Abstract
In the present work, waste eggshells were used as a precursor for the synthesis of aragonite crystals through the wet carbonation method. Cadmium (Cd2+) and lead (Pb2+) were removed by the synthesized aragonite from synthetic wastewater. The influence of initial solution pH, contact time, Cd2+ and Pb2+ concentration, and sorbent dosage were evaluated. The major sorption was observed in the first 100 mins and 360 mins for Pb2+and Cd2+ respectively reaching sorption equilibrium at 720 mins (12 hr). The sorption capacity toward Pb2+ was much higher than toward Cd2+. Both heavy metals displayed high sorption capacities at initial pH 6. The pseudo-second-order kinetic model fits well with the experimental data with a higher correlation coefficient R2. Two isotherm models were also evaluated for the best fit with the experimental data obtained. Langmuir isotherm best fits the sorption of the metals on aragonite synthesized from eggshells. X-ray diffraction (XRD) and Scanning electron microscopy (SEM) results of sorbent after sorption showed that the mechanism of sorption was dominated by surface precipitation. Therefore, aragonite crystals synthesized from waste eggshells can be a potential substitute source for the removal of Cd2+ and Pb2+ from contaminated water.
1. Introduction
Solid waste management, being a current vital issue, is a huge burden regarding sustainability. Indecorous management of solid wastes such as municipal, industrial, and hazardous waste are emerging threats to the environment. The rapid rise in population resulted in an increase in food waste such as eggshell, which is one of the solid wastes available in abundance with serious environmental problems. Globally, approximately 5.92 million tonnes of waste eggshell are generated per year [1]. These waste eggshells have been dumped into landfills without further treatment, causing several environmental consequences [2]. The waste is mainly made up of calcium carbonate (CaCO3) with minor impurities, which has been used in further applications as a source of calcium supplements [3,4,5]. However, the effect of impurities has been minimized with further treatments such as chemical treatment and physical treatment [6,7].
Cadmium (Cd2+) and lead (Pb2+) are prevalent heavy metals used in industries such as the electroplating industry, metal refining industry, and battery industry [8]. Waterbodies are contaminated by heavy metals through industrial wastewater. Cd2+ and Pb2+ are considered threats to the environment due to their cause of acute and chronic damage to human and aquatic life [9]. Lead causes disorder of the brain, anemia, kidney disease, and palsy [9]. Cadmium causes kidney damage, proteinuria, and lung cancer [10]. Therefore, the release of these toxic heavy metals to water bodies should be controlled.
Numerous methods have emerged for removing dissolved heavy metals from contaminated water bodies. Adsorption [6,11,12,13,14,15,16,17,18], chemical precipitation [19], advanced Fenton-chemical precipitation [20], layered double hydroxide precipitation [21], physico-chemical treatment [22], electrocoagulation [23], flotation [24], ion exchange [25], and reverse osmosis [26] are some of these methods. Adsorption is a common method due to better performance, ease of operation, and low cost. Different adsorbents such as waste adsorbents are becoming popular for waste management and creating a sustainable world. The most commonly used waste adsorbents are waste bivalve shells [27,28]. Currently, waste eggshell is also well used for the treatment of wastewater [6,17,29,30].
Calcium carbonate is one of the abundant natural materials that exist in three main polymorphs: calcite, aragonite, and vaterite [31]. Aragonite is one form of the three polymorphics of CaCO3. It has a needle shape morphology with more compact and denser properties [32]. Several studies reported that aragonite is an effective adsorbent with high adsorption capacity toward heavy metals [27,33,34]. Moreover, other studies have found that aragonite exhibited higher adsorption capacity toward Cd2+, whereas calcite had higher absorption capacity toward Pb2+ [27,35,36]. However, the adsorption capacity of aragonite toward Pb2+ was also comparable to that of calcite [27]. The performance of the synthesized aragonite toward both metals was investigated in this study.
The objective of the present work is to study the potential use of synthesized aragonite crystals from eggshells in the sorption of Cd2+ and Pb2+ from synthetic wastewater. The study targeted these two metals due to their large-scale presence in industrial wastewater. The influence of various factors on the sorption capacity of synthesized aragonite was explored. The sorption kinetics and isotherm were also systematically studied.
2. Experimental
2.1. Materials
Waste eggshell was obtained from the Korean Institute of Geoscience and Minerals (KIGAM) campus restaurant in Daejeon, South Korea. Sodium hydroxide with 97% purity, hydrochloric acid with 35–37% concentration, nitric acid with 69–70% purity, Pb(NO3)2 with 99.95% purity, Cd(NO3)2·4 H2O with 98% purity, and magnesium chloride hexahydrate with 98% purity were all purchased from Junsei Chemicals Ltd., Seoul, Korea, while carbon dioxide with 99.9% purity was provided by Jeil Trading CO., Ltd Company, Seoul, Republic of Korea.
2.2. Synthesis of Sorbent (Aragonite)
Calcium hydroxide (Ca(OH)2) synthesized from waste eggshell was used as a precursor. The synthesis of Ca(OH)2 was described in detail in previous work [37]. The wet carbonation method was used for the synthesis of aragonite. One liter aqueous solution of calcium hydroxide and magnesium chloride hexahydrate was prepared with a molar ratio of 1:8 (Mg: Ca). The suspension was stirred continuously at 400 rpm at 80 °C, and pH was monitored continuously. After the stabilization of pH, CO2 gas was introduced to the suspension at 50 mL/min flow rate. The solution pH gradually decreases with the injection of carbon dioxide. The reaction process comes to an end when the pH consistency of the suspension was observed. The synthesized aragonite was then washed, filtered and dried.
2.3. Sorption Experiments
Pb(NO3)2 and Cd(NO3)2 ·4 H2O were dissolved in de-ionized water at specified concentrations to prepare synthetic solutions in a sealed 1000 mL glass vessel. Then, 200 mL of the stock solutions were pre-equilibrated for 24 h prior to the addition of the adsorbent at room temperature. A specified amount of aragonite was added to the flasks. Then, samples were analyzed for remaining metal concentrations by ICP-OES which were collected at designated time intervals. The initial pH adjustments were made by HNO3 (0.1 M) or NaOH (0.1 M) solutions. The influence of three parameters on the adsorption capacity have been studied—adsorbent dosage (0.1 g/L–0.5 g/L), initial pH (2–6), and initial metal concentration (10 mg/L–100 mg/L). First, the dosage effect was investigated with Cd2+ and Pb2+ concentration of 100 mg/L and solution pH (~5.8). A dosage with optimum removal efficiency was selected to study the influence of the other parameters. The influence of initial pH was then studied with Cd2+ and Pb2+ concentration of 100 mg/L and the previously selected dosage of aragonite. Finally, the influence of Cd2+ and Pb2+ concentration was investigated with the previously selected pH and dosage. The selected optimum conditions were used for the sorption kinetics and isotherm studies. Table 1 presents detailed experimental conditions.

Table 1.
The influence of sorbent dosage, initial pH, and Cd2+ and Pb2+ concentration on the sorption of metals on aragonite synthesized from eggshell at different experimental conditions.
The percentage of ion (%) removal was determined at various experimental conditions by Equation (1).
where Co and Ct are initial and final concentration in mg/L, respectively.
The adsorption capacities were also calculated using Equation (2).
where Co is the initial and Ce is the equilibrium metal concentration in mg/L, V is solution volume in liters, and S is the amount of adsorbent used in grams.
2.4. Characterization
Mineralogical and crystallographic phases of synthesized aragonite were investigated by powder X-ray diffraction (XRD) with 2θ ranging from 10° to 90° (BD2745N, Rigaku, Tokyo, Japan). Scanning electron microscopy (SEM), (JSM-6330F, JEOL. Co. Ltd., Tokyo, Japan) was used for crystal structure, shape, and morphological analysis of the synthesized aragonite. The specific surface area of the sorbent was conducted by Brunauer–Emmett–Teller (BET) (Quadrasorb SI, Quantachrome Instruments, Florida, USA). A Particle size analyzer (Malvern Zetasizer Nano ZS90, Cambridge, United Kingdom) was used for particle size distribution analysis. A surface charge study was conducted through zeta potential analysis (Otsuka ELS-Z, Osaka, Japan). The pH of the solution was measured by Orion Versa Star Pro (ThermoFisher Scientific, Waltham, MA, USA) pH meter with a glass electrode. Inductive coupled plasma-optical emission spectroscopy (ICP-OES) (PerkinElmer, Inc. Waltham, MA, USA) was used for the measurement of metal concentrations.
3. Result and Discussion
3.1. Characterizations of Sorbent
Crystal structures and mineralogical phases of waste eggshell and synthesized aragonite were determined by powder XRD. As illustrated in Figure 1a, the raw eggshell has a major phase of rhombohedral calcite with a space group R-3c (Space Group No. 167; JCPDS PDF Card No.86-0174) appeared at 2= 29.48. Figure 1b represents the XRD patterns of aragonite synthesized from eggshells. All the peaks of synthesized sorbent resembled aragonite phase, which implies that the transformation of calcite to a high grade (with minor calcite) aragonite crystals can be obtained from waste eggshell through wet carbonation.
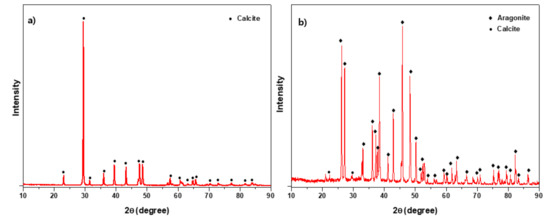
Figure 1.
X-ray diffraction patterns of (a) raw eggshell and (b) aragonite synthesized from eggshell.
Figure 2 represents the surface morphology of the synthesized aragonite. Needle-shaped aragonite particles with an average aspect ratio of 21 were successfully synthesized.

Figure 2.
Scanning electron microscopy images of synthesized aragonite from eggshell.
The particle size distribution of aragonite synthesized from eggshell was found in the range of 10–45 µm, with the highest peak at ~ 20 µm, as can be seen in Figure 3. The presence of nitrogen compounds from amino acids/proteins aid the synthesis of smaller particle size distribution [38,39]. The specific surface area of aragonite synthesized from eggshell was determined as high as ~ 18.7 m2g-1 due to the smaller particle size obtained. Specific surface area increases as particle size decreases, resulting in higher adsorption capacity. Therefore, the higher surface area of the synthesized aragonite aids the performance of the sorbent.
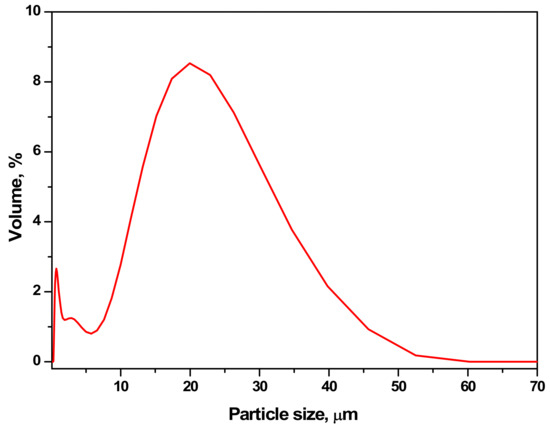
Figure 3.
The particle size distribution of aragonite synthesized from eggshell.
Zeta potential is the key parameter of a sorbent in the adsorption process. It informs the characteristics of the surface charge of particles suspended in an aqueous medium. Figure 4 illustrates the zeta potential values of synthesized aragonite with varied pH. The particles started to dissolve as pH < 6. Furthermore, no positive charge was found on the surface of the synthesized aragonite. The negative zeta potential values of the adsorbent show the suitability of adsorption of positively charged metals.
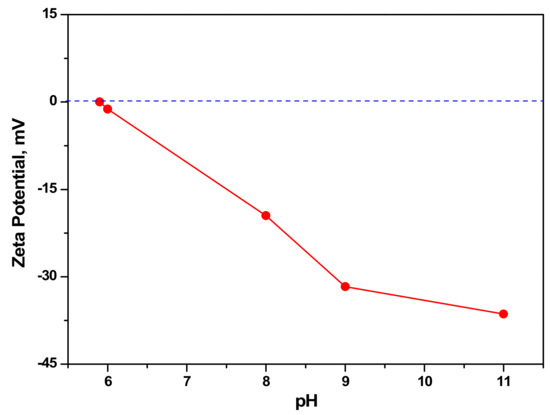
Figure 4.
Zeta potential values of synthesized aragonite from eggshell.
3.2. Influence of Contact Time and Sorption Kinetics
As presented in Figure 5, the sorption phase was very fast initially due to the available active sorption sites on the sorbent followed by a slow sorption phase, indicating the active sites being occupied by the sorbed metals [27]. The major sorption took place in the first 100 mins for Pb2+ and 360 mins for Cd2+, and both metals reached sorption equilibrium at 12 h. This can be explained by the hydration energies of the two metals. The hydration energy of Cd2+ and Pb2+ are −1807 KJ/mol and −1481 KJ/mol respectively as given later in Table 4. The slow sorption process of Cd2+ is due to the higher hydration energy than Pb2+, which tends to stay in the solution.
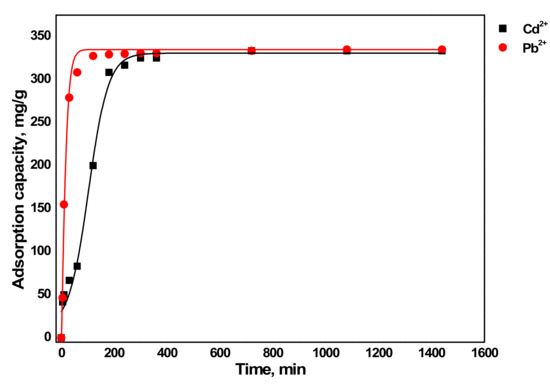
Figure 5.
Influence of contact time on sorption of metals by aragonite.
The sorption mechanism was investigated through modelling the kinetics of metals sorption on synthesized aragonite with the first and pseudo-second-order kinetic models as given in Equations (3) and (4), respectively.
where k1 (min-1) and k2 (g mg-1 min-1) are first and second-order rate constants respectively, t (min) is the time duration of sorption, and qe (mg/g) and qt (mg/g) are adsorption capacity at equilibrium and time t, respectively.
Figure 6 presents the first order and pseudo-second-order kinetic model fitted to the experimental data. The goodness of fit reports whether a model matches well with the experimental data or not. The correlation coefficient R2 value, which is considered as a criterion for determination of good fit and other parameters, is given in Table 2. The R2 values of the first-order kinetic model were observed lower. Moreover, the adsorption capacity of metals (especially Pb2+) calculated from the model was much lower than those determined from the experiment. On the contrary, the R2 values with the pseudo-second-order kinetic model were estimated as 0.986 for Cd2+and 0.999 for Pb2+which were much higher than the R2 values with the first-order kinetic model. Besides, the estimated adsorption capacities (qe) of the model were very close to those obtained from experiments. Therefore, the pseudo-second kinetic model is a good match for the sorption process, indicating that the sorption mechanism is mainly dominated by chemisorption.
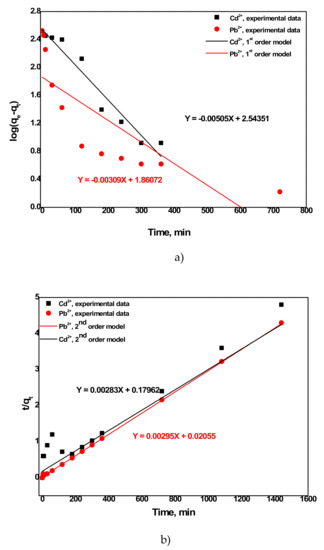
Figure 6.
(a) First-order and (b) pseudo-second-order kinetic models of metals sorption on aragonite.

Table 2.
Kinetic model parameters of metals sorption on aragonite.
3.3. Influence of Sorbent Dosage
The adsorption capacity of a sorbent toward the sorbate is directly affected by an indispensable parameter, sorbent dosage. It determines the amount of sorbent needed for the removal of a certain amount of contaminants. The effect was investigated by varying the amount of synthesized aragonite from 20 to 100 mg in 200 mL heavy metal-containing solution with Cd2+ and Pb2+ concentration of 100 mg/L at solution pH 5.8. As outlined in Figure 7, both the removal efficiency and sorption capacity of Cd2+ was much lower than that of Pb2+. This result was also obtained from the previous study on biosorption of Pb2+, Cd2+, and Zn2+ with aragonite and calcite mollusk shell [27]. As illustrated in the figure, the removal efficiency increased as the sorbent dosage was increased. Contrarily, it is well understood that adsorption capacity of a sorbent decreases as the sorbent dosage increases since only part of active adsorption sites is used [40,41,42]. Although the adsorption capacities of both metals were found high at lower dosages, the removal efficiency of Cd2+ was very low. The removal efficiency of Pb2+ remained almost indistinguishably constant (97.5–99.5%) while the sorption capacity decreased from 1007.5 mg/g to 202 mg/g with increasing the sorbent dosage from 0.1 g/L to 0.5 g/L. The removal efficiency of Cd2+ increased from 41.7% to 86%, while the sorption capacity decreased from 500 mg/g to 180 mg/g with the same increase in dosage. The high sorption of Pb2+ than Cd2+ can be explained by the solubility constant Ksp in addition to other factors discussed later in the sorption mechanism section. The solubility constant Ksp of cerussite and otavite are 7.40×10−14 and 1.0×10−12, respectively (as given later in Table 4). This indicates that cerussite is slightly insoluble than otavite which can be considered for the higher sorption of Pb2+ on synthesized aragonite than Cd2+. One paper studied the sorption of Pb2+ by calcite and aragonite with sizes of 100–200 um or less [43]. It was reported that the sorption of Pb2+ on calcite is higher than aragonite for sizes of 100–200 m, whereas for the smaller size of sorbents, the opposite was found to be true. This result was also found by another study for the sorption of Cd2+ and Pb2+ with aragonite and calcite [27]. However, in the current study, the sorption of Pb2+ on aragonite was also found to be as high as that of calcite. This might be due to the smaller size of the sorbent with a high surface area.
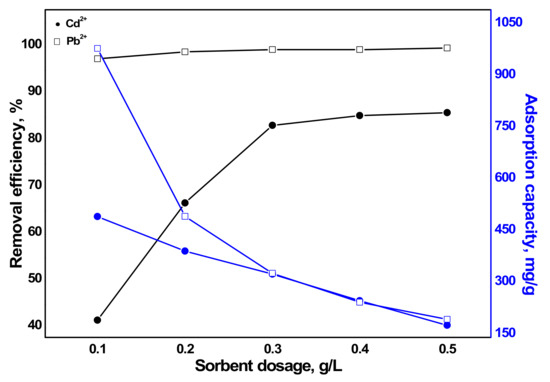
Figure 7.
Influence of sorbent dosage on the removal efficiency and sorption capacity of metals by aragonite (condition: Cd2+ and Pb2+ concentration = 100 mg/L and pH = 5.8).
3.4. Influence of Initial pH
Another important parameter that influences adsorption capacity of a sorbent is the initial pH. The initial pH of a solution can affect the sorbent surface charge and the speciation of sorbates on the surface of the sorbent. The influence of initial pH on the sorption capacity of the metals by aragonite is presented Figure 8. The initial pH of 200 mL heavy metal-containing solution was adjusted to 2–6 with Cd2+ and Pb2+ concentration of 100 mg/L and 60 mg of aragonite to investigate the influence of pH on sorption capacity. At pH 2, H+ is released and competes with the metal ions (Cd2+ and Pb2+) for the same active sorption sites resulting in negligible sorption capacity for both metals [44]. As pH > 2, the amount of adsorbed ions by aragonite started increasing rapidly. Although the sorption capacities at pH > 4 were all high, it reached a maximum capacity of 333.3 mg/L for Cd2+ and 335.8 mg/L for Pb2+ at pH 6.
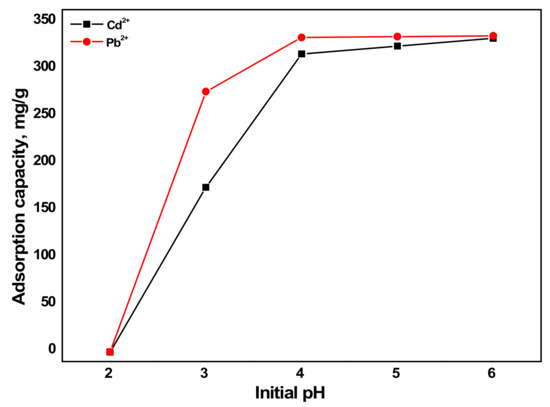
Figure 8.
Influence of initial pH on the sorption capacity of metals by aragonite (condition: Cd2+ and Pb2+ concentration = 100 mg/L and sorbent dosage = 0.3 g/L).
3.5. Influence of Cd2+ and Pb2+ Concentration
Three Cd2+ and Pb2+ concentrations were conducted on 200 mL solution with pH 6 and sorbent dosage 60 mg. The result, as given in Figure 9, depicted that the sorption capacity decreased as Cd2+ and Pb2+ concentration decreased for the same reason explained earlier for the influence of sorbent dosage. As the initial metal concentration decreases, only some part of the active adsorption site will be used due to less sorbate. Besides, the removal efficiency increases with a decrease in the initial metal concentration [45]. However, in this study, the removal efficiencies of the three metal concentrations were almost the same. This was because the sorbent dosage (0.3 g/L) used was higher for the lower Cd2+ and Pb2+ concentrations (10 mg/L and 50 mg/L). Therefore, the effect on the removal efficiency was checked with a lower sorbent dosage (0.1 g/L) as presented in Figure 10. As a result, Pb2+ showed only a slight decrease in removal efficiency with an increase in Cd2+ and Pb2+ concentration since Pb2+ was easily removed even at lower dosages as discussed earlier. Furthermore, Cd2+ has clearly proved that an increase in Cd2+ and Pb2+ concentration resulted in decreased removal efficiency.
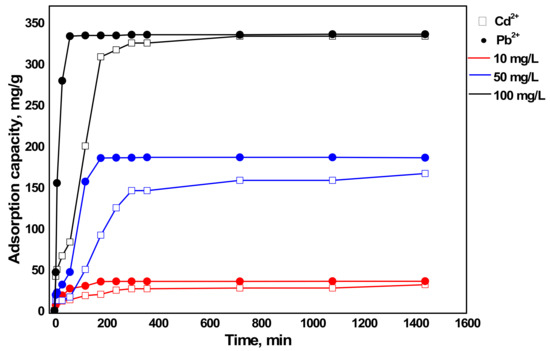
Figure 9.
Influence of Cd2+ and Pb2+ concentration on the sorption capacity of metals by aragonite (condition: sorbent dosage = 0.3 g/L and pH = 6).
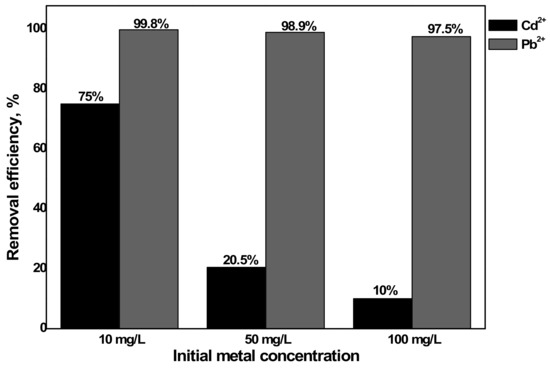
Figure 10.
Influence of Cd2+ and Pb2+ concentration on the removal efficiency of metals by aragonite (condition: sorbent dosage= 0.1 g/L and pH = 6).
3.6. Sorption Isotherm
The sorption isotherm was modeled with the Langmuir and the Freundlich models, as given in Equations (5) and (6), respectively.
where b is the Langmuir constant; qmax (mg/g) and qe (mg/g) are the maximum and equilibrium adsorption capacity, respectively; Ce(mg/L) is the metal concentration in the solution at equilibrium; and kf and n are the Freundlich empirical constants which measure adsorption capacity and intensity, respectively.
The two isotherm models fitted with the experimental data are given in Figure 11. The parameters of each model are also summarized in Table 3. The R2 values with the Freundlich model were found to be slightly lower than Langmuir model. Therefore, the experimental data can be very well fitted with the Langmuir isotherm model.
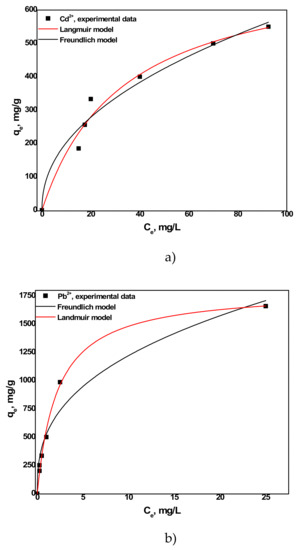
Figure 11.
(a) Langmuir and (b) Freundlich isotherm models of Cd2+and Pb2+ sorption on aragonite.

Table 3.
Isotherm model parameters of Cd2+ and Pb2+ sorption on aragonite.
3.7. Sorption Mechanism
XRD analysis of sorbent after exposed to 100 mg/L of Cd2+ and Pb2+ for 12 h is shown in Figure 12a. The possible phases to be formed were otavite, cerussite, hydrocerussite, and lead hydroxide [27]. However, metal hydroxides were not identified in the XRD patterns since they are not stable in the presence of carbonates. Cerussite and otavite were identified as major peaks with a minor phase of aragonite. An SEM image of the sorbent after exposure to 100 mg/L of Pb2+ and Cd2+ is also presented in Figure 12b. The image showed an appearance of secondary layer on the surface of the sorbent, indicating that surface precipitation has taken place during sorption.
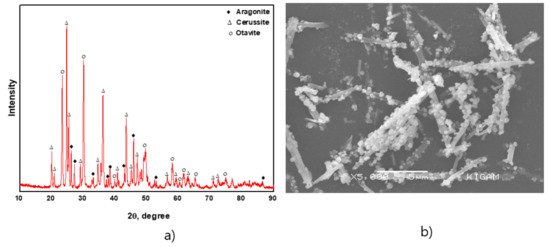
Figure 12.
(a) X-ray diffraction pattern and (b) SEM image of sorbent after sorption.
Three possible processes can be involved at the CaCO3-water interface: Dissolution, sorption and nucleation (crystal growth). The slightly acidic solution (pH = 6), resulted in the dissolution of CaCO3 and released CO3−2 (Equation (7)). Sorption of Cd2+ and Pb2+ also occurred in parallel with the dissolution aragonite (Equation (8)). Then nucleation (crystal growth) of cerussite and otavite resulted where the two metals ions reacted with the released CO3−2 (Equation (9)).
CaCO3(s) → Ca2+ + CO3−2
CaCO3(s) + Pb2+ + Cd2+ + CO3−2 → (Cd, Pb)CO3 + Ca2+
Pb2+ + Cd2+ + CO3−2 → (Cd, Pb)CO3(s)
It has been argued that the mechanism of sorption when heavy metals interact with CaCO3 could be ion exchange, surface precipitation as carbonate and hydroxycarbonate phases, complexation, and adsorption [27,36,43,46]. The similarity of ionic radii of ions favors the mechanism ion exchange where one ion potentially substitutes another similar ion. As presented in Table 4, the ionic radius of the metals Cd2+ and Pb2+ are different from those of Ca2+ in aragonite, which is evidence that a removal mechanism other than ion exchange is involved. Surface precipitation takes place with the dissolution of sorbents and precipitation of compounds on the surface of the sorbent. In this study, dissolution of aragonite took place continuously and metals (Cd2+ and Pb2+) were sorbed where cerussite and otavite precipitated on the surface of aragonite crystals. Therefore, the removal mechanism was dominated by surface precipitation.

Table 4.
Characteristics of possible compounds formed on the surface of the sorbent.
Lattice matching is an important factor for the formation of a layer on the substrate without defects. Aragonite and cerussite have similar crystal systems (orthorhombic) and small lattice mismatches, which can result in the epitaxial growth of cerussite, whereas otavite has a trigonal crystal system that is different from aragonite, resulting in precipitation of otavite in three-dimensional prismatic crystals on the surface of aragonite.
4. Conclusions
Municipal solid waste such as eggshells is a threat to a sustainable environment. However, it can be converted into valuable material like aragonite and used in versatile applications, such as a filler paper and plastics. In the present study, eggshell (mainly composed of CaCO3, calcite) was used as a precursor for the synthesis of aragonite through wet carbonation and applied to the removal of Cd2+ and Pb2+. The conversion of calcite (eggshell) to aragonite is a vital process due to the inefficiency of calcite to remove Cd2+. The influence of various factors was presented in this study. The sorption kinetics and isotherm were also investigated through systematic studies where the pseudo-second-order and Langmuir model well fitted the experimental data. The heavy metals were removed by surface precipitation mechanism where cerussite and otavite were precipitated out on the surface of the sorbent. All results demonstrate the effective and sustainable removal of toxic heavy metals by aragonite synthesized from eggshells. Therefore, eggshells can be a good substitute material of natural CaCO3 for mitigating the contamination of water bodies with toxic heavy metals.
Author Contributions
Conceptualization, data curation, methodology, writing-original draft, L.H.; Visualization, software, N.S. and M.D.K.; Supervision, T.T.; Project administration, supervision, writing—review and editing, J.W.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the National Strategic Project-Carbon upcycling of the National Research Foundation of Korea (NRF) funded by the Ministry of Science and ICT (MSIT), the Ministry of environment (ME), and the Ministry of Trade, Industry, and Energy (MOTIE) (2017M 3D 8A 2084752).
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Gomes, M.G.; Pasquini, D. Utilization of eggshell waste as an adsorbent for dry purification of biodiesel. Environ. Prog. Sustain. 2018, 37, 2093–2099. [Google Scholar] [CrossRef]
- Amanda, L.; Adriana, L.; Mario, D. Eggshell waste as catalyst: A review. J. Environ. Manag. 2017, 197, 351–359. [Google Scholar] [CrossRef]
- Cree, D.; Rutter, A. Sustainable Bio-Inspired Limestone Eggshell Powder for Potential Industrialized. Applications 2015. [Google Scholar] [CrossRef]
- Shiferaw, N.; Habte, L.; Thenepalli, T.; Ahn, J.W. Effect of eggshell powder on the hydration of cement paste. Materials 2019, 12, 2483. [Google Scholar] [CrossRef] [PubMed]
- Hamideh, F.; Akbar, A. Application of eggshell wastes as valuable and utilizable products: A review. Res. Agric. Eng. 2018, 64, 104–114. [Google Scholar] [CrossRef]
- Tizo, M.S.; Blanco, L.A.V.; Cagas, A.C.Q.; Cruz, B.R.B.D.; Encoy, J.C.; Gunting, J.V.; Arazo, R.O.; Mabayo, V.I.F. Efficiency of calcium carbonate from eggshells as an adsorbent for cadmium removal in aqueous solution. Sustain. Environ. Res. 2018, 28, 326–332. [Google Scholar] [CrossRef]
- Baláž, M.; Bujňáková, Z.; Baláž, P.; Zorkovská, A.; Danková, Z.; Briančin, J. Adsorption of cadmium(II) on waste biomaterial. J. Colloid Interface Sci. 2015, 454, 121–133. [Google Scholar] [CrossRef]
- Meena, A.K.; Mishra, G.K.; Rai, P.K.; Rajagopal, C.; Nagar, P.N. Removal of heavy metal ions from aqueous solutions using carbon aerogel as an adsorbent. J. Hazard. Mater. 2005, 122, 161–170. [Google Scholar] [CrossRef]
- Malar, S.; Vikram, S.S.; Favas, P.J.C.; Perumal, V. Lead heavy metal toxicity induced changes on growth and antioxidative enzymes level in water hyacinths [Eichhornia crassipes (Mart.)]. Bot. Stud. 2016, 55, 1–11. [Google Scholar] [CrossRef]
- Boparai, H.K.; Joseph, M.; O’carroll, D.M. Kinetics and thermodynamics of cadmium ion removal by adsorption onto nano zerovalent ion particles. J. Hazard. Mater. 2011, 186, 458–465. [Google Scholar] [CrossRef]
- Argun, M.E.; Dursun, S.; Ozdemir, C.; Karatas, M. Heavy metal adsorption by modified oak sawdust: Thermodynamics and kinetics. J. Hazard. Mater. 2007, 141, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Ali, R.M.; Hamad, H.A.; Hussein, M.M.; Malash, G.F. Potential of using green adsorbent of heavy metal removal from aqueous solutions: Adsorption kinetics, isotherm, thermodynamic, mechanism and economic analysis. Ecol. Eng. 2016, 91, 317–332. [Google Scholar] [CrossRef]
- Uddin, M.K. A review on the adsorption of heavy metals by clay minerals, with special focus on the past decade. Chem. Eng. J. 2017, 308, 438–462. [Google Scholar] [CrossRef]
- Ihsanullah Abbas, A.; Al-Amer, A.M.; Laoui, T.; Al-Marri, M.J.; Nasser, M.S.; Khraisheh, M.; Atieh, M.A. Heavy metal removal from aqueous solution by advanced carbon nanotubes: Critical review of adsorption applications. Sep. Purif. Technol. 2016, 157, 141–161. [Google Scholar] [CrossRef]
- Demirbas, A. Heavy metal adsorption onto agro-based waste materials: A review. J. Hazard. Mater. 2008, 157, 220–229. [Google Scholar] [CrossRef]
- Inyang, M.I.; Gao, B.; Yao, Y.; Xue, Y.; Zimmerman, A.; Mosa, A.; Pullammanappallil, P.; Ok, Y.S.; Cao, X. A review of biochar as a low-cost adsorbent for aqueous heavy metal removal. Crit. Rev. Environ. Sci. Technol. 2016, 46, 406–433. [Google Scholar] [CrossRef]
- Park, H.J.; Jeong, S.W.; Yang, J.K.; Kim, B.G.; Lee, S.M. Removal of heavy metals using waste eggshell. J. Environ. Sci. 2007, 19, 1436–1441. [Google Scholar] [CrossRef]
- Kobya, M.; Demirbas, E.; Senturk, E.; Ince, M. Adsorption of heavy metal ions from aqueous solutions by activated carbon prepared from apricot stone. Bioresour. Technol. 2005, 96, 1518–1521. [Google Scholar] [CrossRef]
- Matlock, M.M.; Howerton, B.S.; Atwood, D.A. Chemical precipitation of heavy metals from acid mine drainage. Water Res. 2002, 36, 4757–4764. [Google Scholar] [CrossRef]
- Fu, F.; Xie, L.; Tang, B.; Wang, Q.; Jiang, S. Application of a novel strategy-Advanced Fenton-chemical precipitation to the treatment of strong stability chelated heavy metal containing wastewater. Chem. Eng. J. 2012, 189, 283–287. [Google Scholar] [CrossRef]
- Ma, L.; Wang, Q.; Islam, S.M.; Liu, Y.; Ma, S.; Kanatzidis, M.G. Highly Selective and Efficient Removal of Heavy Metals by Layered Double Hydroxide Intercalated with the MoS42-Ion. J. Am. Chem. Soc. 2016, 138, 2858–2866. [Google Scholar] [CrossRef] [PubMed]
- Kurniawan, T.A.; Chan, G.Y.S.; Lo, W.H.; Babel, S. Physico-chemical treatment techniques for wastewater laden with heavy metals. Chem. Eng. J. 2006, 118, 83–98. [Google Scholar] [CrossRef]
- Meunier, N.; Drogui, P.; Montané, C.; Hausler, R.; Mercier, G.; Blais, J.F. Comparison between electrocoagulation and chemical precipitation for metals removal from acidic soil leachate. J. Hazard. Mater. 2006, 137, 581–590. [Google Scholar] [CrossRef] [PubMed]
- Polat, H.; Erdogan, D. Heavy metal removal from waste waters by ion flotation. J. Hazard. Mater. 2007, 148, 267–273. [Google Scholar] [CrossRef]
- Zewail, T.M.; Yousef, N.S. Kinetic study of heavy metal ions removal by ion exchange in batch conical air spouted bed. Alex. Eng. J. 2015, 54, 83–90. [Google Scholar] [CrossRef]
- Li, Y.; Xu, Z.; Liu, S.; Zhang, J.; Yang, X. Molecular simulation of reverse osmosis for heavy metal ions using functionalized nanoporous graphenes. Comput. Mater. Sci. 2017, 139, 65–74. [Google Scholar] [CrossRef]
- Du, Y.; Lian, F.; Zhu, L. Biosorption of divalent Pb, Cd and Zn on aragonite and calcite mollusk shells. Environ. Pollut. 2011, 159, 1763–1768. [Google Scholar] [CrossRef]
- Van, H.T.; Nguyen, L.H.; Nguyen, V.D.; Nguyen, X.H.; Nguyen, T.H.; Nguyen, T.V.; Vigneswaran, S.; Rinklebe, J.; Tran, H.N. Characteristics and mechanisms of cadmium adsorption onto biogenic aragonite shells-derived biosorbent: Batch and column studies. J. Environ. Manag. 2019, 241, 535–548. [Google Scholar] [CrossRef]
- Flores-Cano, J.V.; Leyva-Ramos, R.; Mendoza-Barron, J.; Guerrero-Coronado, R.M.; Aragón-Piña, A.; Labrada-Delgado, G.J. Sorption mechanism of Cd(II, from water solution onto chicken eggshell. Appl. Surf. Sci. 2013, 276, 682–690. [Google Scholar] [CrossRef]
- Setiawan, B.D.; Rizqi, O.; Brilianti, N.F.; Wasito, H. Nanoporous of waste avian eggshell to reduce heavy metal and acidity in water. Sustain. Chem. Pharm. 2018, 10, 163–167. [Google Scholar] [CrossRef]
- Seo, K.S.; Han, C.; Wee, J.H.; Park, J.K.; Ahn, J.W. Synthesis of calcium carbonate in a pure ethanol and aqueous ethanol solution as the solvent. J. Cryst. Growth. 2005, 276, 680–687. [Google Scholar] [CrossRef]
- Li, H.Y.; Tan, Y.Q.; Zhang, L.; Zhang, Y.X.; Song, Y.H.; Ye, Y.; Xia, M.S. Bio-filler from waste shellfish shell: Preparation, characterization, and its effect on the mechanical properties on polypropylene composites. J. Hazard. Mater. 2012, 217–218, 256–262. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Zhu, L.; Shan, G. Removal of Cd 2+ from contaminated water by nano-sized aragonite mollusk shell and the competition of coexisting metal ions. J. Colloid Interface Sci. 2012, 367, 378–382. [Google Scholar] [CrossRef] [PubMed]
- Köhler, S.J.; Cubillas, P.; Rodríguez-Blanco, J.D.; Bauer, C.; Prieto, M. Removal of cadmium from wastewaters by aragonite shells and the influence of other divalent cations. Environ. Sci. Technol. 2007, 41, 112–118. [Google Scholar] [CrossRef] [PubMed]
- Cubillas, P.; Köhler, S.; Prieto, M.; Causserand, C.; Oelkers, E.H. How do mineral coatings affect dissolution rates? An experimental study of coupled CaCO3 dissolution-CdCO3 precipitation. Geochim. Cosmochim. Acta 2005, 69, 5459–5476. [Google Scholar] [CrossRef]
- Prieto, M.; Cubillas, P.; Fernández-Gonzalez, Á. Uptake of dissolved Cd by biogenic and abiogenic aragonite: A comparison with sorption onto calcite. Geochim. Cosmochim. Acta 2003, 67, 3859–3869. [Google Scholar] [CrossRef]
- Habte, L.; Shiferaw, N.; Mulatu, D.; Thenepalli, T.; Chilakala, R.; Ahn, J.W. Synthesis of nano-calcium oxide fromwaste eggshell by sol-gel method. Sustainability 2019, 11, 3196. [Google Scholar] [CrossRef]
- Chen, L.; Huang, F.; Li, S.; Shen, Y.; Xie, A.; Pan, J.; Zhang, Y.; Cai, Y. Biomimetic synthesis of aragonite superstructures using hexamethylenetetramine. J. Solid State Chem. 2011, 184, 2825–2833. [Google Scholar] [CrossRef]
- Correia, L.M.; Cecilia, J.A.; Rodríguez-Castellón, E.; Cavalcante, C.L.; Vieira, R.S. Relevance of the Physicochemical Properties of Calcined Quail Eggshell (CaO) as a Catalyst for Biodiesel Production. J. Chem. 2017, 2017. [Google Scholar] [CrossRef]
- Li, Y.; Du, Q.; Wang, X.; Zhang, P.; Wang, D.; Wang, Z.; Xia, Y. Removal of lead from aqueous solution by activated carbon prepared from Enteromorpha prolifera by zinc chloride activation. J. Hazard. Mater. 2010, 183, 583–589. [Google Scholar] [CrossRef]
- Alghamdi, A.A.; Al-Odayni, A.B.; Saeed, W.S.; Al-Kahtani, A.; Alharthi, F.A.; Aouak, T. Efficient adsorption of lead (II), from aqueous phase solutions using polypyrrole-based activated carbon. Materials 2019, 12, 2020. [Google Scholar] [CrossRef] [PubMed]
- Gupta, V.K.; Rastogi, A. Biosorption of lead from aqueous solutions by green algae Spirogyra species: Kinetics and equilibrium studies. J. Hazard. Mater. 2008, 152, 407–414. [Google Scholar] [CrossRef] [PubMed]
- Godelitsas, A.; Astilleros, J.M.; Hallam, K.; Harissopoulos, S.A. Putnis, Interaction of calcium carbonates with lead in aqueous solutions. Environ. Sci. Technol. 2003, 37, 3351–3360. [Google Scholar] [CrossRef] [PubMed]
- Miretzky, P.; Muñoz, C.; Carrillo-Chávez, A. Experimental binding of lead to a low cost on biosorbent: Nopal (Opuntia streptacantha). Bioresour. Technol. 2008, 99, 1211–1217. [Google Scholar] [CrossRef] [PubMed]
- Sprynskyy, M.; Buszewski, B.; Terzyk, A.P.; Namieśnik, J. Study of the selection mechanism of heavy metal (Pb2+, Cu2+, Ni2+, and Cd2+) adsorption on clinoptilolite. J. Colloid Interface Sci. 2006, 304, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Fulghum, J.E.; Bryan, S.R.; Linton, R.W.; Bauer, C.F.; Griffis, D.P. Discrimination between Adsorption and Coprecipitation in Aquatic Particle Standards by Surface Analysis Techniques: Lead Distributions in Calcium Carbonates. Environ. Sci. Technol. 1988, 22, 463–467. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).