Thymelaea hirsuta and Echinops spinosus: Xerophytic Plants with High Potential for First-Generation Biodiesel Production
Abstract
1. Introduction
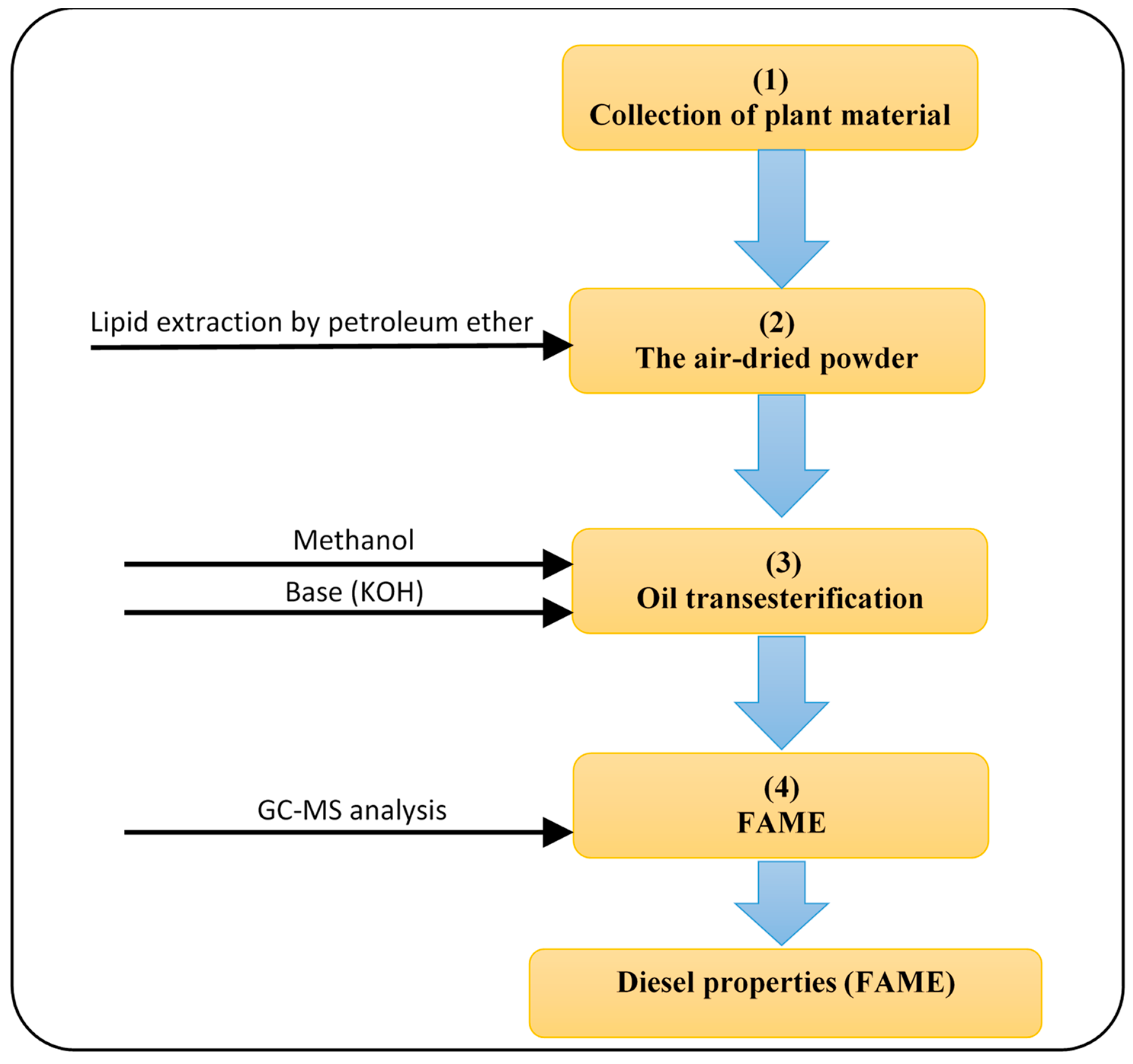
2. Materials and Methods
2.1. Plant Material
2.2. Methods
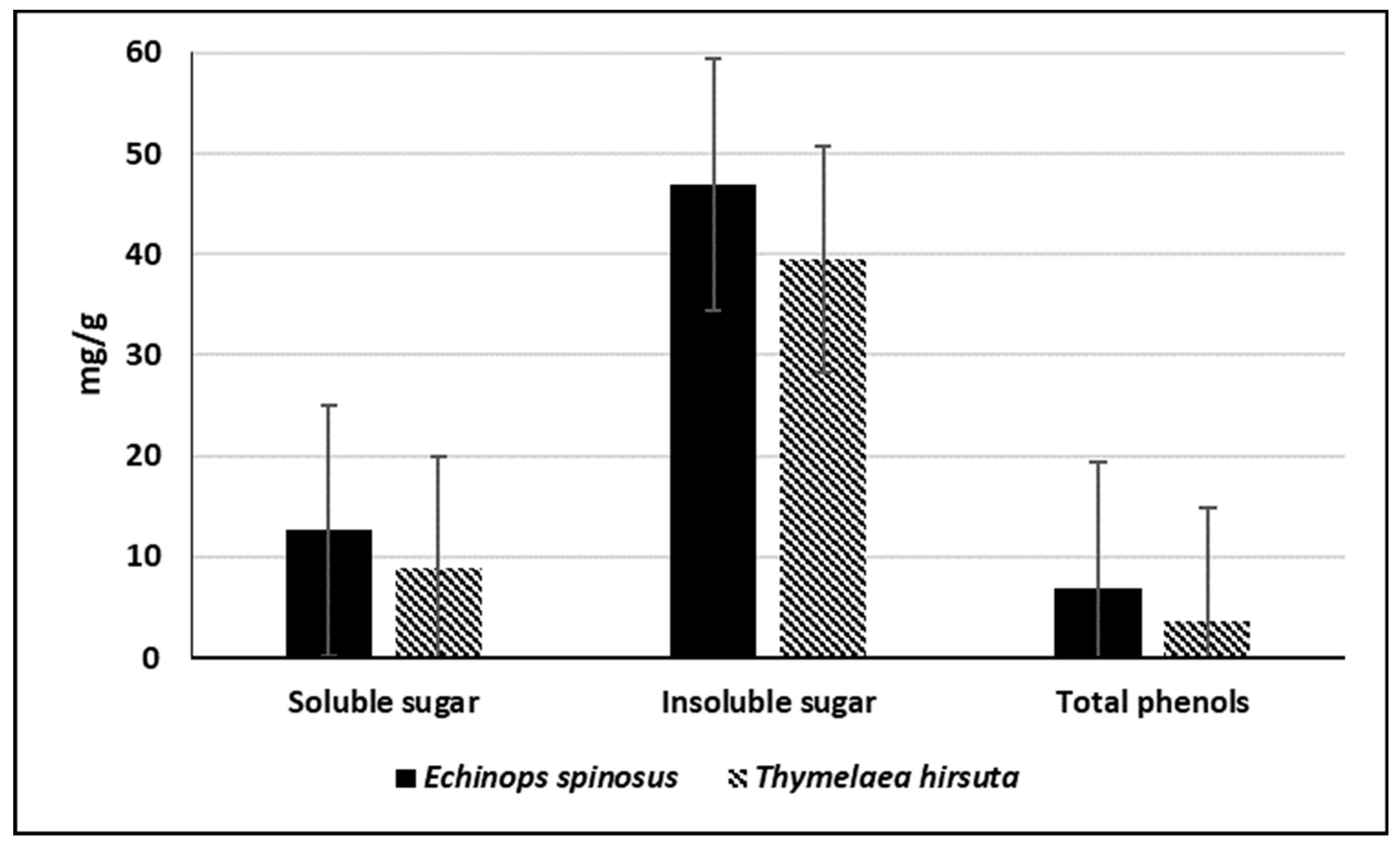
2.2.1. Estimation of Carbohydrates
2.2.2. Estimation of Pectin and Lignin
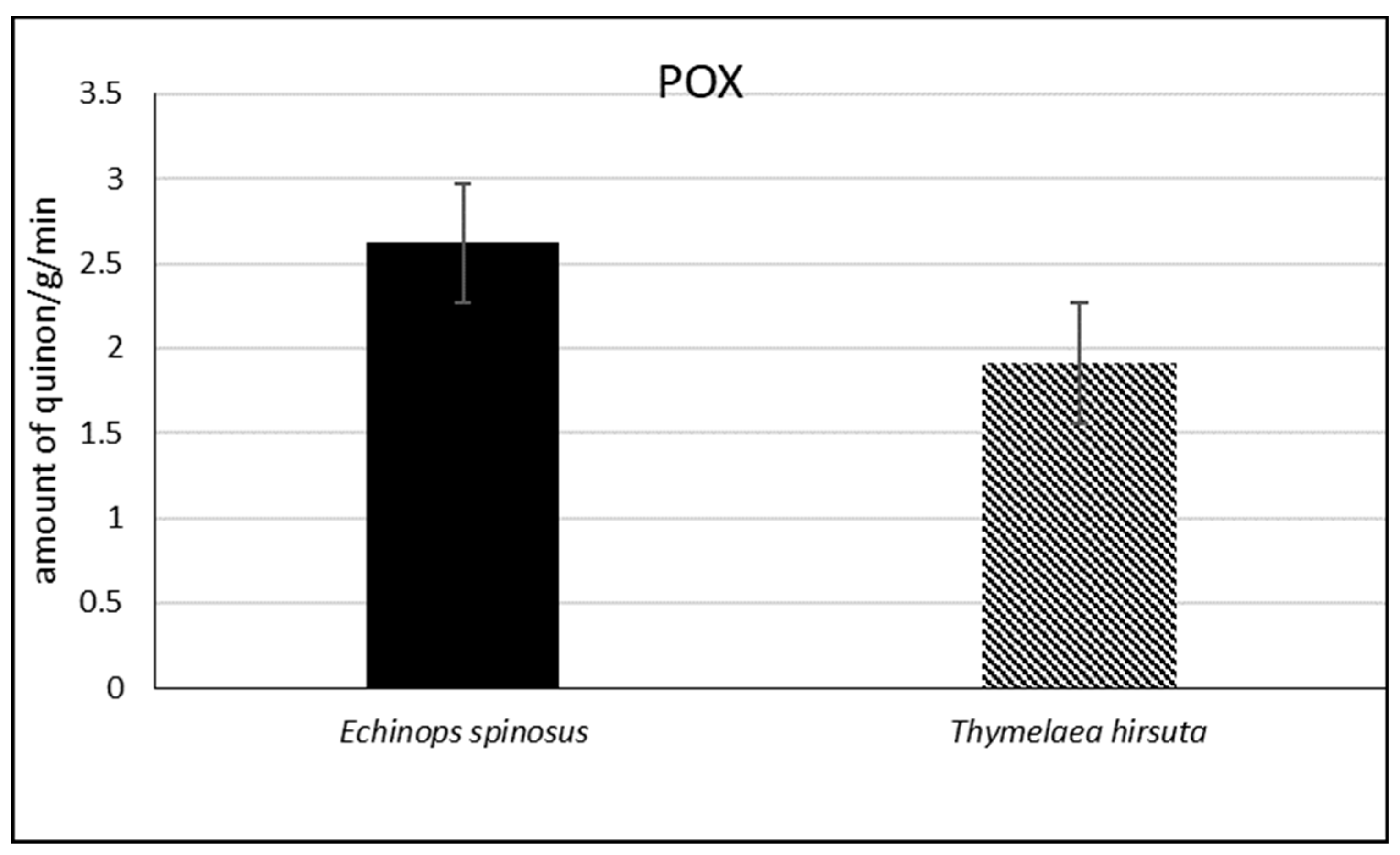
2.2.3. Extraction and Assay of Peroxidase Activity (POX)
2.2.4. Estimation of Total Phenols
2.2.5. Lipid Extraction
2.2.6. Fatty Acid Composition of oil by GC-MS
2.3. The Fuel Properties of Biodiesel
2.4. Statistical Analysis
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Longwell, H.J. The Future of the Oil and Gas Industry. Word Energy 2002, 5, 5. [Google Scholar]
- Tsoskounoglou, M.; Ayerides, G.; Tritopoulou, E. The end of cheap oil: Current status and prospects. Energy Policy 2008, 36, 3797–3806. [Google Scholar] [CrossRef]
- Chhetri, A.B.; Tango, M.S.; Budge, S.M.; Watts, K.C.; Islam, M.R. Non-edible plant oils as new sources for biodiesel production. Int. J. Mol. Sci. 2008, 9, 169–180. [Google Scholar] [CrossRef] [PubMed]
- Soliman, M.; Alhaithloul, H.A.; Hakeem, K.R.; Alharbi, B.M.; El-Esawi, M.; Elkelish, A. Exogenous Nitric Oxide Mitigates Nickel-Induced Oxidative Damage in Eggplant by Upregulating Antioxidants, Osmolyte Metabolism, and Glyoxalase Systems. Plants 2019, 8, 562. [Google Scholar] [CrossRef] [PubMed]
- Ranganathan, S.V.; Narasimhan, S.L.; Muthukumar, K. An overview of enzymatic production of biodiesel. Bioresour. Technol. 2008, 99, 3975–3981. [Google Scholar] [CrossRef] [PubMed]
- Sarbolouki, M.N.; Moacanin, J. Chemicals from biomass—The U.S. prospects for the turn of the century. Sol. Energy 1980, 25, 303–315. [Google Scholar] [CrossRef]
- Mahmudul, H.M.; Hagos, F.Y.; Mamat, R.; Adam, A.A.; Ishak, W.F.W.; Alenezi, R. Production, characterization and performance of biodiesel as an alternative fuel in diesel engines—A review. Renew. Sustain. Energy Rev. 2017, 72, 497–509. [Google Scholar] [CrossRef]
- Singh, S.P.; Singh, D. Biodiesel production through the use of different sources and characterization of oils and their esters as the substitute of diesel: A review. Renew. Sustain. Energy Rev. 2010, 14, 200–216. [Google Scholar] [CrossRef]
- Vasudevan, P.T.; Briggs, M. Biodiesel production—Current state of the art and challenges. J. Ind. Microbiol. Biotechnol. 2008, 35, 421. [Google Scholar] [CrossRef]
- Demirbas, A. Progress and recent trends in biodiesel fuels. Energy Convers. Manag. 2009, 50, 14–34. [Google Scholar] [CrossRef]
- Ge, J.C.; Yoon, S.K.; Choi, N.J. Using Canola Oil Biodiesel as an Alternative Fuel in Diesel Engines: A Review. Appl. Sci. 2017, 7, 881. [Google Scholar] [CrossRef]
- Ge, J.C.; Kim, H.Y.; Yoon, S.K.; Choi, N.J. Optimization of palm oil biodiesel blends and engine operating parameters to improve performance and PM morphology in a common rail direct injection diesel engine. Fuel 2020, 260, 116326. [Google Scholar] [CrossRef]
- Tesfa, B.; Mishra, R.; Gu, F.; Powles, N. Prediction models for density and viscosity of biodiesel and their effects on fuel supply system in CI engines. Renew. Energy 2010, 35, 2752–2760. [Google Scholar] [CrossRef]
- Sajjadi, B.; Raman, A.A.A.; Arandiyan, H. A comprehensive review on properties of edible and non-edible vegetable oil-based biodiesel: Composition, specifications and prediction models. Renew. Sustain. Energy Rev. 2016, 63, 62–92. [Google Scholar] [CrossRef]
- Kuhad, R.C.; Singh, A. Lignocellulose Biotechnology: Current and Future Prospects. Crit. Rev. Biotechnol. 1993, 13, 151–172. [Google Scholar] [CrossRef]
- Patel, A.; Arora, N.; Pruthi, V.; Pruthi, P.A. A novel rapid ultrasonication-microwave treatment for total lipid extraction from wet oleaginous yeast biomass for sustainable biodiesel production. Ultrason. Sonochem. 2019, 51, 504–516. [Google Scholar] [CrossRef]
- Aransiola, E.F.; Ojumu, T.V.; Oyekola, O.O.; Madzimbamuto, T.F.; Ikhu-Omoregbe, D.I.O. A review of current technology for biodiesel production: State of the art. Biomass Bioenergy 2014, 61, 276–297. [Google Scholar] [CrossRef]
- Yang, Z.; Hollebone, B.P.; Wang, Z.; Yang, C.; Landriault, M. Factors affecting oxidation stability of commercially available biodiesel products. Fuel Process. Technol. 2013, 106, 366–375. [Google Scholar] [CrossRef]
- Dunn, R.O. Effect of oxidation under accelerated conditions on fuel properties of methyl soyate (biodiesel). J. Am. Oil Chem. Soc. 2002, 79, 915–920. [Google Scholar] [CrossRef]
- Elkelish, A.A.; Soliman, M.H.; Alhaithloul, H.A.; El-Esawi, M.A. Selenium protects wheat seedlings against salt stress-mediated oxidative damage by up-regulating antioxidants and osmolytes metabolism. Plant Physiol. Biochem. 2019. [Google Scholar] [CrossRef]
- Ramírez-Verduzco, L.F.; Rodríguez-Rodríguez, J.E.; del Jaramillo-Jacob, A.R. Predicting cetane number, kinematic viscosity, density and higher heating value of biodiesel from its fatty acid methyl ester composition. Fuel 2012, 91, 102–111. [Google Scholar] [CrossRef]
- Demirbas, A. Comparison of transesterification methods for production of biodiesel from vegetable oils and fats. Energy Convers. Manag. 2008, 49, 125–130. [Google Scholar] [CrossRef]
- Górnaś, P.; Rudzińska, M. Seeds recovered from industry by-products of nine fruit species with a high potential utility as a source of unconventional oil for biodiesel and cosmetic and pharmaceutical sectors. Ind. Crops Prod. 2016, 83, 329–338. [Google Scholar] [CrossRef]
- Hoekman, S.K.; Broch, A.; Robbins, C.; Ceniceros, E.; Natarajan, M. Review of biodiesel composition, properties, and specifications. Renew. Sustain. Energy Rev. 2012, 16, 143–169. [Google Scholar] [CrossRef]
- Balat, M. Production of bioethanol from lignocellulosic materials via the biochemical pathway: A review. Energy Convers. Manag. 2011, 52, 858–875. [Google Scholar] [CrossRef]
- Chapagain, B.P.; Yehoshua, Y.; Wiesman, Z. Desert date (Balanites aegyptiaca) as an arid lands sustainable bioresource for biodiesel. Bioresour. Technol. 2009, 100, 1221–1226. [Google Scholar] [CrossRef]
- Bambara, L.D.F.; Sawadogo, M.; Roy, D.; Anciaux, D.; Blin, J.; Ouiminga, S.K. Biofuel from Balanites aegyptiaca: Optimization of the Feedstock Supply Chain. Sustainability 2018, 10, 4501. [Google Scholar] [CrossRef]
- Moser, B.R. Biodiesel Production, Properties, and Feedstocks. Vitro Cell. Dev. Biol. Plant 2009, 45, 229–266. [Google Scholar] [CrossRef]
- Elkelish, A.A.; Alnusaire, T.S.; Soliman, M.H.; Gowayed, S.; Senousy, H.H.; Fahad, S. Calcium availability regulates antioxidant system, physio-biochemical activities and alleviates salinity stress mediated oxidative damage in soybean seedlings. J. Appl. Bot. Food Qual. 2019, 258–266. [Google Scholar]
- Elkelish, A.A.; Alhaithloul, H.A.S.; Qari, S.H.; Soliman, M.H.; Hasanuzzaman, M. Pretreatment with Trichoderma harzianum alleviates waterlogging-induced growth alterations in tomato seedlings by modulating physiological, biochemical, and molecular mechanisms. Environ. Exp. Bot. 2019, 103946. [Google Scholar] [CrossRef]
- Elkeilsh, A.; Awad, Y.M.; Soliman, M.H.; Abu-Elsaoud, A.; Abdelhamid, M.T.; El-Metwally, I.M. Exogenous application of β-sitosterol mediated growth and yield improvement in water-stressed wheat (Triticum aestivum) involves up-regulated antioxidant system. J. Plant Res. 2019, 132, 881–901. [Google Scholar] [CrossRef]
- Huang, J.; Xia, J.; Jiang, W.; Li, Y.; Li, J. Biodiesel production from microalgae oil catalyzed by a recombinant lipase. Bioresour. Technol. 2015, 180, 47–53. [Google Scholar] [CrossRef]
- Wahlen, B.D.; Morgan, M.R.; McCurdy, A.T.; Willis, R.M.; Morgan, M.D.; Dye, D.J.; Bugbee, B.; Wood, B.D.; Seefeldt, L.C. Biodiesel from Microalgae, Yeast, and Bacteria: Engine Performance and Exhaust Emissions. Energy Fuels 2013, 27, 220–228. [Google Scholar] [CrossRef]
- El Boulifi, N.; Bouaid, A.; Martinez, M.; Aracil, J. Optimization and oxidative stability of biodiesel production from rice bran oil. Renew. Energy 2013, 53, 141–147. [Google Scholar] [CrossRef]
- Zheng, L.; Hou, Y.; Li, W.; Yang, S.; Li, Q.; Yu, Z. Biodiesel production from rice straw and restaurant waste employing black soldier fly assisted by microbes. Energy 2012, 47, 225–229. [Google Scholar] [CrossRef]
- Fadhil, A.B.; Aziz, A.M.; Al-Tamer, M.H. Biodiesel production from Silybum marianum L. seed oil with high FFA content using sulfonated carbon catalyst for esterification and base catalyst for transesterification. Energy Convers. Manag. 2016, 108, 255–265. [Google Scholar] [CrossRef]
- Lee, H.V.; Juan, J.C.; Taufiq-Yap, Y.H. Preparation and application of binary acid–base CaO–La2O3 catalyst for biodiesel production. Renew. Energy 2015, 74, 124–132. [Google Scholar] [CrossRef]
- Kansedo, J.; Lee, K.T.; Bhatia, S. Cerbera odollam (sea mango) oil as a promising non-edible feedstock for biodiesel production. Fuel 2009, 88, 1148–1150. [Google Scholar] [CrossRef]
- Boulos, L. Medicinal Plants of North Africa; Reference Publications, Inc.: Algonac, MI, USA, 1983. [Google Scholar]
- Boumaraf, M.; Benyahia, S.; Mekkiou, R.; Benayache, S.; Benayache, F. Flavonoids from Ethyl Acetate Extract of Echinops spinosus (Asteraceae). 2016. Available online: https://www.researchgate.net/publication/304970181_Flavonoids_from_ethyl_acetate_extract_of_Echinops_spinosus_Asteraceae (accessed on 1 January 2020).
- Zahran, M.A.; El-Amier, Y.A. Ecology and establishment of fiber producing taxa naturally growing in the Egyptian deserts. Egypt. J. Basic Appl. Sci. 2014, 1, 144–150. [Google Scholar] [CrossRef][Green Version]
- Shaltout, K.H.; Ayyad, M.A. Structure and standing crop of EgyptianThymelaea hirsuta populations. Vegetatio 1988, 74, 137–142. [Google Scholar] [CrossRef]
- Schmidt, J.; Stavisky, N. Uses ofThymelaea hirsuta (Mitnan) with emphasis on hand papermaking. Econ. Bot. 1983, 37, 310–321. [Google Scholar] [CrossRef]
- Kadri, A.; Zarai, Z.; Chobba, I.B.; Gharsallah, N.; Damak, M.; Békir, A. Chemical composition and in vitro antioxidant activities of Thymelaea hirsuta L. essential oil from Tunisia. Afr. J. Biotechnol. 2011, 10, 2930–2935. [Google Scholar]
- Zahran, M.; Boulos, S.T. Potentialities of the fiber plants of the Egyptian flora in national economy. II Thymelaea hirsuta. Bull. Fac. Sci. Mansoura Univ. 1973, 1, 77–87. [Google Scholar]
- Bitew, H.; Hymete, A. The Genus Echinops: Phytochemistry and Biological Activities: A Review. Front. Pharmacol. 2019, 10, 1234. [Google Scholar] [CrossRef]
- Maness, N. Extraction and Analysis of Soluble Carbohydrates. In Plant Stress Tolerance; Sunkar, R., Ed.; Humana Press: Totowa, NJ, USA, 2010; Volume 639, pp. 341–370. ISBN 978-1-60761-701-3. [Google Scholar]
- Jenkins, S.H. The determination of cellulose in straws. Biochem. J. 1930, 24, 1428–1432. [Google Scholar] [CrossRef]
- Nanji, D.R.; Norman, A.G. Studies on pectin. Part II: The estimation of the individual pectic substances in nature. Biochem. J. 1928, 22, 596–604. [Google Scholar]
- Ritter, G.J.; Seborg, R.M.; Mitchell, R.L. Factors Affecting Quantitative Determination of Lignin by 72 Per Cent Sulfuric Acid Method. Ind. Eng. Chem. Anal. Ed. 1932, 4, 202–204. [Google Scholar] [CrossRef]
- Mukherjee, S.P.; Choudhuri, M.A. Implications of water stress-induced changes in the levels of endogenous ascorbic acid and hydrogen peroxide in Vigna seedlings. Physiol. Plant. 1983, 58, 166–170. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Malik, C.P.; Singh, M.B. Plant Enzymology and Histo-Enzymology: A Text Manual; Kalyani Publishers: New Delhi, India, 1980. [Google Scholar]
- Official Methods of Analysis of AOAC International, 20th ed.; Latimer, G.W., AOAC International, Eds.; AOAC International: Gaithersburg, MD, USA, 2016; ISBN 978-0-935584-87-5. [Google Scholar]
- Karaosmanoǧlu, F.; Cıǧızoǧlu, K.B.; Tüter, M.; Ertekin, S. Investigation of the Refining Step of Biodiesel Production. Energy Fuels 1996, 10, 890–895. [Google Scholar] [CrossRef]
- Lang, X.; Dalai, A.K.; Bakhshi, N.N.; Reaney, M.J.; Hertz, P.B. Preparation and characterization of bio-diesels from various bio-oils. Bioresour. Technol. 2001, 80, 53–62. [Google Scholar] [CrossRef]
- Jiang, J.; Jia, X. Profiling of Fatty Acids Composition in Suet Oil Based on GC–EI-qMS and Chemometrics Analysis. Int. J. Mol. Sci. 2015, 16, 2864–2878. [Google Scholar] [CrossRef]
- Francisco, É.C.; Neves, D.B.; Jacob-Lopes, E.; Franco, T.T. Microalgae as feedstock for biodiesel production: Carbon dioxide sequestration, lipid production and biofuel quality. J. Chem. Technol. Biotechnol. 2010, 85, 395–403. [Google Scholar] [CrossRef]
- Kalayasiri, P.; Jeyashoke, N.; Krisnangkura, K. Survey of seed oils for use as diesel fuels. J. Am. Oil Chem. Soc. 1996, 73, 471–474. [Google Scholar] [CrossRef]
- Degirolamo, C.; Rudel, L.L. Dietary Monounsaturated Fatty Acids Appear Not to Provide Cardioprotection. Curr. Atheroscler. Rep. 2010, 12, 391–396. [Google Scholar] [CrossRef] [PubMed]
- Krisnangkura, K. A simple method for estimation of cetane index of vegetable oil methyl esters. J. Am. Oil Chem. Soc. 1986, 63, 552–553. [Google Scholar] [CrossRef]
- Demirbas, A. Biofuels sources, biofuel policy, biofuel economy and global biofuel projections. Energy Convers. Manag. 2008, 49, 2106–2116. [Google Scholar] [CrossRef]
- Ramos, M.J.; Fernández, C.M.; Casas, A.; Rodríguez, L.; Pérez, Á. Influence of fatty acid composition of raw materials on biodiesel properties. Bioresour. Technol. 2009, 100, 261–268. [Google Scholar] [CrossRef]
- Park, J.-Y.; Kim, D.-K.; Lee, J.-P.; Park, S.-C.; Kim, Y.-J.; Lee, J.-S. Blending effects of biodiesels on oxidation stability and low temperature flow properties. Bioresour. Technol. 2008, 99, 1196–1203. [Google Scholar] [CrossRef]
- Kumar, M.; Sharma, M.P. Selection of potential oils for biodiesel production. Renew. Sustain. Energy Rev. 2016, 56, 1129–1138. [Google Scholar] [CrossRef]
- Mendu, V.; Harman-Ware, A.E.; Crocker, M.; Jae, J.; Stork, J.; Morton, S.; Placido, A.; Huber, G.; DeBolt, S. Identification and thermochemical analysis of high-lignin feedstocks for biofuel and biochemical production. Biotechnol. Biofuels 2011, 4, 43. [Google Scholar] [CrossRef]
- Thakur, V.K.; Thakur, M.K. Recent advances in green hydrogels from lignin: A review. Int. J. Biol. Macromol. 2015, 72, 834–847. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Li, Z.; Zhang, X.; Hu, F.; Ryu, D.D.Y.; Bao, J. Screening of Oleaginous Yeast Strains Tolerant to Lignocellulose Degradation Compounds. Appl. Biochem. Biotechnol. 2009, 159, 591–604. [Google Scholar] [CrossRef] [PubMed]
- Ding, S.-Y.; Liu, Y.-S.; Zeng, Y.; Himmel, M.E.; Baker, J.O.; Bayer, E.A. How does plant cell wall nanoscale architecture correlate with enzymatic digestibility? Science 2012, 338, 1055–1060. [Google Scholar] [CrossRef] [PubMed]
- Oasmaa, A.; Alén, R.; Meier, D. Catalytic hydrotreatment of some technical lignins. Bioresour. Technol. 1993, 45, 189–194. [Google Scholar] [CrossRef]
- Anker, Y.; Nakonechny, F.; Niazov, B.; Lugovskoy, S.; Nisnevitch, M. Biofuel Production by Fermentation of Water Plants and Agricultural Lignocellulosic by-Products. MATEC Web Conf. 2016, 70, 12005. [Google Scholar] [CrossRef]
- Fahmi, R.; Bridgwater, A.V.; Donnison, I.; Yates, N.; Jones, J.M. The effect of lignin and inorganic species in biomass on pyrolysis oil yields, quality and stability. Fuel 2008, 87, 1230–1240. [Google Scholar] [CrossRef]
- Yang, J.; Gao, T.; Zhang, Y.; Wang, S.; Li, H.; Li, S.; Wang, S. Degradation of the phenolic β-ether lignin model dimer and dyes by dye-decolorizing peroxidase from Bacillus amyloliquefaciens. Biotechnol. Lett. 2019, 41, 1015–1021. [Google Scholar] [CrossRef]
- Alvarez, S.; Marsh, E.L.; Schroeder, S.G.; Schachtman, D.P. Metabolomic and proteomic changes in the xylem sap of maize under drought. Plant Cell Environ. 2008, 31, 325–340. [Google Scholar] [CrossRef]
- Macrelli, S.; Galbe, M.; Wallberg, O. Effects of production and market factors on ethanol profitability for an integrated first and second generation ethanol plant using the whole sugarcane as feedstock. Biotechnol. Biofuels 2014, 7, 26. [Google Scholar] [CrossRef]
- Moser, B.R.; Vaughn, S.F. Coriander seed oil methyl esters as biodiesel fuel: Unique fatty acid composition and excellent oxidative stability. Biomass Bioenergy 2010, 34, 550–558. [Google Scholar] [CrossRef]
- de Oliveira, J.S.; Leite, P.M.; de Souza, L.B.; Mello, V.M.; Silva, E.C.; Rubim, J.C.; Meneghetti, S.M.P.; Suarez, P.A.Z. Characteristics and composition of Jatropha gossypiifoliaand Jatropha curcas L. oils and application for biodiesel production. Biomass Bioenergy 2009, 33, 449–453. [Google Scholar] [CrossRef]
- Mishra, A.; Patel, M.K.; Jha, B. Non-targeted metabolomics and scavenging activity of reactive oxygen species reveal the potential of Salicornia brachiata as a functional food. J. Funct. Foods 2015, 13, 21–31. [Google Scholar] [CrossRef]
- Navari-Izzo, F.; Milone, M.T.A.; Quartacci, M.F.; Pinzino, C. Metabolic changes in wheat plants subjected to a water-deficit stress programme. Plant Sci. 1993, 92, 151–157. [Google Scholar] [CrossRef]
- Yordanov, I.; Velikova, V.; Tsonev, T. Plant Responses to Drought, Acclimation, and Stress Tolerance. Photosynthetica 2000, 38, 171–186. [Google Scholar] [CrossRef]
- Bamgboye, A.I.; Hansen, A.C. Prediction of cetane number of biodiesel fuel from the fatty acid methyl ester (FAME) composition. Int. Agrophys. 2008, 22, 21–29. [Google Scholar]
- Zahan, K.; Kano, M. Biodiesel Production from Palm Oil, Its By-Products, and Mill Effluent: A Review. Energies 2018, 11, 2132. [Google Scholar] [CrossRef]
- Muthu, H.; SathyaSelvabala, V.; Varathachary, T.K.; Kirupha Selvaraj, D.; Nandagopal, J.; Subramanian, S. Synthesis of biodiesel from Neem oil using sulfated zirconia via tranesterification. Braz. J. Chem. Eng. 2010, 27, 601–608. [Google Scholar] [CrossRef]
- Patel, A.; Arora, N.; Mehtani, J.; Pruthi, V.; Pruthi, P.A. Assessment of fuel properties on the basis of fatty acid profiles of oleaginous yeast for potential biodiesel production. Renew. Sustain. Energy Rev. 2017, 77, 604–616. [Google Scholar] [CrossRef]
- Mohibbe Azam, M.; Waris, A.; Nahar, N.M. Prospects and potential of fatty acid methyl esters of some non-traditional seed oils for use as biodiesel in India. Biomass Bioenergy 2005, 29, 293–302. [Google Scholar] [CrossRef]
- Mohammed-Dabo, I.A.; Ahmad, M.S.; Hamza, A.; Muazu, K.; Aliyu, A. Cosolvent transesterification of Jatropha curcas seed oil. J. Pet. Technol. Altern. Fuels 2012, 3, 42–51. [Google Scholar]
- Khattab, H.; El, M.Z. Environmental alterations in biofuel generating molecules in Zilla spinosa. Z. Für Naturforschung C 2016, 72, 77–91. [Google Scholar] [CrossRef] [PubMed]
- Ashraful, A.M.; Masjuki, H.H.; Kalam, M.A.; Rizwanul Fattah, I.M.; Imtenan, S.; Shahir, S.A.; Mobarak, H.M. Production and comparison of fuel properties, engine performance, and emission characteristics of biodiesel from various non-edible vegetable oils: A review. Energy Convers. Manag. 2014, 80, 202–228. [Google Scholar] [CrossRef]
- Ali, E.N.; Tay, C.I. Characterization of Biodiesel Produced from Palm Oil via Base Catalyzed Transesterification. Procedia Eng. 2013, 53, 7–12. [Google Scholar] [CrossRef]
- Guil-Laynez, J.L.; Guil-Guerrero, J.L.; Guil-Laynez, Á. Bioprospecting for seed oils in tropical areas for biodiesel production. Ind. Crops Prod. 2019, 128, 504–511. [Google Scholar] [CrossRef]
- Karavalakis, G.; Hilari, D.; Givalou, L.; Karonis, D.; Stournas, S. Storage stability and ageing effect of biodiesel blends treated with different antioxidants. Energy 2011, 36, 369–374. [Google Scholar] [CrossRef]
- Lin, C.-Y. Blending Biodiesel in Fishing Boat Fuels for Improved Fuel Characteristics. Front. Energy Res. 2014, 2, 1–5. [Google Scholar] [CrossRef]
- Akbar, E.; Yaakob, Z.; Kamarudin, S.K.; Ismail, M.; Salimon, J. Characteristic and Composition of Jatropha Curcas Oil Seed from Malaysia and its Potential as Biodiesel Feedstock Feedstock. Eur. J. Sci. Res. 2009, 29, 396–403. [Google Scholar]
- Rashid, U.; Anwar, F.; Knothe, G. Evaluation of biodiesel obtained from cottonseed oil. Fuel Process. Technol. 2009, 90, 1157–1163. [Google Scholar] [CrossRef]
- Shahabuddin, M.; Kalam, M.A.; Masjuki, H.H.; Bhuiya, M.M.K.; Mofijur, M. An experimental investigation into biodiesel stability by means of oxidation and property determination. Energy 2012, 44, 616–622. [Google Scholar] [CrossRef]
- Loh, S.-K.; Chew, S.-M.; Choo, Y.-M. Oxidative stability and storage behavior of fatty acid methyl esters derived from used palm oil. J. Am. Oil Chem. Soc. 2006, 83, 947–952. [Google Scholar] [CrossRef]



| Properties | Echinops spinosus | Thymelaea hirsuta | Standards | |
|---|---|---|---|---|
| Europe (EN14214:2008) | USA (ASTM D6751) | |||
| Oil content (g/100 g) | 76.1 | 30.2 | -- | -- |
| Cetane number | 229.99 | 379.29 | >47 | >51 |
| Saponification number (mg/g) | 27.97 | 16.07 | -- | -- |
| Iodine value (g I2/100 g) | 50.75 | 29.16 | <120 | -- |
| Cold filter plugging point (°C) | 2.93 | 261.52 | -- | -- |
| Degree of unsaturation | 142.12 | 16.23 | -- | -- |
| Induction period (h) | 4.3 | 19.8 | >3 | >6 |
| Higher heating value (MJ/kg) EN14213 (biodiesel for heating purposes) > 35.0 | 47.52 | 48.33 | -- | -- |
| PK | RT | % | Fatty Acids Profile | Molecular Weight (M) |
|---|---|---|---|---|
| Saturated | ||||
| 1 | 15.6457 | 0.6515 | Pelargonic acid (C9:0) | 168.236 |
| 2 | 12.638 | 11.1106 | Palmitic acid (C16:0) | 270.457 |
| 3 | 13.8877 | 13.9789 | Margaric acid (C17:0) | 270.5 |
| 4 | 14.5708 | 0.6286 | Cerotic acid (C26:0) | 396.7 |
| Monounsaturated | ||||
| 5 | 12.638 | 0.4791 | Palmitoleic acid (C16:1) | 254.41 |
| 6 | 14.8514 | 1.0044 | Gadoleic acid (C20:1) | 310.5 |
| Polyunsaturated | ||||
| 7 | 13.84 | 70.318 | Linolelaidic acid (C18:2) | 294.47 |
| PK | RT | % | Fatty Acids Profile | Molecular Weight (M) |
|---|---|---|---|---|
| Saturated | ||||
| 1 | 16.2335 | 2.2571 | Caprylic acid (C8:0) | 144.21 |
| 2 | 13.9407 | 4.1722 | Pelargonic acid (C9:0) | 168.236 |
| 3 | 13.2523 | 0.5486 | Undecanoic acid (C11:0) | 170.29 |
| 4 | 12.9822 | 18.9054 | Palmitic acid (C16:0) | 270.457 |
| 5 | 17.1179 | 5.8693 | Stearic acid (C18:0) | 284.5 |
| 6 | 14.825 | 22.6803 | Arachidic acid (C20:0) | 326.565 |
| 7 | 15.6352 | 28.0823 | Behenic acid (C22:0) | 354.619 |
| 8 | 16.4189 | 6.3399 | Lignoceric acid (C24:0) | 382.673 |
| 9 | 16.9537 | 1.7649 | Melissic acid (C30:0) | 466.835 |
| Monounsaturated | ||||
| 10 | 15.8205 | 1.6744 | Oleic acid (C18:1) | 282.5 |
| 11 | 17.3455 | 0.8553 | Erucic acid (C22:1) | 338.6 |
| Polyunsaturated | ||||
| 12 | 13.8454 | 6.8504 | Linolelaidic acid (C18:2) | 294.47 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Helal, N.M.; Alharby, H.F.; Alharbi, B.M.; Bamagoos, A.A.; Hashim, A.M. Thymelaea hirsuta and Echinops spinosus: Xerophytic Plants with High Potential for First-Generation Biodiesel Production. Sustainability 2020, 12, 1137. https://doi.org/10.3390/su12031137
Helal NM, Alharby HF, Alharbi BM, Bamagoos AA, Hashim AM. Thymelaea hirsuta and Echinops spinosus: Xerophytic Plants with High Potential for First-Generation Biodiesel Production. Sustainability. 2020; 12(3):1137. https://doi.org/10.3390/su12031137
Chicago/Turabian StyleHelal, Nesma M., Hesham F. Alharby, Basmah M. Alharbi, Atif. A. Bamagoos, and Ahmed M. Hashim. 2020. "Thymelaea hirsuta and Echinops spinosus: Xerophytic Plants with High Potential for First-Generation Biodiesel Production" Sustainability 12, no. 3: 1137. https://doi.org/10.3390/su12031137
APA StyleHelal, N. M., Alharby, H. F., Alharbi, B. M., Bamagoos, A. A., & Hashim, A. M. (2020). Thymelaea hirsuta and Echinops spinosus: Xerophytic Plants with High Potential for First-Generation Biodiesel Production. Sustainability, 12(3), 1137. https://doi.org/10.3390/su12031137





