Abstract
Nannochloris atomus (QUCCCM31) is a local marine microalga showing potential to serve as renewable feedstock for biodiesel production. The investigation of the impact of temperature variation and nitrogen concentrations on the biomass and lipid productivities evidenced that biomass productivity increased with the temperature to reach an optimum of 195 mgL−1 d−1 at 30 °C. Similarly, the lipid content was strongly influenced by the elevation of temperature; indeed, it increased up to ~3 folds when the temperature increased from 20 to 40 °C. When both stresses were combined, triacylglycerols and lipid productivity reached a maximum of 45% and 88 mgL−1 d−1, respectively at 40 °C. Cultures under high temperatures along with Nitrogen-Depleted (ND) favored the synthesis of Fatty Acids Methyl Ester (FAMEs) suitable for high quality biodiesel production, whereas cultures conducted at low temperature coupled with Nitrogen-Limited (NL) led to a production of polyunsaturated fatty acids (PUFAs). Our results support the feasibility of cultivating the thermotolerant isolate QUCCCM31 year-round to meet the sustainability challenges of algal biomass production by growing under temperature and nitrogen variations. The presence of omega 3 and 9 fatty acids as valuable co-products will help in reducing the total process cost via biorefinery.
1. Introduction
Due to the expanding human population, the global energy demand per capita is expected to increase to 37% of consumption by 2040 [1]. Qatar is amongst the countries where the energy sector is heavily dependent on the fossil fuels. Although, the existing crude oil reserves are estimated to be 25,244 (million barrels) (Annual Statistical Bulletin 2015), they are reportedly diminishing and have raised major environmental concerns. Therefore, alternative renewable energy needs to be exploited to achieve a sustainable bio-economy, which copes with the agenda of 2030 sustainable development goals and Qatar’s national vision.
Biodiesel, obtained from biological feedstocks, emerged as one of the future fuels, owing to its less polluting, biodegradable and renewable characteristics [2]. The main criteria for algal biodiesel production is selection of strain with high growth rate and considerable amounts of lipids [3]. Several methods are adopted for microalgal lipid extraction. The traditional techniques involve organic solvent extraction using different methods such as the Bligh and Dyer and Folch method with a mixture of chloroform and methanol as solvents, nHexane extraction etc. [4,5]. These methods are usually associated with treatments such as sonication, bead beating, and microwave oven techniques to disrupt the algal cell wall and facilitate the transfer of intracellular lipids from microalgae to the extraction solvent [5,6]. However, these techniques present some limitations [7,8]. Recently, the supercritical fluid extraction emerged as a novel extraction technique with the ability to enhance both the disruptive and diffusive mechanisms essential for efficient algal oil extraction. It allows the preservation of the quality of bioactive compounds with less energy cost and lower environmental impact [9,10].
In the last decades, the production of algae-based biodiesel increased with an estimated yield of 58,700 to 136,900 Lha−1year−1 [11]. Despite this, the commercial production is only limited to few strains [12]. This is because today, the outdoor scale production undergoes a variety of temperature fluctuations between 10 and up to 45 °C due to the greenhouse effect, which, unfortunately, hindered the algal growth [13]. On the other hand, the high lipid content needed for biodiesel production is usually obtained under unfavorable biomass growth conditions [14]. To overcome this, intensive efforts are devoted to select suitable strains, which can grow in extreme conditions with high temperature tolerance, especially in arid places such as Qatar’s environment. In parallel, strategies are applied to enhance both lipid and biomass productivities, an important factor for biodiesel production [13,15].
Previous studies proved that temperatures affect both the algae growth and lipid accumulation [16]. Indeed, several researches demonstrated that most microalgae species are able to carry out photosynthesis and cellular division over a wide range of temperatures generally stated between 15 and 30 °C with optimal conditions between 20 and 25 °C [17]. Converti et al. [18] highlighted also the effect of temperature on lipid content and the work results showed that an increase in cultivation temperature from 20 to 25 °C doubled the lipid content of Nannochloropsis oculata (from 7.9% to 15.9%) [18]. Along with the temperature, nitrogen limitation is one of the most efficient strategies used to enhance algal lipid accumulation [19]. It is easy to manipulate and has low cost compared with other factors influencing the intracellular lipid content [20]. Earlier research work indicated that strains such as Dunaliella, Chlorella and Nannochloropsis species are known to respond to nitrogen starvation by increasing lipid production, one the most critical factors for biodiesel production [21]. However, for user acceptance, the obtained microalgal biodiesel needs to comply with existing standards, such as ASTM Biodiesel Standard D 6751 (USA) or Standard EN 14214 (European Union) [22].
Qatar is a country where the energy sector is heavily dependent on fossil fuels. Our research work aims on minimizing this dependence by investigating a local algae and improvement of its biochemical composition for biodiesel production. While many studies have investigated the production of biodiesel from algal biomass, there is scant information in the literature, to the best of our knowledge, reporting the use of microalgae isolated from desert environment. Nannochloris atomus (QUCCCM31) is a marine microalgae isolated from Qatar and showing advantages such as high growth rate and resistance to salinity up to 60 ppt [23]. Due to huge differences in Qatar’s temperature between winter and summer, and the lack of control over temperature in open raceway ponds, the ability of algae isolates to grow at different ranges of temperature is of vital importance to avoid culture collapse, which can significantly influence the profitability of algal cultivation systems. The primary objective of our study was to mimic the outdoor seasonal temperature conditions throughout the year and determine the impact of the temperature’s fluctuations on growth, biomass and lipid productivity; 3 different temperatures from the low annual average (20 °C) followed by the average annual temperature (30 °C) then (40 °C) as the high annual average temperature were investigated. The secondary objective of the work aimed to optimize the conditions leading to maximum biomass and lipid productivities of the algae by studying the effect of different nitrogen regimes. Furthermore, FAME profiles resulting from the various cultivation conditions were investigated to evaluate the effect of the conditions applied on the quality of biodiesel by estimating the fuel properties.
2. Materials and Methods
2.1. Chemicals
All solvents used were HPLC grade. Fatty Acid methyl esters standards were obtained from Sigma-Supelco (# 47885-U).
2.2. Strain, Growth Medium and Pre-Cultivation Conditions
Nannochloris atomus QUCCCM31 was obtained from the Qatar University Culture Collection for Cyanobacteria and Microalgae (QUCCCM) [23].
The strain was cultured in 2X f/2 media with 0.15gL−1 [24] for biomass production using an illuminated Innova 44 Shaker Incubator (New Brunswick Scientific) with agitation set to 200 RPM and a photon flux of 200 μmol photons m−2s−1 in a cycle of 12:12h light/dark. Optical density (OD) was followed daily and cultures were harvested once the early stationary phase was reached. The biomass was centrifuged for 5min at 400–600 g, after which the pellet was sub-cultured in nitrate replete (Normal f/2), washed with nitrate limited (NL: f/2 media with only 10% of the NaNO3 concentration) or nitrate depleted (ND: f/2 media without NaNO3) before sub-culturing, to eliminate any residual nitrogen in the media. All cultivations were performed in duplicate.
2.3. Photobioreactor Setup and Experimental Conditions: Nitrate Limitation and Depletion
The strain was cultivated in aseptic 1L photobioreactors (DASGIP parallel bioreactor system, Eppendorf, Inc., USA). The culture pellet was re-suspended in a volume of either NR, NL or ND medium, to an initial OD750nm of 0.3. The culture was continuously sparged with 3 Lh−1 air enriched with 1.5% CO2 concentration controlled to maintain a pH of 7.5. The temperature was set to 20, 30 or 40 °C, as per the experimental conditions. Illumination was provided by 3 internal DASGIP LED Sticks, with a 3-channel emission-spectrum (Channel A, 660, 780 nm; Channel B, 572, 625, 640 nm; Channel C, 453 nm). Set-points were 2.00, 1.244 and 2.00 µmol photons s−1 for channels A, B and C respectively, under 12:12h light/dark cycles, which is equivalent to a light intensity of 600 µmolm−2 s−1. Mixing was set to 200 rpm (pitch-blade impeller). The Culture density (OD750nm) was measured every other day using a UV/Vis spectrophotometer (Jenway 7310, UK) and 5 mL samples were taken in parallel for biomass dry weight analysis.
The nine investigated conditions: Control (f/2), 3 different temperatures (20, 30 and 40 °C) in (f/2) media and 2 nitrogen concentrations (NL and ND) at the 3 different temperatures previously indicated, were performed in biological duplicate. Cultures were cultivated under the above-mentioned conditions until reaching stationary phase after which cells were harvested by centrifugation at 5000 rpm for 15 min, washed with 0.5 M ammonium formate to eliminate the residual salt and freeze-dried prior to analysis.
2.4. Determination of Specific Growth Rate and Biomass Productivity
The specific growth rate was determined using the following Equation (1):
where (t1) and (t2) are the beginning and end of logarithmic growth phase and (Nt1) and (Nt2) are the microalgal density at the time of t1 and t2, respectively.
The biomass productivity was assessed according the Zhu et al. [25] protocol which was developed for marine algae. Briefly, 5 mL of the algal cells’ samples were taken at inoculation (T0) and after reaching the stationary phase (Tend). The algal suspension was filtered using a vacuum pump through pre-weighed glass fiber filters (Whatman GF/F, 47 mm), the filters were washed twice with 0.5 M ammonium formate to remove the salts. Finally, the filters were dried at 90 °C until constant weight, cooled down in a vacuum desiccator and then reweighed.
The biomass productivity was calculated by subtracting both dry weights and dividing it by the volume of samples multiplied by the number of culture days. Equation (2) according to Zhu et al. [25].
Dry weight of the biomass was assessed at the time of inoculation (t0) and at time of harvesting (tend) after drying the algal cells at 90 °C. Biomass productivity is determined as per
in which (Px) is the biomass productivity in gL−1d−1, (Cx) the difference of dry weights in g, (V) the culture volume in L and (t) the duration of the cultivation in days. All measurements were performed in duplicate.
2.5. Lipid Quantitative and Qualitative Analysis
Total lipids were extracted using a modified Folch Method, and Fatty Acid Methyl Esters (FAMEs) were extracted using a one-step trans-esterification method, both as described by Saadaoui et al. [23]. Indeed, total lipids were extracted using modified Folch Method [26]. Briefly, 10mg of freeze-dried microalgae biomass was treated first with 0.88% NaCl prior to being incubated at 4 °C in an adequate volume of methanol. After overnight incubation, 2 V of chloroform was added to the mixture and was subjected to Tissuelyser (Qiagen, Germany) at 30Hz during 4min for cell disruption. The tubes were later centrifuged at maximum speed and the supernatant is transferred into pre- weighed glass tube. Several extractions using methanol/chloroform (V/2 V) were performed to ensure a complete extraction. Later, an adequate volume of 0.88% NaCl will be added to separate the organic and aqueous phases, and then vortex for 10 min for phase separation. The organic phase was washed twice with an authentic upper phase to eliminate any debris of contaminants.
Preparation of authentic upper phase: chloroform/methanol/water (16 mL/8 mL/6 mL); mix it well by vortex for 30 s; centrifuge the tube for 5min then remove the upper layer (methanol-water), this is your authentic upper phase.
Lipid content measurement was done gravimetrically: After separation of the layers, the organic phase was collected and dried using nitrogen flu. The weight of the tube after drying will be recorded and subtracted from the weight of the empty tube to determine the % of total lipid/mg of dry biomass.
Lipid productivity was calculated using the following equation [27]:
where PLipid is lipid productivity, Px is biomass productivity (g L−1d−1) and Cf is lipid percentage.
Fatty Acid Methyl Esters (FAMEs) were extracted using one-step trans-esterification method to avoid any loss of the lipids. Briefly, 10 mg of dried biomass was added to 4 mL of a sulfuric acid 95% and methanol solution (H2SO4: CH3OH=1:10) then sonicated (Branscon 1510, Mexico) for 10 min prior to being heated at 80 °C for 2 h. Subsequently, the solution was transferred into a centrifuge tube containing 1 mL of distilled water and 3 mL of hexane: chloroform (4:1) mixture. The tube was well mixed and centrifuged at 5000 rpm for 5 min. Finally, the top layer containing the FAMEs fraction was collected and filtrated prior to analysis.
The extracted FAMEs were later analyzed using a GC-FID (Shimadzu 2010 plus, Japan). 2 μL of the sample extract was injected into gas chromatography with an oven temperature program between 100–240 at 5 °C/min holding time. The sample was separated using a 100 m column; helium gas is used as a carrier gas. FAME peaks were identified by comparison of their retention times with a 37 supelco standards from marine oil by GC–FID post run analysis and quantified by area.
2.6. Determination of Calorific Values (CV)
The calorific values (CV) of dried algal cells were determined using a Parr 6300 bomb calorimeter (Parr Instrument Company, USA). Microalgal cells were harvested from the different media conditions. The algal samples (100 mL) were filtered onto cellulose nitrate filters (Whatman GF/CTM Ø 47 mm) and dried before combustion in the calorimeter. Benzoic acid was used as calorific standard. The calorific value of biomass was calculated as indicated in Equation (4), Illman et al. [28].
with 2325 (kcal) being benzoic acid equivalent energy, T(end) and T(0) are the initial and final temperature of combustion, 45 is CV of Nichrome wire and cotton thread (kcal) and m is the total biomass weight.
2.7. Assessment of Biodiesel Quality
The quality of biodiesel was determined by assessing the saponification value (SV), iodine value (IV), cetane number (CN) and degree of unsaturation (DU) and compared to the international biodiesel standard (EN14214, ASTM D6751-02).
The values were calculated by using the equations below [29] where, F is the percentage of each fatty acid, M is the molecular mass of fatty acid, D is the number of double bonds, MUFA is monounsaturated fatty acids and PUFA is polyunsaturated fatty acid in wt %.
The kinematic viscosity at 40 °C (υ) of each FAME can be calculated by using Equation (9) and summation of all FAME-derived fuel properties provides the final υ of the biodiesel as published in Ramírez-Verduzco et al. [30]:
Predictive oxidative stability was calculated, where possible, based on C18:2 and C18:3 content as suggested by Park et al. [31] following Equation (10):
where X is the content of linoleic and linolenic acids (wt%) (0 < X < 100); and Y is the oxidation stability in hours.
2.8. Statistical Analysis
All experiments were performed in duplicate, unless stated otherwise. The reported values are the mean of the individual samples, while the error bars represent the range. One-way ANOVA was used to determine significance difference (α = 0.05%) between the means of independent conditions.
3. Results and Discussion
3.1. Assessment of the Temperature Effect on the Biomass Productivity
Microalgae are exposed to a variety of changes in the environment. Temperature is considered as a crucial factor influencing the algae culture and biomass productivity especially for outdoor cultivation. Seasonal cycles vary according to the climatic and geographical location of the habitat in which they are growing, and different strains of the same species may respond differently. Due to huge differences in Qatar’s climate between winter and summer, the ability of algae isolates to grow at different ranges of temperatures is of a vital importance for biomass production.
Figure 1 illustrates the effect of temperature on QUCCM31 growth rates and biomass productivity. The optimum growth rate and biomass productivity was found at 30 °C with a value of 1.05 (day−1) and 195 ± 4.2 mgL−1d−1 respectively, followed by a growth rate equal to 0.96 (day−1) and productivity of 157 ± 2.1 mgL−1d−1 at 40 °C, which confirm the thermotolerant capacity of the strain. A dramatic decrease (62%) of biomass productivity was seen at 20 °C in comparison to cultivation at 30 °C. The obtained growth pattern was observed by Sorokin et al. [32] where they reported the same findings and claimed that algae growth is slow at low temperatures, reaches an optimum growth at an adequate temperature and becomes slower at high temperatures [23]. Below optimal growth temperatures, an increase in temperature has a positive effect on photosynthesis and cell division. In order to explain the growth variations of algal strains under different temperatures, different strategies are developed. Torzillo and Vonshak [33] showed that temperature influences the photosynthetic process by altering the activities of important enzymes involved, among them Rubisco, which is a key enzyme responsible for carbon assimilation in photosynthetic organisms [34,35]. In addition, it also affects cell membrane fluidity, influencing the uptake of nutrients, which alter the growth of the algal strains [36]. On the other hand, for temperatures exceeding the optimal temperature, microalgae growth rate and biomass productivity decreases. This is generally explained by heat stress which can affect the functionalities of enzymes (inactivation, denaturation) or modify proteins which are involved in photosynthetic processes, thereby inhibiting the growth [37].
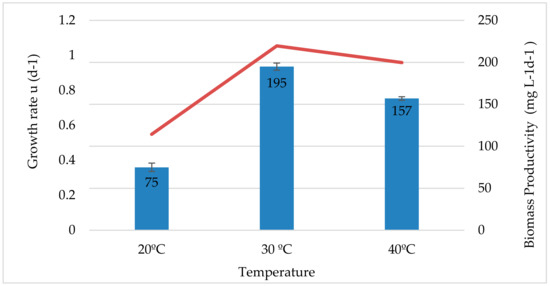
Figure 1.
Growth rate μ (d−1) and biomass productivity (mg L−1 d−1) of Nannochloris atomus at different cultivation temperatures.
The effect of temperature on the growth of Nannochloris atomus has not been a major focus of past research during the past decades. For that, our research work is considered as interesting as it contributes to the knowledge base and will lead to developing data on this very promising microalga. Results of the temperature fluctuations in Qatar’s environments show that algae cultivated outdoors are exposed to strong temperature variations with maximum temperatures of 40 °C. Consequently, a very temperature-robust algae strain is required for outdoor algae cultivation. The strain revealed the capacity to have a consistent growth at high temperature specific to Qatar’s climate and proved its flexible nature to adapt to the wide range of environmental conditions in Qatar, allowing its suitability to be cultivated year-round. Therefore, high biomass accumulation can be achieved during the day time and the low temperature observed at nights or during winter can help in avoiding the biomass loss [37].
3.2. Effect of Temperature Variations on Lipid Quantity, Productivity and Quality
Temperature is one of the parameters that is not only affecting algae growth but also influencing the quantity and quality of fatty acid. The total lipid content of the strain at different temperatures is shown in Figure 2. The results obtained showed that the total lipid content of the algae varied significantly (p ≤ 0.05) between the highest (40 °C) and lowest (20 °C) culture temperatures. With increasing temperature, the total lipid content increased 0.8 folds between 20 and 30 °C, and 1.33 folds between 20 and 40 °C. The strain showed the highest lipid content of 30%, with a lipid productivity of 47.1 ± 3.77 mgL−1d−1, at 40 °C, while the minimum lipid content (22.5%) was observed at 20 °C with a lipid productivity of 16.9 ± 3.27 mgL−1d−1. The studied Qatar Nannochloris atomus isolate exhibited higher amounts of lipid at all temperatures as compared to the findings of Pereira et al. [38], who reported 21% (w/w) in Nannochloris atomus isolated from the west coast of Saudi Arabia, a region that has similar environmental conditions to Qatar. Such results prove that temperature conditions are able to drive the production of lipids in QUCCCM31 cells and hence confirm the suitability of growing QUCCCM31 for high lipid production under Qatar’s diverse climate conditions. Enhancement of lipid production in cells under temperature variations is amply studied, however, similar to the growth, the effect of temperature on micro algal lipid content is shown to be strain dependent [16]. Nannochloris atomus QUCCCM31 showed the ability to accumulate increased amounts of lipids with increased cultivation temperatures, which is confirmed by several previous investigations of other microalgae species such as Chlorella, Spirogyra, Chlamydomonas, Botryococcus, Scenedesmus, Neochloris, Haematococcus, Nannochloropsis, and Ulva species [39]. Therefore, our data emphasizes on sustainable biomass and lipid production using QUCCCM31 over the entire seasons with a maximum production at high temperature, which is an important characteristic of Qatar’s climate. Several explanations were proposed to illustrate the effect of temperature on algal lipid. Some studies suggested that temperature presents a stressful condition to the microalgae to which they react by synthetizing the triacylglycerols (TAGs) as storage product; hence, the amount of total lipid increases. However, there are also species such as Chlorella vulgaris, which showed a decrease of 2.5 folds in lipids when the temperature increased from 25 °C to 30 °C. The alteration of the photosystem II activity was considered responsible for such lipid production behavior [18].
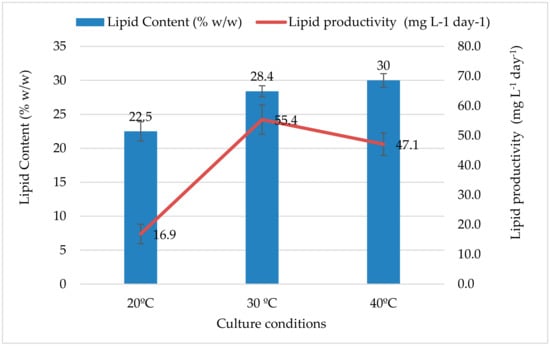
Figure 2.
Effect of Temperature Variation on QUCCCM31 Lipid Content and Productivity.
In conjunction with the lipid content, different temperatures of cultivation affect also the fatty acid composition (Table 1) and the numbers of double bonds in fatty acids (Figure 3). The results show that the saturated fatty acids (SFAs) presented the majority of FAMEs followed by the monounsaturated fatty acids (MUFAs) where the polyunsaturated fatty acids (PUFAs) were the lowest fraction. In parallel, the number of double bonds per fatty acid molecule decreased with increasing cultivation temperature. In general, temperature 40 °C stimulated the production of SFAs with a relative percentage of 45.2% of the total fatty acids and dominance of C16–C18 (97%), while 30 °C presented the highest amount of MUFAs with a maximum percentage of 40.6% and dominance of Oleic Acid C18:1n9 (38%). The oleic acid is the preferred fatty acid for biodiesel production and the existence of single bond provides the methyl ester a good cold flow and viscosity properties without compromising with the oxidative stability [40]. Besides, the temperature 20 °C presented the maximum percentage of polyunsaturated fatty acids (PUFAs) at 32.2% of total fatty acids. The majority of PUFAs was composed of essential fatty acids such as linoleic acid (LA, C18:2n-6) and α-linolenic acid (ALA, C18:3n-3). Other essential PUFAs in human nutrition such as arachidonic acid (AA, C20:4), eicosapentaenoic acid (EPA, C20:5n3), docosahexaenoic acid (DHA 22:6n3) and nervonic acid (C24:1) were also present. Such data correlates with previous findings stating an increase in MUFAs and decrease in PUFAs with temperature increase [41,42]. This is a common strategy in many organisms in an attempt to tolerate various temperatures. All cell types synthesize fatty acids. Besides their role as a major form of carbon and energy storage, they are main components of cell membranes and their composition controls the movement of materials into and out of the cell and ensure protection and structural stability for the organism. The common data might be explained by the fact that lower temperatures stimulate the expression of unsaturated fatty acids to help in maintaining the membrane fluidity and functions [41]. On the other hand, an elevation in temperature is accompanied by an increase in the saturated FA content in order to preserve the integrity of the algal cell membrane [43]. Respectively, we can conclude from our findings that the strain behaves differently at different temperatures for culture. High temperature culture conditions can, hence, be used to promote the production of high total lipid yield with desired saturation and carbon chain length (C16 to C18) suitable for biodiesel production, while cultures at low temperature seen at night or during the winter can be applied for the production of polyunsaturated fatty acids used for several nutraceutical and pharmaceutical applications.

Table 1.
Fatty acids composition of the strain Nannochloris atomus under 20, 30, at 40 °C. Data shown is the mean ± range, n = 2.
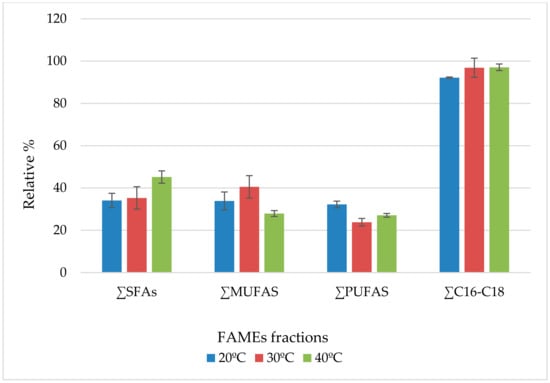
Figure 3.
Relative percentage of different fractions of fatty acid at the 3 different investigated temperatures.
3.3. Combined Effect of Temperature and Nitrogen Starvation on Biomass Productivity
In the second part of the work, a two-stage culture strategy was used wherein initially the culture was cultivated in nitrate-replete conditions followed by a nitrate-starvation phase. The aim was to increase the biomass productivity in the first step under normal conditions, followed by cultivation step for a lipid-accumulation. This is a very crucial parameter in the commercial production of microalgae biomass, which reduces the cost for down-stream processing. Accordingly, the combined effect of different nitrogen regimes and temperatures on biomass productivity of QUCCCM31 was studied.
Figure 4 summarizes the variations in biomass productivity under the different conditions investigated. Our study showed that there was no biomass loss while growing the culture in both nitrogen regimes comparing to the nitrogen replete medium. Moreover, at the three applied temperatures, the biomass productivity was higher in nitrogen depleted and limited compared to the replete media. We also noticed that, similarly to the normal conditions, the highest biomass productivity was observed at 30 °C for both nitrogen regimes with a maximum of 226mg L−1d−1 at nitrogen depleted.
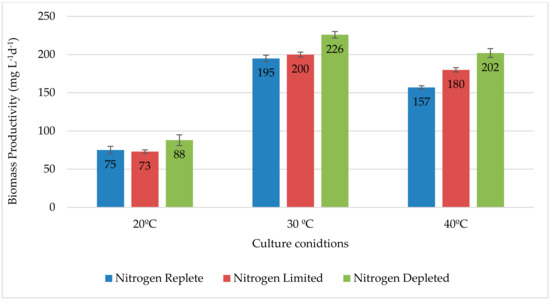
Figure 4.
Biomass Productivity of Strain QUCCCM31 under Nitrogen limitation and depletion.
A considerable biomass yield is a main concern for biodiesel production. Upon nitrogen starvation, the ability of strains to increase in biomass is observed for many microalgal strains [44,45,46]. Davis et al. [47] reported that growth of Chlorella pyrenoidosa continued in a medium that was depleted or deficient in nutrients, particularly nitrogen. A similar effect was observed recently in the species C. vulgaris [46]. Our Nannochloris isolate exhibited the same data with a continued growth at limitation or depletion conditions. These findings are supported by two explanations. The two-phase cultivation method followed in this study, where the algae were cultivated under normal conditions to reach a high biomass production, and then transferred in nitrogen limited or free medium for metabolite production. Culture in replete media enhance the chlorophyll accumulation, which is a nitrogen-rich compound and is easily accessible. After transfer to nitrogen limited or free media, the cells start to use the chlorophyll as an intracellular source which will allow their continuous growth despite exhaustion of external nitrogen source [44,48,49]. On the other hand, the nitrogen stress allows accumulation of large amount of lipids inside the cells, which results in an increase of the dry weight observed later by an increment in the biomass productivity [50].
3.4. Combined Effect of Temperature and Nitrogen Regime on Lipid Quantity and Quality
A number of previous studies have demonstrated that biochemical composition of microalgae can be manipulated by simultaneous changes in the chemical parameters of the culture medium. The secondary objective of the work aimed to optimize the lipid productivities through different nitrogen regimes and study the impact of interactions’ effects between the nitrogen and temperature fluctuations to understand the behavior of algal cells in large-scale systems. To achieve that, after first culturing QUCCCM31 in replete media, a nitrate-starvation phase was combined with temperature variations to improve the lipid production over an entire year. Subsequent to the biomass productivity, the impact of the combined parameters (temperature and nitrogen stress) on lipid quantity and quality was investigated.
The results showed that variation of temperature and nitrogen concentration had a significant effect on the lipid contents (Figure 5a) and productivity (Figure 5b) of Nannochloris atomus. In general, the temperature stress combined to both nitrogen regimes (NL and ND) increased the lipid content and productivity compared to the control culture. Between the two nitrogen regimes applied in this study, the nitrogen depletion revealed the highest lipid content compared to the nitrogen limitation with a maximum of 45% (w/w) at 40 °C compared to 41.2% and 32% at 30 °C and 20 °C respectively.
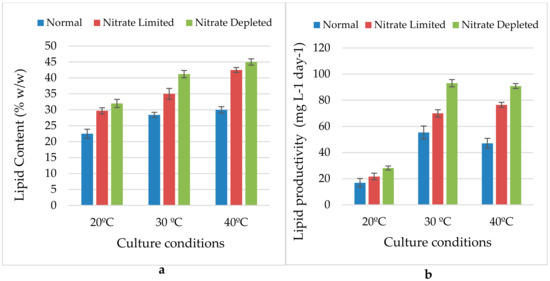
Figure 5.
Effect of temperature variation and nitrogen regimes on lipid content (a) and lipid productivities (b) of QUCCCM31.
Table 2 summarizes the percentage increase of biomass productivity and lipid content between the different conditions investigated. At 20 °C, the percentage increase of lipid content and productivity was 32% and 28.5%, respectively, for the nitrogen limitation, compared to 42.2% and 66.9%, respectively, for the nitrogen depleted. For the 30 °C, the percentage increase of lipid content and productivity was 23.2% and 26.4%, respectively, for the nitrogen limitation, versus 45.1% and 68.1%, respectively, for the nitrogen depleted. The temperature 40 °C condition induced higher accumulation of lipid content and productivity with a maximum percentage increase of 41.7% and 62.4%, respectively, for the nitrogen limitation, and 50% and 93%, respectively, at nitrogen depletion.

Table 2.
Effect of temperature variation and nitrogen regimes on QUCCCM31 Lipid productivity.
Based on data from the literature, the average daily lipid productivity in microalgae strains grown in N-replete media at 30 °C was calculated as ~50 mgL−1 day −1 [51]. In the first part of the study, we concluded that the temperature raised the lipid accumulation in our strain. The findings of the second part indicate that when combined with nitrogen stress, a greater positive effect on lipid productivity was observed than when subjected only to temperature variations, which is a very important factor affecting the biodiesel production. Our results are in correlation with other previous research work which highlighted that limited feeding of nitrate in batch cultivation of Nannochloris atomus successfully increased the intracellular lipid yield [44,45,52,53]. Another report observed a 22% drop in lipid yield in Nannochloris atomus when nitrate concentration increased from 0.9 mMl−1 to 9.9 mMl−1 [54]. Moreover, other studies conducted by Ordog et al. [55] also demonstrated that both temperature and nitrogen had a significant effect on lipid productivity of 3 different species of Chlorella.
Nitrogen is the most critical nutrient affecting lipid metabolism in algae. Numerous species of microalgae use the available carbon dioxide and solar energy and exhibit a significant accumulation of lipids, particularly TAG, in response to nitrogen deficiency [16,37]. Rodolfi et al. [56] also conducted a detailed and large-scale study and proved that nutrient starvation boost lipid production in several diatoms, green algae, and red algae. Different strategies were highlighted to explain the accumulation of lipids under nitrogen stress. Studies carried out by Li et al. [57] suggested that the increase under the nitrogen stress might be linked to the shifting from starch as the primary storage of carbon and energy into neutral lipids as a secondary storage product. This shifting is also explained by Roessler [58], showing that these storage lipids are mainly composed of saturated and monounsaturated fatty acids, which can be efficiently stored into the cell and can participate in rebuilding them following any stress. Other research work done by Ratledge [59] explained this accretion in lipids by the cessation of enzymes related to the cell growth and activation of other enzymes responsible for the accumulation of lipids. The present study confirms that our local isolate QUCCCM31 is an oleaginous thermotolerant strain-producing lipid in response to temperature stress and, additionally, the nitrogen stress enhanced the lipid expression. From the perspective of economics, these investigated conditions can be applied to grow the strain Nannochloris atomus QUCCCM31 in order to accumulate lipids, which is a key factor for biodiesel production.
The quantity and quality of fatty acids content, as well as the entire biochemical composition, varies in response to culture conditions [60]. In order to analyze the combined effect of temperature and nitrogen on the fatty acid composition, trans-esterified total lipids were analyzed by GC-FID and the results expressed in terms of relative percentage of each fatty acid are presented in Table 3.

Table 3.
Effect of nitrogen limitation and depletion on Fatty acid composition of QUCCCM31.
Similarly, to the temperature effect on lipid quality, nutrient deprivation tends to result in a FAMEs profile enriched with SFA and MUFAs (Table 3). Indeed, saturated fatty acids were high at 40 °C ND, while the MUFAs presented the second-high fraction at 20 and 30 °C ND. It was previously stated that C16:0 and C18:0 are the major SFAs and C18:1 is the most dominant MUFA [56]. These data are similar to our present study where the main saturated fatty acid was palmitic acid (C16:0), whereas oleic acid (18:1) raised the MUFAs’ amount. The obtained FAME compositions are in agreement with those reported for Nannochloris species by Talebi et al. [61] and Reitan et al.’s [52] findings, which showed an increment in SFAs and MUFAs under nutrient depletion. The same trend was observed in Dunaliella tertiolecta and Stephanodiscus minutulus where the amounts in MUFAs’ and SFAs’ values were directly related to changes in the amount of nitrogen in the media [62]. In contrast to the SFAs’ and MUFAs’ fractions that were high under the depletion conditions, cultures in nitrogen limitations at low temperatures enhanced the amount of PUFAs. PUFAs are one of the most nutritionally important and essential fatty acids due to their multiple health benefits [63]. A considerable increment in PUFAs (%) was observed reaching a maximum accumulation at 20 °C NL with 42.8%, while it was a maximum of 32.2% in replete media (~1.3X) (Table 3). Moreover, the high value of PUFAs/SFAs index proved the nutritional value of Nannochloris atomus cultivated under the previously cited conditions [64]. Linoleic (18:2) and linolenic (18:3) acids were dominant in PUFAs and contribute to the overall increase. This nutritional value is enhanced by the existence of eicosapentaenoic acid (EPA, C20:5n3), docosahexaenoic acid (DHA 22:6n3) and nervonic acid (C24:1). In line with our findings, Wang et al. [65] stated that N-limitation led to an increase of PUFAs for Phaeodactylum tricornutum, Isochrysis aff. galbana clone T-Iso, Rhodomonas baltica, and Nannochloropsis oceanica. This is also in accordance with previous research data reporting that the nutrient limitation induced the synthesis of PUFAs [66].
In recent years, species of green algae are considered as a source of essential fatty acids that are necessary not only for improving the organism’s nutrition, but also for production of biodiesel. Apart from adjusting carbon allocation to favor lipid accumulation under nitrogen starvation, microalgae also modify their lipid metabolism in response to changing environmental conditions. Changes in specific fatty acids in response to N-deprivation appear to be strain specific [67]. Moreover, it is speculated that microalgae modify their fatty acid composition as a strategy to adapt to the changing temperatures and nitrogen. At high temperatures and nitrate depletion, the amount of SFAs and MUFAs increase to maintain the integrity and fluidity of the cell membrane phospholipids layer [33], [36]; while the trend for increasing fatty acid unsaturation with decreasing temperature and nitrogen limitation is linked to the biosynthesis or conversion of existing polar membrane lipids into neutral lipid storage [55].
Lipid quality is a critical variable evaluating the algae species. Finding the appropriate conditions to stimulate the synthesis of different fatty acids of interest is crucial for developing an efficient biological production process. From a practical point of view, culturing QUCCCM31 under nitrogen depletion can be applied for good quality of biodiesel production, while the nitrogen limitation can be applied to grow Nannochloris to enhance PUFAs for pharmaceutical applications. The existence of PUFA-chain from algae constitute a robust scientific and technological basis for the industrial development of high-value products from algae. This is very important in the biodiesel production as it helps to make the process economically feasible by reducing the cost through biorefinery process. The main targeted application could be the use of high purified omega-3 fatty acids (DHA/EPA) and omega-9 (nervonic acid) for nutrition and pharmaceutical applications.
3.5. Determination of Biomass Calorific Values (CV)
The full biomass sample of strain Nannochloris atomus obtained from the different culture conditions was analyzed for calorific value and the data are outlined in Table 4.

Table 4.
Calorific value of dried biomass cultivated under different culture conditions.
In accordance with the other results, it was clear that the response of the algal strains to different temperature and nitrogen regimes was variable. Under temperature variations, the maximum calorific value was seen at 40 °C with a value of 19.27 ± 0.596 kJg−1. When different nitrogen regimes are combined to temperature variations, there was a significant increase in the calorific value from 7 kJg−1 to 15 kJ g−1 under 20 ºC, 16 kJg−1 to 28 kJg−1 under 30 °C and 19 kJg−1 to 29 kJg−1 under 40 °C. The highest calorific value was obtained with the strain grown at 40 °C in ND medium (29 kJg−1). We also noticed that there is significant correlation at the 5% level between lipid content and the calorific value (Figure 6). This is because lipids are high energy-rich biomolecules, and their accumulation yielded to an increase in the calorific value.
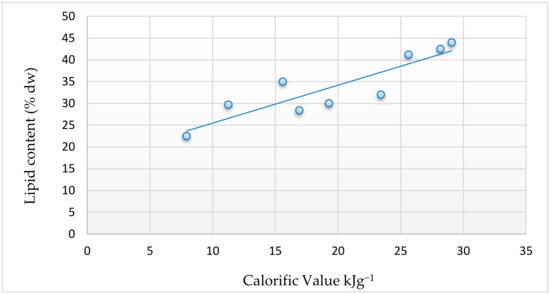
Figure 6.
Relation between lipid content and calorific value of strain QUCCCM31 under different culture conditions.
Earlier findings showed that microalgae, when grown under normal conditions, presents calorific values between 18 and 21 kJg−1 [59]. With variation of nutrient concentration as well as other environmental conditions, some microalgae accumulates high amount of lipids and the calorific value increases from 21 to 29 kJg−1 for some Chlorella species [28], and can reach a value of 35.58 kJg−1 for B. braunii [68]. In the present research work, the data obtained when the nitrogen and temperature stress were combined were found to conform with Illman et al. [28] and Scragg et al.’s [69] findings. Although the calorific value of petroleum diesel is 42 kJg−1 [28], biomass with a calorific value greater than 20 kJg−1 are seen to be satisfactory [70]. This is because biodiesel from microalgae biomass gives a cleaner product without contamination of sulphur or phosphorus pollutants, unlike the petroleum diesel, which is preferable for fuel quality [71]. Therefore, the algal biomass of strain QUCCCM31, when cultivated under specific conditions, proved its suitability as a high-energy source, and a viable alternative to be used as a diesel replacement.
3.6. Assessment of Biodiesel Quality
FAMEs are used to estimate the properties of the biodiesel and previous studies have shown that the profile of FAMEs in microalgal biomass are affected by many factors [40]. This has also been elucidated in our study where it was proved that the different temperatures and nitrogen regimes applied affected amply the composition of FAMEs.
Despite the actual emphasis on use of algal lipids as biodiesel feedstocks, only a few literature reports of actual biodiesel produced from algae exist, and even fewer reports of relevant fuel properties. The Table 5 summarizes a comparison between the biodiesel properties produced from our local isolate Nannochloris atomus under different culture conditions and data reported by other research work. This table summarizes the properties of biodiesel fuel such as CN, IV, SV, DU, and kinematic viscosity derived from the FAME compositions of Nannochloris atomus compared to the international biodiesel standard (EN14214, ASTM D6751-02) and other algae strains. It was observed that most of the nine conditions investigated presented biodiesel properties within the range of standard values.

Table 5.
Biodiesel properties calculated from the FAME profile of strain Nannochloris atomus at different culture conditions.
The cetane number (CN) is widely used as a fuel quality parameter. It is related to the time between injection and ignition. The shorter this time is, the higher is the CN [76]. Fuels with low cetane number can cause damage to the engines and increase the formation of white smoke emission due to incomplete combustion. The CN is highly influenced by the FAMEs’ profile. According to UNE-EN 14214 and ASTM D6751-02 standards, the CN for biodiesel should be a minimum of 51. Most of the conditions studied in our experiment presented a CN matching with the standard values for biodiesel. Our findings evidenced that CN values of Nannochloris atomus FAMEs increased with temperature combined with nitrate depletion. Indeed, the highest CN was observed in the case of 30 and 40 °C under nitrate depleted regimes (~62). This is due to the effect of temperature variations combined to nitrate starvation on the saturation of the fatty acids [22]. However, CN value of 20 °C nitrate limited condition is slightly below the range. This is explained by the fact that low cetane numbers have been associated with more highly unsaturated components, mainly the esters of linoleic (C18:2) and linolenic (C18:3) acids [40]. Biodiesel is most likely used with conventional petroleum diesel in different blend concentration depending on CN and density of the biodiesel. Therefore, biodiesel with higher cetane numbers can be blended at higher concentrations with petroleum diesel. Conditions of nitrate depleted at 30 °C and 40 °C are the best concerning the CN comparing with the other investigated culture conditions and even with regards to other species of algae strains presented in Table 5.
Iodine value (IV) is another parameter that estimates the properties of biodiesel. It measures the unsaturation within a mixture of fatty acid which is important for viscosity and cloud points. The lower the IV, the better the fuel will be as a biodiesel [77,78]. In the UNE-EN 14214 and ASTM D6751-02 standards, the iodine value is limited to 120 g I2/100 g. All the conditions investigated presented an IV value matching with the standards. Conditions with less unsaturation are giving a better iodine number. This is the case, in this study, of the culture at high temperature in nitrogen-depleted media where the IV is very low. Other species, Micractinium pusillum CCAP 248/1 investigated in the literature, showed lower IV = 28. This can be explained by a possible low percentage of unsaturation probably due to the culture conditions.
Oxidation stability is also one of the major issues affecting the use of biodiesel. It corresponds to the minimum time that biodiesel can stay before degradation and is extremely affected by the content of polyunsaturated methyl esters [79]. As per the standard values defined by UNE-EN 14214 and ASTM D6751-02, a minimum induction period of six hours is determined for biodiesel samples. Except the conditions of 20 °C nitrate limited; all the other conditions meet the standards. Among them, 30 °C and 40 °C ND tend to give biodiesel with better stability.
Additionally, to these parameters, biodiesel must have an appropriate kinematic viscosity (υ) for a proper operation of the engine. It is an important parameter to ensure that fuel supply reaches injectors at different operating temperatures. From our study, the microalgae-based biodiesel issued from the nine different conditions studied presented a kinematic viscosity within the range of ASTM D6751-02 set standard.
As elucidated above, temperature and nitrogen starvation had a major impact on FAME profiles in QUCCCM31 isolate. It is, thus, not surprising that the investigated biodiesel properties were also tightly linked with the applied culture conditions. In general, besides the cultures issued from conditions in nitrate limitation media at 20 °C, the fuel properties of biodiesel issued from the eight other different conditions were found to be conformed to the UNE-EN 14214 and ASTM D6751-02 standards. However, cultures in ND media under 30 °C and 40 °C were the most efficient conditions improving the quality of biodiesel properties and gave better results to some of the strains described in literature (Table 5). This is very interesting for large-scale biodiesel production in places such as Qatar’s climate where the temperatures are high almost all the year. Nonetheless, the nitrogen stress should be taken into account. Nannochloris atomus QUCCCM31 oil appeared to be a good feedstock for biodiesel production, which can be partially substituted for petro-diesel under most operating conditions, regarding performance parameters, without any modifications having to be made to the engine.
4. Conclusions
The present study investigated the impact of seasonal temperature variations seen in Qatar on the growth and lipid productivity of the locally isolated marine microalgae Nannochloris atomus QUCCCM31. In parallel various nitrogen, starvation strategies were combined to temperatures to enhance the lipid productivity and quality.
The strain showed its ability to meet the sustainability challenges of algal biomass production by growing under temperature and nitrogen variations. The two-stage cultivation processes followed by nitrogen starvation represented a feasible strategy to increase the lipid content without affecting the biomass productivity, which is very important at commercial level for biodiesel production.
The analysis of FAMEs’ profiles of the Nannochloris atomus QUCCCM31 under the different conditions applied showed that saturated fatty acids are more dominant at high temperature, and N-depleted where low temperatures coupled to N-limitation enhanced the production of PUFAs. The presence of omega-3 and omega-9 fatty acids as valuable co-products increased the potential use of the QUCCCM31.
Biodiesel properties of the strains issued from most conditions studied was conformed to the EN 14214 and ASTM D6751-02 standards. Nonetheless, when the strain is cultivated in nitrogen-depleted media under 30 and 40 °C, the quality of the biodiesel synthesized was improved.
These data are essential to optimize the cultivation of commercially interesting microalgae for biodiesel production and, hence, to grow them in a cost-effective and profitable way.
Author Contributions
T.B.: Conception & Design, performance of the experiments, collection and assembly of data, Analysis and interpretation of the data, drafting the article. I.S.: obtaining the funding, Conception & Design, Critical revision of the article for important intellectual content, Final approval of the article. R.R.: Collection and assembly of data (Growth Analysis), critical revision of the article. K.S. and H.A.J.: Critical revision of the article and M.A.M.: Technical. All authors have read and agreed to the published version of the manuscript.
Funding
This report was made possible by the NPRP award (NPRP8-1087-1-207) from the Qatar National Research Fund (a member of The Qatar Foundation).
Acknowledgments
This report was made possible by the NPRP award (NPRP8-1087-1-207) from the Qatar National Research Fund (a member of The Qatar Foundation). The statements made herein are solely the responsibility of the authors. The author would like to acknowledge the support from Ms. Mariam Al Emadi, Research Services Manager at the Centre, to fulfill all requirements for this research. The author is also thankful to Ms. Ghamza Al Ghasal and Mr. Mahroof Eroth from Qatar University for their assistance during the work. The publication of this article was funded by the Qatar National Library.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Leader, T.; Team, A.I.; Analysis, I.E.; Analysis, R.; Gas, N.; Analysis, B.; Analysis, E.; Lynn, D.; Building, F. Annual Energy Outlook 2014; U.S. Energy Inf. Administration: Washington, DC, USA, 2014; pp. 1–269. [Google Scholar]
- Larkum, A.W.D. Limitations and prospects of natural photosynthesis for bioenergy production. Curr. Opin. Biotechnol. 2010, 21, 271–276. [Google Scholar] [CrossRef] [PubMed]
- Metting, F.B. Biodiversity and application of microalgae. J. Ind. Microbiol. Biotechnol. 1996, 17, 477–489. [Google Scholar] [CrossRef]
- Encarnação, T.; Arranja, C.T.; Cova, T.F.G.G.; Pais, A.A.C.C.; Campos, M.G.; Sobral, A.J.F.N.; Burrows, H.D. Monitoring oil production for biobased feedstock in the microalga Nannochloropsis sp.: A novel method combining the BODIPY BD-C12 fluorescent probe and simple image processing. J. Appl. Phycol. 2018, 30, 2273–2285. [Google Scholar] [CrossRef]
- Bertozzini, E.; Galluzzi, L.; Penna, A.; Magnani, M. Application of the standard addition method for the absolute quantification of neutral lipids in microalgae using Nile red. J. Microbiol. Methods 2011, 87, 17–23. [Google Scholar] [CrossRef]
- Bobde, K.; Momin, H.; Bhattacharjee, A.; Aikat, K. Energy assessment and enhancement of the lipid yield of indigenous Chlorella sp. KA-24NITD using Taguchi approach. Renew. Energy 2019, 131, 1226–1235. [Google Scholar] [CrossRef]
- Ranjan, A.; Patil, C.; Moholkar, V.S. Mechanistic assessment of microalgal lipid extraction. Ind. Eng. Chem. Res. 2010, 49, 2979–2985. [Google Scholar] [CrossRef]
- Molino, A.; Mehariya, S.; Di Sanzo, G.; Larocca, V.; Martino, M.; Leone, G.P.; Marino, T.; Chianese, S.; Balducchi, R.; Musmarra, D. Recent developments in supercritical fluid extraction of bioactive compounds from microalgae: Role of key parameters, technological achievements and challenges. J. CO2 Util. 2020, 36, 196–209. [Google Scholar] [CrossRef]
- Alexandre, A.M.R.C.; Serra, A.T.; Matias, A.A.; Duarte, C.M.M.; Bronze, M.R. Supercritical fluid extraction of Arbutus unedo distillate residues—Impact of process conditions on antiproliferative response of extracts. J. CO2 Util. 2020, 37, 29–38. [Google Scholar] [CrossRef]
- Leone, G.P.; Balducchi, R.; Mehariya, S.; Martino, M.; Larocca, V.; Sanzo, G.D.; Iovine, A.; Casella, P.; Marino, T.; Karatza, D.; et al. Selective extraction of ω-3 fatty acids from nannochloropsis sp. using supercritical CO2 Extraction. Molecules 2019, 24, 2406. [Google Scholar] [CrossRef]
- Da Rosa, B.V.; Kuhn, K.R.; Ugalde, G.A.; Zabot, G.L.; Kuhn, R.C. Antioxidant compounds extracted from Diaporthe schini using supercritical CO2 plus cosolvent. Bioprocess Biosyst. Eng. 2020, 43, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Demirbas, A.; Fatih Demirbas, M. Importance of algae oil as a source of biodiesel. Energy Convers. Manag. 2011, 52, 163–170. [Google Scholar] [CrossRef]
- Vadlamani, A.; Viamajala, S.; Pendyala, B.; Varanasi, S. Cultivation of Microalgae at Extreme Alkaline pH Conditions: A Novel Approach for Biofuel Production. ACS Sustain. Chem. Eng. 2017, 5, 7284–7294. [Google Scholar] [CrossRef]
- Béchet, Q.; Shilton, A.; Guieysse, B. Modeling the effects of light and temperature on algae growth: State of the art and critical assessment for productivity prediction during outdoor cultivation. Biotechnol. Adv. 2013, 31, 1648–1663. [Google Scholar] [CrossRef]
- Rai, M.P.; Gupta, S. Effect of media composition and light supply on biomass, lipid content and FAME profile for quality biofuel production from Scenedesmus abundans. Energy Convers. Manag. 2017, 141, 85–92. [Google Scholar] [CrossRef]
- Saadaoui, I.; Bounnit, T.; Muraikhi, M.; Rasheed, R.; Alghasal, G.; Al Jabri, H. Improvement of both lipid and biomass productivities of Qatar Chlorocystis isolate for biodiesel production and food security. Phycol. Res. 2018, 66, 182–188. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, J. Effects of heat stress on photosynthetic electron transport in a marine cyanobacterium Arthrospira sp. J. Appl. Phycol. 2016, 28, 757–763. [Google Scholar] [CrossRef]
- Converti, A.; Casazza, A.A.; Ortiz, E.Y.; Perego, P.; Del Borghi, M. Effect of temperature and nitrogen concentration on the growth and lipid content of Nannochloropsis oculata and Chlorella vulgaris for biodiesel production. Chem. Eng. Process. Process Intensif. 2009, 48, 1146–1151. [Google Scholar] [CrossRef]
- Courchesne, N.M.D.; Parisien, A.; Wang, B.; Lan, C.Q. Enhancement of lipid production using biochemical, genetic and transcription factor engineering approaches. J. Biotechnol. 2009, 141, 31–41. [Google Scholar] [CrossRef]
- Riches, C.J.; Robinson, P.K.; Rolph, C.E. Effect of heavy metals on lipids from the freshwater alga Selenastrum capricornutum. Biochem. Soc. Trans. 2015, 24, 174S. [Google Scholar] [CrossRef]
- Lombardi, A.T.; Wangersky, P.J. Particulate lipid class composition of three marine phytoplankters Chaetoceros gracilis, Isochrysis galbana (Tahiti) and Dunaliella tertiolecta grown in batch culture. Hydrobiologia 1995, 306, 1–6. [Google Scholar] [CrossRef]
- Divya, V.B. Tyagi Biodiesel: Source, Production, Composition, Properties and Its Benefits. J. Oleo Sci. 2006, 55, 487–502. [Google Scholar]
- Saadaoui, I.; Al Ghazal, G.; Bounnit, T.; Al Khulaifi, F.; Al Jabri, H.; Potts, M. Evidence of thermo and halotolerant Nannochloris isolate suitable for biodiesel production in Qatar Culture Collection of Cyanobacteria and Microalgae. Algal Res. 2016, 14, 39–47. [Google Scholar] [CrossRef]
- Guillard, R.R.L. Culture of Phytoplankton for Feeding Marine Invertebrates. In Culture of Marine Invertebrates Animals; Smith, M.L., Chanley, M.H., Eds.; Springer: Boston, MA, USA, 1975; pp. 29–60. [Google Scholar]
- Zhu, C.J.; Lee, Y.K. Determination of biomass dry weight of marine microalgae. J. Appl. Phycol. 1997, 9, 189–194. [Google Scholar] [CrossRef]
- Folch, J.; Lees, M.; Sloane Stanley, G.H. A simple method for the isolation and purification of total lipids from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar]
- Griffiths, M.J.; Harrison, S.T.L. Lipid productivity as a key characteristic for choosing algal species for biodiesel production. J. Appl. Phycol. 2009, 21, 493–507. [Google Scholar] [CrossRef]
- Illman, A.M.; Scragg, A.H.; Shales, S.W. Increase in Chlorella strains calorific values when grown in low nitrogen medium. Enzym. Microb. Technol. 2000, 27, 631–635. [Google Scholar] [CrossRef]
- Francisco, É.C.; Neves, D.B.; Jacob-Lopes, E.; Franco, T.T. Microalgae as feedstock for biodiesel production: Carbon dioxide sequestration, lipid production and biofuel quality. J. Chem. Technol. Biotechnol. 2010, 85, 395–403. [Google Scholar] [CrossRef]
- Ramírez-Verduzco, L.F.; Rodríguez-Rodríguez, J.E.; Jaramillo-Jacob, A.D.R. Predicting cetane number, kinematic viscosity, density and higher heating value of biodiesel from its fatty acid methyl ester composition. Fuel 2012, 91, 102–111. [Google Scholar] [CrossRef]
- Park, J.Y.; Kim, D.K.; Lee, J.P.; Park, S.C.; Kim, Y.J.; Lee, J.S. Blending effects of biodiesels on oxidation stability and low temperature flow properties. Bioresour. Technol. 2008, 99, 1196–1203. [Google Scholar] [CrossRef]
- Sorokin, C.; Krauss, R.W. Effects of Temperature & Illuminance on Chlorella Growth Uncoupled From Cell Division. Plant Physiol. 1962, 37, 37–42. [Google Scholar] [PubMed]
- Torzillo, G.; Vonshak, A. Environmental Stress Physiology with Reference to Mass Cultures. In Handbook of Microalgal Culture: Applied Phycology and Biotechnology; Wiley: Chichester, UK, 2013; pp. 90–100. [Google Scholar]
- Feller, U.; Crafts-Brandner, S.J.; Salvucci, M.E. Moderately High Temperatures Inhibit Ribulose-1,5-Bisphosphate Carboxylase/Oxygenase (Rubisco) Activase-Mediated Activation of Rubisco. Plant Physiol. 1998, 116, 539–546. [Google Scholar] [CrossRef] [PubMed]
- Salvucci, M.E.; Crafts-Brandner, S.J. Mechanism for deactivation of Rubisco under moderate heat stress. Physiol. Plant. 2004, 122, 513–519. [Google Scholar] [CrossRef]
- Doubnerová, V.; Ryšlavá, H. What can enzymes of C4 photosynthesis do for C3 plants under stress? Plant Sci. 2011, 180, 575–583. [Google Scholar] [CrossRef] [PubMed]
- Hu, Q. Environmental Effects on Cell Composition. In Handbook of Microalgal Culture; Richmond, A., Ed.; Wiley: Chichester, UK, 2007. [Google Scholar] [CrossRef]
- Pereira, H.; Barreira, L.; Custódio, L.; Alrokayan, S.; Mouffouk, F.; Varela, J.; Abu-Salah, K.M.; Ben-Hamadou, R. Isolation and fatty acid profile of selected microalgae strains from the red sea for biofuel production. Energies 2013, 6, 2773–2783. [Google Scholar] [CrossRef]
- Singh, S.P.; Singh, P. Effect of temperature and light on the growth of algae species: A review. Renew. Sustain. Energy Rev. 2015, 50, 431–444. [Google Scholar] [CrossRef]
- Knothe, G. “Designer” biodiesel: Optimizing fatty ester composition to improve fuel properties. Energy Fuels 2008, 22, 1358–1364. [Google Scholar] [CrossRef]
- Jiang, H.; Gao, K. Effects of lowering temperature during culture on the production of polyunsaturated fatty acids in the marine diatom Phaeodactylum tricornutum (Bacillariophyceae). J. Phycol. 2004, 40, 651–654. [Google Scholar] [CrossRef]
- Rousch, J.M.; Bingham, S.E.; Sommerfeld, M.R. Changes in fatty acid profiles of thermo-intolerant and thermo-tolerant marine diatoms during temperature stress. J. Exp. Mar. Bio. Ecol. 2003, 295, 145–156. [Google Scholar] [CrossRef]
- Tsydendambaev, V.D.; Ivanova, T.V.; Khalilova, L.A.; Kurkova, E.B.; Myasoedov, N.A.; Balnokin, Y.V. Fatty acid composition of lipids in vegetative organs of the halophyte Suaeda altissima under different levels of salinity. Russ. J. Plant Physiol. 2013, 60, 661–671. [Google Scholar] [CrossRef]
- Da Silva, A.F.; Lourenço, S.O.; Chaloub, R.M. Effects of nitrogen starvation on the photosynthetic physiology of a tropical marine microalga Rhodomonas sp. (Cryptophyceae). Aquat. Bot. 2009, 91, 291–297. [Google Scholar] [CrossRef]
- Pruvost, J.; Van Vooren, G.; Le Gouic, B.; Couzinet-Mossion, A.; Legrand, J. Systematic investigation of biomass and lipid productivity by microalgae in photobioreactors for biodiesel application. Bioresour. Technol. 2011, 102, 150–158. [Google Scholar] [CrossRef]
- Přibyl, P.; Cepák, V.; Zachleder, V. Production of lipids in 10 strains of chlorella and parachlorella, and enhanced lipid productivity in chlorella vulgaris. Appl. Microbiol. Biotechnol. 2012, 94, 549–561. [Google Scholar] [CrossRef]
- Davis, D.K.; Greene, R.P.; Estaville, L.E.; Saku, J.C.; Matthews, O.P.; Sternberg, R.; Vanderbeck, R.M.; Moseley, W.G.; O’Brien, W.E.; Gatrell, J.D.; et al. Physiology and Biochemistry of Algae. Arch. Biochem. Biophys. 2002, 10, 44–77. [Google Scholar]
- Pal, D.; Khozin-Goldberg, I.; Cohen, Z.; Boussiba, S. The effect of light, salinity, and nitrogen availability on lipid production by Nannochloropsis sp. Appl. Microbiol. Biotechnol. 2011, 90, 1429–1441. [Google Scholar] [CrossRef] [PubMed]
- Msanne, J.; Xu, D.; Konda, A.R.; Casas-Mollano, J.A.; Awada, T.; Cahoon, E.B.; Cerutti, H. Metabolic and gene expression changes triggered by nitrogen deprivation in the photoautotrophically grown microalgae Chlamydomonas reinhardtii and Coccomyxa sp. C-169. Phytochemistry 2012, 75, 50–59. [Google Scholar] [CrossRef] [PubMed]
- Chi, Z.; Liu, Y.; Frear, C.; Chen, S. Study of a two-stage growth of DHA-producing marine algae Schizochytrium limacinum SR21 with shifting dissolved oxygen level. Appl. Microbiol. Biotechnol. 2009, 81, 1141–1148. [Google Scholar] [CrossRef] [PubMed]
- Stansell, G.R.; Gray, V.M.; Sym, S.D. Microalgal fatty acid composition: Implications for biodiesel quality. J. Appl. Phycol. 2012, 24, 791–801. [Google Scholar] [CrossRef]
- Reitan, K.I.; Rainuzzo, J.R.; Olsen, Y. Effect of nutrient limitation on fatty acid and lipid content of marine microalgae. J. Phycol. 1994, 30, 972–979. [Google Scholar] [CrossRef]
- Viêgas, C.V.; Hachemi, I.; Freitas, S.P.; Mäki-Arvela, P.; Aho, A.; Hemming, J.; Smeds, A.; Heinmaa, I.; Fontes, F.B.; Da Silva Pereira, D.C.; et al. A route to produce renewable diesel from algae: Synthesis and characterization of biodiesel via in situ transesterification of Chlorella alga and its catalytic deoxygenation to renewable diesel. Fuel 2015, 155, 144–154. [Google Scholar] [CrossRef]
- Takagi, M.; Watanabe, K.; Yamaberi, K.; Yoshida, T. Limited feeding of potassium nitrate for intracellular lipid and triglyceride accumulation of Nannochloris sp. UTEX LB1999. Appl. Microbiol. Biotechnol. 2000, 54, 112–117. [Google Scholar] [CrossRef] [PubMed]
- Ördög, V.; Stirk, W.A.; Bálint, P.; Aremu, A.O.; Okem, A.; Lovász, C.; Molnár, Z.; van Staden, J. Effect of temperature and nitrogen concentration on lipid productivity and fatty acid composition in three Chlorella strains. Algal Res. 2016, 16, 141–149. [Google Scholar] [CrossRef]
- Rodolfi, L.; Zittelli, G.C.; Bassi, N.; Padovani, G.; Biondi, N.; Bonini, G.; Tredici, M.R. Microalgae for oil: Strain selection, induction of lipid synthesis and outdoor mass cultivation in a low-cost photobioreactor. Biotechnol. Bioeng. 2009, 102, 100–112. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Horsman, M.; Wang, B.; Wu, N.; Lan, C.Q. Effects of nitrogen sources on cell growth and lipid accumulation of green alga Neochloris oleoabundans. Appl. Microbiol. Biotechnol. 2008, 81, 629–636. [Google Scholar] [CrossRef]
- Roessler, P.G. Environmental control of glycerolipid metabolism in microalgae: Commercial implications and future research directions. J. Phycol. 1990, 26, 393–399. [Google Scholar] [CrossRef]
- Ratledge, C. Fatty acid biosynthesis in microorganisms being used for Single Cell Oil production. Biochimie 2004, 86, 807–815. [Google Scholar] [CrossRef]
- Nomura, M.; Kamogawa, H.; Susanto, E.; Kawagoe, C.; Yasui, H.; Saga, N.; Hosokawa, M.; Miyashita, K. Seasonal variations of total lipids, fatty acid composition, and fucoxanthin contents of Sargassum horneri (Turner) and Cystoseira hakodatensis (Yendo) from the northern seashore of Japan. J. Appl. Phycol. 2013, 25, 1159–1169. [Google Scholar] [CrossRef]
- Talebi, A.F.; Mohtashami, S.K.; Tabatabaei, M.; Tohidfar, M.; Bagheri, A.; Zeinalabedini, M.; Hadavand Mirzaei, H.; Mirzajanzadeh, M.; Malekzadeh Shafaroudi, S.; Bakhtiari, S. Fatty acids profiling: A selective criterion for screening microalgae strains for biodiesel production. Algal Res. 2013, 2, 258–267. [Google Scholar] [CrossRef]
- Chen, M.; Tang, H.; Ma, H.; Holland, T.C.; Ng, K.Y.S.; Salley, S.O. Effect of nutrients on growth and lipid accumulation in the green algae Dunaliella tertiolecta. Bioresour. Technol. 2011, 102, 1649–1655. [Google Scholar] [CrossRef]
- Koller, M.; Muhr, A.; Braunegg, G. Microalgae as versatile cellular factories for valued products. Algal Res. 2014, 6, 52–63. [Google Scholar] [CrossRef]
- Lawton, C.L.; Delargy, H.J.; Brockman, J.; Smith, F.C.; Blundell, J.E. The degree of saturation of fatty acids influences post-ingestive satiety. Br. J. Nutr. 2000, 83, 473–482. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Fosse, H.K.; Li, K.; Chauton, M.S.; Vadstein, O.; Reitan, K.I. Influence of nitrogen limitation on lipid accumulation and EPA and DHA content in four marine microalgae for possible use in aquafeed. Front. Mar. Sci. 2019, 6, 95. [Google Scholar] [CrossRef]
- Sukenik, A. Ecophysiological considerations in the optimization of eicosapentaenoic acid production by Nannochloropsis sp. (Eustigmatophyceae). Bioresour. Technol. 1991, 35, 263–269. [Google Scholar] [CrossRef]
- Roleda, M.Y.; Slocombe, S.P.; Leakey, R.J.G.; Day, J.G.; Bell, E.M.; Stanley, M.S. Effects of temperature and nutrient regimes on biomass and lipid production by six oleaginous microalgae in batch culture employing a two-phase cultivation strategy. Bioresour. Technol. 2013, 129, 439–449. [Google Scholar] [CrossRef]
- Chaffin, J.D.; Mishra, S.; Kuhaneck, R.M.; Heckathorn, S.A.; Bridgeman, T.B. Environmental controls on growth and lipid content for the freshwater diatom, Fragilaria capucina: A candidate for biofuel production. J. Appl. Phycol. 2012, 24, 1045–1051. [Google Scholar] [CrossRef]
- Scragg, A.H.; Illman, A.M.; Carden, A.; Shales, S.W. Growth of microalgae with increased calorific values in a tubular bioreactor. Biomass Bioenergy 2002, 23, 67–73. [Google Scholar] [CrossRef]
- Mallick, N.; Mandal, S.; Singh, A.K.; Bishai, M.; Dash, A. Green microalga Chlorella vulgaris as a potential feedstock for biodiesel. J. Chem. Technol. Biotechnol. 2012, 87, 137–145. [Google Scholar] [CrossRef]
- Mittelbach, M. Diesel fuel derived from vegetable oils, VI: Specifications and quality control of biodiesel. Bioresour. Technol. 1996, 56, 7–11. [Google Scholar] [CrossRef]
- Knothe, G. Fuel Properties of Highly Polyunsaturated Fatty Acid Methyl Esters. Prediction of Fuel Properties of Algal Biodiesel. Energy Fuels 2012, 26, 5265–5273. [Google Scholar] [CrossRef]
- Radakovits, R.; Jinkerson, R.E.; Fuerstenberg, S.I.; Tae, H.; Settlage, R.E.; Boore, J.L.; Posewitz, M.C. Draft genome sequence and genetic transformation of the oleaginous alga Nannochloropis gaditana. Nat. Commun. 2012, 3, 686. [Google Scholar] [CrossRef]
- Xu, H.; Miao, X.; Wu, Q. High quality biodiesel production from a microalga Chlorella protothecoides by heterotrophic growth in fermenters. J. Biotechnol. 2006, 126, 499–507. [Google Scholar] [CrossRef] [PubMed]
- Selvarajan, R.; Felföldi, T.; Tauber, T.; Sanniyasi, E.; Sibanda, T.; Tekere, M. Screening and evaluation of some green algal strains (Chlorophyceae) isolated from freshwater and soda lakes for biofuel production. Energies 2015, 8, 7502–7521. [Google Scholar] [CrossRef]
- Meher, L.C.; Vidya Sagar, D.; Naik, S.N. Technical aspects of biodiesel production by transesterification—A review. Renew. Sustain. Energy Rev. 2006, 10, 248–268. [Google Scholar] [CrossRef]
- Knothe, G.; Matheaus, A.C.; Ryan, T.W. Cetane numbers of branched and straight-chain fatty esters determined in an ignition quality tester. Fuel 2003, 82, 971–975. [Google Scholar] [CrossRef]
- Kyriakidis, N.B.; Katsiloulis, T. Calculation of iodine value from measurements of fatty acid methyl esters of some oils: Comparison with the relevant American Oil Chemists’ Society method. JAOCS J. Am. Oil Chem. Soc. 2000, 77, 1235–1238. [Google Scholar] [CrossRef]
- Ayodeji, A.; Opeyemi, A.; Modupe, O.; Bibi, I. Oxidation Stability of Fatty Acid Methyl Ester under Three Different Conditions. J. Energy Technol. Policy 2016, 6, 6–11. [Google Scholar]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).