Abstract
The growing global concern with environmental issues has raised the interest in the research into natural biopolymers as a coagulant aid in order to reduce the use of inorganic coagulants. This paper investigated the feasibility of sesbania seed gum (SSG) as a plant-based coagulant aid and ferric chloride as a coagulant in drinking water treatment. Acid extraction method marked the highest and most promising extraction yield at 20.8%, as compared to other extraction methods. Further, the SSG extracted carried a weak negative charge of −3.02 mV, which is classified as a near neutral coagulant aid. Hydroxyl and carboxyl functional groups, which aid in coagulation–flocculation, were found in the SSG. These physiochemical analyses results evinced good characteristics of SSG as a coagulant aid. On the other hand, response surface methodology (RSM) with three-factor Box–Behnken design (BBD) was employed to evaluate and optimize the reaction condition of the coagulation–flocculation process in drinking water treatment. A quadratic polynomial model was fitted to the data with a high value of R2 (0.9901). Model validation experiments revealed the good correspondence between actual and predicted values. In drinking water treatment, a promising 98.3% turbidity reduction was achieved with 10.2 mg/L of FeCl3 and 4.52 mg/L of SSG. Therefore, SSG exhibited potential as a coagulant aid in drinking water treatment.
1. Introduction
The coagulation–flocculation treatment system has been widely used to remove pollutants in the water. It is a simple and highly effective treatment system that uses inorganic coagulants i.e., alum and ferric chloride, to remove pollutants [1,2]. However, the inorganic coagulant used has raised controversial issues due to its toxicity, which could potentially be a hazard to human health. Since the 1960s, the detrimental effects of inorganic chemical coagulants on human health have been published [3]. The residual aluminum that is found in the treated water could cause serious health issues such as Alzheimer’s disease. Additionally, some clinical observations and epidemiological studies have proven that there is a high positive correlation between Alzheimer’s disease and the residual aluminum that is present in drinking water [4,5,6,7].
Biopolymers extracted from plants, animals, and microbes were found to be emerging alternative coagulant aids that reduce the use of existing inorganic coagulants [8]. Interestingly, these biopolymers exhibited intrinsic properties that have the flocculating ability. They are generally biodegradable, safe, and eco-friendly [9,10]. Moreover, they generate five times less sludge as compared to inorganic coagulants [3]. Lately, plant-based biopolymers are one of the natural coagulant aids that have become the focus of most researchers [1,11]. However, the application of natural coagulant aids is still at its infancy stage. The plants that have received the greatest degree of attention include Moringa oleifera, cactus, and starch [12,13]. In 2017, Cactus opuntia was first studied as a coagulant aid with aluminum sulfate as coagulant for the urban wastewater treatment, and 93.65% of turbidity was achieved [14]. The authors claimed that the use of Cactus opuntia as coagulant aid offers several advantages such as availability, cost, and strength of the flocs formed. On the other hand, rice starch was used as plant-based coagulant aid for drinking water treatment [13]. The result revealed that maximum turbidity reduction of 89% was achieved at pH 3.
Sesbania grandiflora is known as a fast-growing tree legume [15] that is native to Asia, e.g., Malaysia, China, India, Philippines etc., and is widespread in humid tropical regions around the world. Its fast-growing ability allows the tree to produce ripe pods within just nine months of planting the seed. Additionally, the seed collection process is relatively easy and large amounts of seeds can be hand harvested for processing purposes. Therefore, valorization of sesbania seeds as a coagulant aid in drinking water treatment is highly favorable. The aim of this research was to investigate the feasibility of using sesbania seed gum (SSG) as a coagulant aid in drinking water treatment. The three objectives were (1) extraction of SSG, (2) characterization of SSG, and (3) determination of the optimum coagulating condition of the SSG in drinking water treatment. The physicochemical characteristic properties were examined to determine the role of SSG in the water treatment process, i.e., coagulation–flocculation process. The characterization studies included functional group evaluation, elemental composition analysis, and zeta potential analysis. Lastly, the treatment of drinking water was evaluated through the design of experiment (DOE) to determine the behavior of SSG in the treatment process, as well as to understand the mechanism during the water treatment process.
2. Materials and Methods
2.1. Material
The sesbania seeds were purchased from the local market. The chemicals and solvents used were of analytical grade and purchased from Sigma-Aldrich. All the chemicals were used without further purification.
2.2. Method
Preparation of SSG
The biopolymer extraction methods, which include heating, soaking, advanced heating, and acid extraction, were evaluated and modified from Prajapati, et al. [16], as shown in Figure 1.
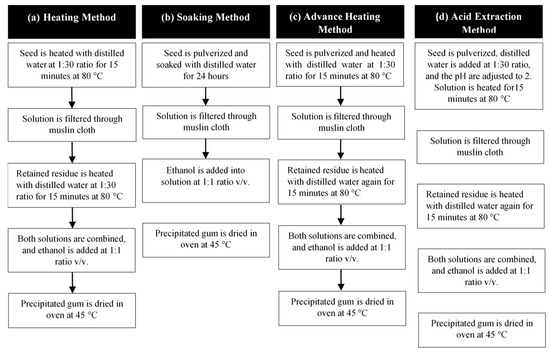
Figure 1.
Flowchart of different sesbania seed gum (SSG) extraction methods, (a) heating, (b) soaking, (c) advance heating, and (d) acid extraction.
2.3. Sampling of River Water
Samples were collected from the inlet of Sultan Idris Shah II Water Treatment Plant, Parit (4.483440, 100.913978) on the 6th of March 2018. The average turbidity and pH of the collected river water samples were 285 ± 6 NTU and 6.4 ± 0.2, respectively. The samples were transported to the laboratory and stored at 4 degrees Celsius.
2.4. Assay of Turbidity Reduction
Jar test was conducted in accordance to the American Society for Testing and Materials (ASTM) standard method jar test (D2035). Briefly, river water was poured into the beaker and ferric chloride was added into the water. The water was then stirred with a rapid mix at a 150 rpm for 1 min. The SSG was added subsequently and the water was stirred with slow mix at 30 rpm for 15 min. The mixtures were left undisturbed for settling purposes (range from 1–15 min). A total of 10 mL of supernatant was withdrawn using the syringe, and the turbidity was measured using the HACH 2100 Q turbidity meter. The suspensions were left undisturbed and the supernatants were collected using a 10 mL syringe for final turbidity measurement. Equation (1) was used to calculate the percentage of turbidity reduction. Each result obtained and shown was the average value of triplicate measurements and all the jar test experiments were conducted at room temperature (22 °C ± 2 °C).
where T1 is the initial turbidity and is the final turbidity in NTU.
2.5. Physicochemical Properties
The functional group in the coagulation–flocculation process was evaluated using Fourier transform infrared spectroscopy (FTIR). The analysis was performed using the Perkin Elmer spectrum. The dry sample powder was mixed with potassium bromide (KBr) and pressed into pellets. The adsorption bands were recorded at characteristic wave numbers between 4000 cm−1 to 450 cm−1. Besides, the surface charge of the SSG was identified through zeta potential analysis. The sample was prepared by dissolving 1 mg/ml of SSG in 0.1 mM of sodium chloride at pH 7. Zeta potential of the sample was analyzed using Malvern Zetasizer Nano (ZSP) at 25 °C with 173° measurement angle. A total of three runs were conducted as replication and a minimum of 10 runs in each replication were performed. Lastly, the elemental compositions of SSG, river water before treatment, and after treatment were analyzed through energy dispersive X-ray analysis (EDX) technique.
2.6. Design of Experiment for Optimization
Response surface methodology (RSM) was employed to identify the influencing factors, the interaction between the influencing factors, and the corresponding optimum value of each factor. It is a statistical approach that uses screening, characterization, and optimization analyses. This approach overcomes the limitation of the traditional one-factor-at-a-time-method, where the interaction between the factors is hard to identify [17,18]. In this experiment, the Box–Behnken design (BBD), as the standard design of RSM [19], was adopted to statistically optimize the influencing factors and evaluate the effect of the influencing factors on the turbidity reduction in drinking water treatment. The dosage of FeCl3, dosage of SSG, and the settling time were chosen as the influencing factors. Each designed factor was set to 3 levels (low, central, and high) and their respective ranges are tabulated in Table 1. The range of each factor was determined through the preliminary single-factor experiments.

Table 1.
Low and high limit of designed factors.
Design Expert software (version 10.0.1, Stat-Ease, Inc., Minneapolis, MN, USA) was used for the analysis of variance (ANOVA). Further, the interactions between the influencing factors were determined by the designed mathematical regression models. Turbidity reduction (%) in drinking water treatment was chosen as the response and a design matrix consisting of 15 experimental runs, including three replicated runs in the center, was constructed. The response data were further analyzed by multiple regression to fit the quadratic polynomial model [19], as illustrated in Equation (2).
where is the measured response (turbidity reduction) and is a constant or an intercept. The terms , , and represent the coefficient of the linear, interaction, and quadratic terms, respectively.
2.7. Verification of Model
The optimal condition for the turbidity reduction from SSG depended on the dosage of ferric chloride, dosage of SSG, and settling time, which were obtained using the predictive equations of RMS. The predicted values were further compared with the experimental value to validate the designed optimization model.
3. Results
3.1. Extraction of SSG
Four different extraction methods were carried out to extract the SSG from sesbania seed. The results of the four different extraction methods are summarized in Table 2. Among all the extraction methods, acid extraction method achieved the highest yield of 20.8%, followed by advance heating extraction method with 5.15% yield. Contrastingly, the yield achieved by the heating and soaking extraction method was too little to be quantified, which can be neglected. One of the factors was due to the temperature in the extraction process. In acid and advance heating extraction methods, extraction of SSG from sesbania seed occurred at 80 °C. According to Nazir, et al. [20], the water achieved better penetration into the solid matrix to solubilize the compound in a high temperature condition. Therefore, high temperature enhances the penetration of SSG into the water, which results in a higher yield in the extraction process. Further, physical crushing was required to break down the cell wall of sesbania seeds to achieve effective extraction. This may be attributed to the strong cell wall of the seeds, which act as the barrier for the penetration process of SSG into the water. Therefore, the seed must be pulverized before the extraction process. On the other hand, it is postulated that the acidic environment further aids in the breaking process of the cell wall. As a result, the SSG inside the sesbania seed was released easily. In view of both economic and yield aspects, the acid method is suggested to be used as it extracted the highest amount of SSG (2079 mg). Despite the fact that the acid extraction method required the adjustment of pH, the yield from this method was excellent. As compared to the advance heating method, the yield of SSG from the acid extraction method was approximately four times higher (404%). Therefore, the acid extraction method was chosen to be used hereafter.

Table 2.
Summary of four different extraction methods.
3.2. Characterization of SSG
3.2.1. Functional Group of SSG
The functional group is extremely important as it determines the flocculating ability of a biopolymer. According to Zhang, et al. [21], the presence of carboxyl, hydroxyl, and amino groups in a biopolymer help in the coagulating and flocculation process. It is reported that these functional groups contributed to the flocculation of suspended particles in the water through the bridging mechanism. Table 3 shows the functional groups presented in the SSG. Besides, the illustration of the peak is given in Supplementary Data 1 (S1). A broad O–H stretching peak at 3410 cm−1 is attributed to the presence of glucose and fructose units in the polysaccharide framework. The strong peak observed at 2925 cm−1 represents the C–H stretch presence in galactose and rhamnose. Whilst the peak at 1648 cm−1 is assigned to the carboxyl groups. Lastly, the peaks found between 1200–1000 cm−1 are postulated to be C–O stretch in aromatic compounds of galactose, rhamnose, and galacturonic acid [22]. The functional group results were similar to okra carbohydrate molecule, where the chemical structure exhibited methyl, carbonyl, and hydroxyl functional groups [23]. The presence of various functional groups, especially hydroxyl and carboxyl functional groups in SSG, revealed its potential as a good natural coagulant aid for turbidity reduction.

Table 3.
Functional groups in SSG.
3.2.2. Zeta Potential and Electrophoretic Mobility of SSG
The strength and the application of the biopolymer are greatly affected by the zeta potential (ZP) and electrophoretic mobility (EM). ZP and EM analyses were carried out too and the results are shown in Figure 2. SSG was found to be a weak anionic biopolymer with −3.02 mV of ZP and −0.24 μm s−1 V−1 cm. The triplicates result showed it was consistent (Figure 2). As the surface charge of SSG is slightly negative, the main mechanism governing the flocculation is suggested to be adsorption and neutralization. During the coagulation–flocculation process, SSG helps in the adsorption and bridging of the polymers with the colloids to increase the size of the floc produced. This ability is attributed to the long-chain properties of the polymer. The polymer chains are adsorbed on the surface of the particle through chemical bonding or physical attachment and, thus, larger floc is formed. In addition, the mechanism of adsorption occurs through hydrogen bonding between the oxygen atoms associated with hydrated metal ions at the surface of the particle [31].
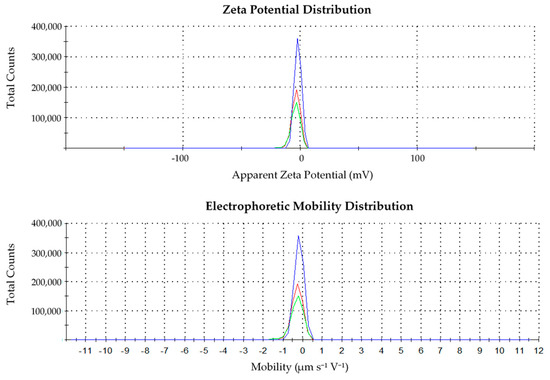
Figure 2.
Zeta potential and electrophoretic mobility distribution of extracted SSG.
3.2.3. Elemental Composition
The elemental compositions of SSG and river water are presented in Table 4. Carbon, potassium, oxygen, magnesium, phosphorus, and sulfur were the element compositions that found in SSG. Carbon had the highest weightage, which was 65.31%, followed by oxygen which was 30.35%. The remaining elements’ concentrations were found to be relatively low (< 5%). This explains the presence of carbon and oxygen structures, as per the FTIR results. Often, carbon and oxygen are the major compositions in bioflocculants such as guar gum [32] and Streptomyces platensis [33]. Bello, et al. [34] and Xiong, et al. [35] reported that polymers with a high composition of carbon generally exhibit good adsorption ability. Therefore, the high composition of carbon in SSG is deemed to enhance the formation of floc during the treatment process. Further, the low anionic properties of SSG may be attributed to the presence of magnesium and potassium elements. The positive charge of these elements neutralizes the anionic functional group, which leads to the weak anionic properties of SSG. On the other hand, carbon and oxygen were the main elemental components in the river water, which were 41.42% and 36.40%, respectively. Besides that, other elemental components such as calcium, sodium, potassium, etc. were found in the river water. These are some general elemental compositions that can be found in fresh river water (Dekov et al., 1997). There was a magnesium presence in aftertreatment samples, where it is postulated the magnesium adhered onto the colloids in the river water through van der Waals attraction and formed flocs by neutralization of particle charge. Similarly, the aftertreatment sample showed an increase in potassium composition, which indicates the adsorption of SSG into the colloidal sample.

Table 4.
Summary of weight of each element in SSG.
3.3. Optimization of the Performance of SSG as Coagulant Aid in River Water Treatment Using RSM
Response surface optimization is more advantageous as compared to traditional single-factor optimization. A total of 15 runs were generated from the Design Expert software to optimize the three individual influencing factors. An analysis of variance (ANOVA) table for fitting the second-order polynomial models to the experimental data was obtained after the analysis and is shown in Table 5.

Table 5.
ANOVA table for screening of influencing factors using SSG.
3.3.1. Model Adequacy Checking
Model adequacy checking is essential to verify the designed model and to examine the fitted model to ensure the accuracy of the prediction model to the true system [36]. Two common methods used for model adequacy checking are through an analysis of variance (ANOVA) table and through diagnostic plots [37]. The R² and adjusted-R² from the ANOVA table (Table 5) confirm the adequacy and suitability of the model. In statistics, R² is calculated from the division of variance explained by the model through total variance. Therefore, the R² obtained (0.9901), which was close to 1, indicates that only 0.99% of the total variations were not explained by the model, and the remaining 99.01% of the variation was perfectly explained. On the other hand, the adjusted-R², 0.9721, denotes a high correlation of the actual and predicted values. The predicted R² was also in good agreement with adjusted R², in which the difference was less than 0.2. Moreover, the p-value of lack of fit (0.0709) in this model implies that the lack of fit was not significant. The insignificance of lack of fit indicated that the proposed model fit the experimental data and the independent variables or parameters have considerable effects on the response [19]. To summarize, the R² and the insignificant lack of fit have proven the reliability of the model.
Diagnostic plots help to visualize the model adequacy and ensure the correlation between the predicted and experimental results [37,38]. For predicted vs. actual diagnostic plots (Figure 3a), the data lie near the straight line, indicating that the designed model is capable of predicting the optimal condition of each designed influencing factor to achieve desired turbidity reduction. A normal probability plot is designed to detect nonnormality and provide a quick way to visually inspect if the pattern of residuals follows a normal distribution [39]. The normal probability plot of the residuals should approximately follow a straight line. As shown, a linear pattern of probability plot is observed in Figure 3b. This result indicates that the normal distribution appears to be a good model for the data obtained. Therefore, this result has further proven the reliability of the designed model.
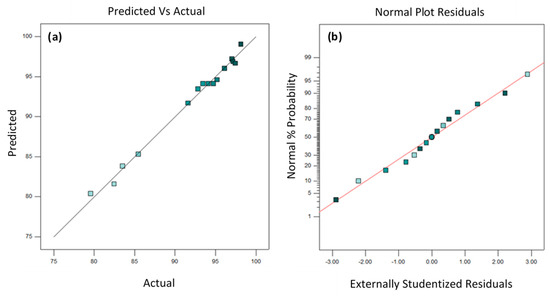
Figure 3.
Diagnostic plots: (a) predicted vs. actual plot; (b) normal plot of residuals for turbidity reduction in drinking water treatment.
3.3.2. Analysis of the Response Surfaces
Since the designed model has shown an insignificant lack of fit and all data were well-fitted, the designed model allowed the prediction of the effects of the three influencing factors on turbidity reduction in drinking water treatment. The significance of each influencing factor was evaluated by testing the null hypothesis. The factor is significant when the null hypothesis is rejected (p-value less than a significant level). As shown in the ANOVA table (Table 5), the dosage of FeCl3 and dosage of SSG are significant since their p-values are less than 0.05, i.e., < 0.0001 and 0.0096, respectively. Evidently, the factors attribute to its role as a coagulant aid in the process.
Based on the sum of squares when SSG was used as a coagulant aid (Table 5), the highest contribution effect toward turbidity reduction was the linear terms, followed by the quadratic term and the interaction term. For turbidity reduction, the total contribution of the linear, quadratic, and interaction terms were 74.10% (70.65% from the dosage of Fe, 2.36% from the dosage of SSG, and 1.09% from the settling time), 25.47% (24.72% from the quadratic effect of dosage of Fe, 0.19% from the quadratic effect of dosage of SSG, and 0.56% from the quadratic effect of settling time), and 1.03% (0.10% from the dosage of Fe and dosage of SSG, 0.25% from the dosage of Fe and settling time, and 0.67% form the dosage of SSG and settling time), respectively. This result reveals that the turbidity reduction is mainly affected by the individual effect of the influencing factors, and shows a good agreement with the 3D surface result (Figure 4), where the FeCl3 had the highest effect on the turbidity reduction. This is attributed to the role of FeCl3 as a coagulant. FeCl3 as a coagulant helps to destabilize the colloidal particles through the charge neutralization and promotes the formation of floc through the attraction of opposite charge particles. Consequently, the particles attract to each other due to the opposite-charge attraction and the disappearing of the electrostatic repulsion force between the particles. Therefore, the increase of FeCl3 dosage enhanced the turbidity reduction, until it reached an optimal point of 10–12 mg/L, after which additional dosage of FeCl3 reduced the turbidity reduction, as shown in Figure 4. This was caused by the repulsive force that formed between the colloidal particles in river water due to the accumulation of positive charges on the particle surface [40]. On the other hand, overdose leads to the destabilization of the particles and the agglomerated particles will be suspended in the water and, therefore, are hard to remove by the gravity settling. Apart from coagulant, SSG as coagulant aid plays a significant role in turbidity reduction. During the coagulation-flocculation process, the SSG and colloidal particles are adsorbed onto each other during the particle stabilization process. A complexes structure will form after, which would bridge two or more colloidal particles to form a bigger floc before settling [29]. Moreover, the increase SSG’s dosage enables more biopolymers to form larger flocs with colloidal particles in river water for effective sedimentation. This also explained the fact where designed settling time (1–15 min) does not significantly impact to the turbidity reduction. When larger and denser flocs formed, majority of the flocs settled within one minute. The result reveals the advantage of using SSG as coagulant aid, which the settling time of the flocs can be significantly reduced.
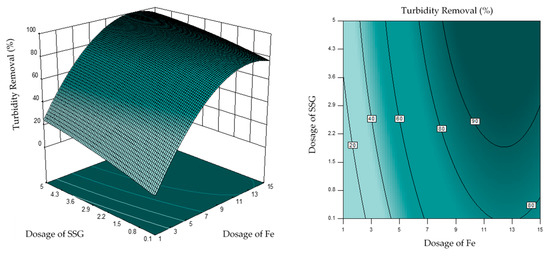
Figure 4.
Three-dimensional response surface and contour for turbidity reduction as a function of dosage of FeCl3 and dosage of SSG.
3.3.3. Optimum Condition of the Influencing Factors
Upstream river water is the primary source of drinking water. To meet the standard limit of the drinking water, coagulation-flocculation process that is generally used in the first stage of the treatment process is required to reduce the turbidity of water. Second-order models for turbidity reduction in terms of coded variables are obtained from the designed optimization model and shown in Equation (3). The equation in terms of coded factors is used to make predictions about the response for given levels of each factor. The coefficient represents the change in response when one coded unit of the factor level is changed while the positive sign indicates the ability of the factors to help in turbidity reduction.
where y = turbidity reduction; = dosage of FeCl3 = dosage of SSG; = settling time.
The optimum dosages for FeCl3 and SSG to achieve 98.3% of turbidity reduction were 10.2 mg/L and 4.52 mg/L, respectively, occurring at settling time of 2.5 min. SSG achieved high flocculating performance in a short amount of time (2.5 min) with the presence of hydroxyl and carboxyl groups and nearly neutral charge. This may be attributed to the strong affinity possessed by SSG due to the presence of hydroxyl and carboxyl functional groups that attract colloids and conform to the colloidal surface in the split second it touches their surface. Therefore, larger flocs are formed and settled in a short time. A single experiment with the same optimum condition was conducted, except that the dosage of the SSG was set to 0.1 mg/L. The predicted turbidity reduction was 80.5%, while the experimental result obtained was 79.2%. The result shows a significant decrease of turbidity reduction (from 98.3% to 79.2%) when the use of SSG is low. This evinces and proves the feasibility of the SSG as coagulant aid to enhance the turbidity reduction through enhancement of floc.
3.3.4. Validation of the Developed Model
To validate the developed model, four runs were conducted with the predicted turbidity reductions that were chosen from the optimization results. The results of the validation test are shown in Table 6. The validation results demonstrated high accuracy of the developed result as the percentage deferred for each of the four runs was less than 4%, which achieved up to a 95% confidence interval. Therefore, the predicted results of optimization are accurate and reliable.

Table 6.
Validation test for the predicted turbidity reduction.
3.4. Proposed Mechanism for SSG Coagulant Aid
The governing coagulating–flocculating mechanisms developed by using SSG as a coagulant aid for the water treatment process are illustrated in Figure 5. When ferric chloride is added into water, Fe3+; ions are released due to the dissociation of the coagulant. The strong, positively charged metal ions strongly are adsorbed on the surface and neutralize the surface charge of the particles adsorbed onto the surface of the colloidal particle in the water, neutralizing the surface charge during the rapid mixing (Figure 5a). Consequently, small flocs are formed due to the absence of electrostatic repulsion force. This process is further facilitated by SSG bridging and adsorption of microaggregates, as shown in Figure 5b. The SSG polymers form a bridge between both particles by simultaneously capturing and binding particles to their surface. Moreover, the SSG and colloidal particles are adsorbed onto each other during the particle stabilization process. A complex structure will form after, which would bridge two or more colloidal particles to form a bigger floc before settling. Further, sufficient concentration of ferric ions contributes to the bridging process by providing sufficient charge to the particles. In the optimum concentration of FeCl3 and SSG, the effects of charge neutralization and the bridging are the strongest. The SSG may adsorb on the surface through hydrogen bonding interaction between oxygen atoms associated with hydrated metal ions at the surface of the particle [31]. Additionally, the electrostatic attraction between the SSG (negatively charged) and ferric ions (positively charged) helps in enhancing the adsorption of the particles.
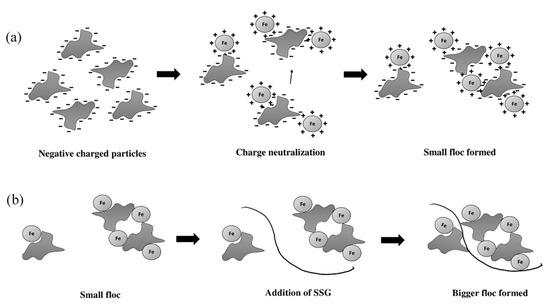
Figure 5.
Proposed mechanism model for (a) charge neutralization, and (b) bridging.
4. Conclusions
This work demonstrated the effectiveness of SSG as a coagulant aid combined with FeCl3 in drinking water treatment. SSG was successfully extracted using the acid extraction method. The flocculating properties of SSG were confirmed by zeta potential, FTIR, and EDX characterizations. The extracted SSG exhibited high flocculation ability with 4.52 mg/L of dosage in 285 NTU of river water. RSM with Box–Behnken design was successfully adopted for the optimization of the turbidity reduction. The optimization studies noted the optimum conditions to achieve 98.3% of turbidity reduction are with FeCl3 10.2 mg/L FeCl3, 4.52 mg/L SSG, and 2.5 min settling time. Charge neutralization, adsorption, and bridging are the mechanisms that govern the removal of colloids in the river water. This study revealed that SSG performs well as a plant-based natural coagulant aid in removing colloids for drinking water treatment.
Supplementary Materials
The following are available online at https://www.mdpi.com/2071-1050/12/6/2273/s1, S1. The FTIR result of SSG.
Author Contributions
Conceptualization, S.-C.C., F.-K.C., and Y.-C.H.; data curation, S.-C.C., M.R.U.M., and W.S.; formal analysis, S.-C.C., F.-K.C., M.A.M., M.R.U.M., N.I., J.-W.L., and Y.-C.H.; funding acquisition, M.A.M. and Y.-C.H.; methodology, S.-C.C., F.-K.C., and Y.-C.H.; project administration, M.R.U.M., N.I., and Y.-C.H.; resources, M.A.M. and W.S.; software, S.-C.C. and J.-W.L.; supervision, F.-K.C. and Y.-C.H.; validation, F.-K.C., M.A.M., N.I., and Y.-C.H.; visualization, S.-C.C., M.A.M., W.S., and Y.-C.H.; writing—original draft, S.-C.C.; writing—review and editing, F.-K.C., M.A.M., M.R.U.M., N.I., W.S., J.-W.L., and Y.-C.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by PETRONAS under the YUTP grants (0153AA-E34 and 015LC0-169) and iRMC Bold2025 (Grant Code: RJO 1043 6494) from Universiti Tenaga Nasional, Malaysia.
Acknowledgments
This work was supported by the Institute of Self-Sustainable Building and Civil and Environmental Engineering Department. Further, technical assistance from Mdm. Norhayama Bt Ramli is acknowledged.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Vijayaraghavan, G.; Sivakumar, T.; Kumar, A.V. Application of plant based coagulants for waste water treatment. Int. J. Adv. Eng. Res. Stud. 2011, 1, 88–92. [Google Scholar]
- Adelodun, B.; Ajibade, F.O.; Ogunshina, M.S.; Choid, K.-S. Dosage and settling time course optimization of Moringa oleifera in municipal wastewater treatment using response surface methodology. Desalin. Water Treat. 2019, 167, 45–56. [Google Scholar] [CrossRef]
- Choy, S.Y.; Prasad, K.N.; Wu, T.Y.; Raghunandan, M.E.; Ramanan, R.N. Performance of conventional starches as natural coagulants for turbidity removal. Ecol. Eng. 2016, 94, 352–364. [Google Scholar] [CrossRef]
- Ahmed, T.; Bhatti, Z.A.; Maqbool, F.; Mahmood, Q.; Faridullah; Qayyum, S.; Mushtaq, N. A comparative study of synthetic and natural coagulants for silver nanoparticles removal from wastewater. Desalin. Water Treat. 2016, 57, 18718–18723. [Google Scholar] [CrossRef]
- Colomina, M.T.; Peris-Sampedro, F. Aluminum and Alzheimer’s disease. In Neurotoxicity of Metals; Springer: Cham, Switzerland, 2017; pp. 183–197. [Google Scholar]
- Klotz, K.; Weistenhöfer, W.; Neff, F.; Hartwig, A.; van Thriel, C.; Drexler, H. The health effects of aluminum exposure. Dtsch. Ärzteblatt Int. 2017, 114, 653. [Google Scholar] [CrossRef]
- Iqbal, A.; Hussain, G.; Haydar, S.; Zahara, N. Use of new local plant-based coagulants for turbid water treatment. Int. J. Environ. Sci. Technol. 2019, 16, 6167–6174. [Google Scholar] [CrossRef]
- Al-Hamadani, Y.A.; Yusoff, M.S.; Umar, M.; Bashir, M.J.; Adlan, M.N. Application of psyllium husk as coagulant and coagulant aid in semi-aerobic landfill leachate treatment. J. Hazard. Mater. 2011, 190, 582–587. [Google Scholar] [CrossRef]
- Sanghi, R.; Bhattacharya, B.; Dixit, A.; Singh, V. Ipomoea dasysperma seed gum: An effective natural coagulant for the decolorization of textile dye solutions. J. Environ. Manag. 2006, 81, 36–41. [Google Scholar] [CrossRef]
- Šćiban, M.; Klašnja, M.; Antov, M.; Škrbić, B. Removal of water turbidity by natural coagulants obtained from chestnut and acorn. Bioresour. Technol. 2009, 100, 6639–6643. [Google Scholar] [CrossRef]
- Banch, T.J.; Hanafiah, M.M.; Alkarkhi, A.F.; Amr, A.; Salem, S. Factorial design and optimization of landfill leachate treatment using tannin-based natural coagulant. Polymers 2019, 11, 1349. [Google Scholar] [CrossRef]
- Ahmadi, N.; Chaibakhsh, N.; Zanjanchi, M.A. Use of Descurainia sophia L. As a natural coagulant for the treatment of dye-containing wastewater. Environ. Prog. Sustain. Energy 2016, 35, 996–1001. [Google Scholar] [CrossRef]
- Chua, S.-C.; Chong, F.-K.; Yen, C.-H.; Ho, Y.-C. Valorization of conventional rice starch in drinking water treatment and optimization using response surface methodology (RSM). Chem. Eng. Commun. 2019, 1–11. [Google Scholar] [CrossRef]
- Rachdi, R.; Srarfi, F.; Shimi, N.S. Cactus Opuntia as natural flocculant for urban wastewater treatment. Water Sci. Technol. 2017, 76, 1875–1883. [Google Scholar] [CrossRef] [PubMed]
- Mund, N.K.; Dash, D.; Barik, C.R.; Goud, V.V.; Sahoo, L.; Mishra, P.; Nayak, N.R. Evaluation of efficient glucose release using sodium hydroxide and phosphoric acid as pretreating agents from the biomass of Sesbania grandiflora (L.) Pers.: A fast growing tree legume. Bioresour. Technol. 2017, 236, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Prajapati, V.D.; Maheriya, P.M.; Jani, G.K.; Patil, P.D.; Patel, B.N. Lepidium sativum Linn.: A current addition to the family of mucilage and its applications. Int. J. Biol. Macromol. 2014, 65, 72–80. [Google Scholar] [CrossRef] [PubMed]
- Du, E.; Li, J.; Zhou, S.; Li, M.; Liu, X.; Li, H. Insight into the Degradation of two benzophenone-type UV filters by the UV/H2O2 advanced oxidation process. Water 2018, 10, 1238. [Google Scholar] [CrossRef]
- Harun, N.Y.; Jian, T.M.; Jusoh, N.; Ramli, R.M. Application of response surface methodology to investigate the effect of different variables on fusion slagging index. In AIP Conference Proceedings; AIP Publishing: Melville, NY, USA, 2019; p. 020033. [Google Scholar]
- Chen, L.; Chen, Q.; Rao, P.; Yan, L.; Shakib, A.; Shen, G. Formulating and optimizing a novel biochar-based fertilizer for simultaneous slow-release of nitrogen and immobilization of cadmium. Sustainability 2018, 10, 2740. [Google Scholar] [CrossRef]
- Nazir, S.; Wani, I.A.; Masoodi, F.A. Extraction optimization of mucilage from Basil (Ocimum basilicum L.) seeds using response surface methodology. J. Adv. Res. 2017, 8, 235–244. [Google Scholar] [CrossRef]
- Zhang, Z.; Xia, S.; Zhao, J.; Zhang, J. Characterization and flocculation mechanism of high efficiency microbial flocculant TJ-F1 from Proteus mirabilis. Colloids and Surfaces B: Biointerfaces 2010, 75, 247–251. [Google Scholar] [CrossRef]
- Rahul, R.; Kumar, S.; Jha, U.; Sen, G. Cationic inulin: A plant based natural biopolymer for algal biomass harvesting. Int. J. Biol. Macromol. 2015, 72, 868–874. [Google Scholar] [CrossRef]
- Ghori, M.U.; Mohammad, M.A.; Rudrangi, S.R.S.; Fleming, L.T.; Merchant, H.A.; Smith, A.M.; Conway, B.R. Impact of purification on physicochemical, surface and functional properties of okra biopolymer. Food Hydrocoll. 2017, 71, 311–320. [Google Scholar] [CrossRef]
- Awang, N.A.; Aziz, H.A. Hibiscus rosa-sinensis leaf extract as coagulant aid in leachate treatment. Appl. Water Sci. 2012, 2, 293–298. [Google Scholar] [CrossRef]
- Lawrie, G.; Keen, I.; Drew, B.; Chandler-Temple, A.; Rintoul, L.; Fredericks, P.; Grøndahl, L. Interactions between alginate and chitosan biopolymers characterized using FTIR and XPS. Biomacromolecules 2007, 8, 2533–2541. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Gao, Y.; Zhai, Y.; Liu, F.; Gao, G. Synthesis of sesbania gum supported dithiocarbamate chelating resin and studies on its adsorption performance for metal ions. Carbohydr. Polym. 2008, 73, 359–363. [Google Scholar] [CrossRef]
- Jun, B.-M.; Kim, Y.; Han, J.; Yoon, Y.; Kim, J.; Park, C.M. Preparation of Activated Biochar-Supported Magnetite Composite for Adsorption of Polychlorinated Phenols from Aqueous Solutions. Water 2019, 11, 1899. [Google Scholar] [CrossRef]
- Meili, L.; Godoy, R.; Soletti, J.; Carvalho, S.; Ribeiro, L.; Silva, M.; Vieira, M.; Gimenes, M. Cassava (Manihot esculenta Crantz) stump biochar: Physical/chemical characteristics and dye affinity. Chem. Eng. Commun. 2019, 206, 829–841. [Google Scholar] [CrossRef]
- Shak, K.P.Y.; Wu, T.Y. Optimized use of alum together with unmodified Cassia obtusifolia seed gum as a coagulant aid in treatment of palm oil mill effluent under natural pH of wastewater. Ind. Crop. Prod. 2015, 76, 1169–1178. [Google Scholar] [CrossRef]
- Betatache, H.; Aouabed, A.; Drouiche, N.; Lounici, H. Conditioning of sewage sludge by prickly pear cactus (Opuntia ficus Indica) juice. Ecol. Eng. 2014, 70, 465–469. [Google Scholar] [CrossRef]
- Basaran, H.K.; Tasdemir, T. Determination of flocculation characteristics of natural stone powder suspensions in the presence of different polymers. Physicochem. Problems Mineral Process. 2014, 50, 169–184. [Google Scholar]
- Ahmad, R.; Haseeb, S. Absorptive removal of Pb2+, Cu2+ and Ni2+ from the aqueous solution by using groundnut husk modified with Guar Gum (GG): Kinetic and thermodynamic studies. Groundw. Sustain. Dev. 2015, 1, 41–49. [Google Scholar] [CrossRef]
- Agunbiade, M.; Pohl, C.; Ashafa, O. Bioflocculant production from Streptomyces platensis and its potential for river and waste water treatment. Braz. J. Microbiol. 2018. [Google Scholar] [CrossRef] [PubMed]
- Bello, O.S.; Adegoke, K.A.; Akinyunni, O.O. Preparation and characterization of a novel adsorbent from Moringa oleifera leaf. Appl. Water Sci. 2017, 7, 1295–1305. [Google Scholar] [CrossRef]
- Xiong, C.; Jia, Q.; Chen, X.; Wang, G.; Yao, C. Optimization of polyacrylonitrile-2-aminothiazole resin synthesis, characterization, and its adsorption performance and mechanism for removal of Hg (II) from aqueous solutions. Ind. Eng. Chem. Res. 2013, 52, 4978–4986. [Google Scholar] [CrossRef]
- Raissi, S.; Farsani, R.-E. Statistical process optimization through multi-response surface methodology. World Acad. Sci. Eng. Technol. 2009, 51, 267–271. [Google Scholar]
- Swamy, G.J.; Muthukumarappan, K. Optimization of continuous and intermittent microwave extraction of pectin from banana peels. Food Chem. 2017, 220, 108–114. [Google Scholar] [CrossRef] [PubMed]
- Chua, S.-C.; Malek, M.A.; Chong, F.-K.; Sujarwo, W.; Ho, Y.-C. Red Lentil (Lens culinaris) Extract as a Novel Natural Coagulant for Turbidity Reduction: An Evaluation, Characterization and Performance Optimization Study. Water 2019, 11, 1686. [Google Scholar] [CrossRef]
- Rawlings, J.O.; Pantula, S.G.; Dickey, D.A. Applied Regression Analysis: A Research Tool; Springer Science & Business Media: Berlin, Germany, 2001. [Google Scholar]
- Chu, W. Dye removal from textile dye wastewater using recycled alum sludge. Water Res. 2001, 35, 3147–3152. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).