Thermodynamic Analysis and Comparison of Two Small-Scale Solar Electrical Power Generation Systems
Abstract
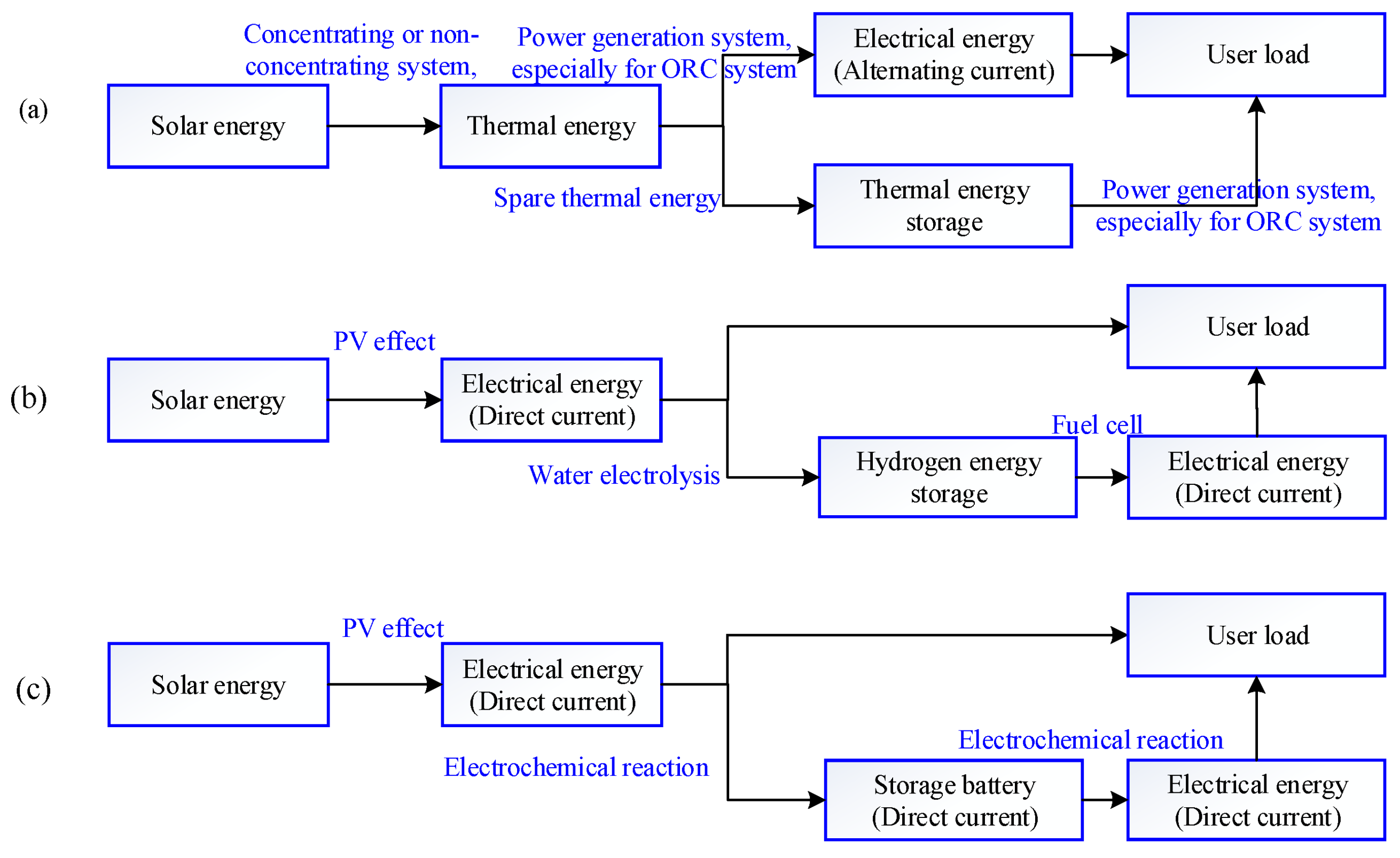
1. Introduction
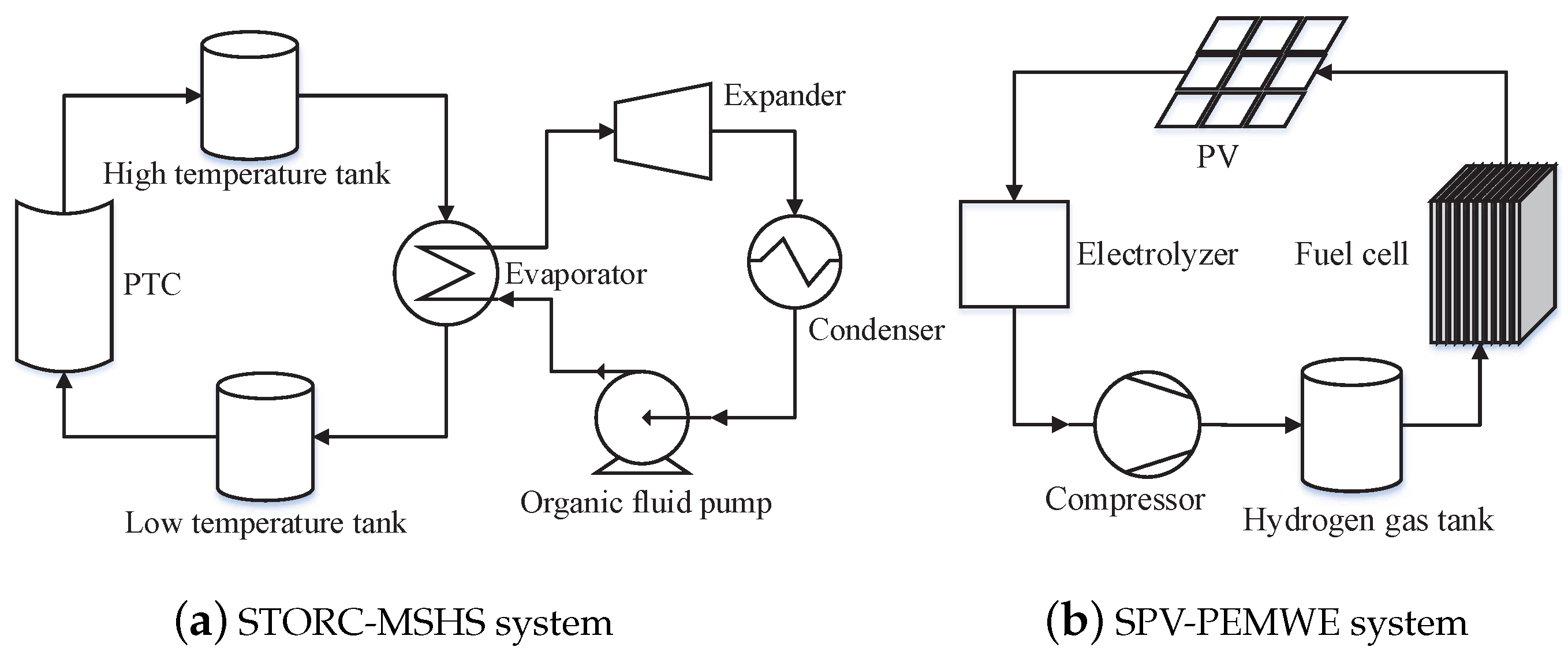
2. System Description
3. Model Establishing
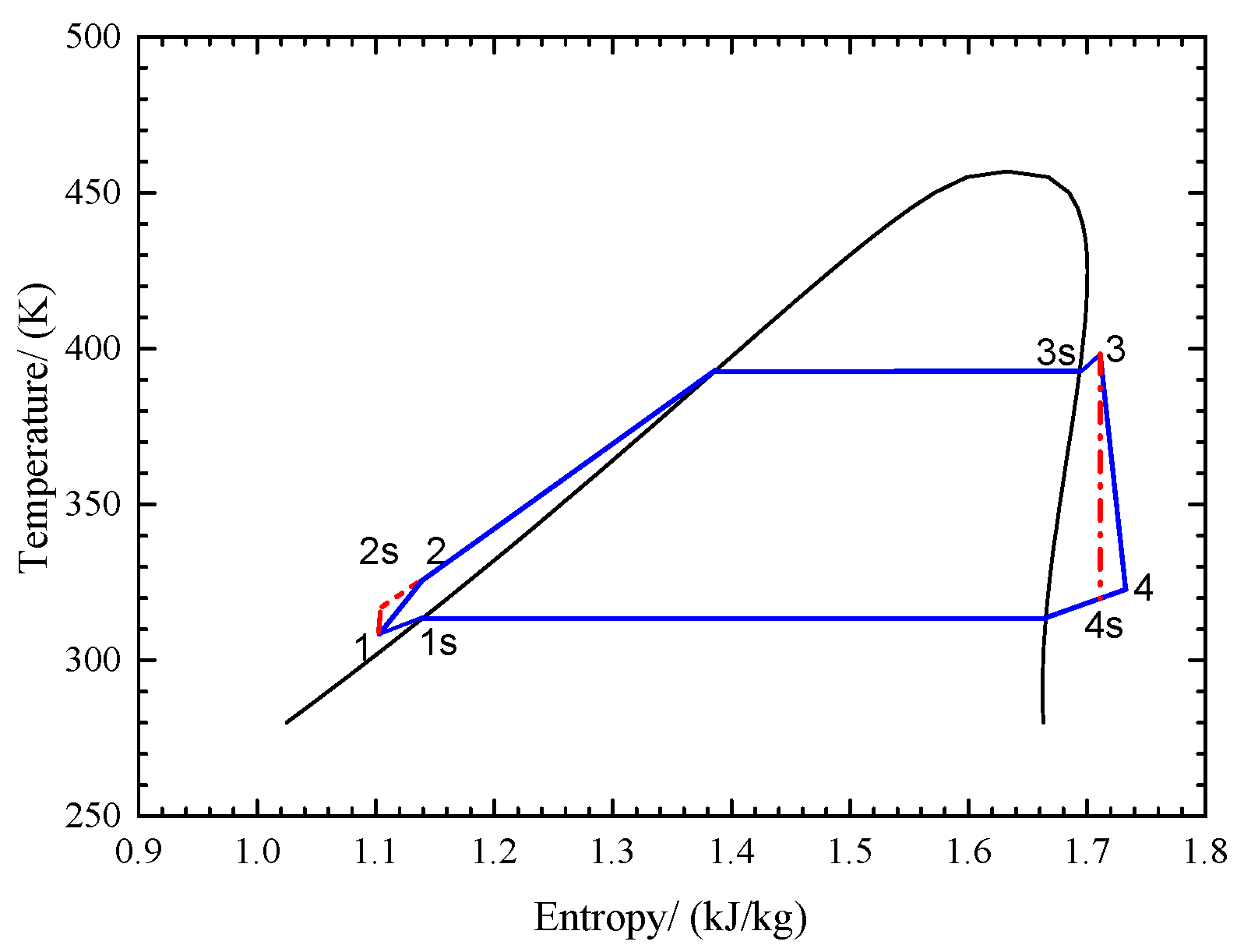
3.1. STORC-MSHS System Modelling
3.2. SPV-PEMWE System Modeling
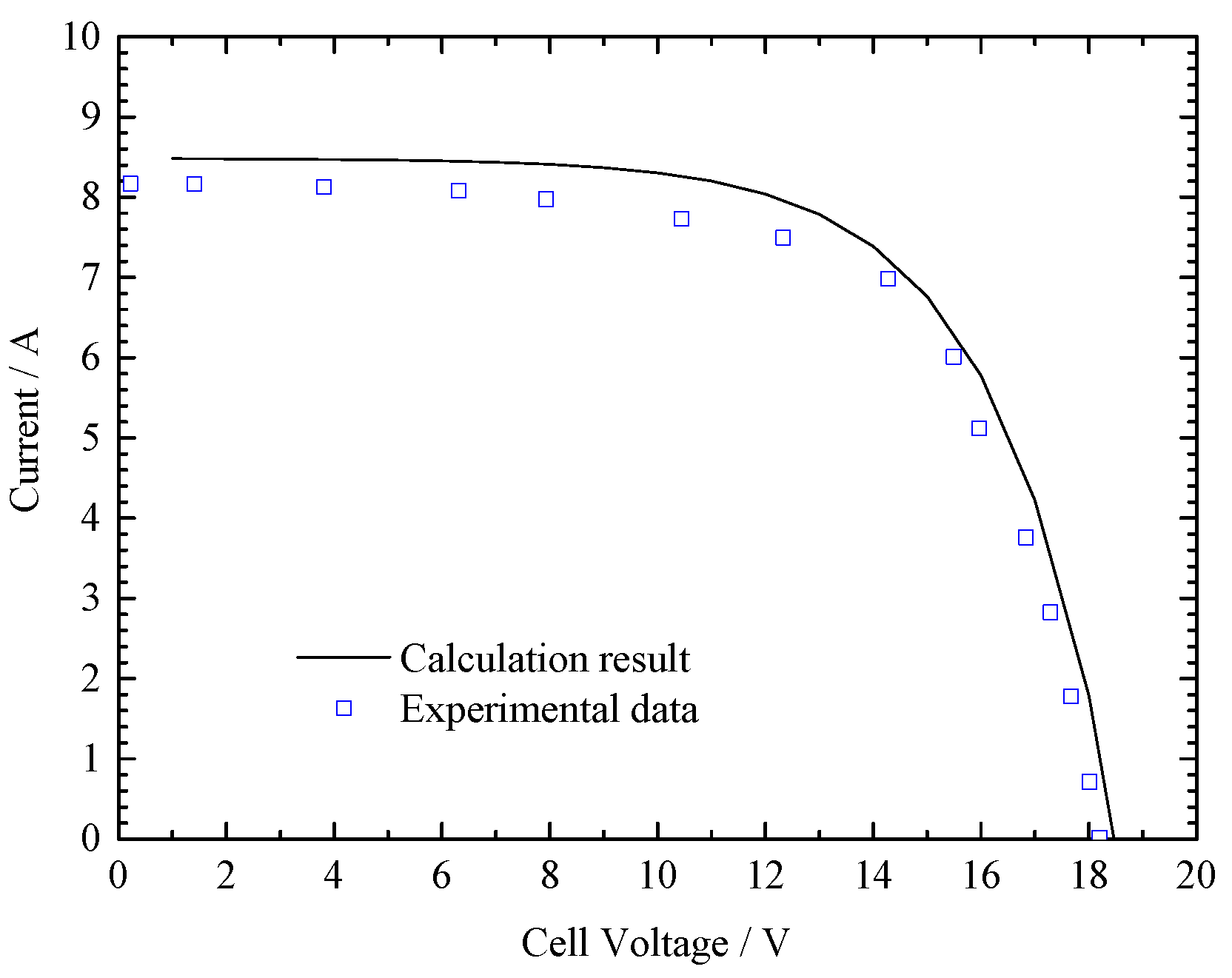
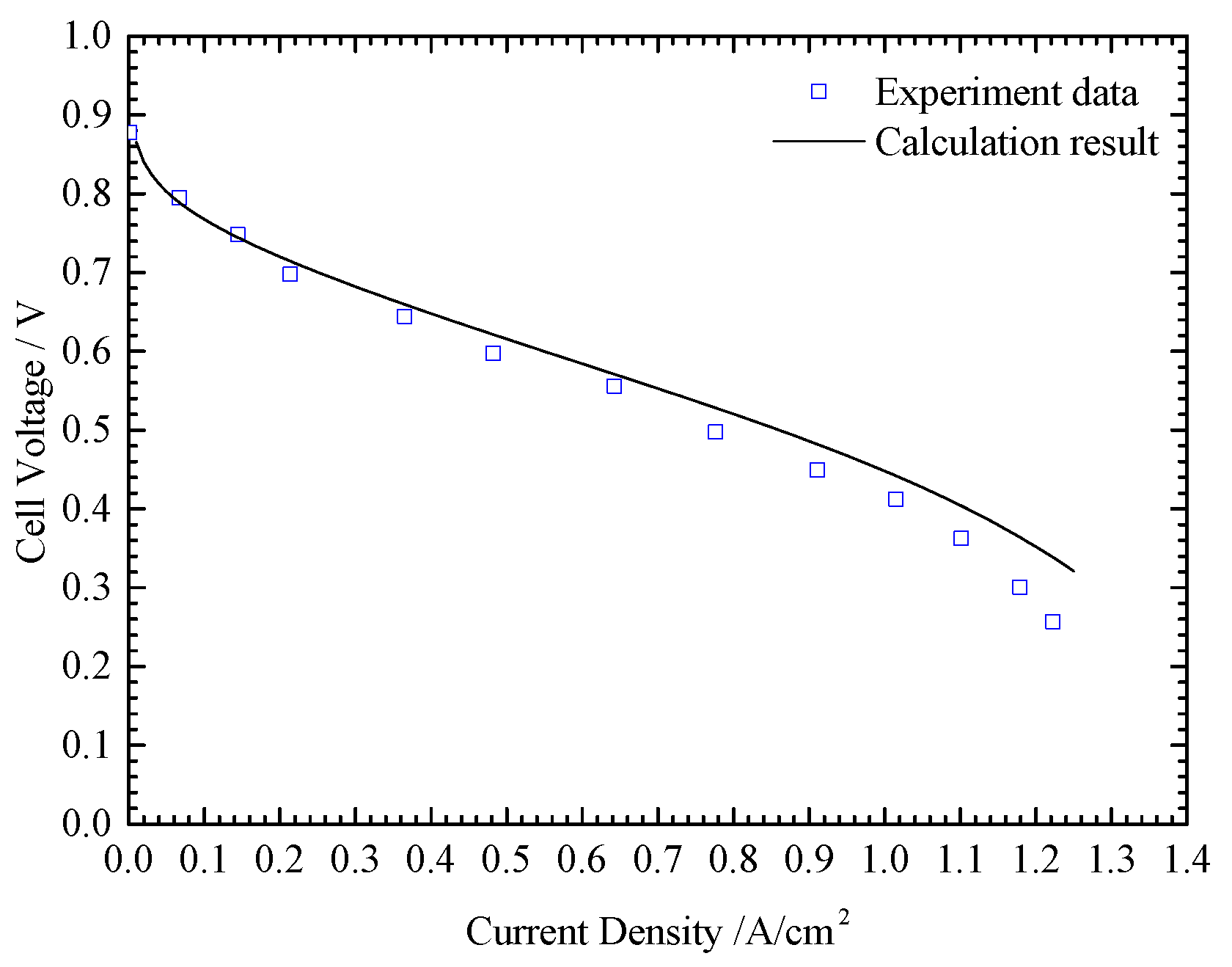
3.3. Model Validation
4. Results and Discussion
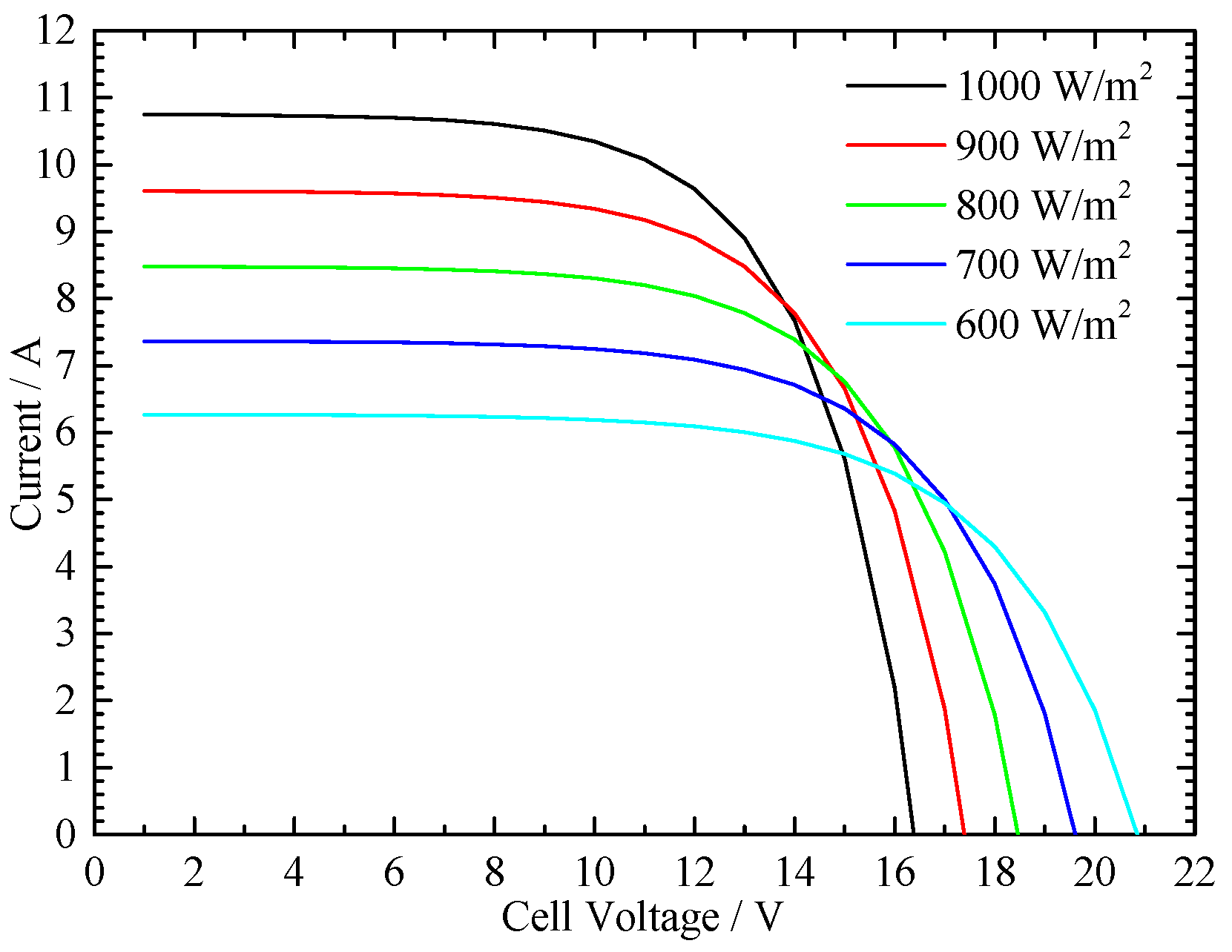
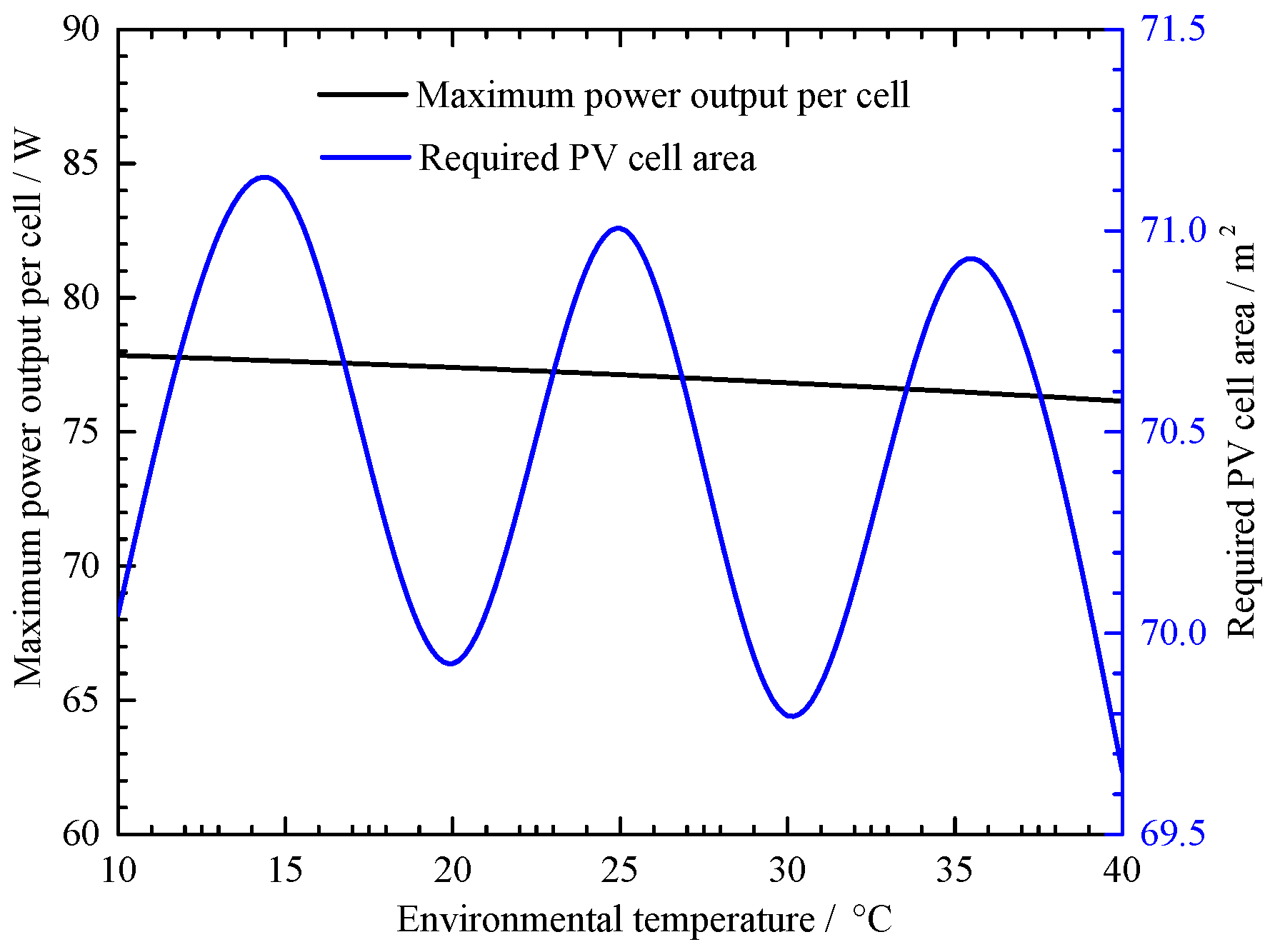
4.1. Irradiation Value and Environmental Temperature Effect on the PV Cell Performance
4.2. Irradiation and Environmental Temperature Effect on the PTC and MSHS Performance
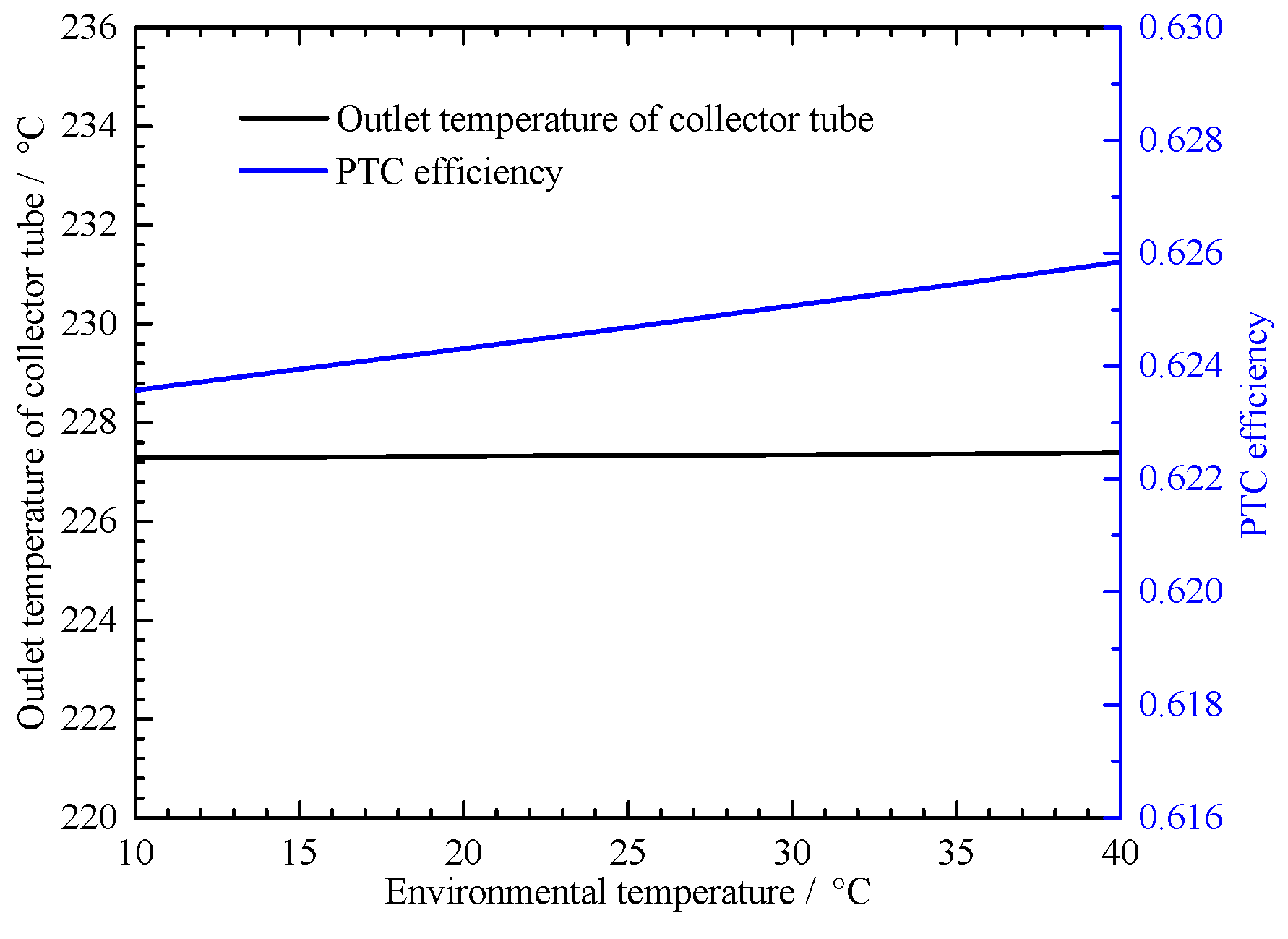
4.2.1. Solar Irradiation and Environmental Temperature Effect on the PTC Performance
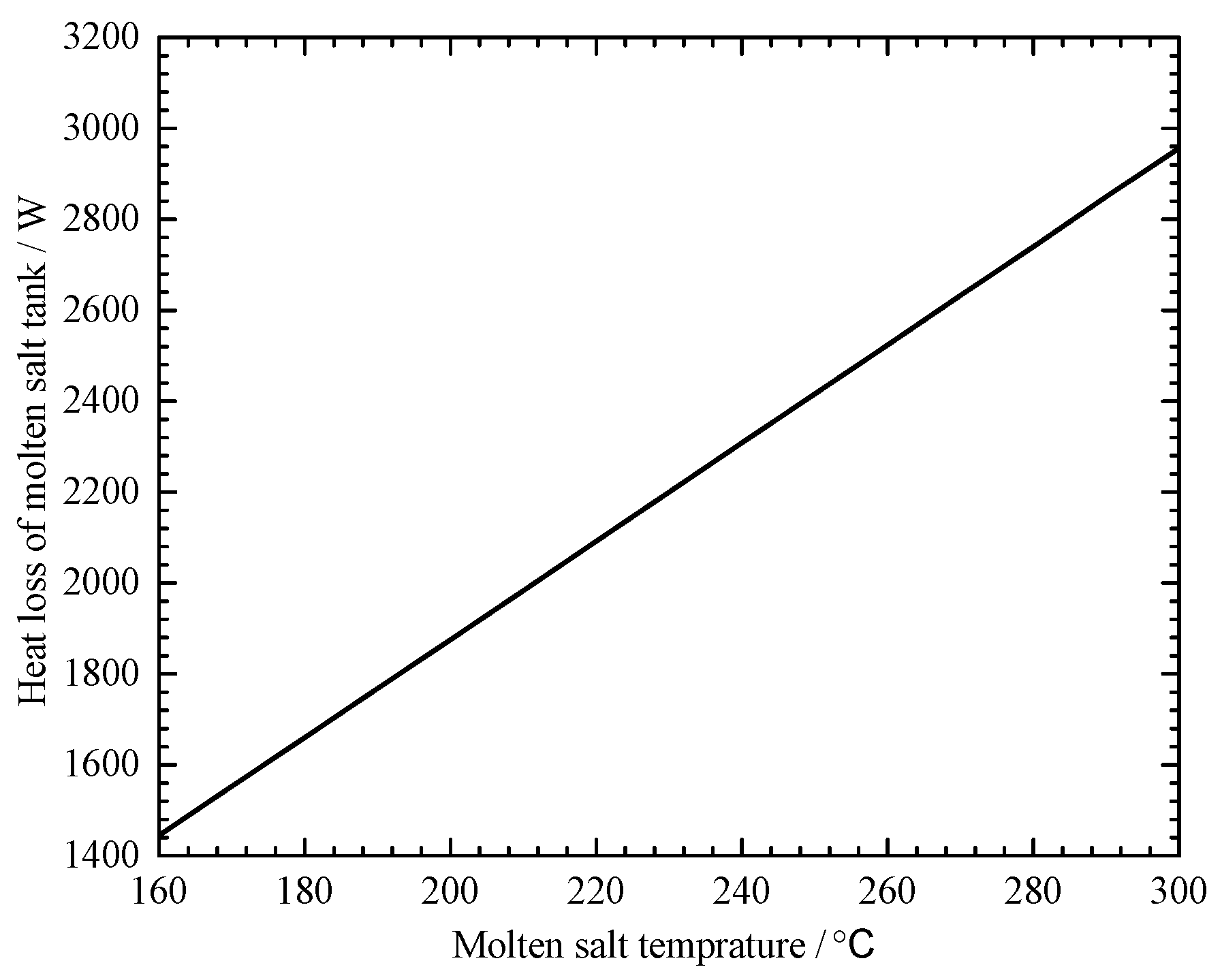
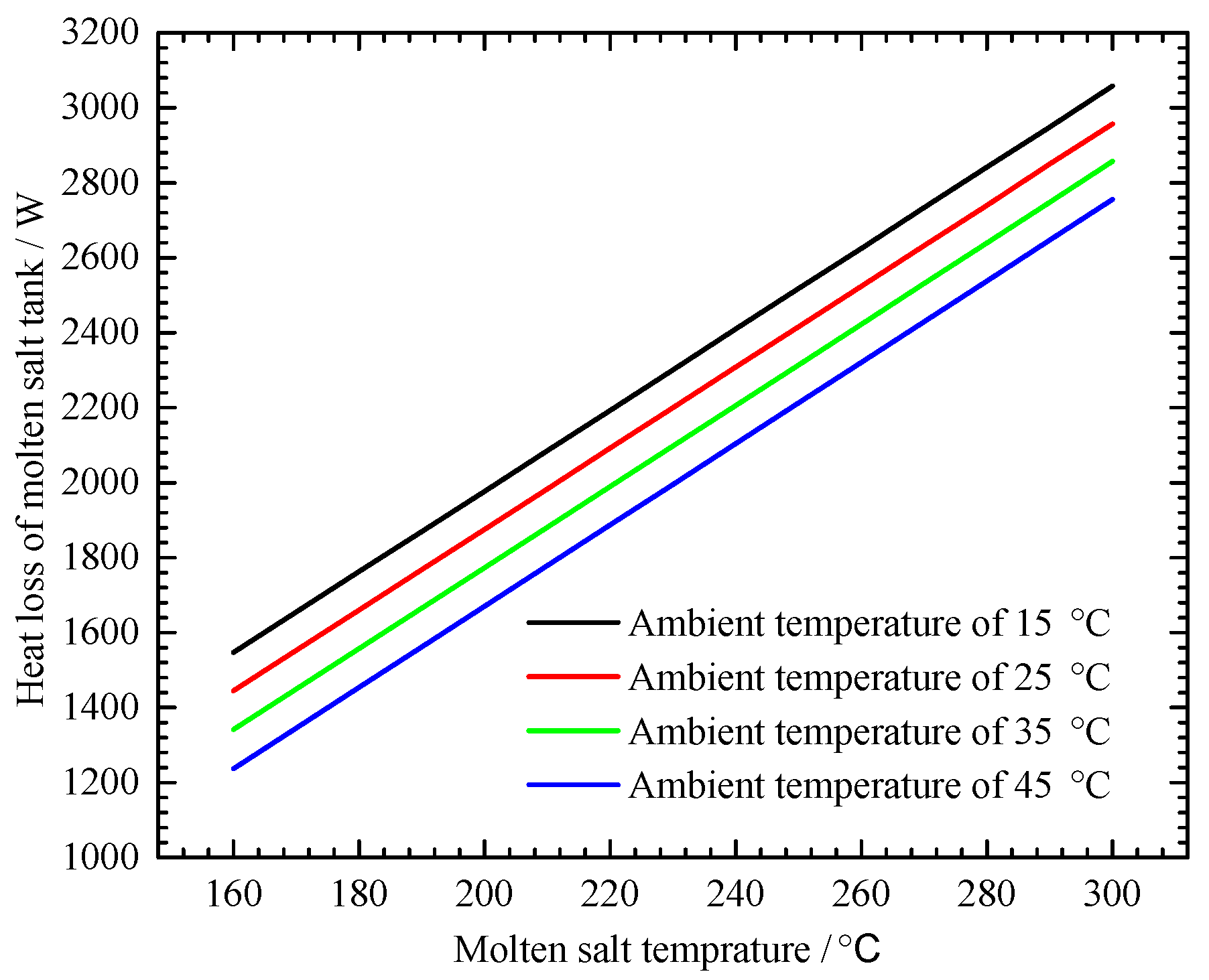
4.2.2. Environmental Temperature Effect on the MSHS Performance
4.3. Energy Storage Type Effect on the System Performance
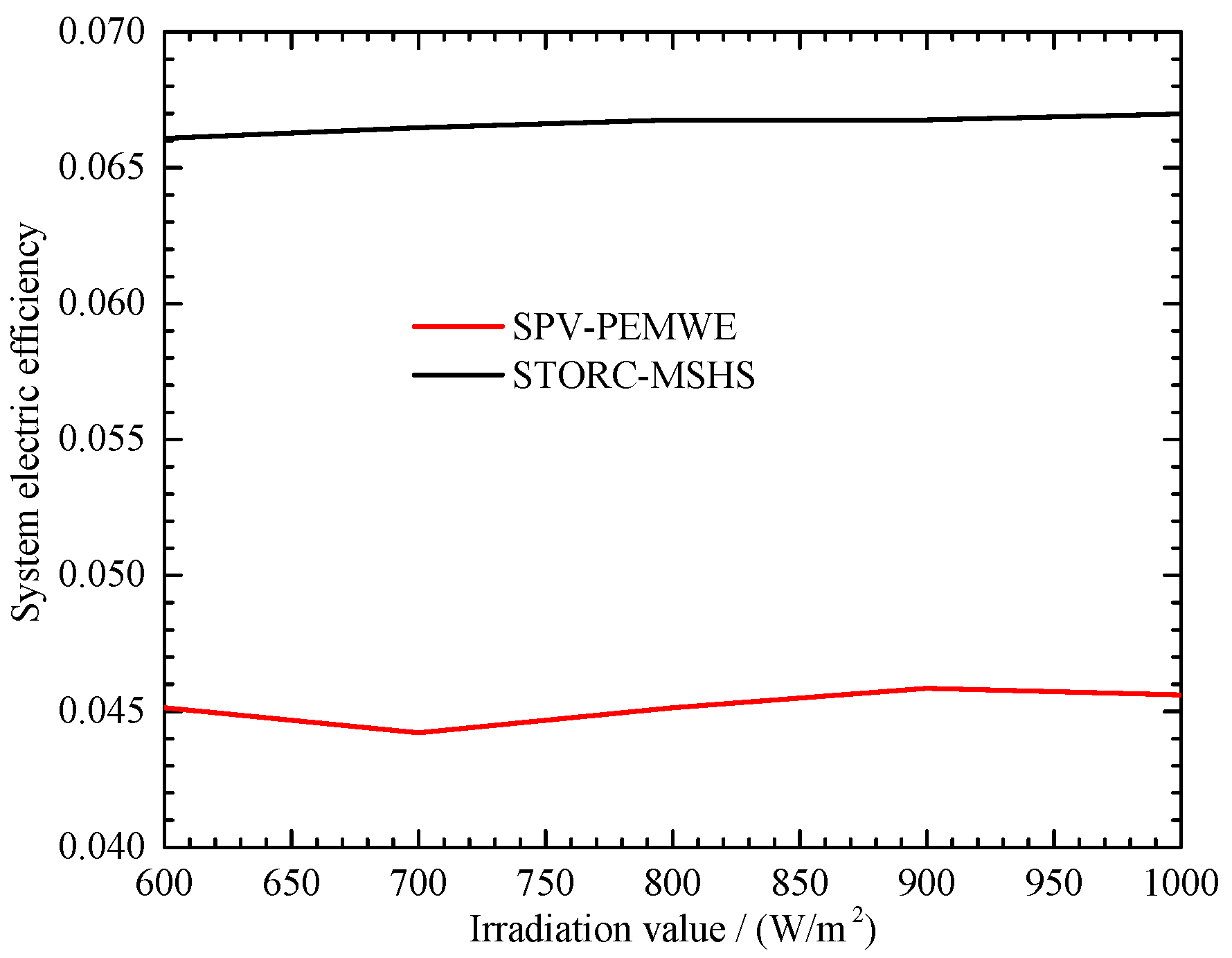
4.4. Solar Irradiation Impact on System Efficiency
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chacartegui, R.; Vigna, L.; Becerra, J.A.; Verda, V. Analysis of two heat storage integrations for an Organic Rankine Cycle parabolic trough solar power plant. Energy Convers. Manag. 2016, 125, 353–367. [Google Scholar] [CrossRef]
- Bufi, E.A.; Camporeale, S.M.; Fornarelli, F.; Fortunato, B.; Pantaleo, A.M.; Sorrentino, A.; Torresi, M. Parametric multi-objective optimization of an Organic Rankine Cycle with thermal energy storage for distributed generation. Energy Procedia 2017, 126, 429–436. [Google Scholar] [CrossRef]
- Barbir, F. PEM electrolysis for production of hydrogen from renewable energy sources. Solar Energy 2005, 78, 661–669. [Google Scholar] [CrossRef]
- Nyholm, E.; Goop, J.; Odenberger, M.; Johnsson, F. Solar photovoltaic-battery systems in Swedish households—Self-consumption and self-sufficiency. Appl. Energy 2016, 183, 148–159. [Google Scholar] [CrossRef]
- Ashouri, M.; Astaraei, F.R.; Ghasempour, R.; Ahmadi, M.H.; Feidt, M. Thermodynamic and economic evaluation of a small-scale organic Rankine cycle integrated with a concentrating solar collector. Int. J. Low-Carbon Technol. 2015, 12, 54–65. [Google Scholar] [CrossRef]
- Ferrara, F.; Gimelli, A.; Luongo, A. Small-scale concentrated solar power (CSP) plant: ORCs comparison for different Organic fluids. Energy Procedia 2014, 45, 217–226. [Google Scholar] [CrossRef]
- Rayegan, R.; Tao, Y.X. A procedure to select working fluids for Solar Organic Rankine Cycles (ORCs). Renew. Energy 2011, 36, 659–670. [Google Scholar] [CrossRef]
- Wang, J.; Yan, Z.; Zhao, P.; Dai, Y. Off-design performance analysis of a solar-powered Organic Rankine Cycle. Energy Convers. Manag. 2014, 80, 150–157. [Google Scholar] [CrossRef]
- Mcmahan, A.C. Design and Optimization of Organic Rankine Cycle Solar-Thermal Power Plants. Master’s Thesis, University of Wisconsin, Madison, WI, USA, 2006. Available online: https://minds.wisconsin.edu/handle/1793/7889 (accessed on 8 December 2020).
- Quoilin, S.; Orosz, M.; Hemond, H.F.; Lemort, V. Performance and design optimization of a low-cost solar Organic Rankine Cycle for remote power generation. Solar Energy 2011, 85, 955–966. [Google Scholar] [CrossRef]
- Sonsaree, S.; Asaoka, T.; Jiajitsawat, S.; Aguirre, H.; Tanaka, K. A small-scale solar Organic Rankine Cycle power plant in Thailand: Three types of non-concentrating solar collectors. Solar Energy 2018, 162, 541–560. [Google Scholar] [CrossRef]
- Caldino-Herrera, U.; Castro, L.; Jaramillo, O.A.; Garcia, J.C.; Urquiza, G.; Flores, F. Small Organic Rankine Cycle coupled to parabolic trough solar concentrator. Energy Procedia 2017, 129, 700–707. [Google Scholar] [CrossRef]
- Borunda, M.; Jaramillo, O.A.; Dorantes, R.; Reyes, A. Organic Rankine Cycle coupling with a parabolic trough solar power plant for cogeneration and industrial processes. Renew. Energy 2016, 86, 651–663. [Google Scholar] [CrossRef]
- Wan, Z.; Wei, J.; Qaisrani, M.A.; Fang, J.; Tu, N. Evaluation on thermal and mechanical performance of the hot tank in the two-tank molten salt heat storage system. Appl. Therm. Eng. 2020, 167, 114775. [Google Scholar] [CrossRef]
- Rahman, S.; Tam, K. A feasibility study of photovoltaic-fuel cell hybrid energy system. IEEE Trans. Energy Convers. 1988, 3, 50–55. [Google Scholar] [CrossRef]
- Lehman, P.A.; Chamberlin, C.E. Design of a photovoltaic-hydrogen-fuel cell energy system. Int. J. Hydrog. Energy 1991, 16, 349–352. [Google Scholar] [CrossRef]
- Torres, L.A.; RODRíGUEZ, F.; Sebastian, P.J. Simulation of a solar-hydrogen-fuel cell system: Results for different locations in Mexico. Int. J. Hydrog. Energy 1998, 23, 1005–1009. [Google Scholar] [CrossRef]
- El-Shatter, T.F.; Eskandar, M.N.; El-Hagry, M.T. Hybrid PV/fuel cell system design and simulation. Renew. Energy 2002, 27, 479–485. [Google Scholar] [CrossRef]
- Arsalis, A.; Alexandrou, A.N.; Georghiou, G.E. Thermoeconomic modeling of a completely autonomous, zero-emission photovoltaic system with hydrogen storage for residential applications. Renew. Energy 2018, 126, 354–369. [Google Scholar] [CrossRef]
- Jia, J.; Seitz, L.C.; Benck, J.D.; Huo, Y.; Chen, Y.; Ng, J.W.D.; Bilir, T.; Harris, J.S.; Jaramillo, T.F. Solar water splitting by photovoltaic-electrolysis with a solar-to-hydrogen efficiency over 30%. Nat. Commun. 2016, 7, 13237. [Google Scholar] [CrossRef]
- Li, J.; Hang, G.; Qingpeng, M.; Yuting, W.; Fang, Y.; Chongfang, M. Thermoeconomic analysis on a molten salt parabolic trough-based concentrated solar Organic Rankine Cycle system. Int. J. Energy Res. 2020, 44, 3395–3411. [Google Scholar] [CrossRef]
- Forristall, R. Heat Transfer Analysis and Modeling of a Parabolic Trough Solar Receiver Implemented in Engineering Equation Solver. Technical Report. 2003. Available online: https://pdfs.semanticscholar.org/950c/d9393159ced22726a89ea9e905ffe5f089d3.pdf?_ga=2.149750006.1708398994.1607399928-807880032.1607399928 (accessed on 8 December 2020).
- Su, J.; Yu, S.; Zhao, W.; Wu, M.; Shen, Y.; He, H. Investigation on engineering analytical model of silicon solar cells. Acta Energiae Solaris Sinca 2001, 022, 409–412. [Google Scholar]
- Zhang, H.; Lin, G.; Chen, J. Evaluation and calculation on the efficiency of a water electrolysis system for hydrogen production. Int. J. Hydrog. Energy 2010, 35, 10851–10858. [Google Scholar] [CrossRef]
- Spiegel, C. PEM fuel cell modeling and simulation using Matlab. J. Am. Chem. Soc. 2008, 110, 567–571. [Google Scholar]
- Millet, P.; Ngameni, R.; Grigoriev, S.A.; Fateev, V.N. Scientific and engineering issues related to PEM technology: Water electrolysers, fuel cells and unitized regenerative systems. Int. J. Hydrog. Energy 2011, 36, 4156–41633. [Google Scholar] [CrossRef]
- Siegel, N.P.; Ellis, M.W.; Nelson, D.J.; Spakovsky, M.R.V. A two-dimensional computational model of a PEMFC with liquid water transport. J. Power Sources 2004, 128, 173–184. [Google Scholar] [CrossRef]





| Variable | Description | Unit |
|---|---|---|
| Required electricity energy per day | J/day | |
| Specific heat of molten salt at constant pressure | J·kg−1K−1 | |
| m | Total mass | kg |
| T | Temperature | K |
| s | Entropy | J·kg−1K−1 |
| h | Enthalpy | J·kg−1 |
| Isentropic efficiency | - | |
| x | Parameter | - |
| I | Current | A |
| V | Voltage | V |
| G | Irradiation value | W/m2 |
| Temperature differencel | °C | |
| Solar irradiation value difference | W/m | |
| A | Area | m |
| E | Potential | V |
| Electrical conductivity | S/m | |
| Thermal conductivity | J·mK | |
| Activity | - | |
| R | Universal gas constant | - |
| P | Partial pressure | Pa |
| j | Current density | A/cm |
| Thickness | m | |
| q | Gas generation rate | mol/s |
| F | Faraday constant | - |
| r | Internal resistance | |
| Gibbs free energy | J·mol |
| Symbol | Description | Symbol | Description |
|---|---|---|---|
| H | Hot tank | C | Cold tank |
| ref | Reference | m | Molten salt |
| oc | Open circuit | sc | Shortcut circuit |
| air | Ambient air | PV | PV array |
| cm | Per square centimeter | cell | Per cell |
| out | Output | rev | Reversible |
| mem | Membrane | we | Water electrolysis |
| s | Saturation | act | Activity |
| an | Anode side | ca | Cathode side |
| con | Concentration | ohmic | Ohmic |
| FC | Fuel cell | liq | Liquid state |
| PTC | ORC | ||
|---|---|---|---|
| Aperture length (m) | 5.77 | Evaporation pressure (MPa) | 1.2 |
| Focal distance (m) | 1.71 | Superheating degree (K) | 5 |
| Absorber inner diameter (mm) | 64 | Condensation temperature (K) | 316.15 |
| Absorber outer diameter (mm) | 70 | Sub-cooling degree (K) | 3 |
| Glass envelop inner diameter (mm) | 120 | Expander isentropic efficiency (%) | 70 |
| Glass envelop outer diameter (mm) | 125 | Isentropic efficiency of the pump (%) | 90 |
| Pressure in annular space (Pa) | <133.2 | Mechanical efficiency (%) | 90 |
| - | - | Generator efficiency (%) | 95 |
| PV Cell | PEMWE | PEMFC | |||
|---|---|---|---|---|---|
| Standard shortcut current density (A) | 10 | Cell temperature (K) | 353.15 | Cell temperature (K) | 333.15 |
| Standard open circuit voltage (V) | 18 | Anode exchange current density (A/cm) | Hydrogen pressure (atm) | 3 | |
| Maximum power density (W) | 8.3 | Cathode exchange current density (A/cm) | Air pressure (atm) | 3 | |
| Maximum voltage (V) | 14.2 | Activation area (cm) | 25 | Standard ambient pressure (kPa) | 101.325 |
| Filling factor | 0.75 | General gas constant | 8.314 | Cell area (cm) | 25 |
| Current density per square centimeter (A/cm) | 0.24 | Faraday constant | 96,485 | Internal resistant () | 0.19 |
| Open circuit voltage (V) | 0.6 | Anode charge transfer coefficient | 0.5 | Conversion efficiency (%) | 0.5 |
| Standard temperature (K) | 298.15 | Cathode charge transfer coefficient | 0.5 | Amplification constant | 0.085 |
| - | - | Anode partial pressure (Pa) | 101,325 | Exchange current density (A/cm) | |
| - | - | Cathode partial pressure (Pa) | 101,325 | Limiting current density (A/cm) | 1.4 |
| - | - | Membrane activity | 1 | Gibbs free energy (kJ/mol) | 228.17 |
| - | - | Membrane thickness (mm) | Mass transport constant | 1.1 | |
| - | - | Electrolyzer utilization efficiency | 0.85 | Hydrogen utilization coefficient | 0.85 |
| Tank height (m) | 1.9 | Thickness of insulation layer (m) | 0. 35 |
| Inner diameter (m) | 2.8 | Thermal conductivity (W·m·k) | 0.2116 |
| Outer diameter (m) | 2.816 | Emissivity of insulation layer | 0.95 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.; Guo, H.; Meng, Q.; Wu, Y.; Ye, F.; Ma, C. Thermodynamic Analysis and Comparison of Two Small-Scale Solar Electrical Power Generation Systems. Sustainability 2020, 12, 10268. https://doi.org/10.3390/su122410268
Li J, Guo H, Meng Q, Wu Y, Ye F, Ma C. Thermodynamic Analysis and Comparison of Two Small-Scale Solar Electrical Power Generation Systems. Sustainability. 2020; 12(24):10268. https://doi.org/10.3390/su122410268
Chicago/Turabian StyleLi, Junfen, Hang Guo, Qingpeng Meng, Yuting Wu, Fang Ye, and Chongfang Ma. 2020. "Thermodynamic Analysis and Comparison of Two Small-Scale Solar Electrical Power Generation Systems" Sustainability 12, no. 24: 10268. https://doi.org/10.3390/su122410268
APA StyleLi, J., Guo, H., Meng, Q., Wu, Y., Ye, F., & Ma, C. (2020). Thermodynamic Analysis and Comparison of Two Small-Scale Solar Electrical Power Generation Systems. Sustainability, 12(24), 10268. https://doi.org/10.3390/su122410268





