrossRef]
- Kattge, J.; Diaz, S.; Lavorel, S.; Prentice, I.C.; Leadley, P.; Bönisch, G.; Garnier, E.; Westoby, M.; Reich, P.B.; Wright, I.J. TRY–a global database of plant traits. Glob. Chang. Biol. 2011, 17, 2905–2935. [Google Scholar] [CrossRef]
- Kuenzer, C.; Ottinger, M.; Wegmann, M.; Guo, H.; Wang, C.; Zhang, J.; Dech, S.; Wikelski, M. Earth observation satellite sensors for biodiversity monitoring: Potentials and bottlenecks. Int. J. Remote Sens. 2014, 35, 6599–6647. [Google Scholar] [CrossRef]
- Chapungu, L.; Nhamo, L.; Gatti, R.C. Estimating biomass of savanna grasslands as a proxy of carbon stock using multispectral remote sensing. Remote Sens. Appl. Soc. Environ. 2020, 17, 100275. [Google Scholar] [CrossRef]
- Pettorelli, N.; Ryan, S.; Mueller, T.; Bunnefeld, N.; Jędrzejewska, B.; Lima, M.; Kausrud, K. The Normalized Difference Vegetation Index (NDVI): Unforeseen successes in animal ecology. Clim. Res. 2011, 46, 15–27. [Google Scholar] [CrossRef]
- Wang, R.; Gamon, J.; Montgomery, R.; Townsend, P.; Zygielbaum, A.; Bitan, K.; Tilman, D.; Cavender-Bares, J. Seasonal variation in the NDVI–species richness relationship in a prairie grassland experiment (Cedar Creek). Remote Sens. 2016, 8, 128. [Google Scholar] [CrossRef]
- Walker, R.; Stoms, D.; Estes, J.; Cayocca, K. Relationships between Biological Diversity and Multi-temporal Vegetation Index Data in California; American Society for Photogrametry & Remote Sensing (ASPRS) Convention: Albuquerque, NM, USA, 1992; p. 562. [Google Scholar]
- Jørgensen, A.; Nøhr, H. The use of satellite images for mapping of landscape and biological diversity in the Sahel. Int. J. Remote Sens. 1996, 17, 91–109. [Google Scholar] [CrossRef]
- Rafique, R.; Zhao, F.; de Jong, R.; Zeng, N.; Asrar, G. Global and regional variability and change in terrestrial ecosystems net primary production and NDVI: A model-data comparison. Remote Sens. 2016, 8, 177. [Google Scholar] [CrossRef]
- Zhang, M.; Lin, H.; Wang, G.; Sun, H.; Cai, Y. Estimation of Vegetation Productivity Using a Landsat 8 Time Series in a Heavily Urbanized Area, Central China. Remote Sens. 2019, 11, 133. [Google Scholar] [CrossRef]
- Skidmore, A.; Oindo, B.; Said, M. Biodiversity assessment by remote sensing. In Proceedings of the 30th International symposium on remote sensing of the environment: Information for risk management and sustainable development, Honolulu, Hawai, 10–14 November 2003; p. 4. [Google Scholar]
- Chapungu, L.; Yekeye, T. Estimating tree species diversity in small scale farming areas for effective environmental management. The case of Bindura and Shamva Districts, Zimbabwe. Sacha J. Environ. Stud. 2013, 3, 23–33. [Google Scholar]
- Sutherland, W.J. Ecological Census Techniques: A Handbook; Cambridge University Press: Cambridge, UK, 2006. [Google Scholar]
- Ludwig, J.A.; Reynolds, J. Statistical Ecology: A Primer in Methods and Computing; John Wiley & Sons: New York, NY, USA, 1988; Volume 1. [Google Scholar]
- GloVis. Global Visualization Viewer (GloVis); U.S. Department of the Interior, U.S. Geological Survey: Reston, VA, USA, 2005. Available online: https://glovis.usgs.gov/ (accessed on 17 March 2020).
- Chander, G.; Markham, B.L.; Helder, D.L. Summary of current radiometric calibration coefficients for Landsat MSS, TM, ETM+, and EO-1 ALI sensors. Remote Sens. Environ. 2009, 113, 893–903. [Google Scholar] [CrossRef]
- Tucker, C.J. Red and photographic infrared linear combinations for monitoring vegetation. Remote Sens. Environ. 1979, 8, 127–150. [Google Scholar] [CrossRef]
- Mpandeli, S.; Nhamo, L.; Moeletsi, M.; Masupha, T.; Magidi, J.; Tshikolomo, K.; Liphadzi, S.; Naidoo, D.; Mabhaudhi, T. Assessing climate change and adaptive capacity at local scale using observed and remotely sensed data. Weather Clim. Extrem. 2019, 26, 100240. [Google Scholar] [CrossRef]
- Matsushita, B.; Yang, W.; Chen, J.; Onda, Y.; Qiu, G. Sensitivity of the enhanced vegetation index (EVI) and normalized difference vegetation index (NDVI) to topographic effects: A case study in high-density cypress forest. Sensors 2007, 7, 2636–2651. [Google Scholar] [CrossRef] [PubMed]
- Young, A.H.; Knapp, K.R.; Inamdar, A.; Rossow, W.B.; Hankins, W. The International Satellite Cloud Climatology Project, H-Series Climate Data Record Product; Earth System Science Data: Asheville, NC, USA, 2017. [Google Scholar] [CrossRef]
- Massey, F.J., Jr. The Kolmogorov-Smirnov test for goodness of fit. J. Am. Stat. Assoc. 1951, 46, 68–78. [Google Scholar] [CrossRef]
- Hamed, K.H.; Rao, A.R. A modified Mann-Kendall trend test for autocorrelated data. J. Hydrol. 1998, 204, 182–196. [Google Scholar] [CrossRef]
- Von Storch, H. Misuses of statistical analysis in climate research. In Analysis of Climate Variability; von Storch, H., Navarra, A., Eds.; Springer: Berlin/Heidelberg, Germany, 1999; pp. 11–26. [Google Scholar]
- Mann, H.B. Nonparametric tests against trend. Econom. J. Econom. Soc. 1945, 13, 245–259. [Google Scholar] [CrossRef]
- Kendall, M.G. Rank Correlation Methods; Griffin: London, UK, 1945. [Google Scholar]
- Ahmad, I.; Tang, D.; Wang, T.; Wang, M.; Wagan, B. Precipitation trends over time using Mann-Kendall and spearman’s rho tests in swat river basin, Pakistan. Adv. Meteorol. 2015, 2015, 15. [Google Scholar] [CrossRef]
- Hoke, M.; Ross, B.; Wickesberg, R.; Lütkenhöner, B. Weighted averaging—theory and application to electric response audiometry. Electroencephalogr. Clin. Neurophysiol. 1984, 57, 484–489. [Google Scholar] [CrossRef]
- Gwitira, I.; Murwira, A.; Shekede, M.D.; Masocha, M.; Chapano, C. Precipitation of the warmest quarter and temperature of the warmest month are key to understanding the effect of climate change on plant species diversity in S outhern A frican savannah. Afr. J. Ecol. 2014, 52, 209–216. [Google Scholar] [CrossRef]
- Lutz, J.; Volkholz, J.; Gerstengarbe, F.-W. Climate projections for southern Africa using complementary methods. Int. J. Clim. Chang. Strateg. Manag. 2013, 5, 130–151. [Google Scholar] [CrossRef]
- Kusangaya, S.; Warburton, M.L.; Van Garderen, E.A.; Jewitt, G.P. Impacts of climate change on water resources in southern Africa: A review. Phys. Chem. EarthParts A/B/C 2014, 67, 47–54. [Google Scholar] [CrossRef]
- Rocchini, D.; Balkenhol, N.; Carter, G.A.; Foody, G.M.; Gillespie, T.W.; He, K.S.; Kark, S.; Levin, N.; Lucas, K.; Luoto, M. Remotely sensed spectral heterogeneity as a proxy of species diversity: Recent advances and open challenges. Ecol. Inform. 2010, 5, 318–329. [Google Scholar] [CrossRef]
- Andrus, R.A.; Harvey, B.J.; Rodman, K.C.; Hart, S.J.; Veblen, T.T. Moisture availability limits subalpine tree establishment. Ecology 2018, 99, 567–575. [Google Scholar] [CrossRef] [PubMed]
- Russell, A.E.; Kivlin, S.N.; Hawkes, C.V. Tropical tree species effects on soil pH and biotic factors and the consequences for macroaggregate dynamics. Forests 2018, 9, 184. [Google Scholar] [CrossRef]
- Cazzolla Gatti, R.; Dudko, A.; Lim, A.; Velichevskaya, A.I.; Lushchaeva, I.V.; Pivovarova, A.V.; Volkov, I.V. The last 50 years of climate-induced melting of the Maliy Aktru glacier (Altai Mountains, Russia) revealed in a primary ecological succession. Ecol. Evol. 2018, 8, 7401–7420. [Google Scholar] [CrossRef] [PubMed]
- Cazzolla Gatti, R.; Callaghan, T.; Velichevskaya, A.; Dudko, A.; Fabbio, L.; Battipaglia, G.; Liang, J. Accelerating upward treeline shift in the Altai Mountains under last-century climate change. Sci. Rep. 2019, 9, 1–13. [Google Scholar] [CrossRef]
- Fukami, T.; Wardle, D.A. Long-term ecological dynamics: Reciprocal insights from natural and anthropogenic gradients. Proc. R. Soc. B Biol. Sci. 2005, 272, 2105–2115. [Google Scholar] [CrossRef]
- Garnier, E.; Cortez, J.; Billès, G.; Navas, M.L.; Roumet, C.; Debussche, M.; Laurent, G.; Blanchard, A.; Aubry, D.; Bellmann, A.; et al. Plant functional markers capture ecosystem properties during secondary succession. Ecology. 2004, 85, 2630–2637. [Google Scholar] [CrossRef]
- Lavorel, S.; Garnier, E. Predicting changes in community composition and ecosystem functioning from plant traits: Revisiting the Holy Grail. Functional Ecology. 2002, 16, 545–556. [Google Scholar] [CrossRef]
- Lavorel, S.; Grigulis, K.; McIntyre, S.; Williams, N.S.; Garden, D.; Dorrough, J.; Berman, S.; Quétier, F.; Thébault, A.; Bonis, A. Assessing functional diversity in the field–methodology matters! Functional Ecology. 2008, 22, 134–147. [Google Scholar] [CrossRef]
- Blois, J.L.; Williams, J.W.; Fitzpatrick, M.C.; Jackson, S.T.; Ferrier, S. Space can substitute for time in predicting climate-change effects on biodiversity. Proc. Natl. Acad. Sci. USA 2013, 110, 9374–9379. [Google Scholar] [CrossRef]
- Pickett, S.T. Space-for-time substitution as an alternative to long-term studies. In Long-Term Studies in Ecology; Springer: New York, NY, USA, 1989; pp. 110–135. [Google Scholar]

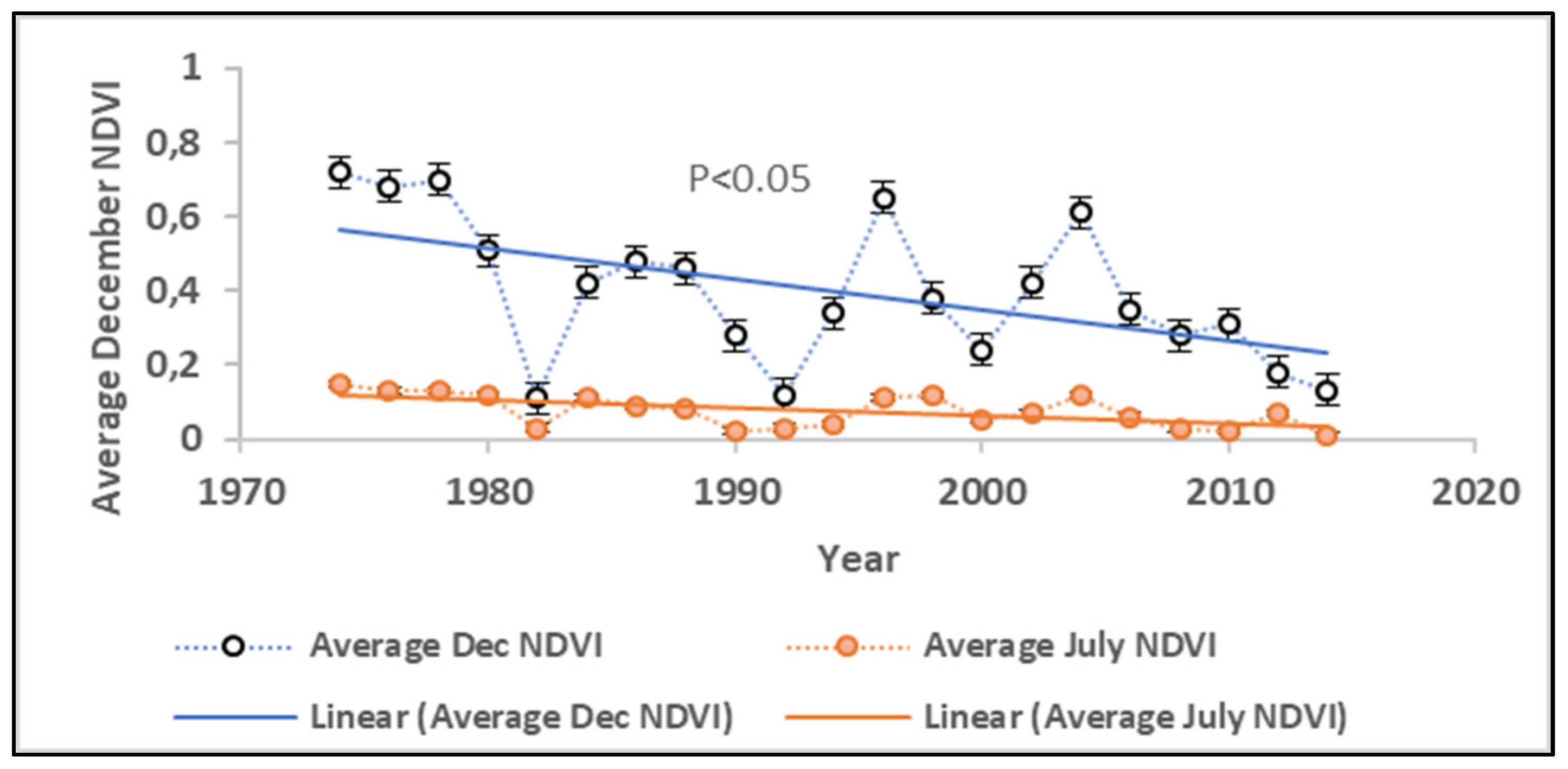
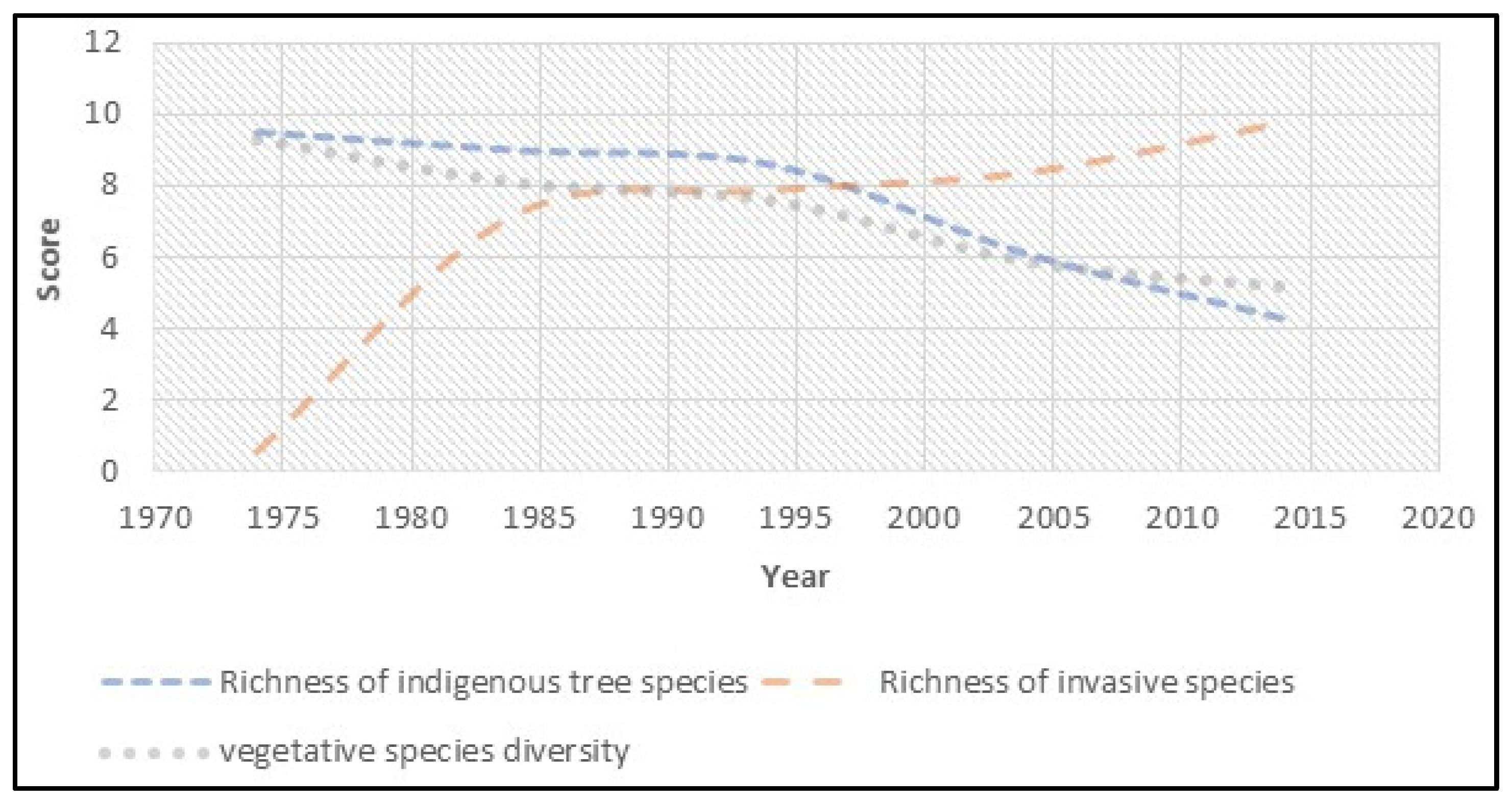
| Year | Indigenous Species | Invasive Species | Plant Species Diversity |
|---|---|---|---|
| 1974 | 9.5 | 0.6 | 9.3 |
| 1978 | 9.1 | 4.8 | 8.6 |
| 1982 | 9 | 6.1 | 8.3 |
| 1986 | 9 | 7.2 | 8.1 |
| 1990 | 8.8 | 7.9 | 7.9 |
| 1994 | 8.6 | 7.9 | 7.6 |
| 1998 | 6.8 | 8.0 | 6.8 |
| 2002 | 6.1 | 8.4 | 5.9 |
| 2006 | 5.9 | 8.7 | 5.6 |
| 2010 | 5 | 9.2 | 5.4 |
| 2014 | 4.3 | 9.8 | 5.2 |
| Climatic Variable | m + c | P-Value | Trend Description |
|---|---|---|---|
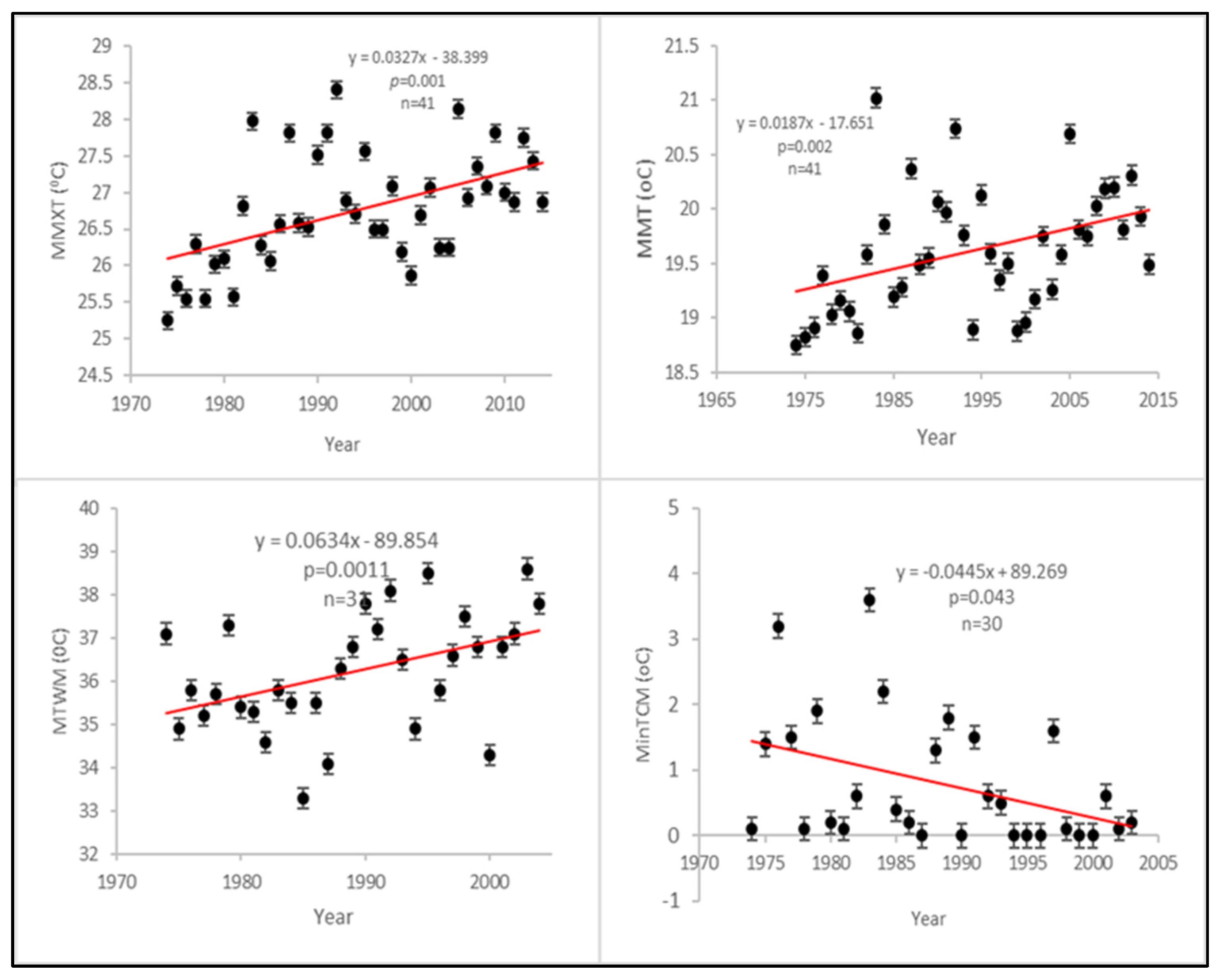
| Mean monthly max. temp | 0.0327−38.399 | 0.001 | Significant change/increasing trend |
| Mean monthly temp | 0.0187−17.651 | 0.002 | Significant change/increasing trend |
| Max. temp, warmest month | 0.863−89.854 | 0.011 | Significant change/increasing trend |
| Min. temp, coldest month | −0.0445 + 89.269 | 0.043 | Significant change/declining trend |
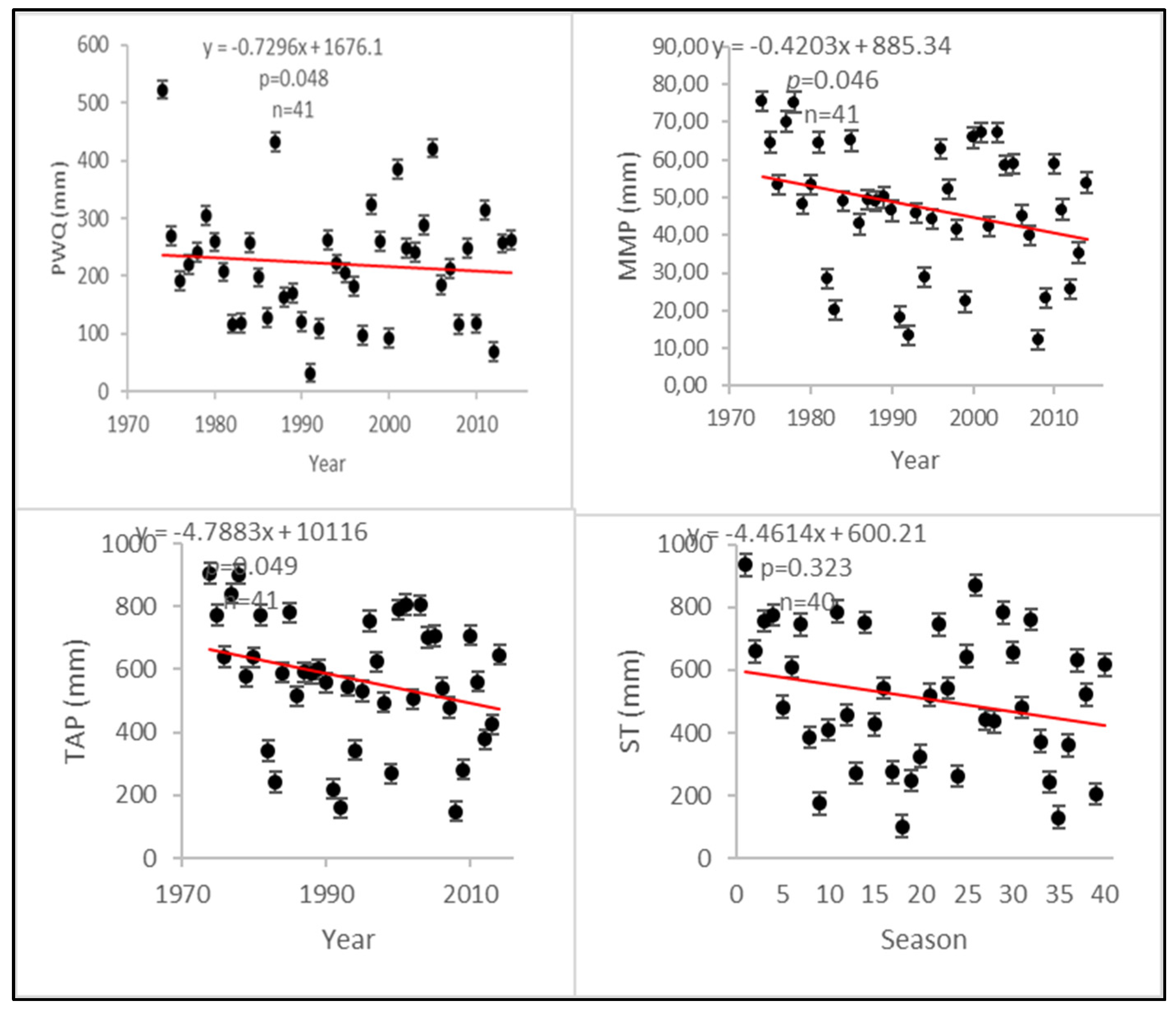
| Total annual precipitation | −4.7883 + 10116 | 0.049 | Significant change/declining trend |
| Mean monthly precipitation | 0.4203 + 88534 | 0.046 | Significant change/declining trend |
| Precipitation: warmest quarter | 3.4206 + 7137.3 | 0.048 | Significant change/declining trend |
| Seasonal max. precipitation | 4.4614 + 60021 | 0.323 a | Not significant/declining trend |
| Regression Variables | m + c | Correlation Coefficient (r) | p Value | R2 |
|---|---|---|---|---|
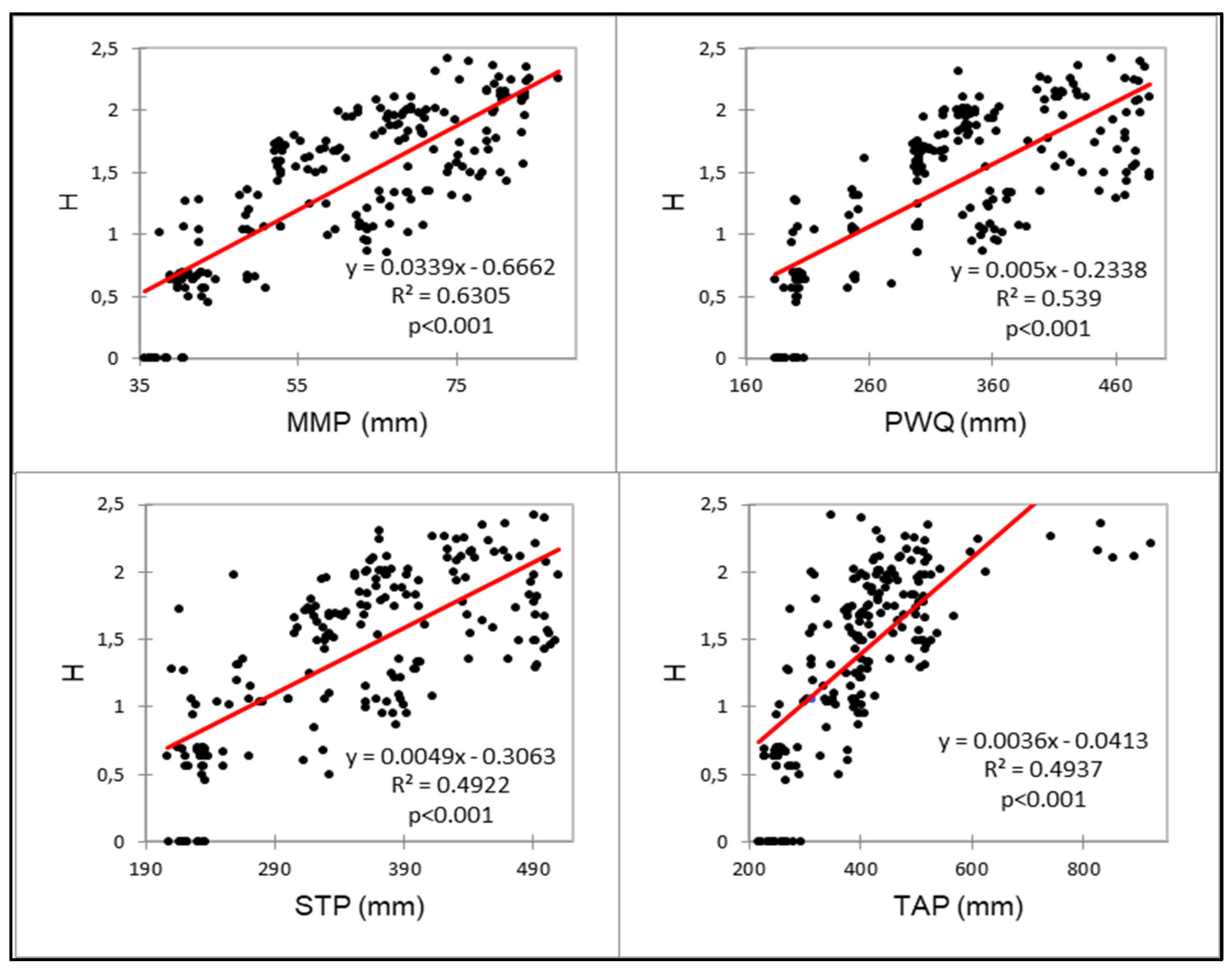
| H and mean monthly precipitation | 0.0339–0.6662 | 0.794 | <0.001 | 0.6736 |
| H and precipitation: warmest quarter | 0.0005–0.2338 | 0.734 | <0.001 | 0.6455 |
| H and seasonal max. precipitation | 0.0049–0.3063 | 0.702 | <0.001 | 0.5841 |
| H and total annual precipitation | 0.0036–0.0413 | 0.703 | <0.001 | 0.6261 |
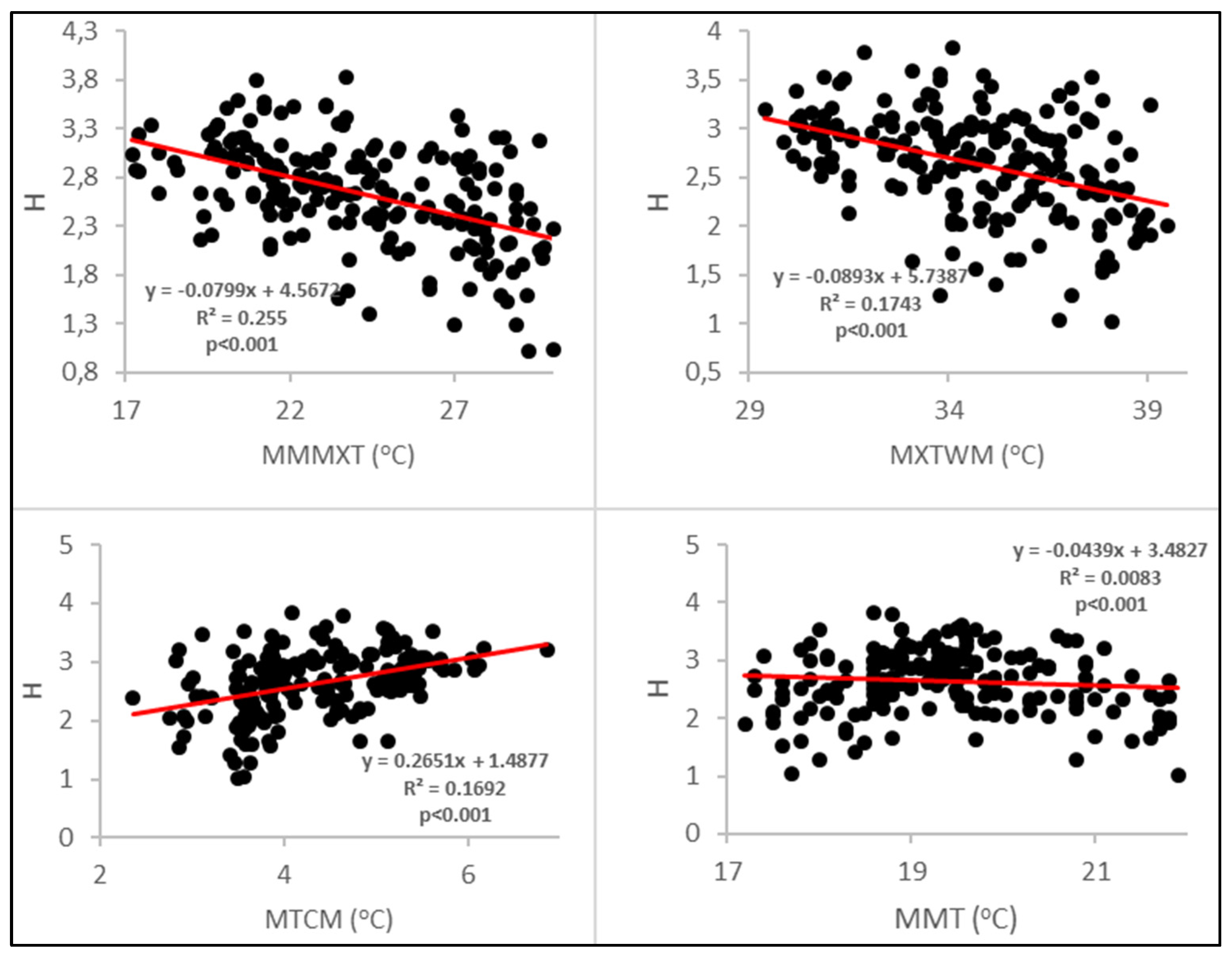
| H and min. temp, coldest month | 0.2651 + 1.4877 | 0.706 | <0.001 | 0.4979 |
| H and max. temp, warmest month | −0.0893 + 5.6494 | −0.799 | <0.001 | 0.6706 |
| H and mean monthly max. temp | −0.0799 + 4.4873 | −0.776 | <0.001 | 0.6223 |
| H and mean monthly temp | −0.0439 + 3.4388 | −0.126 | <0.001 | 0.6943 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chapungu, L.; Nhamo, L.; Gatti, R.C.; Chitakira, M. Quantifying Changes in Plant Species Diversity in a Savanna Ecosystem Through Observed and Remotely Sensed Data. Sustainability 2020, 12, 2345. https://doi.org/10.3390/su12062345
Chapungu L, Nhamo L, Gatti RC, Chitakira M. Quantifying Changes in Plant Species Diversity in a Savanna Ecosystem Through Observed and Remotely Sensed Data. Sustainability. 2020; 12(6):2345. https://doi.org/10.3390/su12062345
Chicago/Turabian StyleChapungu, Lazarus, Luxon Nhamo, Roberto Cazzolla Gatti, and Munyaradzi Chitakira. 2020. "Quantifying Changes in Plant Species Diversity in a Savanna Ecosystem Through Observed and Remotely Sensed Data" Sustainability 12, no. 6: 2345. https://doi.org/10.3390/su12062345
APA StyleChapungu, L., Nhamo, L., Gatti, R. C., & Chitakira, M. (2020). Quantifying Changes in Plant Species Diversity in a Savanna Ecosystem Through Observed and Remotely Sensed Data. Sustainability, 12(6), 2345. https://doi.org/10.3390/su12062345








