Handle with Care—Microplastic Particles in Intestine Samples of Seals from German Waters
Abstract
1. Introduction
2. Materials and Methods
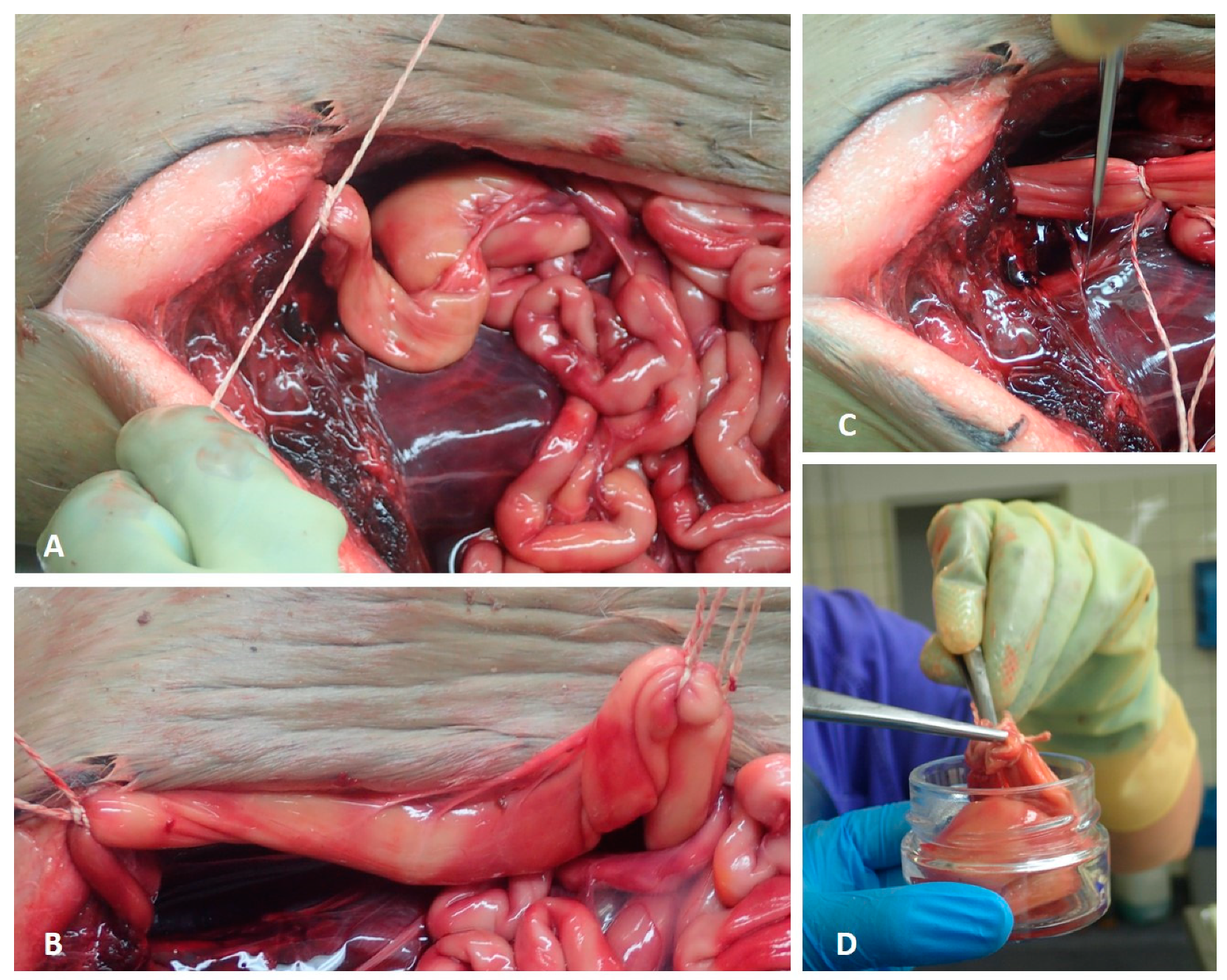
2.1. Sample Collection
2.1.1. Preparation
2.1.2. Washing Procedure
2.1.3. Isolation
2.1.4. Pre-Trials to Verify the Procedure
2.1.5. Polymer Identification
2.2. Evaluation of the Methodical Efficiency
3. Results
3.1. Evaluation of the Methodical Efficiency
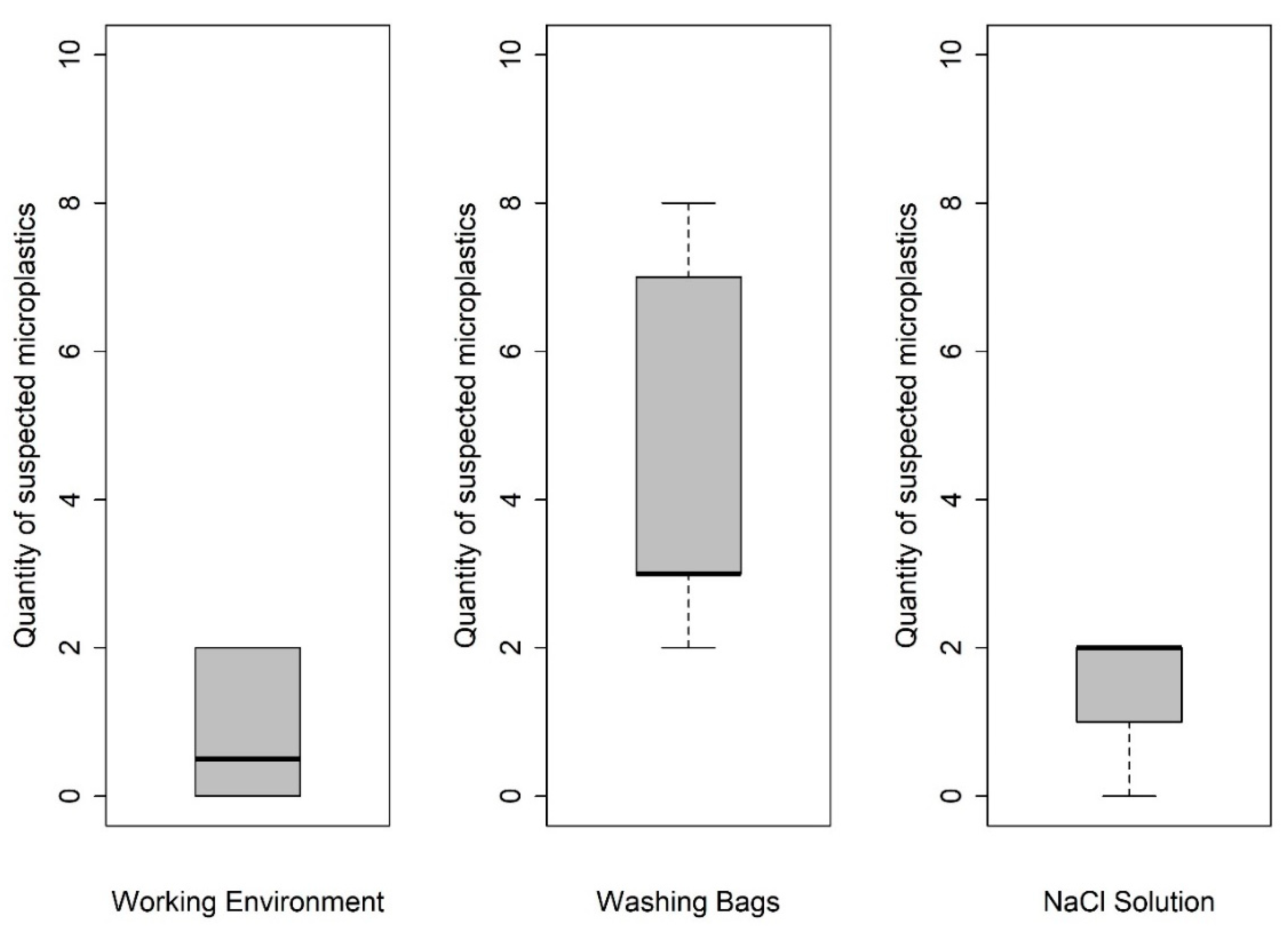
3.1.1. Procedural Efficiency of Washing Sachets
3.1.2. Efficiency of Contamination Control Measures
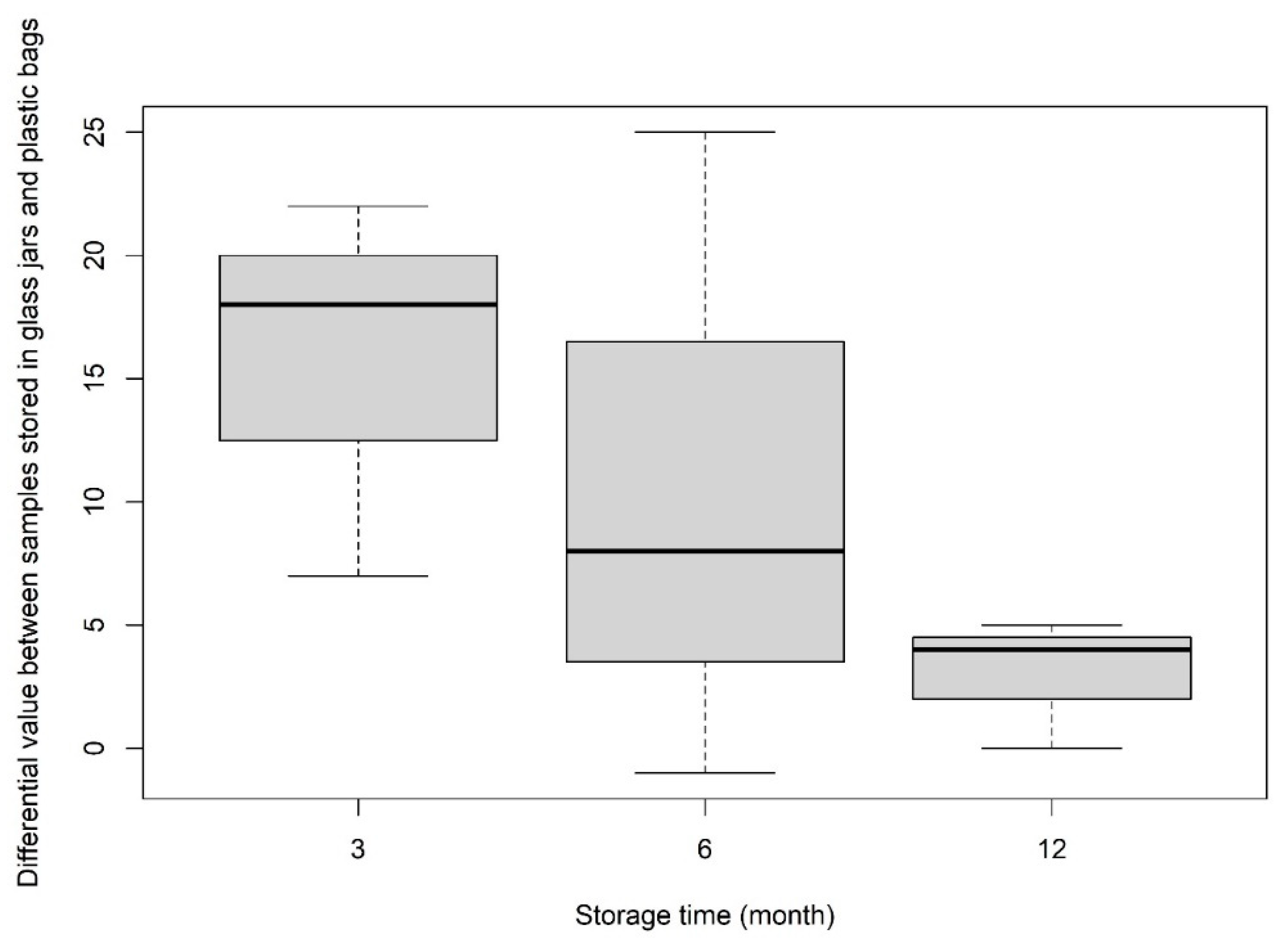
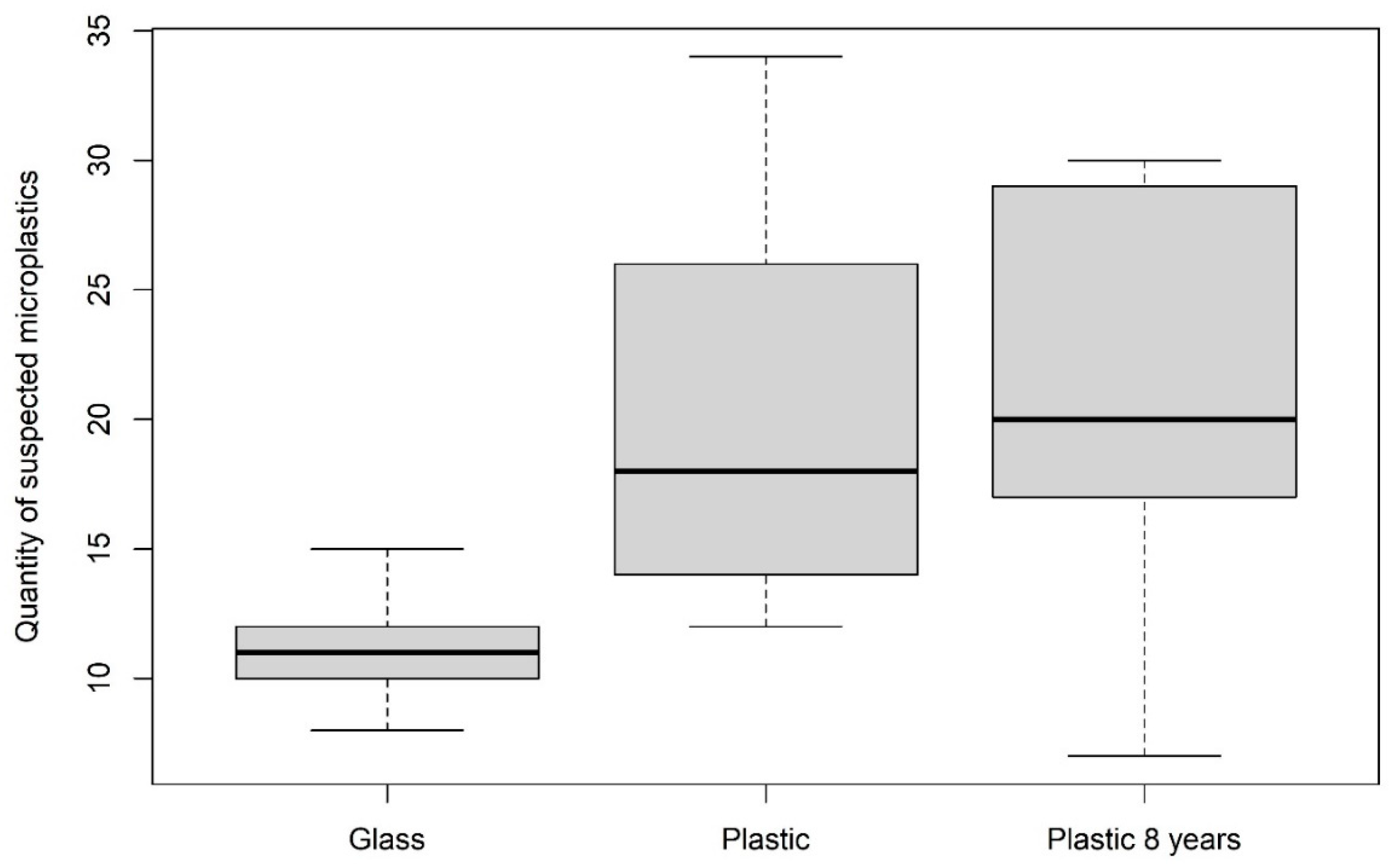
3.1.3. Comparison of Suitable Storage Methods for Avoiding Contamination
3.2. Protocol Validation
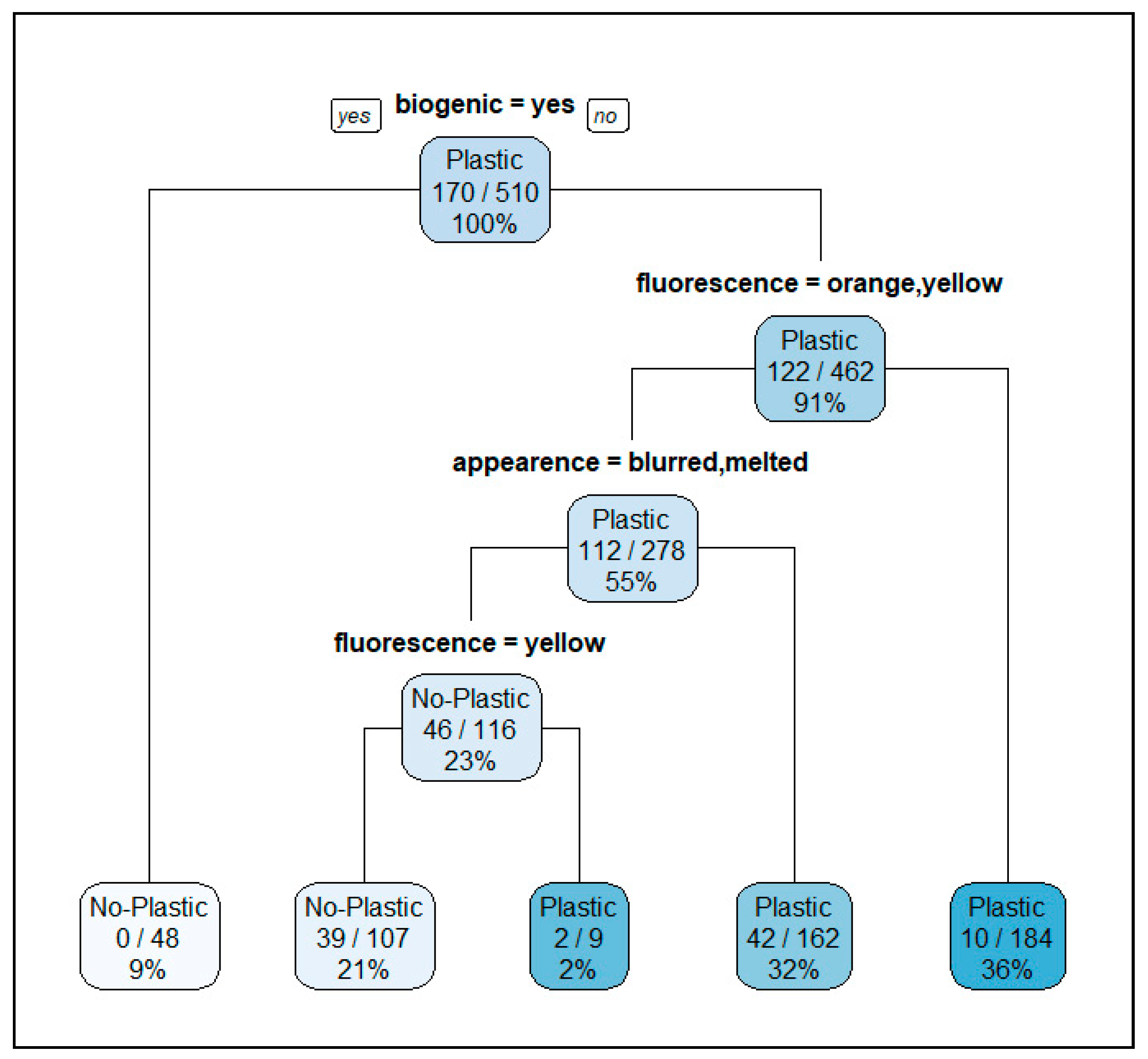
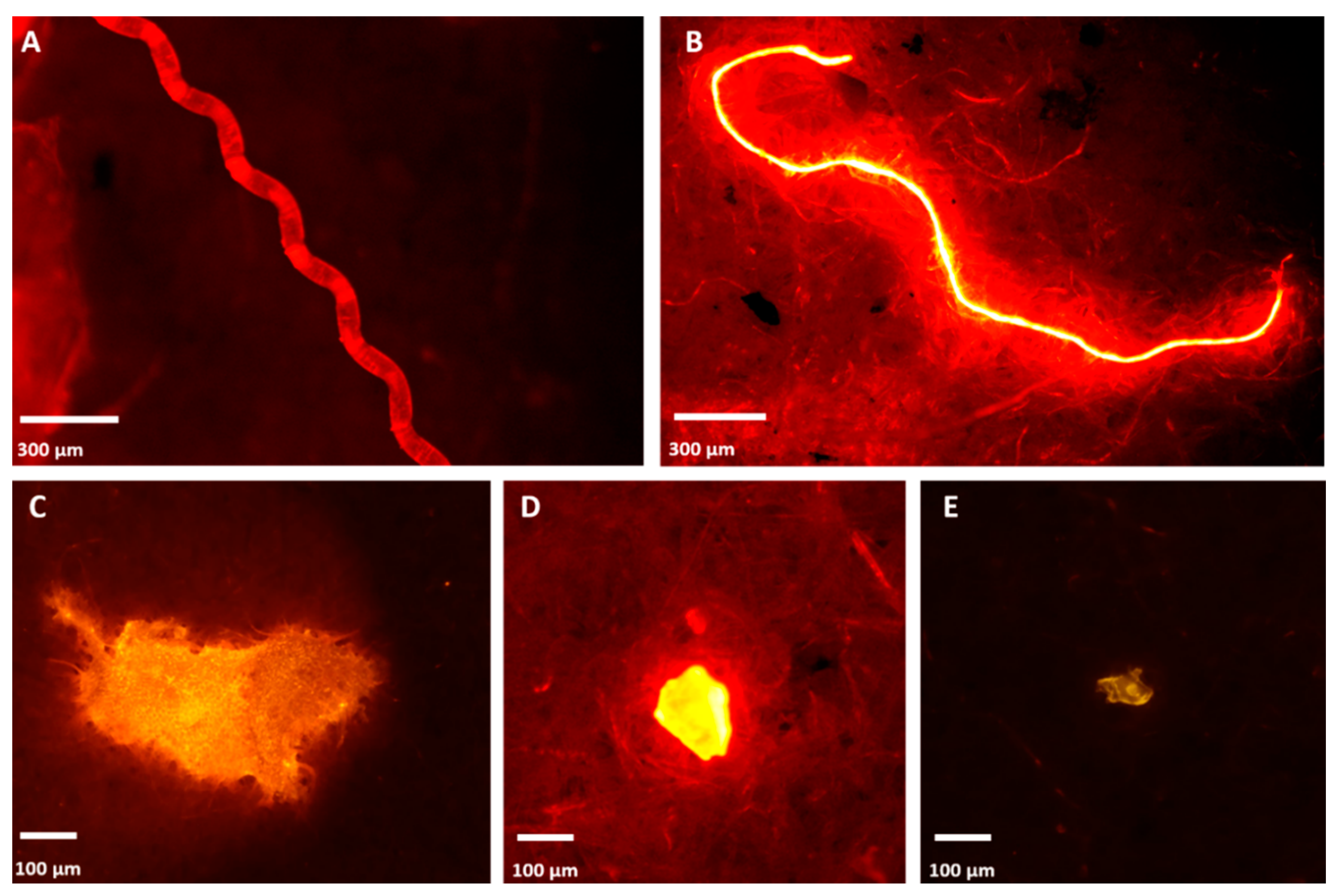
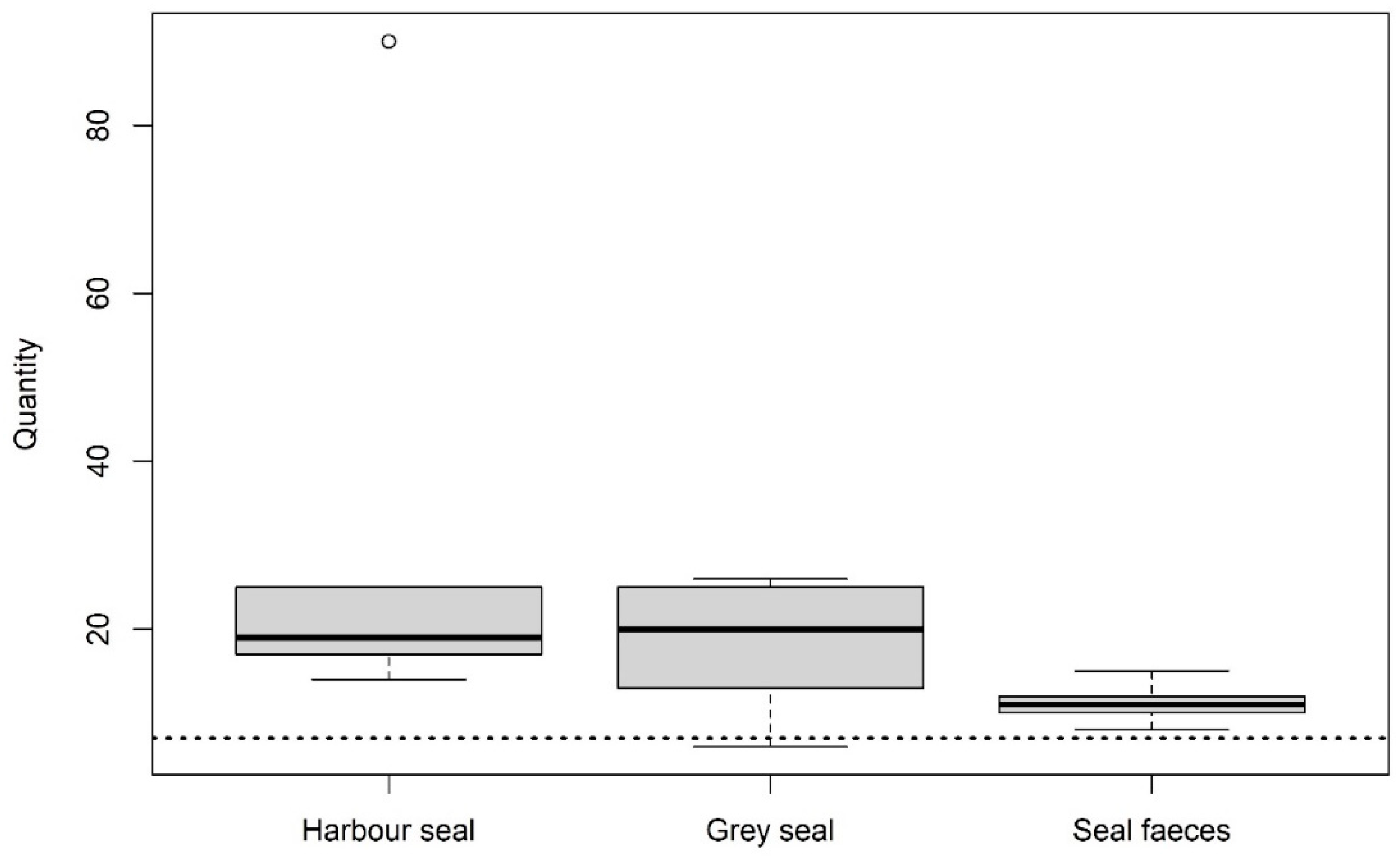
3.3. Isolation of Potential MP in Intestinal and Faecal Samples
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Galgani, F.; Hanke, G.; Maes, T. Global distribution, composition and abundance of marine litter. In Marine Anthropogenic Litter; Bergmann, M., Gutow, L., Klages, M., Eds.; Springer: Cham, Switzerland, 2015; pp. 29–56. [Google Scholar] [CrossRef]
- Thompson, R.C.; Olsen, Y.; Mitchell, R.P.; Davis, A.; Rowland, S.J.; John, A.W.G.; McGonigle, D.; Russell, A.E. Lost at Sea: Where is all the plastic? Science 2004, 304, 838. [Google Scholar] [CrossRef] [PubMed]
- Thiel, M.; Hinojosa, I.A.; Miranda, L.; Pantoja, J.F.; Rivadeneira, M.M.; Vásquez, N. Anthropogenic marine debris in the coastal environment: A multi-year comparison between coastal waters and local shores. Mar. Pollut. Bull. 2013, 71, 307–316. [Google Scholar] [CrossRef] [PubMed]
- Ryan, P.G.; Moore, C.J.; van Franeker, J.A.; Moloney, C.L. Monitoring the abundance of plastic debris in the marine environment. Philos. Trans. R. Soc. B Biol. Sci. 2009, 364, 1999–2012. [Google Scholar] [CrossRef] [PubMed]
- Arthur, C.; Baker, J.; Bamford, H. NOAA technical memorandum NOS-OR&R-30. In Proceedings of the International Research Workshop on the Occurrence, Effects, and Fate of Microplastic Marine Debris, Tacoma, WA, USA, 9–11 September 2008; Available online: https://repository.library.noaa.gov/view/noaa/2509 (accessed on 13 December 2020).
- Gregory, M.R.; Andrady, A.L. Plastics in the marine environment. In Plastics and the Environment; Andrady, A.L., Ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2003; pp. 389–390. [Google Scholar]
- Andrady, A.L. Persistence of plastic litter in the oceans. In Marine anthropogenic Litter; Bergmann, M., Gutow, L., Klages, M., Eds.; Springer: Cham, Switzerland, 2015; pp. 57–72. [Google Scholar] [CrossRef]
- Browne, M.A.; Galloway, T.; Thompson, R. Microplastic-an emerging contaminant of potential concern? Integr. Environ. Assess. Manag. 2007, 3, 559–561. [Google Scholar] [CrossRef]
- Cole, M.; Lindeque, P.; Halsband, C.; Galloway, T.S. Microplastics as contaminants in the marine environment: A review. Mar. Pollut. Bull. 2011, 62, 2588–2597. [Google Scholar] [CrossRef]
- Carpenter, E.J.; Anderson, S.J.; Harvey, G.R.; Miklas, H.P.; Peck, B.B. Polystyrene spherules in coastal waters. Science 1972, 178, 749–750. [Google Scholar] [CrossRef]
- Morris, A.W.; Hamilton, E.I. Polystyrene spherules in the Bristol channel. Mar. Pollut. Bull. 1974, 5, 26–27. [Google Scholar] [CrossRef]
- Hermsen, E.; Mintenig, S.M.; Besseling, E.; Koelmans, A.A. Quality criteria for the analysis of microplastic in biota samples: A critical review. Environ. Sci. Technol. 2018, 52, 10230–10240. [Google Scholar] [CrossRef]
- Zhang, S.; Wang, J.; Liu, X.; Qu, F.; Wang, X.; Wang, X.; Li, Y.; Sun, Y. Microplastics in the environment: A review of analytical methods, distribution, and biological effects. TrAC Trends Anal. Chem. 2019, 111, 62–72. [Google Scholar] [CrossRef]
- Farrell, P.; Nelson, K. Trophic level transfer of microplastic: Mytilus Edulis (L.) to Carcinus Maenas (L.). Environ. Pollut. 2013, 177, 1–3. [Google Scholar] [CrossRef]
- Rummel, C.D.; Löder, M.G.J.; Fricke, N.F.; Lang, T.; Griebeler, E.-M.; Janke, M.; Gerdts, G. Plastic ingestion by pelagic and demersal fish from the North sea and Baltic sea. Mar. Pollut. Bull. 2016, 102, 134–141. [Google Scholar] [CrossRef] [PubMed]
- Setälä, O.; Fleming-Lehtinen, V.; Lehtiniemi, M. Ingestion and transfer of microplastics in the planktonic food web. Environ. Pollut. 2014, 185, 77–83. [Google Scholar] [CrossRef] [PubMed]
- Rebolledo, B.E.L.; Van Franeker, J.A.; Jansen, O.E.; Brasseur, S.M.J.M. Plastic ingestion by harbour seals (Phoca Vitulina) in The Netherlands. Mar. Pollut. Bull. 2013, 67, 200–202. [Google Scholar] [CrossRef] [PubMed]
- Unger, B.; Rebolledo, E.L.B.; Deaville, R.; Gröne, A.; IJsseldijk, L.L.; Leopold, M.F.; Siebert, U.; Spitz, J.; Wohlsein, P.; Herr, H. Large amounts of marine debris found in sperm whales stranded along the North sea coast in early 2016. Mar. Pollut. Bull. 2016, 112, 134–141. [Google Scholar] [CrossRef] [PubMed]
- Unger, B.; Herr, H.; Benke, H.; Böhmert, M.; Burkhardt-Holm, P.; Dähne, M.; Hillmann, M.; Wolff-Schmidt, K.; Wohlsein, P.; Siebert, U. Marine debris in harbour porpoises and seals from German waters. Mar. Environ. Res. 2017, 130, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Nelms, S.E.; Barnett, J.; Brownlow, A.; Davison, N.J.; Deaville, R.; Galloway, T.S.; Lindeque, P.K.; Santillo, D.; Godley, B.J. Microplastics in marine mammals stranded around the British coast: Ubiquitous but transitory? Sci. Rep. 2019, 9, 1075. [Google Scholar] [CrossRef]
- Donohue, M.J.; Masura, J.; Gelatt, T.; Ream, R.; Baker, J.D.; Faulhaber, K.; Lerner, D.T. Evaluating exposure of northern fur seals, callorhinus ursinus, to microplastic pollution through fecal analysis. Mar. Pollut. Bull. 2019, 138, 213–221. [Google Scholar] [CrossRef]
- Löder, M.G.J.; Gerdts, G. Methodology used for the detection and identification of microplastics—A critical appraisal. In Marine Anthropogenic Litter; Bergmann, M., Gutow, L., Klages, M., Eds.; Springer International Publishing: Berlin/Heidelberg, Germany, 2015; pp. 201–227. [Google Scholar] [CrossRef]
- Cole, M.; Webb, H.; Lindeque, P.K.; Fileman, E.S.; Halsband, C.; Galloway, T.S. Isolation of microplastics in biota-rich seawater samples and marine organisms. Sci. Rep. 2014, 4, 4528. [Google Scholar] [CrossRef]
- Woodall, L.C.; Sanchez-Vidal, A.; Canals, M.; Paterson, G.L.J.; Coppock, R.; Sleight, V.; Calafat, A.; Rogers, A.D.; Narayanaswamy, B.E.; Thompson, R.C. The deep sea is a major sink for microplastic debris. R. Soc. Open Sci. 2014, 1, 140317. [Google Scholar] [CrossRef]
- Hidalgo-Ruz, V.; Gutow, L.; Thompson, R.C.; Thiel, M. Microplastics in the marine environment: A review of the methods used for identification and quantification. Environ. Sci. Technol. 2012, 46, 3060–3075. [Google Scholar] [CrossRef]
- Foekema, E.M.; De Gruijter, C.; Mergia, M.T.; van Franeker, J.A.; Murk, A.J.; Koelmans, A.A. Plastic in North sea fish. Environ. Sci. Technol. 2013, 47, 8818–8824. [Google Scholar] [CrossRef] [PubMed]
- Roux, C.; Huttunen, J.; Rampling, K.; Robertson, J. Factors affecting the potential for fibre contamination in purpose-designed forensic search rooms. Sci. Justice 2001, 41, 135–144. [Google Scholar] [CrossRef][Green Version]
- Vandermeersch, G.; Van Cauwenberghe, L.; Janssen, C.R.; Marques, A.; Granby, K.; Fait, G.; Kotterman, M.J.J.; Diogène, J.; Bekaert, K.; Robbens, J.; et al. A critical view on microplastic quantification in aquatic organisms. Environ. Res. 2015, 143, 46–55. [Google Scholar] [CrossRef] [PubMed]
- Lusher, A.; Hernandez-Milian, G. Microplastic extraction from marine vertebrate digestive tracts, regurgitates and scats: A protocol for researchers from all experience levels. Bio Protoc. 2018, 8. [Google Scholar] [CrossRef]
- Catarino, A.I.; Thompson, R.; Sanderson, W.; Henry, T.B. Development and optimization of a standard method for extraction of microplastics in mussels by enzyme digestion of soft tissues. Environ. Toxicol. Chem. 2017, 36, 947–951. [Google Scholar] [CrossRef]
- Claessens, M.; Van Cauwenberghe, L.; Vandegehuchte, M.B.; Janssen, C.R. New Techniques for the detection of microplastics in sediments and field collected organisms. Mar. Pollut. Bull. 2013, 70, 227–233. [Google Scholar] [CrossRef]
- Tamminga, M.; Hengstmann, E.; Fischer, E.K. Nile red staining as a subsidiary method for microplastic quantification: A comparison of three solvents and factors influencing application reliability. SDRP J. Earth Sci. Environ. Stud. 2017, 2. [Google Scholar] [CrossRef]
- Fischer, E. Distribution of Microplastics in Marine Species of the Wadden Sea along the Coastline of Schleswig-Holstein, Germany; Hamburg University: Hamburg, Germany, 2019. [Google Scholar]
- Mintenig, S.M.; Int-Veen, I.; Löder, M.G.J.; Primpke, S.; Gerdts, G. Identification of microplastic in effluents of waste water treatment plants using focal plane array-based micro-fourier-transform infrared imaging. Water Res. 2017, 108, 365–372. [Google Scholar] [CrossRef]
- Primpke, S.; Lorenz, C.; Rascher-Friesenhausen, R.; Gerdts, G. An automated approach for microplastics analysis using focal plane array (FPA) FTIR microscopy and image analysis. Anal. Methods 2017, 9, 1499–1511. [Google Scholar] [CrossRef]
- Jung, M.R.; Horgen, F.D.; Orski, S.V.; Rodriguez, V.; Beers, K.L.; Balazs, G.H.; Jones, T.T.; Work, T.M.; Brignac, K.C.; Royer, S.J.; et al. Validation of ATR FT-IR to identify polymers of plastic marine debris, including those ingested by marine organisms. Mar. Pollut. Bull. 2018, 127, 704–716. [Google Scholar] [CrossRef]
- Lenz, R.; Enders, K.; Stedmon, C.A.; Mackenzie, D.M.A.; Nielsen, T.G. A critical assessment of visual identification of marine microplastic using raman spectroscopy for analysis improvement. Mar. Pollut. Bull. 2015, 100, 82–91. [Google Scholar] [CrossRef] [PubMed]
- Karbalaei, S.; Golieskardi, A.; Hamzah, H.B.; Abdulwahid, S.; Hanachi, P.; Walker, T.R.; Karami, A. abundance and characteristics of microplastics in commercial marine fish from Malaysia. Mar. Pollut. Bull. 2019, 148, 5–15. [Google Scholar] [CrossRef] [PubMed]
- Shim, W.J.; Hong, S.H.; Eo, S.E. Identification methods in microplastic analysis: A review. Anal. Methods 2017, 9, 1384–1391. [Google Scholar] [CrossRef]
- Siebert, U.; Wünschmann, A.; Weiss, R.; Frank, H.; Benke, H.; Frese, K. Post-mortem findings in Harbour porpoises (Phocoena Phocoena) from the German North and Baltic Seas. J. Comp. Pathol. 2001, 124, 102–114. [Google Scholar] [CrossRef] [PubMed]
- Siebert, U.; Wohlsein, P.; Lehnert, K.; Baumgärtner, W. Pathological findings in harbour seals (Phoca Vitulina): 1996–2005. J. Comp. Pathol. 2007, 137, 47–58. [Google Scholar] [CrossRef] [PubMed]
- Hasselmeier, I.; Fonfara, S.; Driver, J.; Siebert, U. Differential hematology profiles of free-ranging, rehabilitated, and captive harbor seals (Phoca Vitulina) of the German North Sea. Aquat. Mamm. 2008, 34, 149–156. [Google Scholar] [CrossRef]
- Tamminga, M.; Stoewer, S.-C.; Fischer, E.K. On the representativeness of pump water samples versus manta sampling in microplastic analysis. Environ Pollut. 2019, 254. [Google Scholar] [CrossRef]
- Therneau, T.; Atkinson, B.; Ripley, B. Rpart: Recursive Partitioning and Regression Trees. R Packageversion 4.1-15. 2019. Available online: https://cran.r-project.org/package=rpart (accessed on 18 August 2020).
- Hengstmann, E.; Tamminga, M.; vom Bruch, C.; Fischer, E.K. Microplastic in beach sediments of the isle of rügen (Baltic Sea)—Implementing a novel glass elutriation column. Mar. Pollut. Bull. 2018, 126, 263–274. [Google Scholar] [CrossRef]
- Nuelle, M.-T.; Dekiff, J.H.; Remy, D.; Fries, E. A new analytical approach for monitoring microplastics in marine sediments. Environ. Pollut. 2014, 184, 161–169. [Google Scholar] [CrossRef]
- Torre, M.; Digka, N.; Anastasopoulou, A.; Tsangaris, C.; Mytilineou, C. Anthropogenic microfibres pollution in marine biota. A new and simple methodology to minimize airborne contamination. Mar. Pollut. Bull. 2016, 113, 55–61. [Google Scholar] [CrossRef]
- Lusher, A.L.; Hernandez-Milian, G.; Berrow, S.; Rogan, E.; O’Conner, I. Incidence of marine debris in cetaceans stranded and bycaught in Ireland: Recent findings and a review of histological knowledge. Environ. Pollut. 2018, 467–476. [Google Scholar] [CrossRef] [PubMed]
- Nelms, S.E.; Galloway, T.S.; Godley, B.J.; Jarvis, D.S.; Lindeque, P.K. Investigating microplastic trophic transfer in marine top predators. Environ. Pollut. 2018, 238, 999–1007. [Google Scholar] [CrossRef] [PubMed]
- Lusher, A.L.; McHugh, M.; Thompson, R.C. Occurrence of microplastics in the gastrointestinal tract of pelagic and demersal fish from the English channel. Mar. Pollut. Bull. 2013, 67, 94–99. [Google Scholar] [CrossRef] [PubMed]
- Steer, M.; Cole, M.; Thompson, R.C.; Lindeque, P.K. Microplastic ingestion in fish larvae in the western English channel. Environ. Pollut. 2017, 226, 250–259. [Google Scholar] [CrossRef] [PubMed]
- Lusher, A.L.; Welden, N.A.; Sobral, P.; Cole, M. Sampling, isolating and identifying microplastics ingested by fish and invertebrates. Anal. Methods 2017, 9, 1346–1360. [Google Scholar] [CrossRef]
- Orr, A.J.; Laake, J.L.; Dhruv, M.I.; Banks, A.S.; DeLong, R.L.; Huber, H.R. Comparison of processing pinniped scat samples using a washing machine and nested sieves. Wildl. Soc. Bull. 2003, 31, 253–257. [Google Scholar]
- Ter Halle, A.; Ladirat, L.; Martignac, M.; Mingotaud, A.F.; Boyron, O.; Perez, E. To what extent are microplastics from the open ocean weathered? Environ. Pollut. 2017, 227, 167–174. [Google Scholar] [CrossRef]
- Andrady, A.L. Microplastics in the marine environment. Mar. Pollut. Bull. 2011, 62, 1596–1605. [Google Scholar] [CrossRef]
- Lang, P.L.; Katon, J.E.; O’Keefe, J.F.; Schiering, D.W. The identification of fibers by infrared and raman microspectroscopy. Microchem. J. 1986, 34, 319–331. [Google Scholar] [CrossRef]
- Cabernard, L.; Roscher, L.; Lorenz, C.; Gerdts, G.; Primpke, S. Comparison of raman and fourier transform infrared spectroscopy for the quantification of microplastics in the aquatic environment. Environ. Sci. Technol. 2018, 52, 13279–13288. [Google Scholar] [CrossRef]
- Helm, R.C. Intestinal length of three California pinniped species. J. Zool. 1983, 199, 297–304. [Google Scholar] [CrossRef]
- Pabst, D.A.; Rommel, S.A.; Mclellan, W.A. The functional morphology of marine mammals. In Biology of Marine Mammals; Reynolds, J.E., Rommel, S.A., Eds.; Smithsonian Institution Press: Washington, DC, USA, 1999; pp. 15–72. [Google Scholar]
- Panti, C.; Baini, M.; Lusher, A.; Hernandez-Milan, G.; Bravo Rebolledo, E.L.; Unger, B.; Syberg, K.; Simmonds, M.P.; Fossi, M.C. Marine litter: One of the major threats for marine mammals. Outcomes from the European cetacean society workshop. Environ. Pollut. 2019, 247, 72–79. [Google Scholar] [CrossRef] [PubMed]
- Harvey, J.T. Assessment of errors associated with harbour seal (Phoca Vitulina) faecal sampling. J. Zool. 1989, 219, 101–111. [Google Scholar] [CrossRef]
- Prime, J.H.; Hammond, P.S. Quantitative assessment of gray seal diet from fecal analysis. In Approaches in Marine Mammal Energetics; The Society for Marine Mammalogy Special Publication: Yarmouth Port, MA, USA, 1987; pp. 165–188. [Google Scholar]
- Prime, J.H.; Hammond, P.S. The diet of grey seals from the south-western north sea assessed from analyses of hard parts found in faeces. J. Appl. Ecol. 1990, 27, 435–447. [Google Scholar] [CrossRef]
- Lusher, A.L.; Hernandez-Milian, G.; O’Brien, J.; Berrow, S.; O’Connor, I.; Officer, R. Microplastic and macroplastic ingestion by a deep diving, oceanic cetacean: The true’s beaked whale mesoplodon mirus. Environ. Pollut. 2015, 199, 185–191. [Google Scholar] [CrossRef]
- Perez-Venegas, D.J.; Seguel, M.; Pavés, H.; Pulgar, J.; Urbina, M.; Ahrendt, C.; Galbán-Malagón, C. First detection of plastic microfibers in a wild population of South American fur seals (Arctocephalus Australis) in the Chilean northern Patagonia. Mar. Pollut. Bull. 2018, 136, 50–54. [Google Scholar] [CrossRef]
- Hernandez-Milian, G.; Lusher, A.; MacGabban, S.; Rogan, E. Microplastics in grey seal (Halichoerus Grypus) intestines: Are they associated with parasite aggregations? Mar. Pollut. Bull. 2019, 146, 349–354. [Google Scholar] [CrossRef]
- Robinson, H.; Thayer, J.; Sydeman, W.J.; Weise, M. Changes in California sea lion diet during a period of substantial climate variability. Mar. Biol. 2018, 165, 169. [Google Scholar] [CrossRef]
- Ory, N.C.; Gallardo, C.; Lenz, M.; Thiel, M. Capture, swallowing, and egestion of microplastics by a planktivorous juvenile fish. Environ. Pollut. 2018, 240, 566–573. [Google Scholar] [CrossRef]
- Rani, M.; Shim, W.J.; Han, G.M.; Jang, M.; Al-Odaini, N.A.; Song, Y.K.; Hong, S.H. Qualitative analysis of additives in plastic marine debris and its new products. Arch. Environ. Contam. Toxicol. 2015, 69, 352–366. [Google Scholar] [CrossRef]
- Siebert, U.; Heidmann, A.; Friedhoff, N.; Kruse, H.; Rigét, F.; Adler, S.; Maser, E. Organochlorine burdens in harbour seals from the German Wadden Sea collected during two phocine distemper epizootics and ringed seals from west Greenland waters. J. Environ. Anal. Toxicol. 2012, 2, 126. [Google Scholar] [CrossRef]
- Ross, P.; Ellis, G.; Ikonomou, M.; Barrett-Lennard, L.; Addison, R. High PCB concentrations in free-ranging pacific killer whales, orcinus orca: Effects of age, sex and dietary preference. Mar. Pollut. Bull. 2000, 40, 504–515. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Philipp, C.; Unger, B.; Fischer, E.K.; Schnitzler, J.G.; Siebert, U. Handle with Care—Microplastic Particles in Intestine Samples of Seals from German Waters. Sustainability 2020, 12, 10424. https://doi.org/10.3390/su122410424
Philipp C, Unger B, Fischer EK, Schnitzler JG, Siebert U. Handle with Care—Microplastic Particles in Intestine Samples of Seals from German Waters. Sustainability. 2020; 12(24):10424. https://doi.org/10.3390/su122410424
Chicago/Turabian StylePhilipp, Carolin, Bianca Unger, Elke K. Fischer, Joseph G. Schnitzler, and Ursula Siebert. 2020. "Handle with Care—Microplastic Particles in Intestine Samples of Seals from German Waters" Sustainability 12, no. 24: 10424. https://doi.org/10.3390/su122410424
APA StylePhilipp, C., Unger, B., Fischer, E. K., Schnitzler, J. G., & Siebert, U. (2020). Handle with Care—Microplastic Particles in Intestine Samples of Seals from German Waters. Sustainability, 12(24), 10424. https://doi.org/10.3390/su122410424





