A Framework to Manage Coastal Squeeze
Abstract
1. Introduction
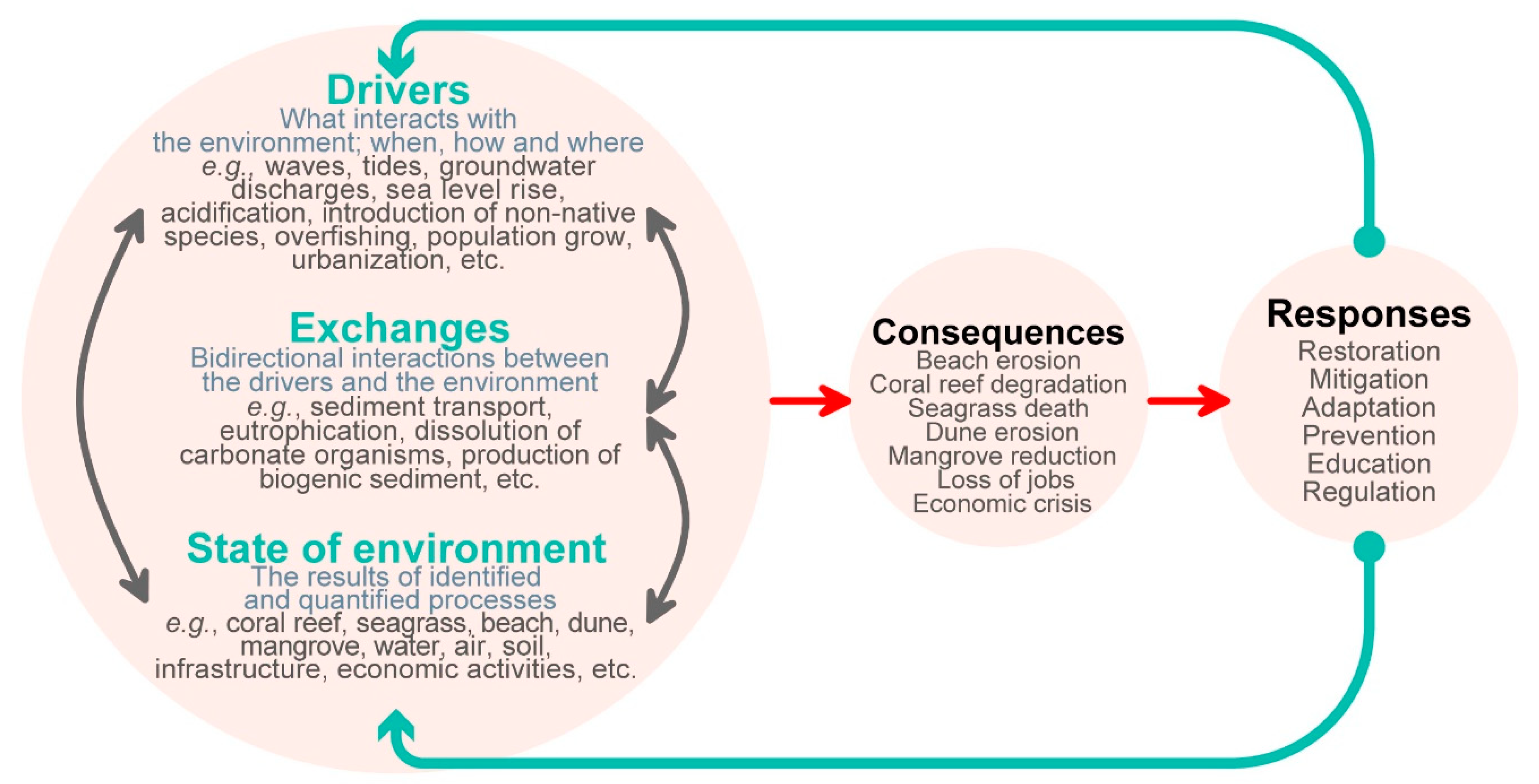
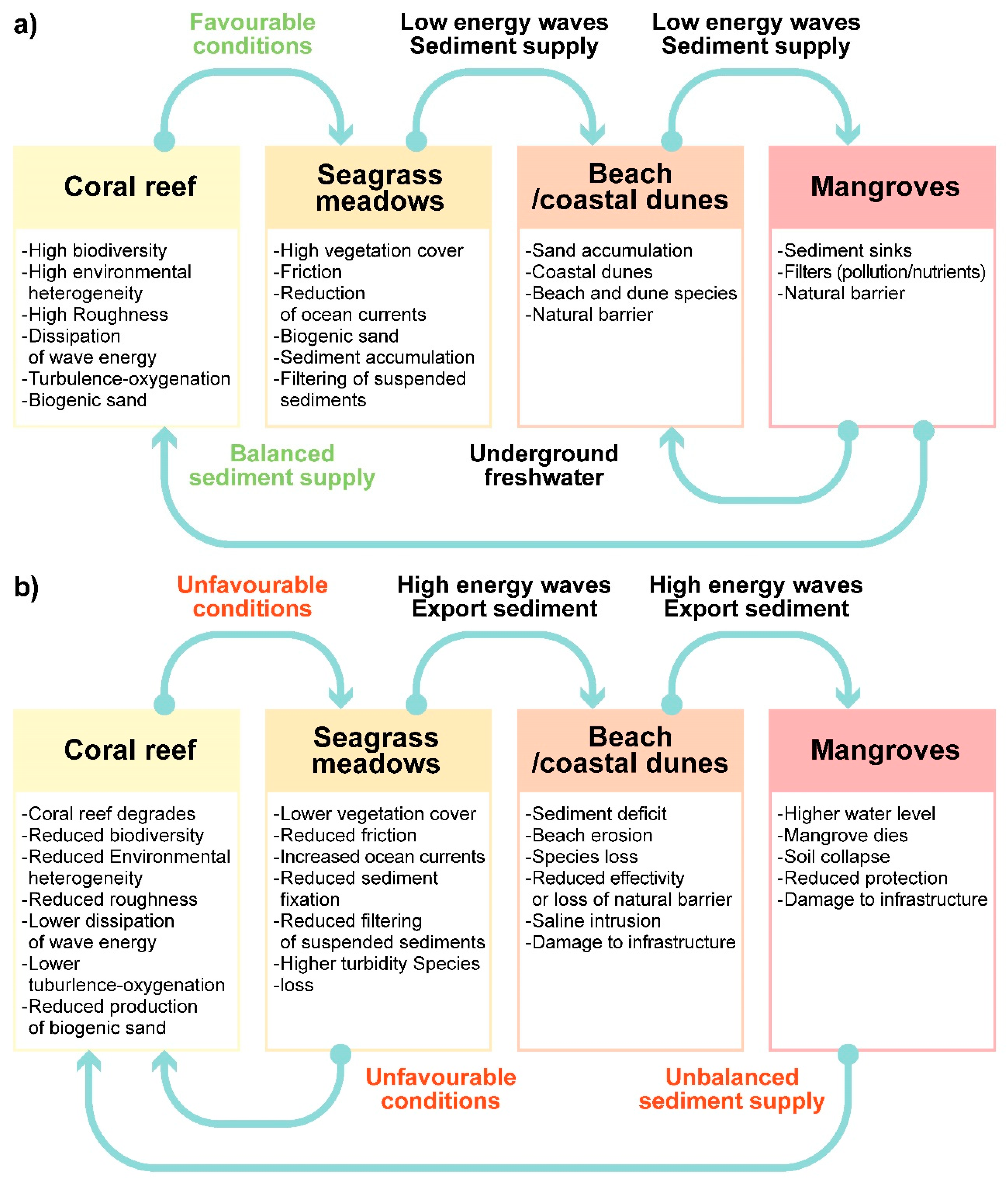
2. The Drivers–Exchanges–State of the Environment (DES) Cycle
2.1. Drivers
Parameters
2.2. Exchanges
2.3. State of the Environment
2.3.1. Spatial Delimitation: Which Coastal Ecosystems Should Be Included?
2.3.2. The Coastal Unit
2.3.3. Parameters
3. The Consequence–Response (CR) Cycle
3.1. Consequences
3.2. Responses
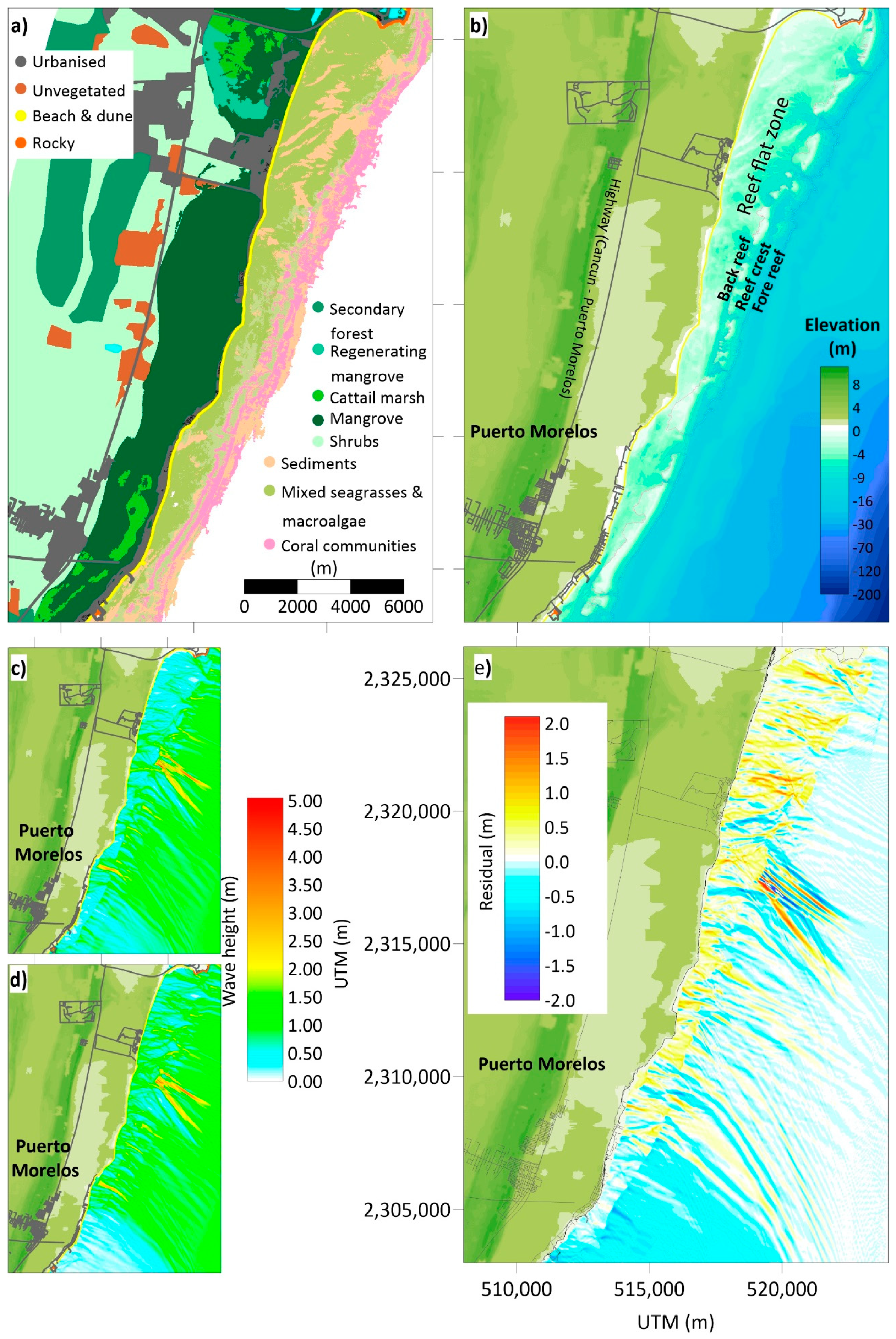
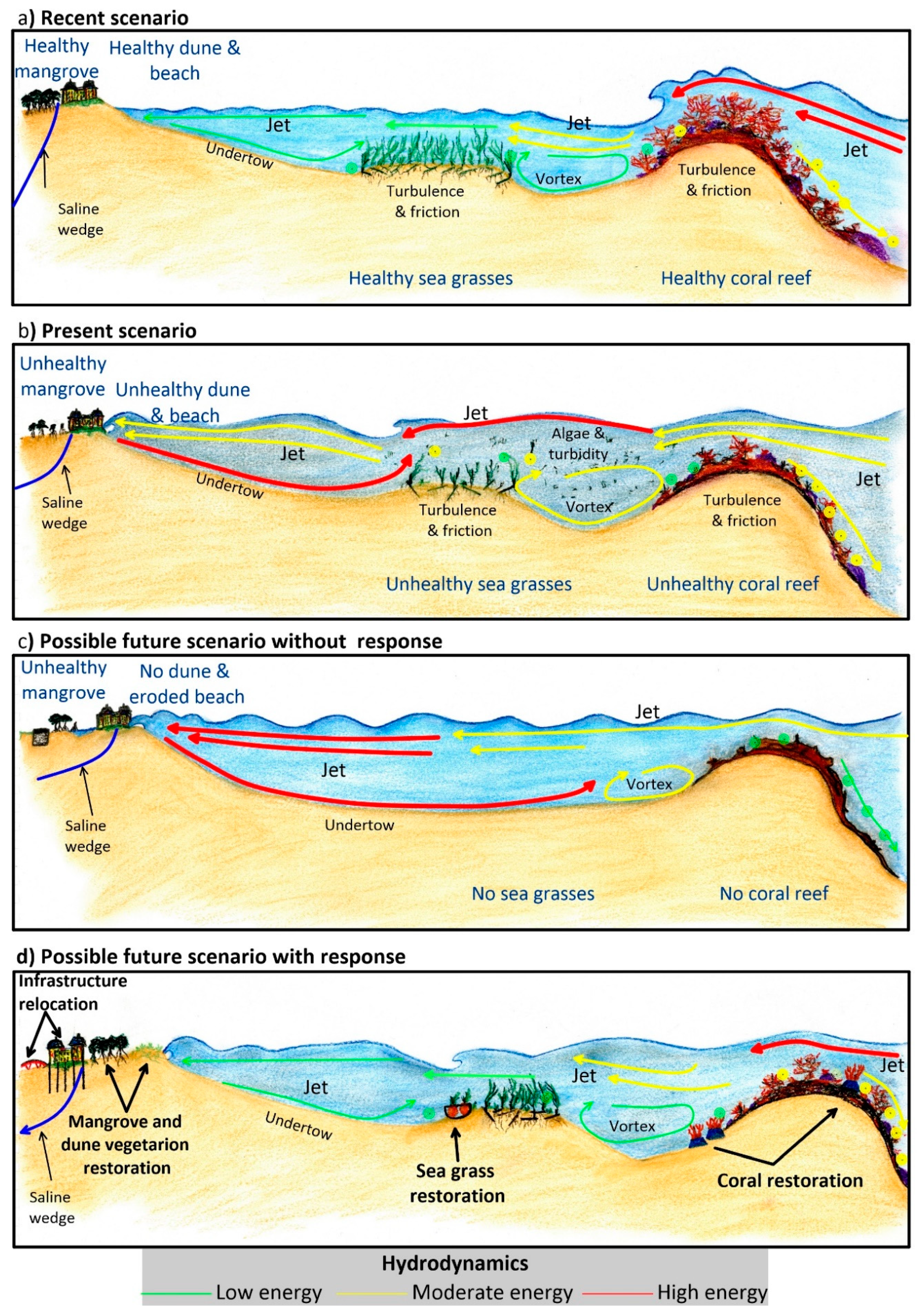
4. An Example of Coastal Squeeze Assessment
4.1. The DES Cycle (the State of the Environment, Exchanges, and Drivers)
4.2. The CR Cycle (Consequences and Responses)
- Executing integral coastal management strategies (regulating activities and use of the space). Over recent decades, the lack of adequate environmental planning for Puerto Morelos has been evident in frequent changes in policy and financial support.
- Implementing mitigation and management plans to deal with the original pressure (in this case, the sargasso influxes) and establishing strategies to respond rapidly and effectively to such emerging problems.
- Restoring seagrasses. By starting with fast-growing and tolerant pioneer species (e.g., Halodule wrightii) in nearshore areas, seagrass restoration will reduce the onshore undertow effect and avoid the resuspension of sediment between the rhizomes of the seagrass beds. Special precautions must be taken with these species, since they do not generate deeply-rooted root-rhizome mats and are, therefore, very easily removed by the action of waves or currents. Therefore, these efforts should be followed-up by enhancing the colonization of more robust and deeper-rooted seagrass species, such as Thalassia testudinum.
- Restoring corals. The first step is to reduce wave dissipation by increasing turbulence and bottom friction effects. These functions can be temporarily mimicked with artificial structures and other restoration techniques, such as coral species transplantation. The increased turbulence will generate more water oxygenation, improving colonization opportunities for back-reef species.
- Restoring dune vegetation. When the beach’s wave energy is less, the slope will be gentle, and the dune accretion process will return. It may be necessary to accelerate this process by nourishing the dune and the beach, recycling local sand from the sea bottom. To stabilize the dune, vegetation will be required. The aerial parts of the plants will control the wind erosion of the dune.
- Improving wastewater treatment. By mitigating the consequences of the poor wastewater system treatment, coral and seagrass degradation will be less. By restoring the groundwater conditions in the subsoil, mangrove species will be less likely to be replaced by cattail marsh.
- Altering infrastructure. Some infrastructure (e.g., buildings, roads) was designed with no thought for environmental sustainability. Relocating it landward may be the only way in which essential ecosystems can be restored. In other cases, infrastructure can be adapted so that it stands on stilts or pillars. The use of continuous foundations must be avoided so that superficial and sub-surface flows are not interrupted. Roads must have sufficient water passes, so that excess water can drain off and flow away, to ensure different water catchments remain connected. It is essential to guarantee mangrove health and its adaptation to new water level conditions.
- Reducing socioeconomic inequality. By encouraging other types of economic activities, the pressure on the coastal ecosystems will lessen, and economic vulnerability will become less acute due to the variability in the influx of tourists.
- Controlling migration into the area. The population of Puerto Morelos tripled from 2010 to 2015, from 10,000 to almost 30,000 inhabitants. Proper, long-term planning will allow the authorities to provide the services and infrastructure the inhabitants deserve.
- Implementing educational programs. The production of information about the ecosystems in Puerto Morelos, easily understandable and freely available, will strengthen the local community’s environmental responsibilities. This type of action will help temper the lack of socio-environmental awareness, respect for laws, and corruption at various levels of society and government.
- Monitoring the coastal unit. Continuous monitoring of the environment will produce reliable information that must be made public, reduce uncertainties, and ensure that appropriate actions are taken to adapt measures when necessary.
5. Discussion and Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
| Definition | Ecosystem/Perspective | Main Causes |
|---|---|---|
| Anthropogenic barriers prevent wetlands from migrating inland, and steep slopes bordering wetlands stall or completely halt wetland migration [61]. | Wetlands | Hard infrastructure |
| Related to coastal steepening or narrowing, the process whereby the cross-shore profile does not retreat or advance [62]. | Cross-shore profile | Sea level rise |
| Coastal habitats and natural features are progressively lost or drowned, caught between coastal defenses and rising sea levels [63]. | Coastal habitats | Sea level rise Hard infrastructure |
| The process where rising sea levels and other factors such as increased storminess push the coastal habitats landward [64,65]. | Coastal habitats | Sea level rise |
| Coastal habitats are progressively reduced in area and lose functionality when caught between a rising sea level and fixed sea defenses or high ground [66]. In this case, there is a loss of the intertidal area [67] and habitats [68]. However, coastal squeeze does not refer to losses due to natural processes [56]. In many estuarine environments, flood and coastal defenses constrain saltmarshes producing losses in intertidal habitat [21]. | Intertidal habitats | Sea level rise Hard infrastructure |
| Sea level rise coupled with shoreline armoring creates a “coastal squeeze” of habitat loss from both directions for many narrow beaches that no longer have an adjacent upland area for subsidence or retreat [69]. | Beach | Sea level rise Hard infrastructure |
| Drivers | Exchanges | State | |
|---|---|---|---|
| Mangrove | Wind Runoff (superficial and groundwater) Salt intrusion Nutrient and pollutant discharge Compaction Bio-modellers (roots, wood, crabs) | Flood control O2 production Organic matter and nutrient source Carbon sink Regulation of freshwater discharge Soil formation Soil retention Water table oscillations Wind intensity reduction | Land-locked mangrove forests dominated by red mangrove—Rhizophora mangle (high flood patterns and moderate salinity) White mangrove- Laguncularia racemosa (moderate flood patterns and low salinity) Black mangrove-Avicennia germinans (infrequent flood patterns and high salinity) Extensive sawgrass (Cladium jamaicense) patches combined with spikerush Eleocharis cellulose and cattail Typha domingensis. Main, paved, and dirt roads have fragmented the original mangrove ecosystem. Golf courses have replaced mangrove areas. |
| Dune | Wind Runoff—sub superficial Sea level Sediments Nutrients Salt intrusion Water table fluctuations Bio-modellers (roots, wood, crabs) | Carbon sink O2 production Organic matter and nutrient production Regulation of saline intrusion Sand stabilization Soil formation Water table oscillations Wind intensity reduction | Windward- Sporobolus virginicus, Sesuvium portulacastrum Dune crest—Croton punctatus, Tournefortia gnaphalodes, Suriana maritima, Scaevola plumerii, Chrysobalanus icaco Leeward—Thrinax radiata, Coccothrinax readii, Pseudophoenix sargentii, Caesalpinia vesicaria, Pithecellobium keyense, Bravaisia berlandieriana, Coccoloba uvifera, Cordia sebestena, Metopium brownei At the north and south of the coastal unit, the coastal dune has been replaced by tourist and housing infrastructure. |
| Beach | Wind Sea level Tides Waves Currents Sediments Bio-modellers (turtles, crabs) | Wave/current energy dissipation Sea turtle nesting Sediment transport | Healthy corals and vast seagrass meadows, and fine white sand (>0.3 mm) with a very gentle slope. Wave energy dissipates as it crosses the reef and seagrasses. The wind-generated waves in the reef lagoon are dissipated by a spilling break, with a small swash zone. The energy of the reflected waves is small. In some areas, beach erosion and instability have been reported. Around a dozen jetties and groynes are found along the beachfront of the unit. |
| Seagrasses | Tides Waves Currents Sediments Nutrients Pollutants Bio-modelers (turtles, fishes) | Sediment retention Sediment production Organic matter and nutrient production Exportation of mature fish Turbidity reduction O2 production Carbon sink | Extensive submarine meadows covering the bottom of the reef lagoon composed of Thalassia testudinum (robust climax species under stable conditions), Syringodium filiforme (dominates in areas with higher nutrient concentrations) and Halodule wrightii (dominates in disturbed areas), accompanied by calcareous sand-producing rhizophytic algae. Water quality in the areas is affected by the submarine springs containing wastewater. The Sargassum influxes also affects the process of photosynthesis. |
| Coral | Tides Waves Currents Sediments Nutrients Acidification Pollutants Bio-modelers (algae, sponges, polychaetes, urchins, fishes) | Wave/current energy dissipation Fish and invertebrate growth and production Organic matter and nutrient production Sediment production O2 production Carbon sink | Optimal development in highly hydrodynamic areas in warm (22–28° C), clear waters. There is a relatively large coral cover on the reef crest and a lesser cover of Acropora palmata in the shallower sector of the reef crest, while Orbicella spp. and Pseudodiploria sp. dominate the back reef. Other benthos includes sponges and calcareous and fleshy algae. The reef status is assessed through indicators such as the abundance of key organisms, density and abundance of reef-building corals, the abundance of fishes, and macroalgae abundance. A wide range of statuses (from healthy to stressed) has been reported. |
References
- Defeo, O.; McLachlan, A.; Schoeman, D.S.; Schlacher, T.A.; Dugan, J.; Jones, A.; Lastra, M.; Scapini, F. Threats to sandy beach ecosystems: A review. Estuar. Coast. Shelf Sci. 2009, 81, 1–12. [Google Scholar] [CrossRef]
- Duke, N.C.; Meynecke, J.O.; Dittmann, S.; Ellison, A.M.; Anger, K.; Berger, U.; Cannicci, S.; Diele, K.; Ewel, K.C.; Field, C.D.; et al. A World Without Mangroves? Science 2007, 317, 41. [Google Scholar] [CrossRef] [PubMed]
- Orth, R.J.; Carruthers, T.J.B.; Dennison, W.C.; Duarte, C.M.; Fourqurean, J.W.; Heck, K.L.; Hughes, A.R.; Kendrick, G.A.; Judson Kenworthy, W.; Olyarnik, S.; et al. A Global Crisis for Seagrass Ecosystems. BioScience 2006, 56, 987–996. [Google Scholar] [CrossRef]
- Pandolfi, J.M.; Bradbury, R.H.; Sala, E.; Hughes, T.P.; Bjorndal, K.A.; Cooke, R.G.; McArdle, D.; McClenachan, L.; Newman, M.J.H.; Paredes, G.; et al. Global Trajectories of the Long-Term Decline of Coral Reef Ecosystems. Science 2003, 301, 955. [Google Scholar] [CrossRef]
- Silva, R.; Martínez, M.L.; Hesp, P.A.; Catalan, P.; Osorio, A.F.; Martell, R.; Fossati, M.; Miot da Silva, G.; Mariño-Tapia, I.; Pereira, P.; et al. Present and future challenges of coastal erosion in Latin America. J. Coast. Res. 2014, 1–16. [Google Scholar] [CrossRef]
- Silva, R.; Chávez, V.; Bouma, T.J.; Van Tussenbroek, B.I.; Arkema, K.K.; Martínez, M.L.; Oumeraci, H.; Heymans, J.J.; Osorio, A.F.; Mendoza, E.; et al. The incorporation of biophysical and social components in coastal management. Estuaries Coasts 2019, 42, 1695–1708. [Google Scholar] [CrossRef]
- Bruun, P. Sea-level rise as a cause of shore erosion. J. Waterw. Harb. Div. 1962, 88, 117–132. [Google Scholar]
- Mendoza, E.; Silva, R.; Enriquez-Ortiz, C.; Mariño-Tapia, I.; Felix, A. Analysis of the hazards and vulnerability of the Cancun Beach System. In Extreme Events; Chavez, M., Ghil, M., Urrutia-Fucugauchi, J., Eds.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2015; pp. 125–136. [Google Scholar] [CrossRef]
- Reguero, B.G.; Secaira, F.; Toimil, A.; Escudero, M.; Díaz-Simal, P.; Beck, M.W.; Silva, R.; Storlazzi, C.; Losada, I.J. The Risk Reduction Benefits of the Mesoamerican Reef in Mexico. Front. Earth Sci. 2019, 7, 125. [Google Scholar] [CrossRef]
- Luijendijk, A.; Hagenaars, G.; Ranasinghe, R.; Baart, F.; Donchyts, G.; Aarninkhof, S. The State of the World’s Beaches. Sci. Rep. 2018, 8, 6641. [Google Scholar] [CrossRef]
- Vousdoukas, M.I.; Ranasinghe, R.; Mentaschi, L.; Plomaritis, T.A.; Athanasiou, P.; Luijendijk, A.; Feyen, L. Sandy coastlines under threat of erosion. Nat. Clim. Chang. 2020, 10, 260–263. [Google Scholar] [CrossRef]
- Esteves, L.; Foord, J.; Walters, G. CHAPTER 8. Assessment of natural resources use for sustainable development—DPSIR framework for case studies in Portsmouth and Thames Gateway, UK. In Environmental Stresses and Resource Use in Coastal Urban and Peri-Urban Regions: DPSIR Approach to SECOA’s 17 Case Studies; Lan, T.D., Olsson, G.A., Alpokay, S., Eds.; Sapienza Università Editrice: Rome, Italy, 2014; pp. 235–282. [Google Scholar]
- Smeets, E.; Weterings, R. Environmental Indicators: Typology and Overview; Tech. Rep. 25; European Environment Agency: Copenhagen, Denmark, 1999; Available online: http://reports.eea.eu.int:80/TEC25/en/tech_25_text.pdf; https://www.eea.europa.eu/publications/TEC25/download; (accessed on 17 December 2020). [Google Scholar]
- Patrício, J.; Elliott, M.; Mazik, K.; Papadopoulou, K.-N.; Smith, C.J. DPSIR—Two Decades of Trying to Develop a Unifying Framework for Marine Environmental Management? Front. Mar. Sci. 2016, 3, 177. [Google Scholar] [CrossRef]
- Escudero Castillo, M.; Mendoza Baldwin, E.; Silva Casarin, R.; Posada Vanegas, G.; Arganis Juaréz, M. Characterization of Risks in Coastal Zones: A Review. CLEAN Soil Air Water 2012, 40, 894–905. [Google Scholar] [CrossRef]
- Cowell, P.J.; Thom, B.G. Morphodynamics of coastal evolution. In Coastal Evolution: Late Quaternary Shoreline Morphodynamics; Woodroffe, C.D., Carter, R.W.G., Eds.; Cambridge University Press: Cambridge, UK, 1995; pp. 33–86. [Google Scholar] [CrossRef]
- Kraus, N.C.; Larson, M.; Kriebel, D. Evaluation of beach erosion and accretion predictors. In Proceedings of the Coastal Sediments ’91, Seattle, WA, USA, 25–27 June 1991; pp. 527–587. [Google Scholar]
- Odériz, I.; Silva, R.; Mortlock, T.R.; Mori, N. ENSO Impacts on Global Wave Climate and Potential Coastal Hazards. J. Geophys. Res. Oceans 2020, e2020JC016464. [Google Scholar] [CrossRef]
- Jones, C.G.; Lawton, J.H.; Shachak, M. Organisms as ecosystem engineers. In Ecosystem Management: Selected Readings; Samson, F.B., Knopf, F.L., Eds.; Springer: New York, NY, USA, 1996; pp. 130–147. [Google Scholar] [CrossRef]
- Bouma, T.J.; Olenin, S.; Reise, K.; Ysebaert, T. Ecosystem engineering and biodiversity in coastal sediments: Posing hypotheses. Helgol. Mar. Res. 2009, 63, 95–106. [Google Scholar] [CrossRef]
- English Nature. Coastal Squeeze, Saltmarsh Loss and Special Protection Areas; English Nature Research Report No. 710; Royal Haskoning for English Nature: Peterborough, UK, 2006; p. 55.
- Lithgow, D.; Martínez, M.L.; Gallego-Fernández, J.B.; Silva, R.; Ramírez-Vargas, D.L. Exploring the co-occurrence between coastal squeeze and coastal tourism in a changing climate and its consequences. Tour. Manag. 2019, 74, 43–54. [Google Scholar] [CrossRef]
- Martínez, M.L.; Mendoza-Gonzalez, G.; Silva, R.; Mendoza, E. Land use changes and sea level rise may induce a “coastal squeeze” on the coasts of Veracruz, Mexico. Glob. Environ. Chang. 2014, 29, 180–188. [Google Scholar] [CrossRef]
- Pontee, N.; Beck, M. Coastal Risk Reduction: Integrating Natural Defenses into a Sustainable Coastal Risk Management Framework, (September 2014), The Nature Conservancy 1–20. 2014. Available online: https://coastalresilience.org/integrating-natural-defenses-into-sustainable-coastal-risk-management-new-report/ (accessed on 17 December 2020).
- Saunders, M.I.; Leon, J.; Phinn, S.R.; Callaghan, D.P.; O’Brien, K.R.; Roelfsema, C.M.; Lovelock, C.E.; Lyons, M.B.; Mumby, P.J. Coastal retreat and improved water quality mitigate losses of seagrass from sea level rise. Glob. Chang. Biol. 2013, 19, 2569–2583. [Google Scholar] [CrossRef]
- Costanza, R.; De Groot, R.; Braat, L.; Kubiszewski, I.; Fioramonti, L.; Sutton, P.; Farber, S.; Grasso, M. Twenty years of ecosystem services: How far have we come and how far do we still need to go? Ecosyst. Serv. 2017, 28, 1–16. [Google Scholar] [CrossRef]
- Zanuttigh, B.; Nicholls, R.J.; Vanderlinden, J.-P.; Thompson, R.C.; Burcharth, H.F. Coastal Risk Management in a Changing Climate; Butterworth-Heinemann: Oxford, UK, 2014; p. 670. [Google Scholar] [CrossRef]
- Monteiro, R.; Ferreira, J.C. Green Infrastructure Planning as a Climate Change and Risk Adaptation Tool in Coastal Urban Areas. J. Coast. Res. 2020, 95, 889–893. [Google Scholar] [CrossRef]
- Ruckelshaus, M.H.; Guannel, G.; Arkema, K.; Verutes, G.; Griffin, R.; Guerry, A.; Silver, J.; Faries, J.; Brenner, J.; Rosenthal, A. Evaluating the Benefits of Green Infrastructure for Coastal Areas: Location, Location, Location. Coast. Manag. 2016, 44, 504–516. [Google Scholar] [CrossRef]
- Silva, R.; Lithgow, D.; Esteves, L.S.; Martínez, M.L.; Moreno-Casasola, P.; Martell, R.; Pereira, P.; Mendoza, E.; Campos-Cascaredo, A.; Winckler-Grez, P.; et al. Coastal risk mitigation by green infrastructure in Latin America. In Proceedings of the Institution of Civil Engineers-Maritime Engineering; Thomas Telford Ltd.: London, UK, 2017; Volume 170, pp. 39–54. [Google Scholar] [CrossRef]
- Barbier, E.B.; Koch, E.W.; Silliman, B.R.; Hacker, S.D.; Wolanski, E.; Primavera, J.; Granek, E.F.; Polasky, S.; Aswani, S.; Cramer, L.A.; et al. Coastal Ecosystem-Based Management with Nonlinear Ecological Functions and Values. Science 2008, 319, 321. [Google Scholar] [CrossRef] [PubMed]
- Temmerman, S.; Meire, P.; Bouma, T.J.; Herman, P.M.J.; Ysebaert, T.; De Vriend, H.J. Ecosystem-based coastal defence in the face of global change. Nature 2013, 504, 79–83. [Google Scholar] [CrossRef] [PubMed]
- Schoonees, T.; Gijón Mancheño, A.; Scheres, B.; Bouma, T.J.; Silva, R.; Schlurmann, T.; Schüttrumpf, H. Hard structures for coastal protection, towards greener designs. Estuaries Coasts 2019, 42, 1709–1729. [Google Scholar] [CrossRef]
- Cerdeira-Estrada, S.; Heege, t.; Kolb, M.; Ohlendorf, S.; Uribe, A.; Müller, A.; Garza, R.; Ressl, R.; Aguirre, R.; Mariño, I.; et al. Benthic habitat and bathymetry mapping of shallow waters in Puerto morelos reefs using remote sensing with a physics based data processing. In Proceedings of the 2012 IEEE International Geoscience and Remote Sensing Symposium, Munich, Germany, 22–27 July 2012; pp. 4383–4386. [Google Scholar]
- Cruz, C.J.; Mendoza, E.; Silva, R.; Chávez, V. Assessing Degrees of Anthropization on the Coast of Mexico from Ecosystem Conservation and Population Growth Data. J. Coast. Res. 2019, 92, 136–144. [Google Scholar] [CrossRef]
- Jordán-Dahlgren, E.; Rodríguez-Martínez, R.E. The Atlantic coral reefs of Mexico. In Latin American Coral Reefs; Cortés, J., Ed.; Elsevier Science: Amsterdam, The Netherlands, 2003; pp. 131–158. [Google Scholar] [CrossRef]
- Rodríguez-Martínez, R.E.; Ruíz-Rentería, F.; Van Tussenbroek, B.; Barba-Santos, G.; Escalante-Mancera, E.; Jordán-Garza, G.; Jordán-Dahlgren, E. Environmental state and tendencies of the Puerto Morelos CARICOMP site, Mexico. Rev. Biol. Trop. 2010, 58 (Suppl. 3), 23–43. [Google Scholar]
- Alcerreca, J.C.; Silva, R.; Mendoza, E. Simple settling velocity formula for calcareous sand. J. Hydraul. Res. 2013, 51, 215–219. [Google Scholar] [CrossRef]
- Ruiz de Alegria-Arzaburu, A.; Mariño-Tapia, I.; Enriquez, C.; Silva, R.; González-Leija, M. The role of fringing coral reefs on beach morphodynamics. Geomorphology 2013, 198, 69–83. [Google Scholar] [CrossRef]
- Coronado, C.; Candela, J.; Iglesias-Prieto, R.; Sheinbaum, J.; López, M.; Ocampo-Torres, F.J. On the circulation in the Puerto Morelos fringing reef lagoon. Coral Reefs 2007, 26, 149–163. [Google Scholar] [CrossRef]
- Silva, R.; Ruíz, G.; Posada, G.; Pérez, D.; Rivillas, G.; Espinal, J.; Mendoza, E. Atlas de Clima Marítimo de la Vertiente Atlántica Mexicana; Universidad Nacional Autónoma de México: Mexico City, Mexico, 2008. [Google Scholar]
- Mariño-Tapia, I.; Enriquez, C.; Silva, R.; Mendoza-Baldwin, E.; Escalante-Mancera, E.; Ruiz-Renteria, F. Comparative morphodynamics between exposed and reef protected beaches under hurrricane conditions. Coast. Eng. Proc. 2014, 1. [Google Scholar] [CrossRef]
- Miret-Villaseñor, D.; Enriquez, C.; Mariño-Tapia, I.; Silva, R.; Ruiz, G. Interactions between Nearshore and Shelf Dynamics under Hurricane Conditions: Implications for Exposed and Reef Protected Beaches. J. Coast. Res. 2019, 92, 55–67. [Google Scholar] [CrossRef]
- Silva, R.; Mendoza, E.; Mariño-Tapia, I.; Martínez, M.L.; Escalante, E. An artificial reef improves coastal protection and provides a base for coral recovery. J. Coast. Res. 2016, 467–471. [Google Scholar] [CrossRef]
- Van Tussenbroek, B.I. Dynamics of seagrasses and associated algae in coral reef lagoons. Hidrobiológica 2011, 21, 293–310. [Google Scholar]
- Chávez, V.; Uribe-Martínez, A.; Cuevas, E.; Rodríguez-Martínez, R.E.; Van Tussenbroek, B.I.; Francisco, V.; Estévez, M.; Celis, L.B.; Monroy-Velázquez, L.V.; Leal-Bautista, R.; et al. Massive Influx of Pelagic Sargassum spp. on the Coasts of the Mexican Caribbean 2014–2020: Challenges and Opportunities. Water 2020, 12, 2908. [Google Scholar] [CrossRef]
- Alvarez-Filip, L.; Estrada-Saldívar, N.; Pérez-Cervantes, E.; Molina-Hernández, A.; González-Barrios, F.J.J.P. A rapid spread of the stony coral tissue loss disease outbreak in the Mexican Caribbean. PeerJ 2019, 7, 341–345. [Google Scholar] [CrossRef]
- Osorio-Cano, J.D.; Alcérreca-Huerta, J.C.; Mariño-Tapia, I.; Osorio, A.F.; Acevedo-Ramírez, C.; Enriquez, C.; Costa, M.; Pereira, P.; Mendoza, E.; Escudero, M.; et al. Effects of Roughness Loss on Reef Hydrodynamics and Coastal Protection: Approaches in Latin America. Estuaries Coasts 2019, 42, 1742–1760. [Google Scholar] [CrossRef]
- Silva, R.; Borthwick, A.G.L.; Taylor, R.E. Numerical implementation of the harmonic modified mild-slope equation. Coast. Eng. 2005, 52, 391–407. [Google Scholar] [CrossRef]
- Martell, R.; Mendoza, E.; Mariño-Tapia, I.; Odériz, I.; Silva, R. How Effective Were the Beach Nourishments at CANCUN? J. Mar. Sci. Eng. 2020, 8, 388. [Google Scholar] [CrossRef]
- Kramer, P.; McField, M.; Álvarez-Filip, L.; Drysdale, I.; Rueda-Flores, M.; Giró, A.; Pott, R. 2015 Report Card for the Mesoamerican Reef. In Healthy Reefs Initiative; Okinawa, Japan. 2015, pp. 1118–1123. Available online: www.healthyreefs.org (accessed on 17 December 2020).
- Van Tussenbroek, B.I.; Hernández Arana, H.A.; Rodríguez-Martínez, R.E.; Espinoza-Avalos, J.; Canizales-Flores, H.M.; González-Godoy, C.E.; Barba-Santos, M.G.; Vega-Zepeda, A.; Collado-Vides, L. Severe impacts of brown tides caused by Sargassum spp. on near-shore Caribbean seagrass communities. Mar. Pollut. Bull. 2017, 122, 272–281. [Google Scholar] [CrossRef]
- James, R.K.; Silva, R.; Van Tussenbroek, B.I.; Escudero-Castillo, M.; Mariño-Tapia, I.; Dijkstra, H.A.; Van Westen, R.M.; Pietrzak, J.D.; Candy, A.S.; Katsman, C.A.; et al. Maintaining Tropical Beaches with Seagrass and Algae: A Promising Alternative to Engineering Solutions. BioScience 2019, 69, 136–142. [Google Scholar] [CrossRef]
- López-Portillo, J.; Lewis, R.R.; Saenger, P.; Rovai, A.; Koedam, N.; Dahdouh-Guebas, F.; Agraz-Hernández, C.; Rivera-Monroy, V.H. Mangrove forest restoration and rehabilitation. In Mangrove Ecosystems: A Global Biogeographic Perspective: Structure, Function, and Services; Rivera-Monroy, V.H., Lee, S.Y., Kristensen, E., Twilley, R.R., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 301–345. [Google Scholar] [CrossRef]
- Pérez-Ceballos, R.; Zaldívar-Jiménez, A.; Canales-Delgadillo, J.; López-Adame, H.; López-Portillo, J.; Merino-Ibarra, M. Determining hydrological flow paths to enhance restoration in impaired mangrove wetlands. PLoS ONE 2020, 15, e0227665. [Google Scholar] [CrossRef]
- Pontee, N. Defining coastal squeeze: A discussion. Ocean Coast. Manag. 2013, 84, 204–207. [Google Scholar] [CrossRef]
- Martínez, M.L.; Silva, R.; López-Portillo, J.; Feagin, R.A.; Martínez, E. Coastal Ecosystems as an Ecological Membrane. J. Coast. Res. 2020, 95, 97–101. [Google Scholar] [CrossRef]
- Maxwell, P.S.; Eklöf, J.S.; Van Katwijk, M.M.; O’Brien, K.R.; De la Torre-Castro, M.; Boström, C.; Bouma, T.J.; Krause-Jensen, D.; Unsworth, R.K.F.; Van Tussenbroek, B.I.; et al. The fundamental role of ecological feedback mechanisms for the adaptive management of seagrass ecosystems—A review. Biol. Rev. 2017, 92, 1521–1538. [Google Scholar] [CrossRef]
- Blanchon, P.; Eisenhauer, A.; Fietzke, J.; Liebetrau, V. Rapid sea-level rise and reef back-stepping at the close of the last interglacial highstand. Nature 2009, 458, 881–884. [Google Scholar] [CrossRef] [PubMed]
- Oumeraci, H.; Kortenhaus, A.; Burzel, A.; Naulin, M.; Dassanayake, D.R.; Jensen, J.; Wahl, T.; Mudersbach, C.; Gönnert, G.; Gerkensmeier, B.; et al. XtremRisK—Integrated flood risk analysis for extreme storm surges at open coasts and in estuaries: Methodology, key results and lessons learned. Coast. Eng. J. 2015, 57, 1–23. [Google Scholar] [CrossRef]
- Brinson, M.M.; Christian, R.R.; Blum, L.K. Multiple States in the Sea-Level Induced Transition from Terrestrial Forest to Estuary. Estuaries 1995, 18, 648–659. [Google Scholar] [CrossRef]
- Soulsby, R.L.; Sutherland, J.; Brampton, A.H. Coastal Steepening—The UK View; Report TR 91; HR Wallingford Ltd.: Wallingford, UK, 1999. [Google Scholar]
- DEFRA. Guidance Note on Managed Realignment: Land Purchase, Compensation and Payment for Alternative Beneficial Land Use; DEFRA: London, UK, 2003.
- Doody, J.P. ‘Coastal squeeze’—An historical perspective. J. Coast. Conserv. 2004, 10, 129–138. [Google Scholar] [CrossRef]
- Doody, J.P. Coastal squeeze and managed realignment in southeast England, does it tell us anything about the future? Ocean Coast. Manag. 2013, 79, 34–41. [Google Scholar] [CrossRef]
- English Nature. Conservation of Dynamic Coasts: A Framework for Managing Natura 2000. Living with the Sea LIFE Project; 1857167430; English Nature: Peterborough, UK, 2003.
- Black, V. Issue 2. Report produced for the Environment Agency, 30pp + appendices. August 2006. Coast. Squeeze Study 2006, 30. in press. [Google Scholar]
- Pontee, N. Reappraising coastal squeeze: A case study from north-west England. In Proceedings of the Institution of Civil Engineers-Maritime Engineering; Thomas Telford Ltd.: London, UK, 2011; Volume 164, pp. 127–138. [Google Scholar] [CrossRef]
- Martin, K.L. Coastal squeeze: New threats to beach-spawning fishes and their critical habitats. In Beach-Spawning Fishes: Reproduction in an Endangered Ecosystem; CRC Press: Boca Raton, FL, USA, 2014; pp. 157–174. [Google Scholar]
- Moreno-Casasola, P.; Espejel, I. Classification and ordination of coastal sand dune vegetation along the Gulf and Caribbean Sea of Mexico. Vegetatio 1986, 66, 147–182. [Google Scholar] [CrossRef]
- Ruíz-Rentería, F.; Van Tussenbroek, B.I.; Jordán-Dahlgren, E. Puerto Morelos, Quintana Roo, Mexico. CARICOMP site. In CARICOMP—Caribbean Coral Reef, Seagrass, and Mangrove Sites; Kjerfve, B., Ed.; UNESCO: Paris, France, 1998; pp. 57–66. [Google Scholar]
- Elizondo, N.C.; Barba-Macías, E.; Hernández-Arana, H.A.; Mendoza-Vega, J.; Tovilla-Hernández, C. Estudio Para la Caracterización y Diagnóstico de Humedales en Puerto Morelos; Ecosur, Conanp, Cinvestav, Seduma Yucatán, Onca Maya A.C.: Mexico City, Mexico, 2012; p. 108. [Google Scholar]
- Zapata-Ramírez, P.A.; Blanchon, P.; Olioso, A.; Hernandez-Nuñez, H.; Sobrino, J.A. Accuracy of IKONOS for mapping benthic coral-reef habitats: A case study from the Puerto Morelos Reef National Park, Mexico. Int. J. Remote Sens. 2013, 34, 3671–3687. [Google Scholar] [CrossRef]
- Franklin, G.L.; Torres-Freyermuth, A.; Medellin, G.; Allende-Arandia, M.E.; Appendini, C.M. The role of the reef–dune system in coastal protection in Puerto Morelos (Mexico). Nat. Hazards Earth Syst. Sci. 2018, 18, 1247–1260. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva, R.; Martínez, M.L.; van Tussenbroek, B.I.; Guzmán-Rodríguez, L.O.; Mendoza, E.; López-Portillo, J. A Framework to Manage Coastal Squeeze. Sustainability 2020, 12, 10610. https://doi.org/10.3390/su122410610
Silva R, Martínez ML, van Tussenbroek BI, Guzmán-Rodríguez LO, Mendoza E, López-Portillo J. A Framework to Manage Coastal Squeeze. Sustainability. 2020; 12(24):10610. https://doi.org/10.3390/su122410610
Chicago/Turabian StyleSilva, Rodolfo, María Luisa Martínez, Brigitta I. van Tussenbroek, Laura Odette Guzmán-Rodríguez, Edgar Mendoza, and Jorge López-Portillo. 2020. "A Framework to Manage Coastal Squeeze" Sustainability 12, no. 24: 10610. https://doi.org/10.3390/su122410610
APA StyleSilva, R., Martínez, M. L., van Tussenbroek, B. I., Guzmán-Rodríguez, L. O., Mendoza, E., & López-Portillo, J. (2020). A Framework to Manage Coastal Squeeze. Sustainability, 12(24), 10610. https://doi.org/10.3390/su122410610








