4D Printing: Materials, Technologies, and Future Applications in the Biomedical Field
Abstract
1. Introduction
2. Additive Manufacturing Techniques
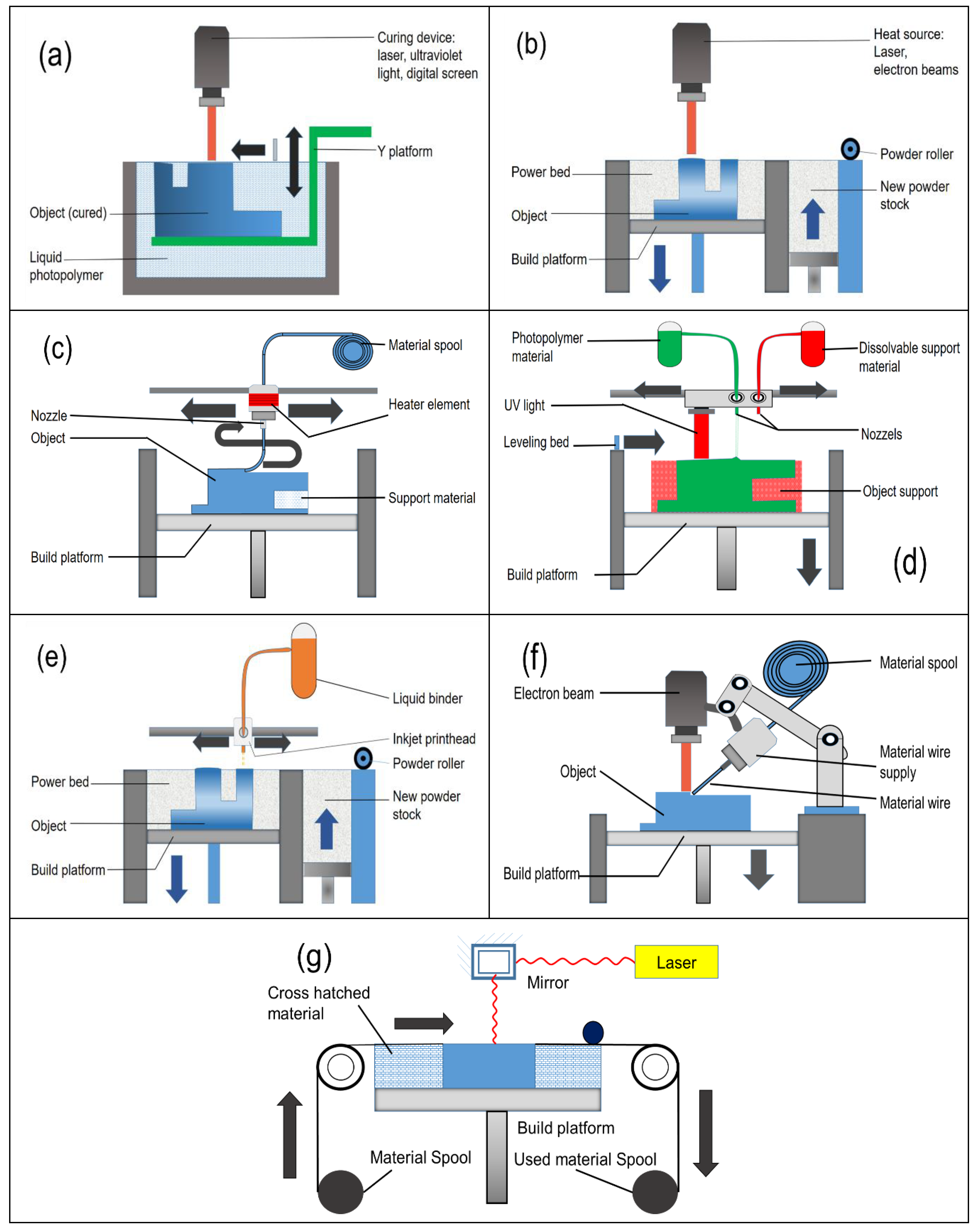
2.1. Vat Polymerization
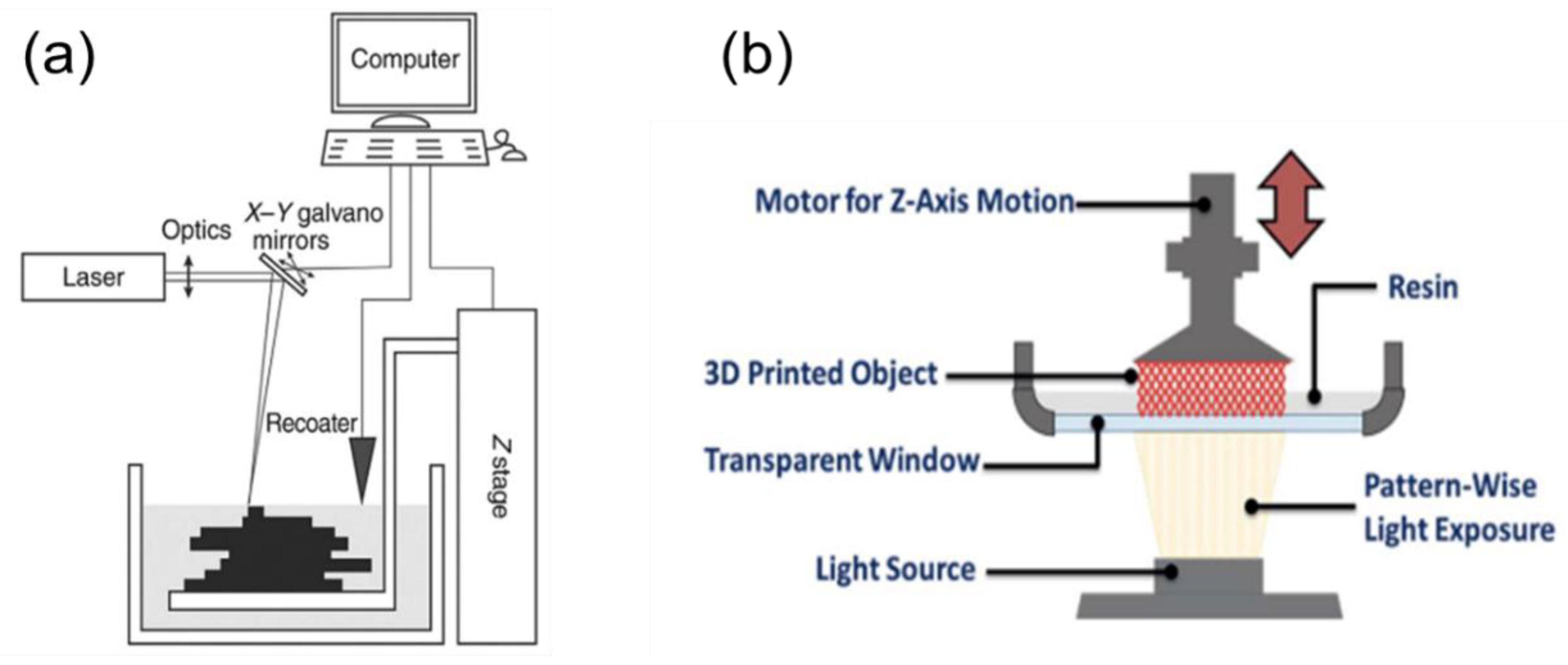
2.1.1. Stereolithography (SLA)
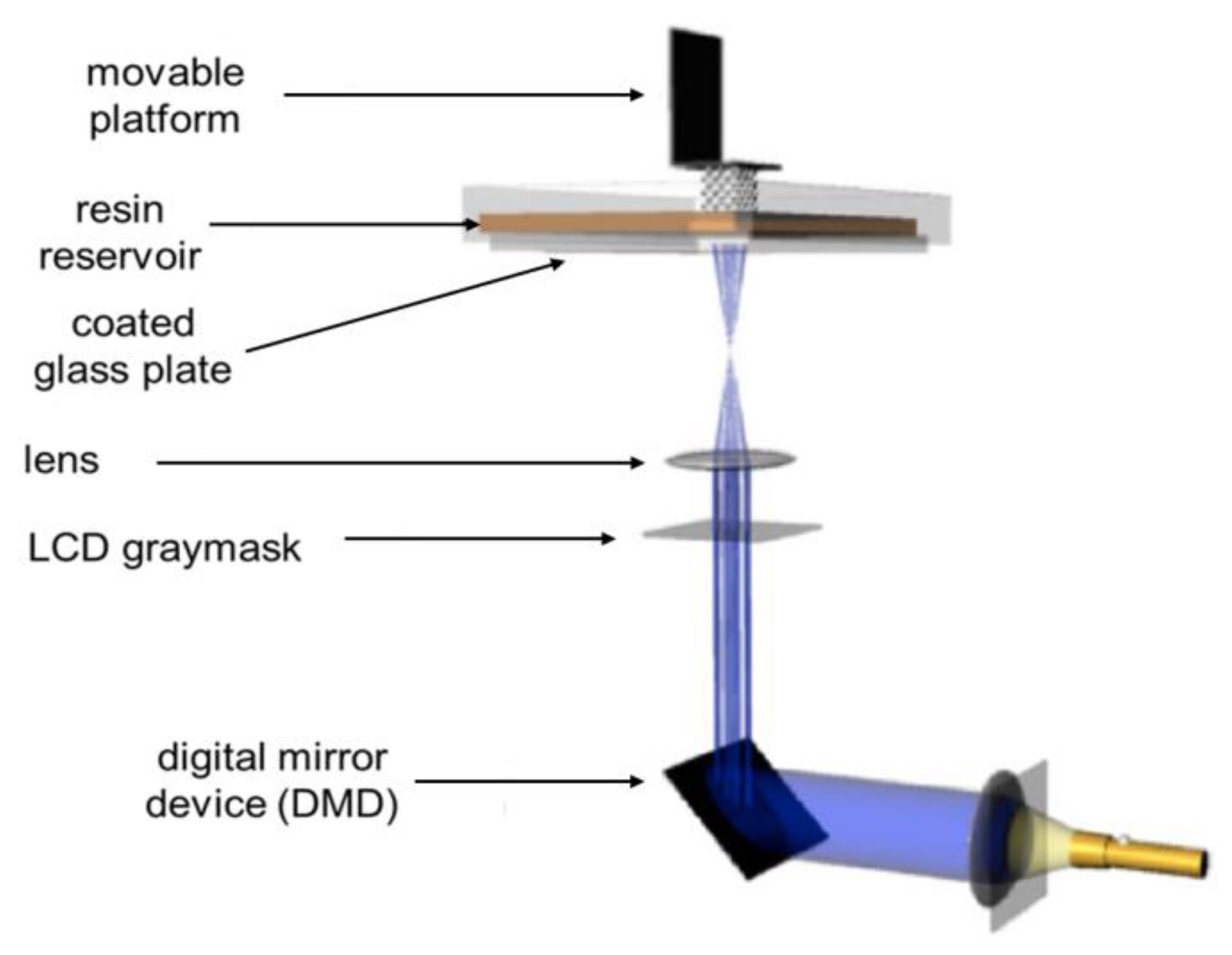
2.1.2. Digital Light Processing (DLP)
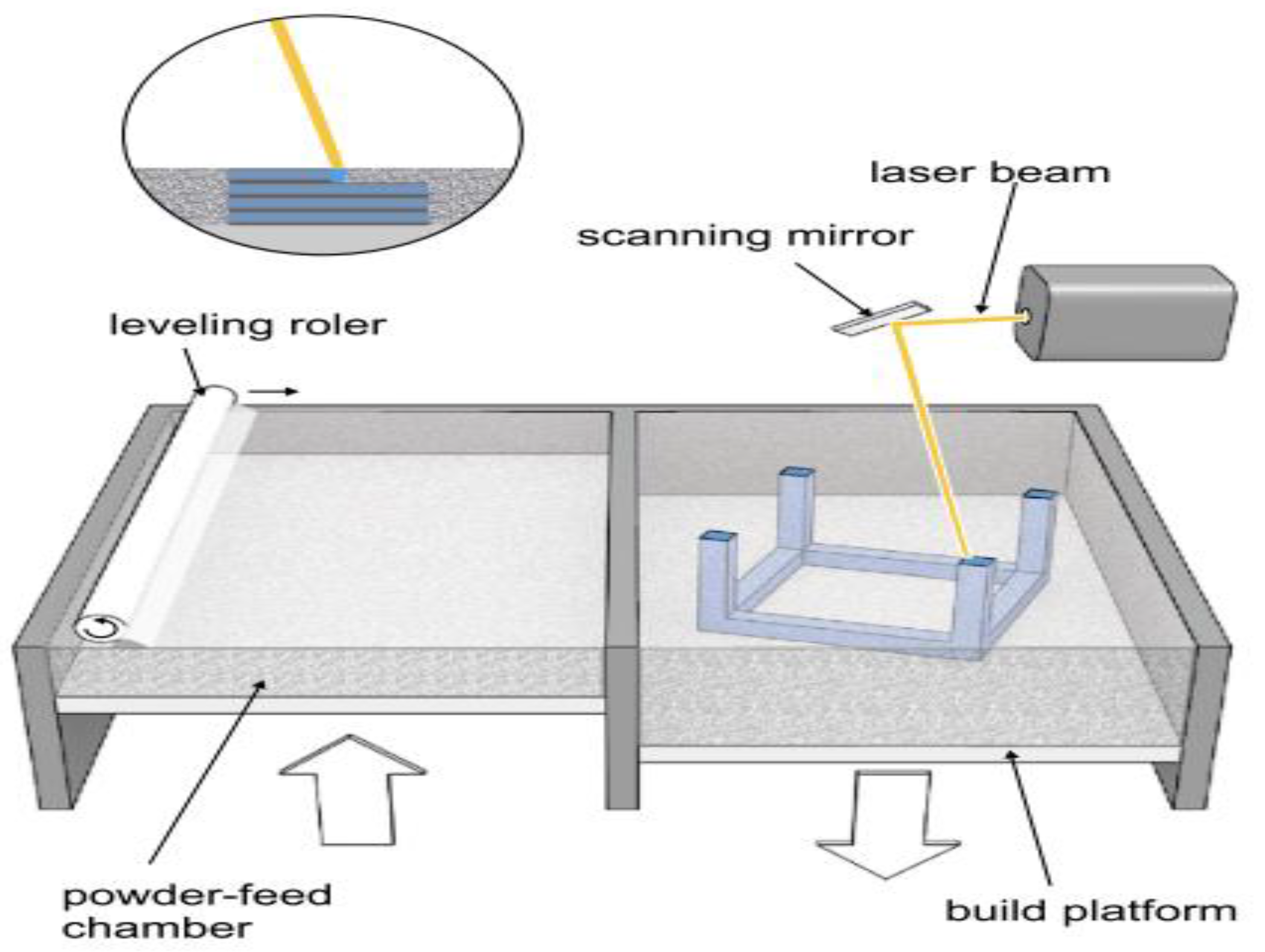
2.2. Powder Bed Fusion
2.2.1. Selective Laser Sintering (SLS)
2.2.2. Selective Laser Melting (SLM)
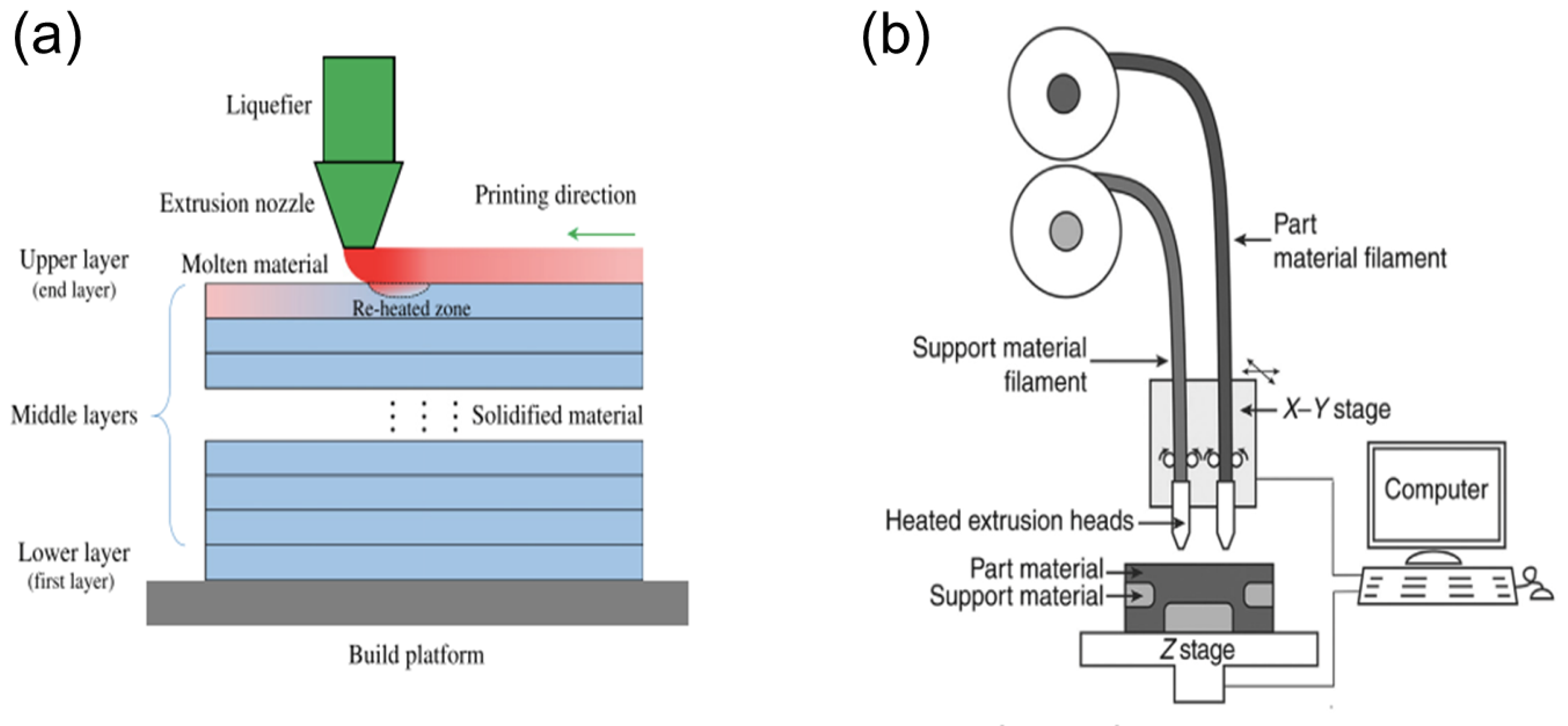
2.3. Material Extrusion
2.3.1. Direct Ink Writing (DIW)
2.3.2. Fused-Deposition Modelling (FDM)
2.4. Material Jetting
2.5. Microscopy Aided Design and Manufacture (MADAME)
3. Smart Printing Materials
3.1. Active Polymers
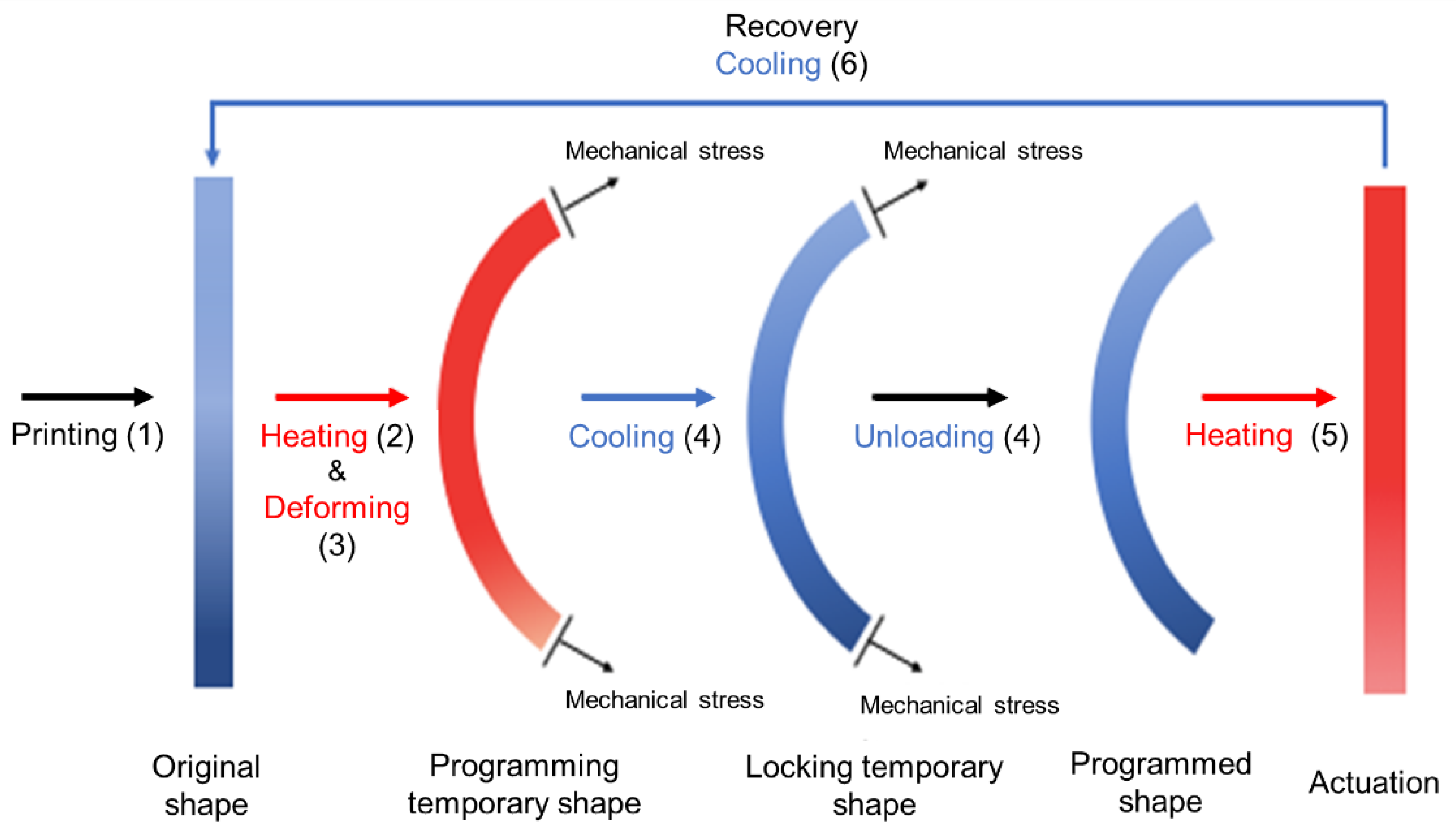
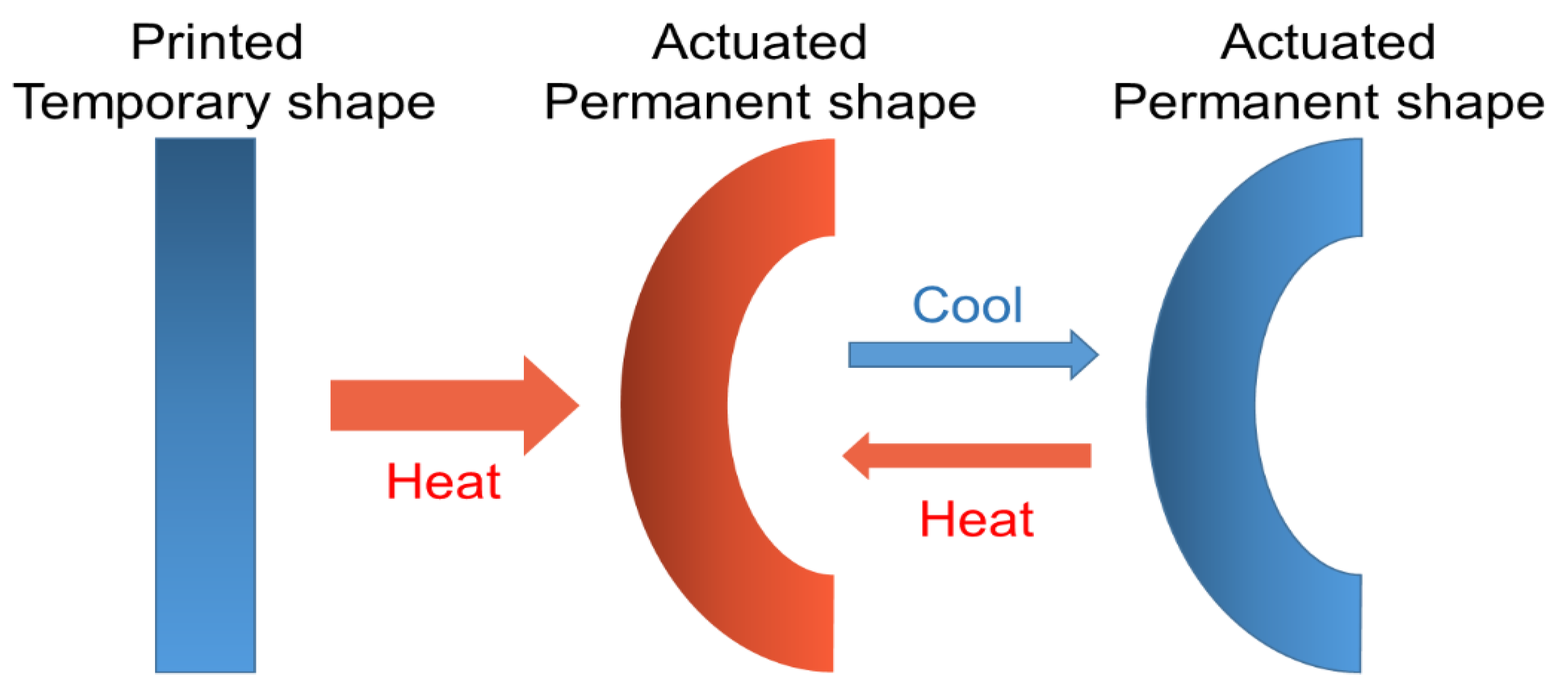
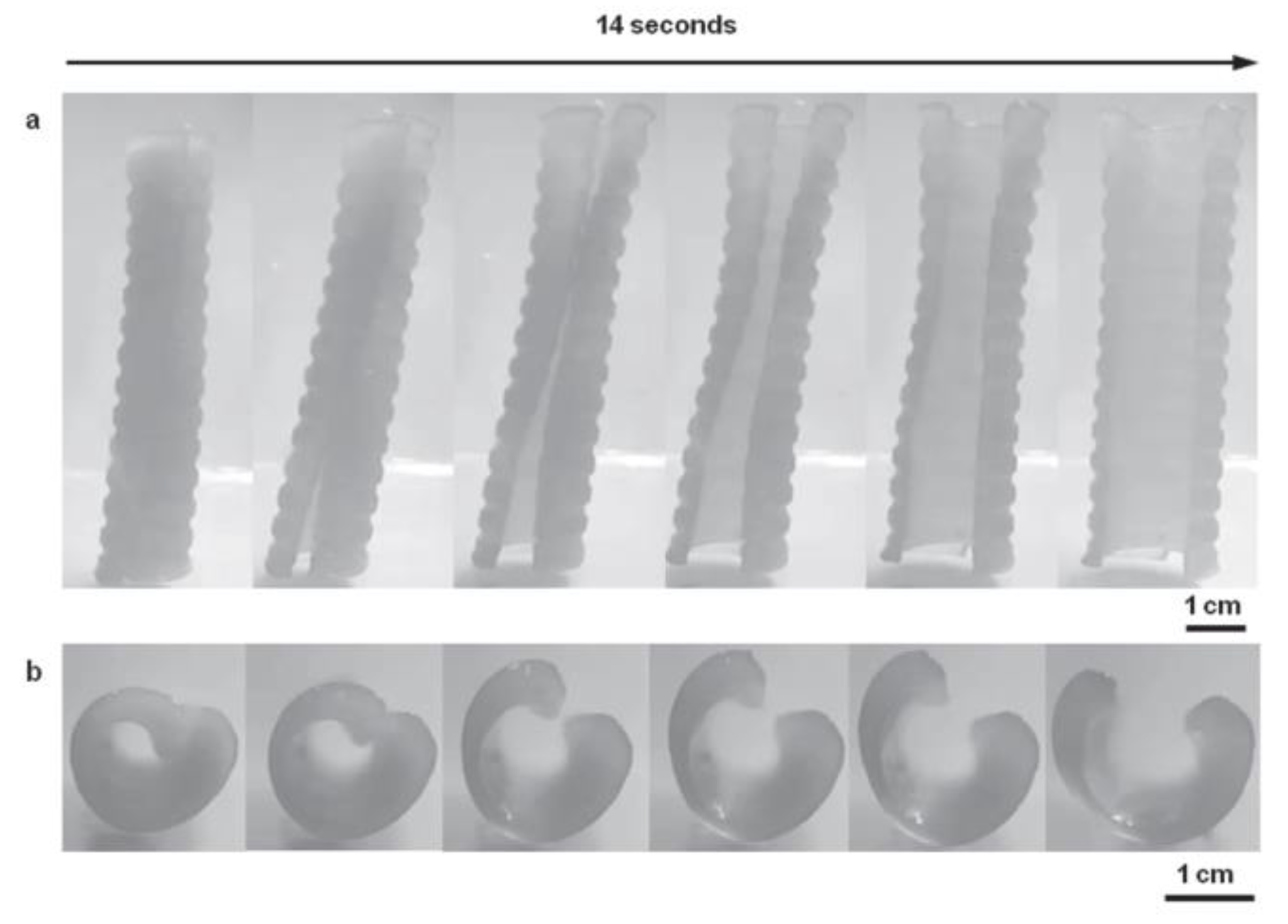
3.1.1. Shape-Memory Polymers
- (1)
- Heating the 3D printed structure above the glass transition temperature (Tg)
- (2)
- Applying mechanical load to form the deformed configuration
- (3)
- Cooling below Tg to "set" the temporary shape
- (4)
- Removing load,
- (5)
- Actuation
- (6)
- Cooling
3.1.2. Multi-Shape Memory Effect (Multi-SME)
3.1.3. Hydrogels
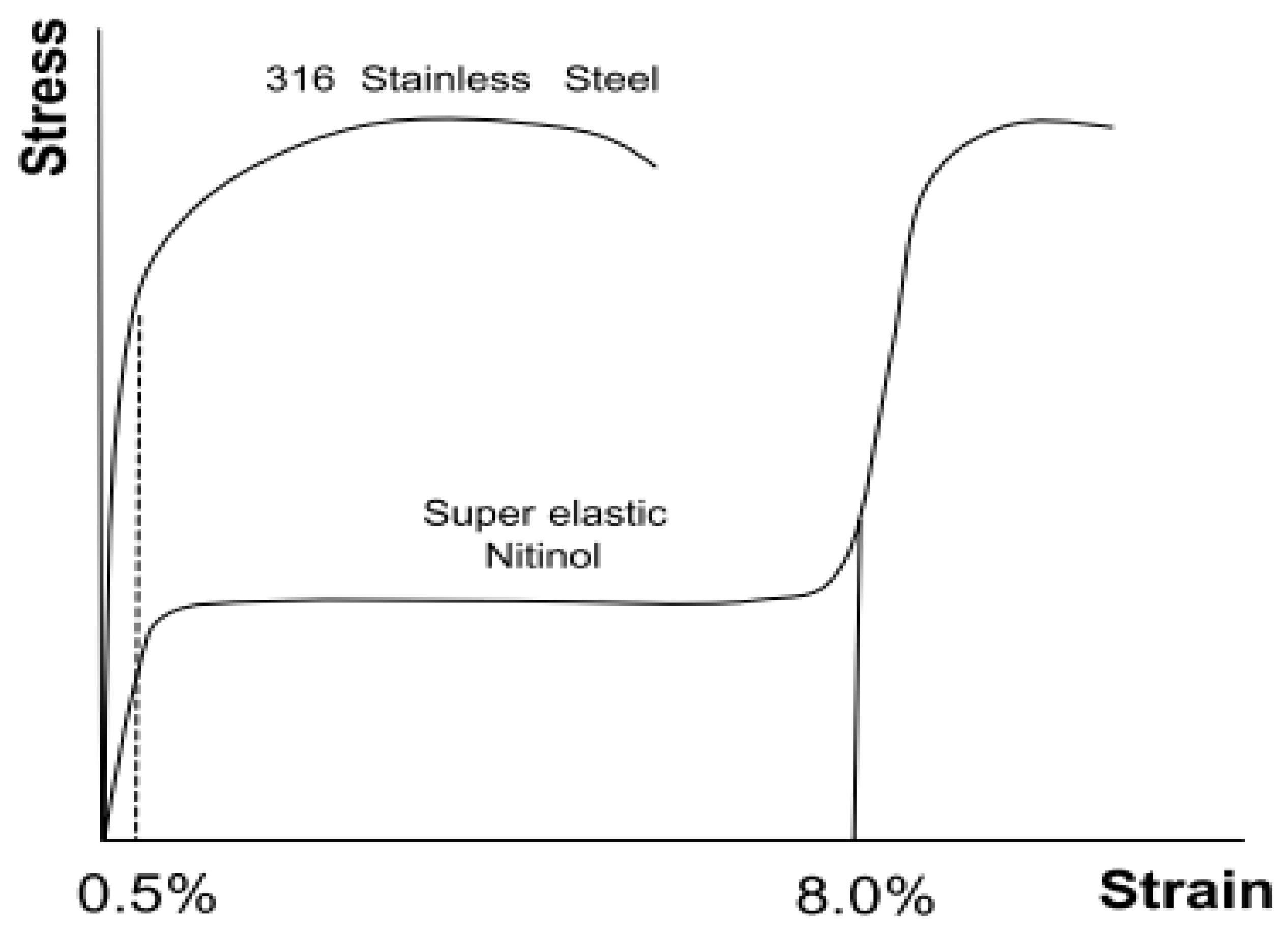
3.2. Shape-Memory Alloys
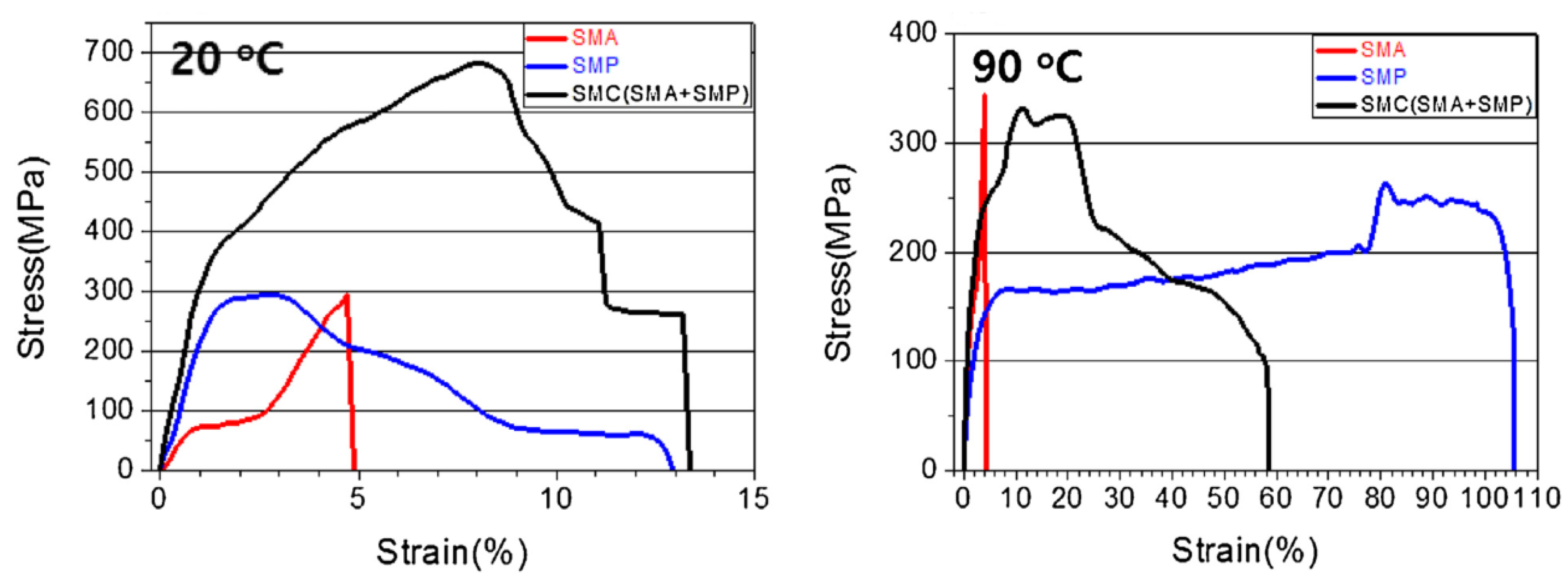
3.3. Shape Memory Composites
4. Recent Developments in the Biomedical Field
4.1. Tissue Engineering
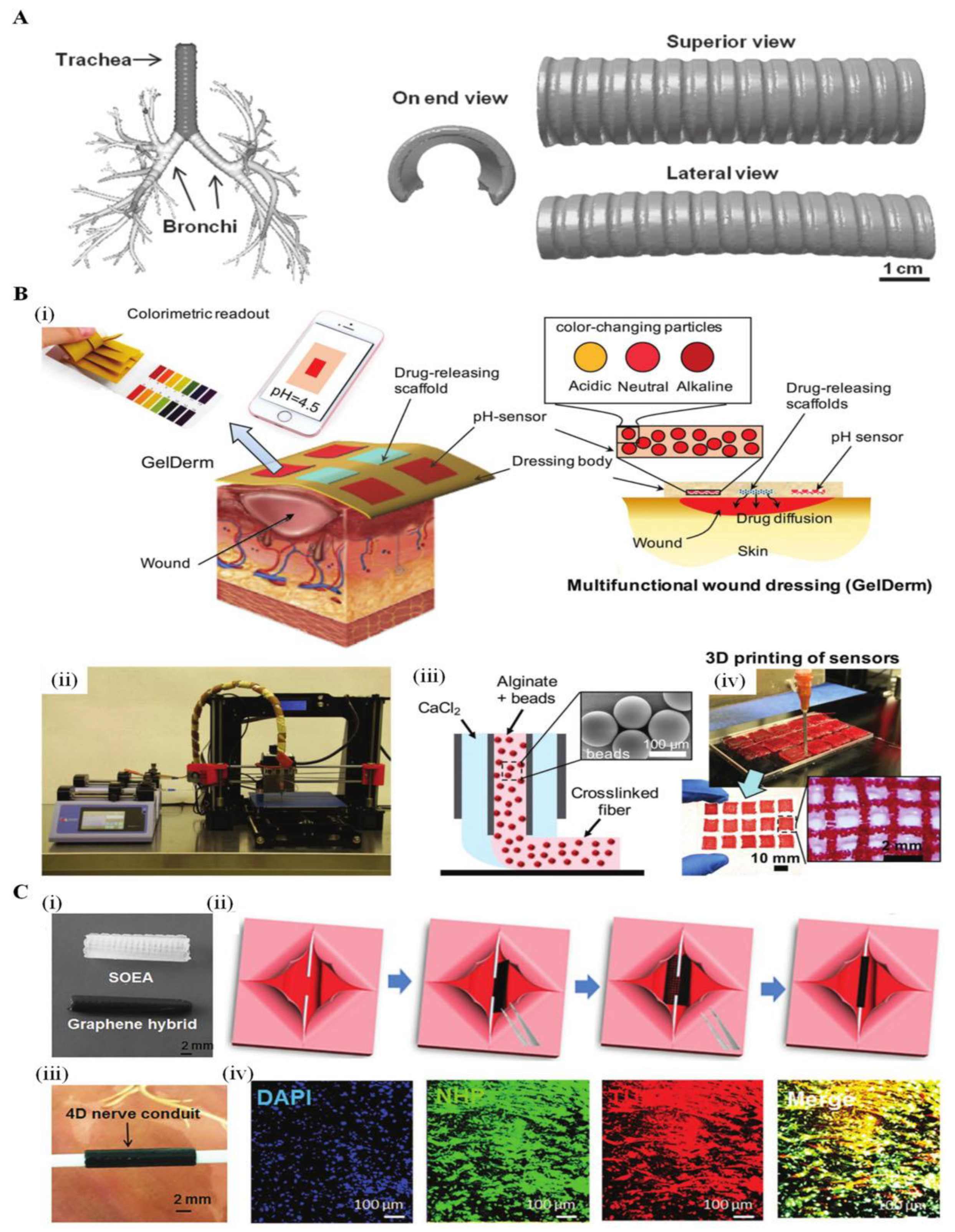
4.1.1. Implantable Organs
4.1.2. Skin Reconstruction
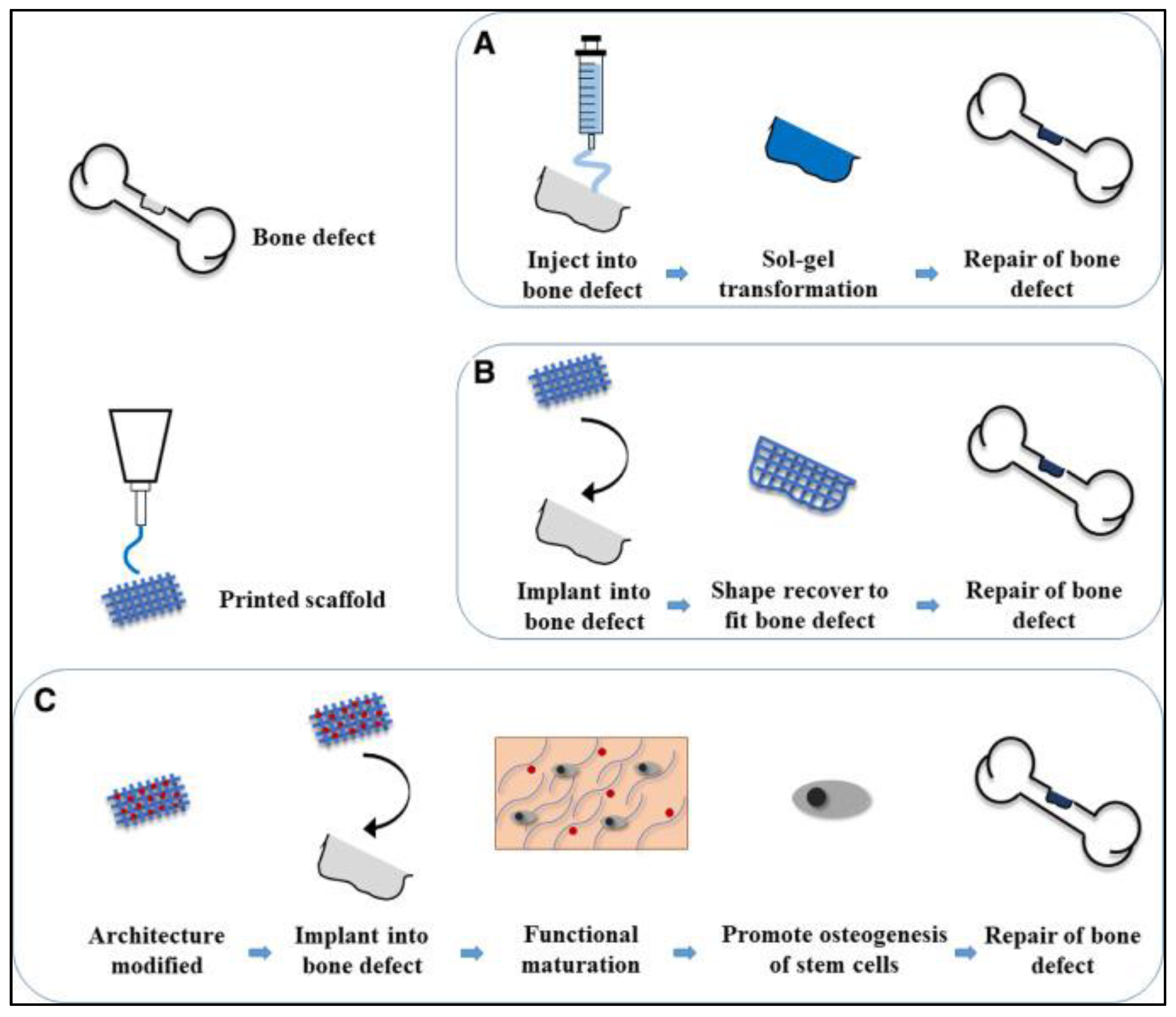
4.1.3. Bone Reconstruction
4.1.4. Stents
4.1.5. Nerves
4.2. Drug Delivery Systems (DDS)
4.3. Minimally Invasive Surgeries
5. Potential Future Applications
6. Limitations and Future Outlook
6.1. Materials Availability
6.2. Cost and Research Limitations
6.3. Practicality and Technical Limitations
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| AM | Additive manufacturing |
| DDS | Drug delivery system |
| SMP | Shape memory polymer |
| SMA | Shape memory alloy |
| SMC | Shape memory composite |
| 3D | Three-dimensional |
| DIW | Direct ink writing |
| 4D | Four-dimensional |
| SLA | Stereolithography apparatus |
| FDM | Fused deposition modelling |
| SLM | Selective laser melting |
| DLP | Direct laser printing |
| SLS | Selective laser sintering |
| PolyJet | Photopolymer Inkjet |
| SME | Shape memory effect |
| MME | Melt material extrusion |
| Tg | Glass transition temperature |
References
- Khoo, Z.X.; Teoh, J.E.M.; Liu, Y.; Chua, C.K.; Yang, S.; An, J.; Leong, K.F.; Yeong, W.Y. 3D printing of smart materials: A review on recent progresses in 4D printing. Virtual Phys. Prototyp. 2015, 10, 103–122. [Google Scholar] [CrossRef]
- Huang, L.; Jiang, R.; Wu, J.; Song, J.; Bai, H.; Li, B.; Zhao, Q.; Xie, T. Ultrafast Digital Printing toward 4D Shape Changing Materials. Adv. Mater. 2017, 29. [Google Scholar] [CrossRef]
- Raviv, R.; Zhao, W.; Mcknelly, C.L.; Papadopoulou, A.; Kadambi, A.; Shi, B.; Hirsch, S.; Dikovsky, D.; Zyracki, M.; Olguin, C.; et al. Active printed materials for complex self-evolving deformations. Sci. Rep. 2014, 4. [Google Scholar] [CrossRef] [PubMed]
- Leist, S.K.; Gao, D.; Chiou, R.; Zhou, J. Investigating the shape memory properties of 4D printed polylactic acid (PLA) and the concept of 4D printing onto nylon fabrics for the creation of smart textiles. Virtual Phys. Prototyp. 2017, 12, 290–300. [Google Scholar] [CrossRef]
- Tibbits, S. 4D Printing: Multi-Material Shape Change. Archit. Des. 2014, 84, 116–121. [Google Scholar] [CrossRef]
- André, J.-C. From Additive Manufacturing to 3D/4D Printing 3: Breakthrough Innovations: Programmable Material, 4D Printing and Bio-Printing, 1st ed.; John Wiley Sons Incorporated: Hoboken, NJ, USA, 2018. [Google Scholar]
- Gladman, A.S.; Matsumoto, E.A.; Nuzzo, R.G.; Mahadevan, L.; Lewis, J.A. Biomimetic 4D printing. Nat. Mater. 2016, 15, 413–418. [Google Scholar] [CrossRef] [PubMed]
- Zarek, M.; Mansour, N.; Shapira, S.; Cohn, D. 4D Printing of Shape Memory-Based Personalized Endoluminal Medical Devices. Macromol. Rapid Commun. 2017, 38. [Google Scholar] [CrossRef]
- Ramesh, S.; Reddy, S.K.; Usha, C.; Naulakha, N.K.; Adithyakumar, C.; Reddy, M.L.K. Advancements in the Research of 4D Printing-A Review. IOP Conf. Ser. Mater. Sci. Eng. 2018, 376, 012123. [Google Scholar] [CrossRef]
- Bodaghi, M.; Damanpack, A.R.; Liao, W.-H. Self-expanding/shrinking structures by 4D printing. Smart Mater. Struct. 2016, 25, 105034. [Google Scholar] [CrossRef]
- Shin, D.-G.; Kim, T.-H.; Kim, D.-E. Review of 4D printing materials and their properties. Int. J. Precis. Eng. Manuf. Technol. 2017, 4, 349–357. [Google Scholar] [CrossRef]
- Javaid, M.; Haleem, A. 4D printing applications in medical field: A brief review. Clin. Epidemiol. Glob. Health Sep. 2018. [Google Scholar] [CrossRef]
- Al’Aref, S.J.; Mosadegh, B.; Dunham, S.; Min, J.K. 3D printing applications in cardiovascular medicine. 3D Print. Appl. Cardiovasc. Med. 2018, 1–278. [Google Scholar] [CrossRef]
- Miao, S.; Cui, H.; Nowicki, M.; Xia, L.; Zhou, X.; Lee, S.-J.; Zhu, W.; Sarkar, K.; Zhang, Z.; Zhang, L.G. Stereolithographic 4D Bioprinting of Multiresponsive Architectures for Neural Engineering. Adv. Biosyst. 2018, 2. [Google Scholar] [CrossRef] [PubMed]
- Sidler, H.J.; Duvenage, J.; Anderson, E.J.; Ng, J.; Hageman, D.J.; Tate, M.L.K. Prospective design, rapid prototyping, and testing of smart dressings, drug delivery patches, and replacement body parts using Microscopy Aided Design and ManufacturE (MADAME). Front. Med. 2018, 5, 348. [Google Scholar] [CrossRef] [PubMed]
- Pilate, F.; Toncheva, A.; Dubois, P.; Raquez, J.-M. Shape-memory polymers for multiple applications in the materials world. Eur. Polym. J. 2016, 80, 268–294. [Google Scholar] [CrossRef]
- Pei, E.; Loh, G.H. Technological considerations for 4D printing: An overview. Prog. Addit. Manuf. 2018, 3, 95–107. [Google Scholar] [CrossRef]
- Teoh, J.E.M.; An, J.; Chua, C.K.; Lv, M.; Krishnasamy, V.; Liu, Y. Hierarchically self-morphing structure through 4D printing. Virtual Phys. Prototyp. 2016, 12, 61–68. [Google Scholar] [CrossRef]
- Miao, S.; Castro, N.; Nowicki, M.; Xia, L.; Cui, H.; Zhou, X.; Zhu, W.; Lee, S.-J.; Sarkar, K.; Vozzi, G.; et al. 4D printing of polymeric materials for tissue and organ regeneration. Mater. Today 2017, 20, 577–591. [Google Scholar] [CrossRef]
- Ding, Z.; Yuan, C.; Peng, X.; Wang, T.; Qi, H.J.; Dunn, M.L. Direct 4D Printing Via Active Composite Materials. Available online: http://advances.sciencemag.org/ (accessed on 10 October 2020).
- Gioumouxouzis, C.I.; Katsamenis, O.L.; Bouropoulos, N.; Fatouros, D.G. 3D printed oral solid dosage forms containing hydrochlorothiazide for controlled drug delivery. J. Drug Deliv. Sci. Technol. 2017, 40, 164–171. [Google Scholar] [CrossRef]
- Sun, L.; Huang, W.M. Thermo/moisture responsive shape-memory polymer for possible surgery/operation inside living cells in future. Mater. Des. 2010, 31, 2684–2689. [Google Scholar] [CrossRef]
- Morouço, P.; Lattanzi, W.; Alves, N. Four-Dimensional Bioprinting as a new Era for Tissue Engineering and Regenerative Medicine. Front. Bioeng. Biotechnol. 2017, 5, 61. [Google Scholar] [CrossRef] [PubMed]
- Introduction to 3D Printing—Additive Processes|Make. Available online: https://make.3dexperience.3ds.com/processes/introduction-to-additive-processes (accessed on 6 December 2020).
- Jamróz, W.; Szafraniec, J.; Kurek, M.; Jachowicz, R. 3D Printing in Pharmaceutical and Medical Applications—Recent Achievements and Challenges. Pharm. Res. 2018, 35, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Boydston, A.J.; Cao, B.; Nelson, A.; Ono, R.J.; Saha, A.; Schwartz, J.J.; Thrasher, C.J. Additive manufacturing with stimuli-responsive materials. J. Mater. Chem. A 2018, 6, 20621–20645. [Google Scholar] [CrossRef]
- Senatov, F.; Zadorozhnyy, M.; Niaza, K.; Medvedev, V.; Kaloshkin, S.; Anisimova, N.; Kiselevskiy, M.; Senatov, F. Shape memory effect in 3D-printed scaffolds for self-fitting implants. Eur. Polym. J. 2017, 93, 222–231. [Google Scholar] [CrossRef]
- Melchels, F.P.; Feijen, J.; Grijpma, D.W. A review on stereolithography and its applications in biomedical engineering. Biomaterials 2010, 31, 6121–6130. [Google Scholar] [CrossRef]
- Vehse, M.; Petersen, S.E.; Sternberg, K.; Schmitz, K.-P.; Seitz, H. Drug Delivery from Poly (ethylene glycol) Diacrylate Scaffolds Produced by DLC Based Micro-Stereolithography. Macromol. Symp. 2014, 346, 43–47. [Google Scholar] [CrossRef]
- Bertsch, A.; Renaud, P. Microstereolithography. In Three-Dimensional Microfabrication Using Two-Photon Polymerization, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 25–56. [Google Scholar]
- Ng, W.L.; Lee, J.M.; Zhou, M.; Chen, Y.-W.; Lee, K.-X.A.; Yeong, W.Y.; Shen, Y.-F. Vat polymerization-based bioprinting—Process, materials, applications and regulatory challenges. Biofabrication 2020, 12, 022001. [Google Scholar] [CrossRef]
- Choong, Y.Y.C.; Maleksaeedi, S.; Eng, H.; Wei, J.; Su, P.-C. 4D printing of high performance shape memory polymer using stereolithography. Mater. Des. 2017, 126, 219–225. [Google Scholar] [CrossRef]
- Saxena, P.; Bissacco, G.; Meinert, K.Æ.; Danielak, A.H.; Ribó, M.M.; Pedersen, D.B. Soft tooling process chain for the manufacturing of micro-functional features on molds used for molding of paper bottles. J. Manuf. Process. 2020, 54, 129–137. [Google Scholar] [CrossRef]
- Arcaute, K.; Mann, B.; Wicker, R. Acta Biomaterialia Stereolithography of spatially controlled multi-material bioactive poly (ethylene glycol) scaffolds. Acta Biomater. 2010, 6, 1047–1054. [Google Scholar] [CrossRef] [PubMed]
- Invernizzi, M.; Turri, S.; Levi, M.; Suriano, R. 4D printed thermally activated self-healing and shape memory polycaprolactone-based polymers. Eur. Polym. J. 2018, 101, 169–176. [Google Scholar] [CrossRef]
- Gorji, N.E.; Saxena, P.; Corfield, M.; Clare, A.; Rueff, J.-P.; Bogan, J.; González, P.G.; Snelgrove, M.; Hughes, G.; O’Connor, R.; et al. A new method for assessing the recyclability of powders within Powder Bed Fusion process. Mater. Charact. 2020, 161, 110167. [Google Scholar] [CrossRef]
- Gurung, D. Technological Comparison of 3D and 4D Printing. 2017. Available online: https://scholar.google.com/scholar?hl=enas_sdt=0%2C23as_ylo=2016q=Technological+comparison+of+3D+and+4D+printing+Dilip+GurungbtnG=%0Ahttps://www.theseus.fi/bitstream/handle/10024/130325/Thesis_Dilip.pdf?sequence=1 (accessed on 10 September 2020).
- Ji, S.; Guvendiren, M. Recent Advances in Bioink Design for 3D Bioprinting of Tissues and Organs. Front. Bioeng. Biotechnol. 2017, 5, 23. [Google Scholar] [CrossRef] [PubMed]
- Goyanes, A.; Buanz, A.B.; Basit, A.W.; Gaisford, S. Fused-filament 3D printing (3DP) for fabrication of tablets. Int. J. Pharm. 2014, 476, 88–92. [Google Scholar] [CrossRef] [PubMed]
- Shishkovsky, I.; Scherbakov, V. 4D manufacturing of intermetallic SMA fabricated by SLM process. Laser 3D Manuf. V 2018, 10523, 1052311. [Google Scholar] [CrossRef]
- Oberoi, G.; Nitsch, S.; Edelmayer, M.; Janjić, K.; Müller, A.S.; Agis, H. 3D Printing—Encompassing the Facets of Dentistry. Front. Bioeng. Biotechnol. 2018, 6, 172. [Google Scholar] [CrossRef]
- Kashyap, D.; Kumar, P.K.; Kanagaraj, S. 4D printed porous radiopaque shape memory polyurethane for endovascular embolization. Addit. Manuf. 2018, 24, 687–695. [Google Scholar] [CrossRef]
- Bodaghi, M.; Damanpack, A.R.; Liao, W.-H. Triple shape memory polymers by 4D printing. Smart Mater. Struct. 2018, 27, 065010. [Google Scholar] [CrossRef]
- Ge, Q.; Dunn, C.K.; Qi, H.J.; Dunn, M.L. Active origami by 4D printing. Smart Mater. Struct. 2014, 23. [Google Scholar] [CrossRef]
- Lee, A.Y.; An, J.; Chua, C.K. Two-Way 4D Printing: A Review on the Reversibility of 3D-Printed Shape Memory Materials. Engineering 2017, 3, 663–674. [Google Scholar] [CrossRef]
- Miao, S.; Zhu, W.; Castro, N.J.; Nowicki, M.; Zhou, X.; Cui, H.; Fisher, J.P.; Zhang, L.G. 4D printing smart biomedical scaffolds with novel soybean oil epoxidized acrylate. Sci. Rep. 2016, 6, 27226. [Google Scholar] [CrossRef] [PubMed]
- Vijayavenkataraman, S.; Lu, W.F.; Fuh, J.Y.H. 3D bioprinting of functional human skin: Production and in vivo analysis Related content 3D bioprinting of skin: A state-of-the-art review on modelling, materials, and processes. Biofabrication 2016, 9. [Google Scholar] [CrossRef]
- Ge, Q.; Qi, H.J.; Dunn, M.L. Active materials by four-dimension printing. Appl. Phys. Lett. 2013, 103, 131901. [Google Scholar] [CrossRef]
- Mao, Y.; Ding, Z.; Yuan, C.; Ai, S.; Isakov, M.; Wu, J.; Wang, T.; Dunn, M.L.; Qi, H.J. 3D Printed Reversible Shape Changing Components with Stimuli Responsive Materials. Sci. Rep. 2016, 6, 24761. [Google Scholar] [CrossRef]
- Ge, Q.; Sakhaei, A.H.; Lee, H.; Dunn, C.K.; Fang, N.X.; Dunn, M.L. Multimaterial 4D Printing with Tailorable Shape Memory Polymers. Sci. Rep. 2016, 6, 31110. [Google Scholar] [CrossRef]
- Leist, S.K.; Zhou, J. Current status of 4D printing technology and the potential of light-reactive smart materials as 4D printable materials materials as 4D printable materials. Virtual Phys. Prototyp. 2016, 11, 249–262. [Google Scholar] [CrossRef]
- Mu, T.; Liu, L.; Lan, X.; Liu, Y.; Leng, J. Shape memory polymers for composites. Compos. Sci. Technol. 2018, 160, 169–198. [Google Scholar] [CrossRef]
- Pyo, Y.; Kang, M.; Jang, J.Y.; Park, Y.; Son, Y.-H.; Choi, M.; Ha, J.W.; Chang, Y.-W.; Lee, C.S. Design of a shape memory composite(SMC) using 4D printing technology. Sens. Actuators A Phys. 2018, 283, 187–195. [Google Scholar] [CrossRef]
- Behl, M.; Razzaq, M.Y.; Lendlein, A. Multifunctional shape-memory polymers. Adv. Mater. 2010, 22, 3388–3410. [Google Scholar] [CrossRef]
- Yang, G.; Liu, X.; Tok, A.I.Y.; Lipik, V. Body temperature-responsive two-way and moisture-responsive one-way shape memory behaviors of poly(ethylene glycol)-based networks. Polym. Chem. 2017, 8, 3833–3840. [Google Scholar] [CrossRef]
- Wu, J.; Yuan, C.; Ding, Z.; Isakov, M.; Mao, Y.; Wang, T.; Dunn, M.L.; Qi, H.J. Multi-shape active composites by 3D printing of digital shape memory polymers. Sci. Rep. 2016, 6, 24224. [Google Scholar] [CrossRef] [PubMed]
- Mao, Y.; Yu, K.; Isakov, M.S.; Wu, J.; Dunn, M.L.; Qi, H.J. Sequential Self-Folding Structures by 3D Printed Digital Shape Memory Polymers. Sci. Rep. 2015, 5, 13616. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Huang, W.M.; Lu, H.; Sun, L. Thermo-/chemo-responsive shape memory/change effect in a hydrogel and its composites. Mater. Des. 2014, 53, 1077–1088. [Google Scholar] [CrossRef]
- Kopeček, J. Hydrogels: From soft contact lenses and implants to self-assembled nanomaterials. J. Polym. Sci. Part A Polym. Chem. 2009, 47, 5929–5946. [Google Scholar] [CrossRef]
- Highley, C.B.; Rodell, C.B.; Burdick, J.A. Direct 3D Printing of Shear-Thinning Hydrogels into Self-Healing Hydrogels. Adv. Mater. 2015, 27, 5075–5079. [Google Scholar] [CrossRef]
- Mehrabi, R.; Kadkhodaei, M.; Elahinia, M. A thermodynamically-consistent microplane model for shape memory alloys. Int. J. Solids Struct. 2014, 51, 2666–2675. [Google Scholar] [CrossRef]
- Boyd, J.G.; Lagoudas, D.C. A thermodynamical constitutive model for shape memory materials. Part I. The monolithic shape memory alloy. Int. J. Plast. 1996, 12, 805–842. [Google Scholar] [CrossRef]
- Morgan, N. Medical shape memory alloy applications—The market and its products. Mater. Sci. Eng. A 2004, 378, 16–23. [Google Scholar] [CrossRef]
- Wu, J.-J.; Huang, L.-M.; Zhao, Q.; Xie, T. 4D Printing: History and Recent Progress. Chin. J. Polym. Sci. 2018, 36, 563–575. [Google Scholar] [CrossRef]
- Ozbolat, I.T.; Yu, Y. Bioprinting toward organ fabrication: Challenges and future trends. IEEE Trans. Biomed. Eng. 2013, 60, 691–699. [Google Scholar] [CrossRef] [PubMed]
- Ng, W.L.; Chua, C.K.; Shen, Y.-F. Print Me an Organ! Why We Are Not There Yet. Prog. Polym. Sci. 2019, 97, 101145. [Google Scholar] [CrossRef]
- Ng, W.L.; Wang, S.; Yeong, W.Y.; Naing, M.W. Skin Bioprinting: Impending Reality or Fantasy? Trends Biotechnol. 2016, 34, 689–699. [Google Scholar] [CrossRef] [PubMed]
- Farrell, A.-M.; Quigley, M. Organ Donation and Transplantation. In Encyclopedia of Applied Ethics; Elsevier: Amsterdam, The Netherlands, 2012; pp. 288–296. [Google Scholar]
- Ahadian, S.; Khademhosseini, A. A perspective on 3D bioprinting in tissue regeneration. Bio-Design Manuf. 2018, 1, 157–160. [Google Scholar] [CrossRef]
- Mota, C.; Camarero-Espinosa, S.; Baker, M.B.; Wieringa, P.; Moroni, L. Bioprinting: From Tissue and Organ Development to in Vitro Models. Chem. Rev. 2020, 120, 10547–10607. [Google Scholar] [CrossRef]
- Gugala, Z.; Lindsey, R.W.; Gogolewski, S. New approaches in the treatment of critical-size segmental defects in long bones. Macromol. Symp. 2007, 253, 147–161. [Google Scholar] [CrossRef]
- Karageorgiou, V.; Kaplan, D.L. Porosity of 3D biomaterial scaffolds and osteogenesis. Biomaterials 2005, 26, 5474–5491. [Google Scholar] [CrossRef]
- Kanczler, J.M.; Oreffo, R.O.C. Osteogenesis and angiogenesis: The potential for engineering bone. Eur. Cells Mater. 2008, 15, 100–114. [Google Scholar] [CrossRef]
- Wan, Z.; Zhang, P.; Liu, Y.; Lv, L.; Zhou, Y. Four-dimensional bioprinting: Current developments and applications in bone tissue engineering. Acta Biomater. 2020, 101, 26–42. [Google Scholar] [CrossRef]
- Senatov, F.; Niaza, K.; Stepashkin, A.; Kaloshkin, S. Low-cycle fatigue behavior of 3d-printed PLA-based porous scaffolds. Compos. Part B Eng. 2016, 97, 193–200. [Google Scholar] [CrossRef]
- Senatov, F.; Niaza, K.; Zadorozhnyy, M.; Maksimkin, A.; Kaloshkin, S.; Estrin, Y. Mechanical properties and shape memory effect of 3D-printed PLA-based porous scaffolds. J. Mech. Behav. Biomed. Mater. 2016, 57, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Chawla, S.; Sharma, A.; Bandyopadhyay, A.; Ghosh, S. Developmental Biology-Inspired Strategies to Engineer 3D Bioprinted Bone Construct. ACS Biomater. Sci. Eng. 2018, 4, 3545–3560. [Google Scholar] [CrossRef]
- Yang, Q.; Gao, B.; Xu, F. Recent Advances in 4D Bioprinting. Biotechnol. J. 2020, 15, e1900086. [Google Scholar] [CrossRef] [PubMed]
- Raman, R.; Cvetkovic, C.; Uzel, S.G.M.; Platt, R.J.; Sengupta, P.; Kamm, R.D.; Bashir, R. Optogenetic skeletal muscle-powered adaptive biological machines. Proc. Natl. Acad. Sci. USA 2016, 113, 3497–3502. [Google Scholar] [CrossRef]
- Kirillova, A.; Maxson, R.; Stoychev, G.; Gomillion, C.T.; Ionov, L. 4D Biofabrication Using Shape-Morphing Hydrogels. Adv. Mater. 2017, 29. [Google Scholar] [CrossRef]
- Wei, H.; Zhang, Q.; Yao, Y.; Liu, L.; Liu, Y.; Leng, J. Direct-write fabrication of 4D active shape-changing structures based on a shape memory polymer and its nanocomposite. ACS Appl. Mater. Interfaces 2017, 9, 876–883. [Google Scholar] [CrossRef]
- Mirani, B.; Pagan, E.; Currie, B.; Siddiqui, M.A.; Hosseinzadeh, R.; Mostafalu, P.; Zhang, Y.S.; Ghahary, A.; Akbari, M. An Advanced Multifunctional Hydrogel-Based Dressing for Wound Monitoring and Drug Delivery. Adv. Health Mater. 2017, 6, 1–15. [Google Scholar] [CrossRef]
- Zhang, Z.; Demir, K.G.; Gu, G.X. Developments in 4D-printing: A review on current smart materials, technologies, and applications. Int. J. Smart Nano Mater. 2019, 10, 205–224. [Google Scholar] [CrossRef]
- Kehoe, S.; Zhang, X.; Boyd, D. FDA approved guidance conduits and wraps for peripheral nerve injury: A review of materials and efficacy. Injury 2012, 43, 553–572. [Google Scholar] [CrossRef]
- Mu, X.; Bertron, T.; Dunn, C.; Qiao, H.; Wu, J.; Zhao, Z.; Saldana, C.; Qi, H.J. Porous polymeric materials by 3D printing of photocurable resin. Mater. Horiz. 2017, 4, 442–449. [Google Scholar] [CrossRef]
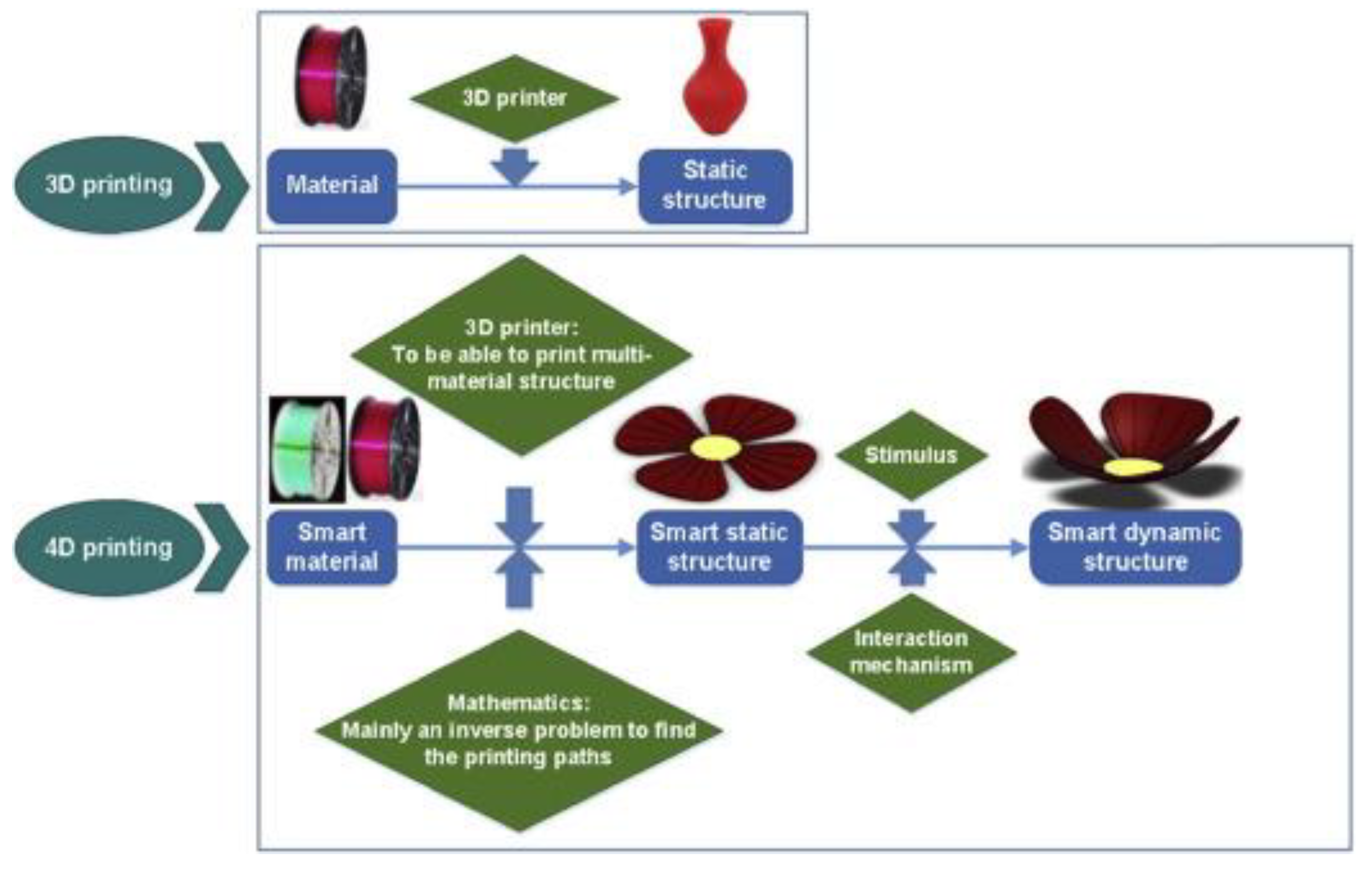

| Characteristics | 3D Printing | 4D Printing |
|---|---|---|
| Build Process |
|
|
| Materials |
|
|
| Shape flexibility |
|
|
| Shape-memory programming |
|
|
| Applications |
|
|
| AM Process | AM Systems | Applicable Materials | Ref. |
|---|---|---|---|
| Liquid solidification | SLA | SMPs | [32] |
| Soybean oil | [46] | ||
| SMCs | [34] | ||
| Direct laser printing (DLP) | SMPs | [35] | |
| Material Extrusion | FDM | SMPs | [26] |
| SMCs | [42] | ||
| Hydrogel extrusion | SMCs | [47] | |
| Material Jetting | PolyJet | SMPs | [48] |
| SMCs | [44,49] | ||
| Powder solidification | SLM | SMAs | [40] |
| SLS | SMPs | [38] | |
| SMCs | [44] |
| Material | Advantages | Disadvantages | Suitable AM Techniques | Actuation Methods |
|---|---|---|---|---|
| SMP | Simple programming [52] | Low tensile strength [11] | FDM [42] | Heat, light, ultrasound, pH, solvents, metal ions [52] |
| Biocompatibility and biodegradability [11] | Prone to degradation [16] Low thermal conductivity [11] | SLA [32] PolyJet [1] | ||
| Low density [27] | Single stimulation mode [52] | Extrusion [2] | ||
| Self-healing capabilities [52] | Slow shape-memory behaviour [52] | SLS [30] | ||
| SMA | Can use for large-scale fabrication [51] | High cost compared with SMPs [51] | SLM [40] | Electricity, heat, magnetism [52] |
| High tensile strength [51] | High density [27] | |||
| High moduli [51] | More complicated programming than SMP [35] | |||
| Wide operating temperature range [51] | Less biocompatible and biodegradable options available [51,52] | |||
| SMC | Good strain recovery [53] | Not well developed [64] | FDM [53] | Electricity, magnetism, light, microwave, UV, water, solvent [52] |
| Can achieve lower density and tensile strength than SMA [53] | SLS [44] PolyJet [1] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bajpai, A.; Baigent, A.; Raghav, S.; Brádaigh, C.Ó.; Koutsos, V.; Radacsi, N. 4D Printing: Materials, Technologies, and Future Applications in the Biomedical Field. Sustainability 2020, 12, 10628. https://doi.org/10.3390/su122410628
Bajpai A, Baigent A, Raghav S, Brádaigh CÓ, Koutsos V, Radacsi N. 4D Printing: Materials, Technologies, and Future Applications in the Biomedical Field. Sustainability. 2020; 12(24):10628. https://doi.org/10.3390/su122410628
Chicago/Turabian StyleBajpai, Ankur, Anna Baigent, Sakshika Raghav, Conchúr Ó. Brádaigh, Vasileios Koutsos, and Norbert Radacsi. 2020. "4D Printing: Materials, Technologies, and Future Applications in the Biomedical Field" Sustainability 12, no. 24: 10628. https://doi.org/10.3390/su122410628
APA StyleBajpai, A., Baigent, A., Raghav, S., Brádaigh, C. Ó., Koutsos, V., & Radacsi, N. (2020). 4D Printing: Materials, Technologies, and Future Applications in the Biomedical Field. Sustainability, 12(24), 10628. https://doi.org/10.3390/su122410628









