An Evaluation of Restocking Practice and Demographic Stock Assessment Methods for Cryptic Juvenile European Eel in Upland Rivers
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Site
2.2. Demographic Assessment Methods
2.3. Statistical Analyses
3. Results
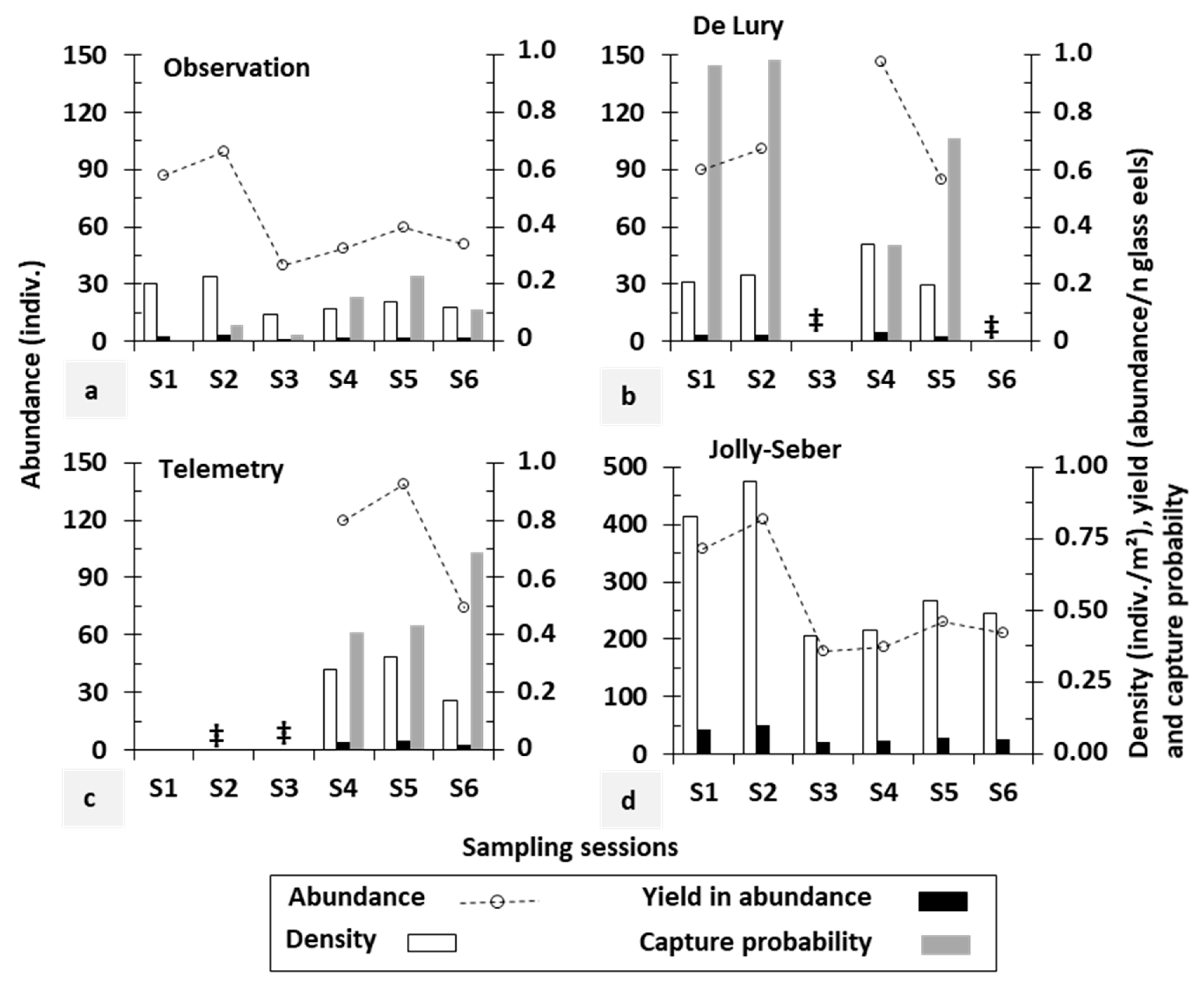
3.1. Comparison between Stock Assessment Methods
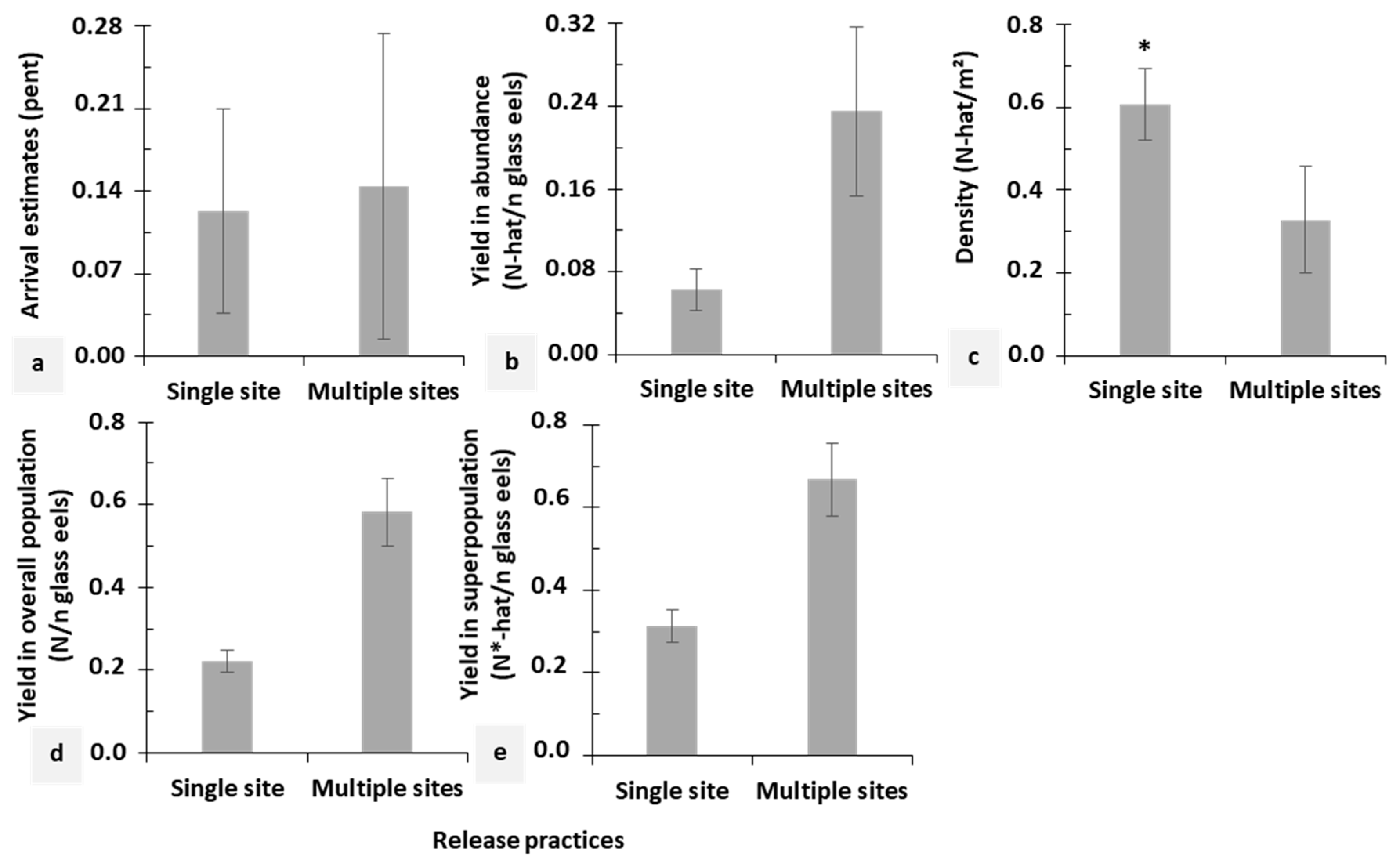
3.2. Comparison between Restocking Practices
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Jacoby, D.; Gollock, M. Anguilla anguilla. The IUCNRed List of Threatened Species. Version 2014.2. 2014. Available online: www.iucnredlist.org (accessed on 28 May 2013).
- Nzau Matondo, B.; Séleck, E.; Dierckx, A.; Benitez, J.-P.; Rollin, X.; Ovidio, M. What happens to glass eels after restocking in upland rivers? A long-term study on their dispersal and behavioural traits. Aquat. Conserv. Mar. Freshw. Ecosyst. 2019, 29, 374–388. [Google Scholar] [CrossRef]
- Tesch, F.W.; Thorpe, J.E. The Eel, 3rd ed.; Blackwell Science: Oxford, UK, 2003. [Google Scholar]
- van Ginneken, V.J.T.; Maes, G.E. The European eel (Anguilla anguilla, Linnaeus), its lifecycle, evolution and reproduction: A literature review. Rev. Fish Biol. Fish. 2005, 15, 367–398. [Google Scholar] [CrossRef]
- Palm, S.; Dannewitz, J.; Prestegaard, T.; Wickstrom, H. Panmixia in European eel revisited: No genetic difference between maturing adults from southern and northern Europe. Heredity 2009, 103, 82–89. [Google Scholar] [CrossRef] [PubMed]
- Als, T.D.; Hansen, M.M.; Maes, G.E.; Castonguay, M.; Riemann, L.; Aarestrup, K.; Munk, P.; Sparholt, H.; Hanel, R.; Bernatchez, L. All roads lead to home: Panmixia of European eel in the Sargasso Sea. Mol. Ecol. 2011, 20, 1333–1346. [Google Scholar] [CrossRef]
- Bonhommeau, S.; Castonguay, M.; Rivot, E.; Sabatié, R.; Le Pape, O. The duration of migration of Atlantic Anguilla anguilla larvae. Fish Fish. 2010, 11, 289–306. [Google Scholar] [CrossRef]
- Bevacqua, D.; Melià, P.; Gatto, M.; De Leo, G.A. A global viability assessment of the European eel. Glob. Chang. Biol. 2015, 21, 3323–3335. [Google Scholar] [CrossRef]
- Arai, T.; Kotake, A.; McCarthy, T.K. Habitat use by the European eel Anguilla anguilla in Irish waters. Estuar. Coast. Shelf Sci. 2006, 67, 569–578. [Google Scholar] [CrossRef]
- Ibbotson, A.; Smith, J.; Scarlett, P.; Aprahamian, M.W. Colonisation of freshwater habitats by the European eel Anguilla anguilla. Freshw. Biol. 2002, 47, 1696–1706. [Google Scholar] [CrossRef]
- Imbert, H.; Labonne, J.; Rigaud, C.; Lambert, P. Resident and migratory tactics in freshwater European eels are size-dependent. Freshw. Biol. 2010, 55, 1483–1493. [Google Scholar] [CrossRef]
- Nzau Matondo, B.; Ovidio, M. Dynamics of upstream movements of the European eel Anguilla anguilla in an inland area of the River Meuse over the last 20 years. Environ. Biol. Fish. 2016, 99, 223–235. [Google Scholar] [CrossRef][Green Version]
- Ovidio, M.; Seredynski, A.; Philippart, J.C.; Nzau Matondo, B. A bit of quiet between the migrations: The resting life of the European eel during their freshwater growth phase in a small stream. Aquat. Ecol. 2013, 47, 291–301. [Google Scholar] [CrossRef]
- Daverat, F.; Limburg, K.E.; Thibault, I.; Shiao, J.-C.; Dodson, J.; Caron, F.; Tzeng, W.-N.; Iizuka, Y.; Wickström, H. Phenotypic plasticity of habitat use by three temperate eel species, Anguilla anguilla, A. japonica and A. rostrata. Mar. Ecol. Prog. Ser. 2006, 308, 231–241. [Google Scholar] [CrossRef]
- Van den Thillart, G.V.; Van Ginneken, F.; Körner, R.; Heijmans, R.; Van der Linden, R.; Gluvers, A. Endurance swimming of European eel. J. Fish Biol. 2004, 65, 312–318. [Google Scholar] [CrossRef]
- Daverat, F.; Tomas, J.; Lahaye, M.; Palmer, M.; Elie, P. Tracking continental habitat shifts of eels using otolith Sr/Ca ratios: Validation and application to the coastal, estuarine and riverine eels of the Gironde-Garonne-Dordogne watershed. Mar. Freshw. Res. 2005, 56, 619–627. [Google Scholar] [CrossRef]
- Panfili, J.; Darnaude, A.M.; Lin, Y.J.; Chevalley, M.; Iizuka, Y.; Tzeng, W.N.; Crivelli, A.J. Habitat residence during continental life of the European eel Anguilla anguilla investigated using linear discriminant analysis applied to otolith Sr:Ca ratios. Mar. Ecol. Prog. Ser. 2012, 15, 175–185. [Google Scholar] [CrossRef]
- MacNamara, R.; McCarthy, T.K.; Wickström, H.; Clevestam, P.D. Fecundity of silver-phase eels (Anguilla anguilla) from different habitat types and geographic locations. ICES J. Mar. Sci. 2016, 73, 135–141. [Google Scholar] [CrossRef]
- ICES. Joint EIFAAC/ICES/GFCM Working Group on Eels (WGEEL); ICES Scientific Reports: Copenhagen, Denmark, 2019; 177p. [Google Scholar]
- Dekker, W. The history of commercial fisheries for European commenced only a century ago. Fish. Manag. Ecol. 2019, 26, 6–19. [Google Scholar] [CrossRef]
- Baras, E.; Philippart, J.C.; Salmon, B. Estimation of migrant yellow eels stock in large rivers through the survey of fish passes: A preliminary in the River Meuse (Belgium). In Stock Assessment in Inland Fisheries; Cowx, I.G., Ed.; Oxford Fishing News Books (Blackweel): London, UK, 1996; pp. 314–325. [Google Scholar]
- Nzau Matondo, B.; Benitez, J.P.; Dierckx, A.; Philippart, J.C.; Ovidio, M. Assessment of the entering stock, migration dynamics and fish pass fidelity of European eel in the Belgian Meuse River. River Res. Appl. 2017, 33, 292–301. [Google Scholar] [CrossRef]
- Nzau Matondo, B.; Ovidio, M. Decreased stock entering the Belgian Meuse is associated with the loss of colonisation behaviour in yellow-phase European eels. Aquat. Living Resour. 2018, 31, 7. [Google Scholar] [CrossRef]
- Aschonitis, V.; Castaldelli, G.; Lanzoni, M.; Rossi, R.; Kennedy, C.; Fano, E.A. Long-term records (1781–2013) of European eel (Anguilla anguilla L.) production un the Comacchio Lagoon (Italy): Evaluation of local and global factors as causes of the population collapse. Aquat. Conserv. Mar. Freshw. Ecosyst. 2017, 27, 502–520. [Google Scholar] [CrossRef]
- Council of the European Communities. Council regulation (EC) no 1100/207 of 18 September 2007 establishing measures for the recovery of the stock of European eel. Off. J. Eur. Union 2007, 248, 17–23. [Google Scholar]
- Simon, J.; Dörner, H.; Scott, R.D.; Schreckenbach, K.; Knösche, R. Comparison of growth and condition of European eels stocked as glass and farm sourced eels in lakes in the first four years after stocking. J. Appl. Ichthyol. 2013, 29, 323–330. [Google Scholar] [CrossRef]
- Ovidio, M.; Tarrago-Bès, F.; Nzau Matondo, B. Short-term responses of glass eels transported from UK to small Belgian streams. Ann. Limnol. Int. J. Limnol. 2015, 51, 219–226. [Google Scholar] [CrossRef][Green Version]
- Pedersen, M.I. Long-term survival and growth of stocked eel, Anguilla anguilla (L.), in a small eutrophic lake. Dana 2000, 12, 71–76. [Google Scholar]
- Shiao, J.C.; Lozys, L.; Iizuka, Y.; Tzeng, W.N. Migratory patterns and contribution of stocking to the population of European eel in Lithuanian waters as indicated by otolith Sr:Ca ratios. J. Fish Biol. 2006, 69, 749–769. [Google Scholar] [CrossRef]
- Lin, Y.J.; Lozys, L.; Shiao, J.C.; Iizuka, Y.; Tzeng, W.N. Growth differences between naturally recruited and stocked European eel Anguilla anguilla from different habitats in Lithuania. J. Fish Biol. 2007, 71, 1773–1787. [Google Scholar] [CrossRef]
- Sjöberg, N.B.; Wickström, H.; Petersson, E. Bidrar den Omflyttadeålen Till Lekbeståndet i Sargassohavet?—Blankålsmärkning Kan Gesvaret. Slutrapport Från Pilotprojekt Till Fonden för Fiskets Utveckling; (Does Restocked Eel Contribute to the Spawning Stock in the Sargasso Sea?—Silver Eel Tagging can Give the Answer); Unpublished Report; Stockholm, 2008; 43p. (In Swedish) [Google Scholar]
- Prigge, E.; Marohn, L.; Hanel, R. Tracking the migratory success of stocked European eels Anguilla anguilla in the Baltic Sea. J. Fish Biol. 2013, 82, 686–699. [Google Scholar] [CrossRef]
- Simon, J.; Dörner, H. Survival and growth of European eels stocked as glass- and farm-sourced eels in five lakes in the first years after stocking. Ecol. Freshw. Fish 2014, 23, 40–48. [Google Scholar] [CrossRef]
- Sjöberg, N.B.; Wickström, H.; Asp, A.; Petersson, E. Migration of eels tagged in the Baltic Sea and Lake Mälaren in the context of the stocking question. Ecol. Freshw. Fish 2017, 26, 517–532. [Google Scholar] [CrossRef]
- Kullmann, B.; Thiel, R. Bigger is better in eel stocking measures? Comparison of growth performance, body condition, and benefit-cost ratio of simultaneously stocked glass and farmed eels in a brackish fjord. Fish. Res. 2018, 205, 132–140. [Google Scholar] [CrossRef]
- Brämick, U.; Fladung, E.; Simon, J. Stocking is essential to meet the silver eel escapement target in a river system with currently low natural recruitment. ICES J. Mar. Sci. 2016, 73, 91–100. [Google Scholar] [CrossRef]
- Félix, P.M.; Costa, J.L.; Monteiro, R.; Castro, N.; Quintella, B.R.; Almeida, P.R.; Domingos, I. Can a restocking event with European (glass) eels cause early changes in local biological communities and its ecological status? Glob. Ecol. Conserv. 2020, 21, e00884. [Google Scholar] [CrossRef]
- Bisgaard, J.; Pedersen, M.I. Mortality and growth of wild and introduced cultured eels Anguilla anguilla (L.) in a Danish stream, with special reference to a new tagging technique. Dana 1991, 9, 57–69. [Google Scholar]
- Pedersen, M.I. Does Stocking of Danish lowland streams with Elvers increase European Eel Populations? Am. Fish. Soc. Symp. 2009, 58, 149–156. [Google Scholar]
- Desprez, M.; Crivelli, A.J.; Lebel, I.; Massez, G.; Gimenez, O. Demographic assessment of a stocking experiment in European Eels. Ecol. Freshw. Fish 2013, 22, 412–420. [Google Scholar] [CrossRef]
- Wickström, H.; Sjöberg, N.H. Traceability of stocked eels—The Swedish approach. Ecol. Freshw. Fish 2014, 23, 33–39. [Google Scholar] [CrossRef]
- Stacey, J.A.; Pratt, T.C.; Verreault, G.; Fox, M.G. A caution for conservation stocking as an approach for recovering Atlantic eels. Aquat. Conserv. Mar. Freshw. Ecosyst. 2015, 25, 569–580. [Google Scholar] [CrossRef]
- De Lury, D.B. On the estimation of biological populations. Biometrics 1947, 3, 145–167. [Google Scholar] [CrossRef]
- Laurent, M.; Lamarque, P. Utilisation de la méthode des captures successives (De Lury) pour l’évaluation des peuplements piscicoles. Bull. Fr. Pêche Piscic. 1975, 259, 66–77. [Google Scholar] [CrossRef][Green Version]
- Arreguin-Sanchez, F. Catchability: A key parameter for fish stock assessment. Rev. Fish Biol. Fish. 1996, 6, 221–242. [Google Scholar] [CrossRef]
- Schwarz, C.J.; Arnason, A.N. A general methodology for the analysis of capture-recapture experiments in open populations. Biometrics 1996, 52, 860–873. [Google Scholar] [CrossRef]
- White, G.C.; Burnham, K.P. Program MARK: Survival estimation from populations of marked animals. Bird Study 1999, 46, 120–139. [Google Scholar] [CrossRef]
- Pledger, S.; Pollock, K.H.; Norris, J.L. Open Capture–Recapture Models with Heterogeneity: II. Jolly–Seber Model. Biometrics 2010, 66, 883–890. [Google Scholar] [CrossRef] [PubMed]
- Huet, M. Aperçu de la relation entre la pente et les populations piscicoles des eaux courantes. Schweiz. Z. Hydrol. 1949, 11, 332–351. [Google Scholar] [CrossRef]
- Elie, P.; Lecomte-Finiger, R.; Cantrelle, I.; Charlon, N. Définition des limites des différents stades pigmentaires durant la phase civelle d’Anguilla anguilla (L.). Vie Milieu 1982, 32, 149–157. [Google Scholar]
- Esteve, C.; Garay, E. Heterotrophic Bacterial Flora Associated with European Eel Anguilla anguilla Reared in Freshwater. Nippon Suisan Gakk 1991, 57, 1369–1375. [Google Scholar] [CrossRef][Green Version]
- Angelidis, P.; Pournara, I.; Photis, G. Glass eels (Anguilla anguilla) growth in a recirculating system. Mediterr. Mar. Sci. 2005, 6, 99–106. [Google Scholar] [CrossRef][Green Version]
- Burnham, K.P.; Anderson, D.R. Model Selection and Multimodel Inference: A Practical Information-Theoretic Approach, 2nd ed.; Springer-Verlag Science and Business Média: New York, NY, USA, 2002; 454p. [Google Scholar]
- R Development Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2016. [Google Scholar]
- Fox, J. Using the R Commander: A Point-and-Click Interface for R; Chapman and CRC Press: Boca Raton, FL, USA, 2017; Available online: http://socserv.mcmaster.ca/jfox/Books/RCommander/ (accessed on 14 January 2020).
- Dinno, A. Dunn’s Test of Multiple Comparisons Using Rank Sums. 2017. Available online: https://cran.r-project.org/web/packages/dunn.test/dunn.test.pdf (accessed on 14 January 2020).
- Acou, A.; Rivot, E.; van Gils, J.A.; Legault, A.; Ysnel, F.; Feunteun, E. Habitat carrying capacity is reached for the European eel in a small coastal catchment: Evidence and implications for managing eel stocks. Freshw. Biol. 2011, 56, 952–968. [Google Scholar] [CrossRef]
- Jonsson, B.; Jonsson, N. Partial migration: Niche shift versus sexual maturation in fishes. Rev. Fish Biol. Fish. 1993, 3, 348–365. [Google Scholar] [CrossRef]
- Miller, M.J.; Feunteun, E.; Tsukamoto, K. Did a “perfect storm” of oceanic changes and continental anthropogenic impacts cause northern hemisphere anguillid recruitment reductions? ICES J. Mar. Sci. 2016, 73, 43–56. [Google Scholar] [CrossRef]
- Bonnineau, C.; Scaion, D.; Lemaire, B.; Belpaire, C.; Thomé, J.-P.; Thonon, M.; Leermaker, M.; Gao, Y.; Debier, C.; Silvestre, F.; et al. Accumulation of neurotoxic organochlorines and trace elements in brain of female European eel (Anguilla anguilla). Environ. Toxicol. Pharmacol. 2016, 45, 346–355. [Google Scholar] [CrossRef] [PubMed]
- Belpaire, C.; Geeraerts, C.; Evans, D.; Ciccotti, E.; Poole, R. The European eel quality database: Towards a pan-European monitoring of eel quality. Environ. Monit. Assess. 2011, 183, 273–284. [Google Scholar] [CrossRef] [PubMed]
- Naismith, I.A.; Knights, B. Studies of sampling methods and of techniques for estimating populations of eels, Anguilla anguilla L. Aquac. Res. 1990, 21, 357–368. [Google Scholar] [CrossRef]
- Lebreton, J.-D.; Burnham, K.P.; Clobert, J.; Anderson, D.R. Modelling survival and testing biological hypotheses using marked animals: A unified approach with case studies. Ecol. Monogr. 1992, 62, 67–118. [Google Scholar] [CrossRef]
- Ricker, W.E. Calcul et interprétation des statistiques biologiques des populations de poissons. Bull. Fish. Res. Board Can. 1980, 191, 409. [Google Scholar]
- Davey, A.J.H.; Jellyman, D.J. Sex determination in freshwater eels and management options for manipulation of sex. Rev. Fish Biol. Fish. 2005, 15, 37–52. [Google Scholar] [CrossRef]
- Bark, A.; Williams, B.; Knights, B. Current status and temporal trends in stocks of European eel in England and Wales. ICES J. Mar. Sci. 2007, 64, 1368–1378. [Google Scholar] [CrossRef]
- Poole, W.R.; Diseru, O.H.; Thorsta, E.B.; Durif, C.M.; Dolan, C.; Sandlund, O.T.; Bergesen, K.; Rogan, G.; Kelly, S.; Vøllestad, L.A. Long-term variation in numbers and biomass of silver eels being produced in two European river systems. ICES J. Mar. Sci. 2018, 75, 1627–1637. [Google Scholar] [CrossRef]
- Melia, P.; Bevacqua, D.; Crivelli, A.J.; De Leo, G.A.; Panfili, J.; Gatto, M. Sex differentiation of the European eel in brackish and freshwater environments: A comparative analysis. J. Fish Biol. 2006, 69, 1228–1235. [Google Scholar] [CrossRef]
- Walsh, C.T.; Pease, B.C.; Booth, D.J. Variation in the sex ratio, size and age of longfinned eels within and among coastal catchments of south-eastern Australia. J. Fish Biol. 2004, 64, 1297–1312. [Google Scholar] [CrossRef]
- Bevacqua, D.; Melià, P.; Schiavina, M.; Crivelli, A.J.; De Leo, G.A.; Gatto, M. A demographic model for the conservation and management of the European eel: An application to a Mediterranean coastal lagoon. ICES J. Mar. Sci. 2019, 76, 2164–2178. [Google Scholar] [CrossRef]
- Castonguay, M.; Dutil, J.D.; Audet, C.; Miller, R. Locomotor activity and concentration of thyroid hormones in migratory and sedentary juvenile American eels. Trans. Am. Fish. Soc. 1990, 119, 946–956. [Google Scholar] [CrossRef]
- Feunteun, E.; Laffaille, P.; Robinet, T.; Briand, C.; Baisez, A.; Olivier, J.M.; Acou, A. A review of upstream migration and movements in inland waters by Anguillid eels: Towards a general theory. In Eel Biology; Aida, K., Tsukamoto, K., Yamauchi, K., Eds.; Springer: Tokyo, Japan, 2003; pp. 181–190. [Google Scholar]
- Edeline, E.; Dufour, S.; Elie, P. Proximate and ultimate control of eel continental dispersal. In Spawning Migration of the European Eel; Springer: Dordrecht, The Netherlands, 2009; pp. 433–461. [Google Scholar]
- Durif, C.; Dufour, S.; Elie, P. The silvering process of Anguilla anguilla: A new classification from the yellow resident to the silver migrating stage. J. Fish Biol. 2005, 66, 1025–1043. [Google Scholar] [CrossRef]
- Cattrijsse, A.; Hampel, H. Life history and habitat use tables. In Subproject 1—‘Nursery Function Westerschelde’; Final Report; University of Gent, Department of Biology, Marine Biology Section: Ghent, Belgium, 2000. [Google Scholar]
- Acou, A.; Lefebvre, F.; Contournet, P.; Poizat, G.; Panfili, J.; Crivelli, A.J. Silvering of female eels (Anguilla anguilla) in two sub-populations of the Rhône delta. Bull. Fr. Pêche Piscic. 2003, 368, 55–68. [Google Scholar] [CrossRef]
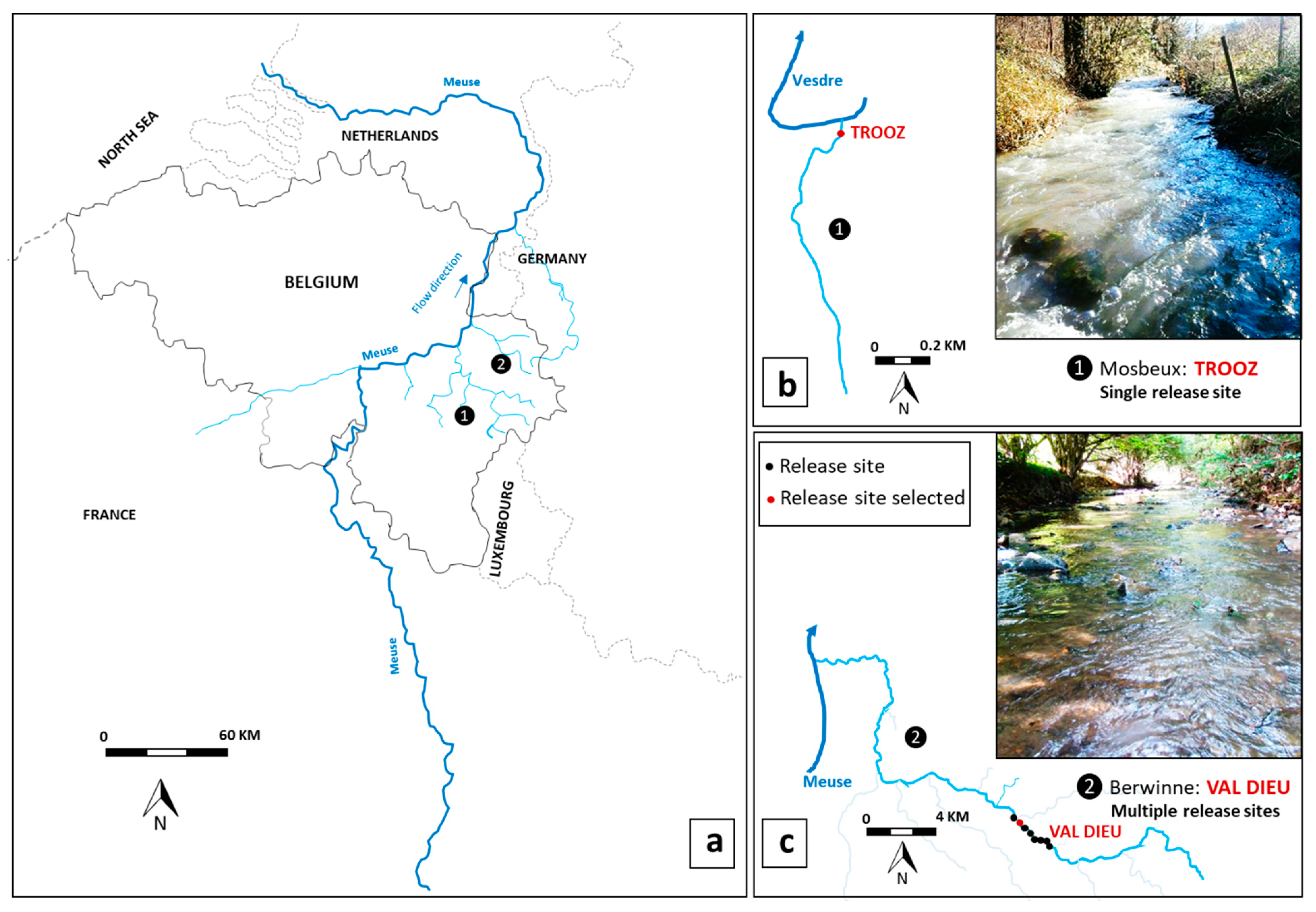

| Parameter | Mosbeux | Berwinne |
|---|---|---|
| Reference site | Trooz | Val Dieu |
| Longitude | 5°41′E | 5°48′E |
| Latitude | 50°34′N | 50°41′N |
| Altitude (m) | 97 | 161 |
| Catchment area (km2) | 19.16 | 118.0 |
| Distance from the North Sea (km) | 359.3 | 341.0 |
| Year of restocking | 2013 | 2017 |
| Origin of glass eels | UK | France |
| Pigment stages | VIA1 and VIA2 | VB, VIA0, VIA1 and VIA2 |
| Length of glass eels (mean ± SE) | 7.0 ± 0.4 cm | 6.7 ± 0.4 cm |
| Weight of glass eels (mean ± SE) | 0.26 ± 0.07 g | 0.23 ± 0.04 g |
| Total number of release site | 1 | 8 |
| Glass eels (number per site) | 4155 | 1586 |
| Daily temperature (°C) | 9.8 | 9.1 |
| Width in May (m) | 2.7 | 5.8 |
| Depth in May (cm) | 15.2 | 20.0 |
| Predominant substratum | Large stones and blocks (60%) | Large stones and blocks (81%) |
| Conductivity (µs cm−1) | 473.5 | 591.2 |
| pH | 7.9 | 7.7 |
| Ammonium (mg L−1) | <0.05 | <0.05 |
| Nitrate (mg L−1) | 2.0 | 2.0 |
| Nitrite (mg L−1) | 0.10 | 0.11 |
| Calcium carbonate (mg L−1) | 90 | 100 |
| Phosphates (mg L−1) | 0.19 | 0.22 |
| Trophic status | Eutrophic | Eutrophic |
| Fish community (species number) | 11 | 9 |
| Predominant species | Bullhead, brown trout | Bullhead, loach |
| Resident eels > 55 cm (number m−1) | 0.003 | 0.005 |
| Sampling Session | Date | Time Interval (Weeks) | Time after Restocking (Weeks) | No. of Eels per Passage | Total Length (cm) | ||
|---|---|---|---|---|---|---|---|
| Age | First | Second | Mean ± SE | ||||
| Mosbeux: Single site | |||||||
| S1 | 29 Oct. 2013 | 0 | 22.7 | 0+ | 73 | 14 | 8.9 ± 1.0 |
| S2 | 7 Apr. 2014 | 22.9 | 45.6 | 0+ | 87 | 12 | 10.0 ± 1.6 |
| S3 | 18 Nov. 2014 | 32.1 | 77.7 | 1+ | 18 | 22 | 12.6 ± 2.1 |
| S4 | 19 May 2015 | 26.0 | 103.7 | 1+ | 27 | 22 | 13.1 ± 2.3 |
| S5 | 27 May 2015 | 1.1 | 104.9 | 2+ | 39 | 21 | 14.0 ± 2.8 |
| S6 | 9 Jun. 2015 | 1.9 | 106.7 | 2+ | 24 | 27 | 13.2 ± 2.7 |
| Berwinne: Multiple sites | |||||||
| S1 | 8 Sep. 2017 | 0 | 24.4 | 0+ | 99 | 43 | 12.1 ± 1.7 |
| S2 | 16 May 2018 | 36.6 | 61.0 | 1+ | 41 | 29 | 14.1 ± 1.7 |
| S3 | 25 Sep. 2018 | 18.9 | 79.9 | 1+ | 85 | 40 | 21.2 ± 4.0 |
| S4 | 15 May 2019 | 33.1 | 113.0 | 2+ | 34 | 19 | 22.1 ± 3.3 |
| Model description | QAICc | ∆QAICc | AICc Weights | Model Likelihood | No. of Parameters | Deviance | −2Log(L) | N ± SE |
|---|---|---|---|---|---|---|---|---|
| Mosbeux: Single site | ||||||||
| { p(.), ϕ(t), pent(t), N(.)} | 386.56 | 0.00 | 0.59 | 1.00 | 11 | −1046.14 | 363.86 | 930 ± 135 |
| { p(.), ϕ(.), pent(t), N(.)}* | 388.10 | 1.53 | 0.27 | 0.47 | 7 | −1036.19 | 373.80 | 926 ± 113 |
| { p(t), ϕ(.), pent(t), N(.)} | 390.52 | 3.96 | 0.08 | 0.14 | 11 | −1042.18 | 367.81 | 1043 ± 173 |
| { p(t), ϕ(t), pent(t), N(.)} | 391.07 | 4.51 | 0.06 | 0.11 | 14 | −1048.05 | 361.94 | 1378 ± 910 |
| Berwinne: Multiple sites | ||||||||
| { p(.), ϕ(.), pent(t), N(.)}* | 346.41 | 0.00 | 0.83 | 1.00 | 5 | −798.51 | 334.68 | 924 ± 132 |
| { p(.), ϕ(t), pent(t), N(.)} | 351.07 | 4.65 | 0.08 | 0.10 | 8 | −800.09 | 334.68 | 857 ± 128 |
| { p(t), ϕ(.), pent(t), N(.)} | 351.50 | 5.08 | 0.07 | 0.04 | 8 | −799.65 | 335.11 | 641 ± 083 |
| { p(t), ϕ(t), pent(t), N(.)} | 353.16 | 6.74 | 0.03 | 0.03 | 10 | −802.21 | 332.55 | 693 ± 125 |
| River and Release Practice | Session | Time Interval (Weeks) | Age | Overall Population (N) | Superpopulation (N*-hat) | Arrival Probability (Pent) | Net Immigration(B-hat) | Net Emigration(B*-hat) | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Estimate ± SE | 95% CI | Estimate ± SE | 95% CI | Estimate ± SE | 95% CI | Estimate ± SE | 95% CI | Estimate ± SE | 95% CI | ||||
| Mosbeux Single site | 926 ± 113 | 742–1191 | 1301 ± 161 | 1022–1656 | |||||||||
| 29 Oct. 2013 | 0 | 0+ | - | - | - | - | - | - | |||||
| 07 Apr. 2014 | 22.9 | 0+ | 0.314 ± 0.039 | 0.244–0.395 | 291 ± 52 | 205–411 | 478 ± 87 | 336–681 | |||||
| 18 Nov. 2014 | 32.1 | 1+ | 0.097 ± 0.029 | 0.052–0.171 | 89 ± 28 | 49–163 | 174 ± 60 | 91–337 | |||||
| 19 May 2015 | 26.0 | 1+ | 0.146 ± 0.030 | 0.097–0.214 | 135 ± 31 | 87–210 | 236 ± 55 | 151–369 | |||||
| 27 May 2015 | 1.1 | 2+ | 0.058 ± 0.031 | 0.020–0.157 | 54 ± 28 | 21–140 | 55 ± 29 | 21–144 | |||||
| 9 Jun. 2015 | 1.9 | 2+ | 0.417×10−6 ± 0.206×10−3 | <0.001–1.00 | 0.389×10−4 ± 0.190 | <0.001–0.384 | 0.404×10−3 ± 0.199 | <0.001–0.402 | |||||
| Berwinne Multiple sites | 924 ± 132 | 716–1244 | 1058 ± 139 | 820–1366 | |||||||||
| 8 Sep. 2017 | 0 | 0+ | - | - | - | - | - | - | |||||
| 16 May 2018 | 36.6 | 1+ | 0.094 ± 0.043 | 0.037–0.219 | 87 ± 37 | 38–195 | 136 ± 62 | 57–320 | |||||
| 25 Sep. 2018 | 18.9 | 1+ | 0.336 ± 0.037 | 0.268–0.412 | 311 ± 51 | 226–428 | 395 ± 65 | 288–543 | |||||
| 15 May 2019 | 33.1 | 2+ | 0.769×10−6 ± 0.513×10−3 | <0.001–1.000 | 0.711×10−3 ± 0.475 | <0.001–0.835 | 0.001 ± 0.714 | <0.001–1 | |||||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nzau Matondo, B.; Benitez, J.-P.; Dierckx, A.; Rollin, X.; Ovidio, M. An Evaluation of Restocking Practice and Demographic Stock Assessment Methods for Cryptic Juvenile European Eel in Upland Rivers. Sustainability 2020, 12, 1124. https://doi.org/10.3390/su12031124
Nzau Matondo B, Benitez J-P, Dierckx A, Rollin X, Ovidio M. An Evaluation of Restocking Practice and Demographic Stock Assessment Methods for Cryptic Juvenile European Eel in Upland Rivers. Sustainability. 2020; 12(3):1124. https://doi.org/10.3390/su12031124
Chicago/Turabian StyleNzau Matondo, Billy, Jean-Philippe Benitez, Arnaud Dierckx, Xavier Rollin, and Michaël Ovidio. 2020. "An Evaluation of Restocking Practice and Demographic Stock Assessment Methods for Cryptic Juvenile European Eel in Upland Rivers" Sustainability 12, no. 3: 1124. https://doi.org/10.3390/su12031124
APA StyleNzau Matondo, B., Benitez, J.-P., Dierckx, A., Rollin, X., & Ovidio, M. (2020). An Evaluation of Restocking Practice and Demographic Stock Assessment Methods for Cryptic Juvenile European Eel in Upland Rivers. Sustainability, 12(3), 1124. https://doi.org/10.3390/su12031124








