Abstract
This study presents an overview of the economic analysis and environmental impact of natural gas conversion technologies. Published articles related to economic analysis and environmental impact of natural gas conversion technologies were reviewed and discussed. The economic analysis revealed that the capital and the operating expenditure of each of the conversion process is strongly dependent on the sophistication of the technical designs. The emerging technologies are yet to be economically viable compared to the well-established steam reforming process. However, appropriate design modifications could significantly reduce the operating expenditure and enhance the economic feasibility of the process. The environmental analysis revealed that emerging technologies such as carbon dioxide (CO2) reforming and the thermal decomposition of natural gas offer advantages of lower CO2 emissions and total environmental impact compared to the well-established steam reforming process. Appropriate design modifications such as steam reforming with carbon capture, storage and utilization, the use of an optimized catalyst in thermal decomposition, and the use of solar concentrators for heating instead of fossil fuel were found to significantly reduced the CO2 emissions of the processes. There was a dearth of literature on the economic analysis and environmental impact of photocatalytic and biochemical conversion processes, which calls for increased research attention that could facilitate a comparative analysis with the thermochemical processes.
1. Introduction
Natural gas is a fossil fuel-based energy resource that is abundant in nature [1,2]. It is a mixture of various components, predominantly methane [3]. Natural gas has wide applications in the industries, residential buildings for heating, electricity generation, as a transportation fuel, and for various commercial purposes [4,5,6,7]. The use of natural gas for electric power generation offers several advantages compared to other fossil fuels [8]. It has high fuel efficiency and its cleaner compare to coal and petroleum [9]. Hence, less CO2 is emitted when natural gas is utilized for power generation compared to coal and petroleum [10]. Consequently, the global consumption of natural gas for power generation has increased over the years [11]. Due to its vital role in the global energy mix, the International Energy Outlook in 2016 projected that the global consumption of natural gas will rise to 203 TcF in 2040 [11]. Compared to other primary energy sources, natural gas accounted for a large proportion of global primary energy consumption [12].
Besides being used as a primary source of energy, the value chain of natural gas can be improved by converting it to various chemical products for sustainable use [5,13]. To achieve this, methane, a vital component of natural gas, is often used as a feedstock to produce value-added chemicals and chemical intermediates such as methanol and syngas [14,15,16]. Additionally, a large proportion of global hydrogen consumption is produced from the conversion of methane by the steam reforming technique [17]. The conversion of methane to value-added products can be achieved using various technological pathways such as reforming, fermentative and photocatalytic processes [18,19]. An extensive review of the nano-catalytic conversion of natural gas to liquid fuels and petrochemical feedstocks has been reported by Gharibi et al. [20]. The authors reported that natural gas can be converted directed or indirectly to various products using different types of nano-catalysts. The direct conversions include pyrolysis, methane dehydro-aromatization, and direct oxidative conversions. While the indirect methods involve the reforming of methane using different types of oxidants such as carbon dioxide, oxygen, and steam. A review reported by Gür [21] revealed the prospects of efficient electricity generation by the conversion of methane in solid oxide fuel cells. The study revealed that the electrochemical conversion of methane in solid oxide fuel offers a great potential for efficient generation of electricity [22]. The efficient conversion of natural gas to value-added products is key to its sustainable utilization. However, it is expedient to consider the economic viability and environmental sustainability of some of the emerging conversion technologies. Although there are several review papers on the conversion of natural gas to various products, none of these studies focused on the economic analysis and environmental implications of the technological processes used for natural gas conversion. This review delves into the literature on the economic feasibility and environmental impacts of various technological processes employed for natural gas conversion to provide a quick overview of the potential economic feasibility and environmental impact of the processes. The literature considered in this review was published between 1998 and 2020.
2. Natural Gas Conversion Technologies
The conversion of natural gas can be done using various technological routes broadly classify as thermochemical, biochemical, and photochemical in Figure 1. The thermochemical route entails the use of thermal energy and catalytic materials for the conversion of natural gas to various products. These methods include methane reforming using steam, carbon dioxide, and oxygen. Recently, research focus is shifting to the advanced form of reforming such as tri-reforming of methane, chemical looping reforming, and photocatalytic reforming [23,24,25,26,27,28]. Amongst the thermochemical processes, the steam methane reforming is the most established and presently being employed for a large proportion of global hydrogen production [17,29,30]. Other emerging reforming technologies using carbon dioxide and oxygen are seriously receiving research attention due to their perceived advantages over the well-established steam methane reforming [31]. One of such advantages is the prospect of the methane dry reforming to help mitigate greenhouse gases through the utilization of carbon dioxide. Besides, it is a potential technological route for producing syngas used as chemical intermediates for Fischer Tropsch synthesis.
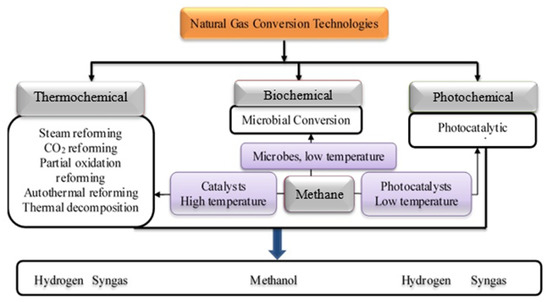
Figure 1.
Schematic representation of natural gas conversion technologies.
The various reforming processes have been investigated using transition metal catalysts such as Ni, Co, Pd, Pt Cu, Ru, and Rh [32,33,34,35]. Among the various catalysts, Ni-based catalysts have been reported to displayed high activity for the steam reforming reaction [36]. Besides, it has a very low cost compared to other metal catalysts. However, it is very prone to catalyst deactivation by sintering and carbon deposition [37]. The stability of the Ni-catalysts during the reforming reaction can be improved using promoters. The use of noble metals in small composition has been reported to be effective in improving the stability of Ni-catalysts during reforming reaction. Rategarpanah et al. [38] reported the influence of noble metal on the activity of Ni-Cu/MgO-Al2O3 catalysts in the thermo-catalytic conversion of methane. The unpromoted Ni-Cu/MgO-Al2O3 was found to be unstable during the thermo-catalytic methane conversion. The addition of the noble metal promoters created a synergistic effect with the Ni-Cu/MgO-Al2O3, thereby preventing the sintering and deactivation of the catalysts and enhancing its stability with time on stream. Besides the use of promoters, a well-synthesized support material could significantly improve the activity and stability of Ni catalysts as demonstrated by Kumar et al. [24]. The stability of Ni-catalyst synthesized on Al2O3, ZrO2, TiO2, SBA-15, MgO, and CeO2-ZrO2 was investigated in tri-reforming of methane at 800 °C [24]. The Ni/SBA-15 was found to display the highest stability throughout the 10 h time on stream. The high stability of the Ni/SBA-15 can be attributed to the confinement of the Ni-species within the pores of the SBA-15 thereby preventing sintering and deactivation [39].
The use of advanced reforming processes such as coupling chemical looping reforming incorporated with CO2 splitting reactions has been reported [40]. This advanced reforming process could offer an advantage of mitigating CO2 emissions in a cyclic two-step syngas production. Additionally, the integration of solar power units with chemical looping reforming has been proven to help meet the high energy required for the reaction [41]. The use of a ceria oxygen carrier for solar enhanced chemical looping methane reforming was reported to increase syngas selectivity, methane conversion, and reactor performance [41]. In a similar study, Wang et al. [42] employed the use of CeO2-ZrO2-CuO oxygen carriers to catalyze syngas production by chemical looping reforming. The addition of Cu and Zr to the CeO2 leads to the distortion of the lattice thereby resulting in better oxygen mobility.
The biochemical conversion entails the use of microorganisms (methane-oxidizing organisms) to convert methane to methanol. The methanotrophic bacterium is commonly used for the conversion of methane to methanol. Studies have shown that the biochemical methane conversion to methanol offers the advantages of high conversion efficiency. More so, the biochemical process is more environmentally friendly compared to the thermochemical process. Besides, the presence of toxic impurities in the natural gas composition could inhibit catalytic activity during thermochemical methane conversion. On the contrary, the presence of impurities does not impede the biochemical process. Henard et al. [43] revealed that methanotrophic bacterium has a high capability of bio-converting methane to lactate, an industrial chemical platform. One of the major constraints with the bioconversion process is the low yield of the products. Moreover, there is often the challenge of insufficient genetic tractability of microorganisms that can be used to convert methane.
The use of abundant solar energy resources could help in overcoming the high thermal energy required for the thermochemical process. The photochemical conversion process enables the conversion of methane to hydrogen and syngas using a photocatalyst that can be excited under visible or ultraviolet irradiation. The photocatalytic reaction often occurs at a very low temperature compared to the thermochemical conversion process. Shoji et al. [44] demonstrated that strontium titanate supported rhodium nanoparticles (Rh/STO) was efficient in the photocatalytic conversion of natural gas to syngas. The Rh/STO photocatalyst exhibited high efficiency in promoting the conversion of methane under ultraviolet light irradiation. An H2:CO ratio close to 1 was obtained from photocatalytic reforming the methane, making it suitable as a technological route to produce oxygenated fuel by FTS using cobalt-based catalysts. Comparatively, each of the natural gas conversion processes has its pros and cons which can be evaluated using the life cycle assessment and life cycle cost analysis. The review of selected published articles on the various technological routes used for converting natural gas to value-added chemicals and chemical intermediated based on their life cycle assessment and life cycle cost analysis are presented in the next section.
3. Economic Analysis of Natural Gas Conversion Technologies
3.1. Emerging Technologies
Performing an economic analysis for emerging technologies is crucial in determining whether such a process is economically viable in terms of profitability in the eventuality of a scale-up process [45]. Prior to the economic analysis of the process, technical design, and analysis of the process are often performed [46]. The details of the design specifications of each of the process units in the production process are employed to carry out detail economic analysis of the process [47]. The economic analysis is usually performed as a function of the process cost which consists of the capital expenditure (CAPEX) or the investment cost and the operating expenditure (OPEX) [48]. The capital expenditure consists of all the costs associated with buying fixed assets with a useful life above one year. The economic analysis of various technologies used for natural gas conversion to valuable products has been reported in the literature, as summarized in Table 1.
3.1.1. Thermal Decomposition of Methane
Due to the unique nature of the thermal decomposition of methane, the economic analysis of the process is strongly dependent on the design specification of the process [49]. One of the key economic indicators of hydrogen production by various natural gas conversion processes is the production cost. As reported by Mueller-Langer et al. [50], the hydrogen production cost can be estimated from the cost of materials, fixed assets, maintenance, water, electricity, and labor. A process that involves the emissions of CO2 might incur an additional cost known as the emissions tax. The mass and energy balance of each of the unit processes for the thermal decomposition of methane as well as the reactor design is crucial in estimating the avoidable CO2 emissions, product carbon, hydrogen production, and cost of equipment. The economic analysis of direct thermal decomposition of methane to hydrogen has been reported by Keipi et al. [51]. Based on the assumption of small-scale production, the study revealed that the production of hydrogen by thermal decomposition of methane has a great potential to be economically competitive with steam methane reforming which is a matured technological route for hydrogen production. The total cost of hydrogen production by thermal decomposition of methane was estimated as 72 EUR/MWh while the total OPEX and CAPEX were calculated as 815 kEUR/annum and 4203 kEUR, respectively. Based on the sensitivity analysis, the product carbon value was found to have the most significant effect on the hydrogen production cost for the thermal decomposition of methane. The cost of natural gas significantly influenced the hydrogen production cost by steam methane reforming. Technically, one major constraint of hydrogen production by thermal decomposition of methane and steam reforming of methane is catalyst deactivation. Studies have shown that the catalyst costs usually account for a large proportion of the operating cost of thermochemical processes. The cost implications of catalyst deactivation were not evaluated in the work of Keipi et al. [51] which could have a significant effect on the operating cost and hence the cost of hydrogen production from both processes. Since thermal decomposition of methane requires high thermal energy, the type of energy source utilize for the process could play a significant role in determining the economic viability of the process. Keipi et al. [52] investigated the economic analysis of hydrogen production by thermal decomposition of methane using four different types of energy sources. The energy sources which include using the energy from methane combustion, electricity, energy carrier catalyst and energy from regenerative heat reactors were found to significantly influence the CAPEX and the OPEX of the thermal decomposition process. The thermal decomposition of methane using energy carrier catalysts was found to have the highest CAPEX and OPEX. This can be attributed to the high cost of the catalysts and the frequent replacement of the catalyst due to deactivation. Several authors have reported that the cost of catalytic materials plays a significant role in the economic analysis of a thermo-catalytic methane decomposition. Various studies have shown that the cost of hydrogen production from the thermal decomposition of methane is strongly dependent on the nature of the catalyst (Figure 2). The cost of hydrogen production is generally low for non-noble metal catalysts such as Ni, Fe, Mo, and so. The cost of hydrogen production was reported as <USD 1/mole H2 for non-noble metal catalysts. While hydrogen production by noble metal catalysts >USD 100/mol H2. The economic analysis of thermal decomposition of methane at temperature <600 °C has been reported by Mondal and Ramesh Chandran [53]. Based on the economic analysis, the CAPEX and the production cost of the hydrogen by the thermal decomposition of methane were calculated as USD 301 million and USD 948/MT, respectively. The fixed cost, raw material cost and the utility cost accounted for 70%, 9% and 17%, respectively.
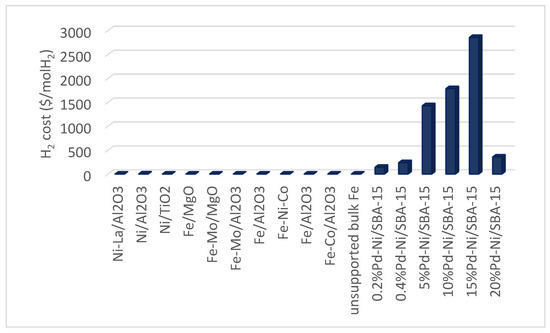
Figure 2.
Hydrogen cost as a function of the type of catalysts.
Various modifications have been employed to improve the efficiency of hydrogen production by thermal decomposition process. Timmerberg et al. [54] employed a plasma reactor for the thermal decomposition of methane to produce hydrogen and carbon black. The economic analysis of the plasma-assisted thermal decomposition of methane shows that hydrogen production cost was estimated as EUR 1.6/kg compared to the hydrogen production cost of EUR 2.2/kg for the conventional reactor used for methane decomposition. The lower hydrogen production cost obtained from the plasma-assisted methane decomposition can be attributed to the improved process conditions offered by the plasma reactor. The improved process condition could have reduced the operating cost of the plasma-assistant thermal methane decomposition for hydrogen and carbon black production. Similarly, the use of plasma to improve the pyrolysis of natural gas to hydrogen and carbon black has been reported by Labanca et al. [55]. The economic analysis of the plasma-assisted natural gas pyrolysis revealed a lower CAPEX, OPEX and lower hydrogen production cost compared to the conventional natural gas pyrolysis reported by Gaudernack et al. [56]. The plasma-assisted natural gas pyrolysis process was analyzed to have CAPEX, and OPEX of USD 0.674 million and 0.228 million, respectively. Which is lower compared to the conventional high temperature natural pyrolysis with CAPEX, and OPEX of USD 29.4 million and 14.3 million, respectively. It is obvious that the use of plasma reactor for the pyrolysis of natural gas to produce hydrogen and carbon black significantly reduce the OPEX.
3.1.2. Other Emerging Technologies for Natural Gas Reforming
The economic analysis of hydrogen production from various emerging thermochemical processes such as auto-thermal methane reforming, syngas chemical looping, chemical looping reforming in comparison with steam methane reforming has been reported by Khojasteh Salkuyeh et al. [57]. The economic analysis revealed that auto-thermal methane reforming has the lowest total capital cost compared to the syngas chemical looping, chemical looping reforming, and steam methane reforming. However, the integration of carbon capture facilities into each of the process significantly increased the total capital cost. The chemical looping reforming without carbon capture facilities was found to have the lowest minimum hydrogen selling price of USD 0.98 kg H2 which is competitive with the USD 1.07/kg H2 calculated for the steam methane reforming under the same scenario. The economic analysis of hydrogen production from auto-thermal reforming of methane from biogas sources has been reported by Montenegro et al. [58]. The hydrogen production cost of 2.5 EUR/kg was calculated for the process which was lower than that of the 5 EUR/kg H2 European targets. The economic analysis of natural gas conversion using oxidative coupling techniques on an industrial scale was reported by Godini et al. [58]. Unlike other studies, the fixed and operating costs of each of the sections of the oxidative coupling of methane process were performed. The compressors and the CO2 removal section accounted for the largest part of the capital investment cost. Besides, factors such as the types of utilities and the operating conditions each section of the plants were found to significantly influence the process of operating cost. The analysis revealed that the operating cost and the annual utility cost for the entire conversion process were estimated as EUR 254 and 228.6 million, respectively. Fei et al. [59] reported an economic analysis of bioconversion of natural gas to lactic acid using methanotrophic bacteria. A minimum selling lactic acid price of USD 5.83/kg, OPEX of USD 47.0 million, and CAPEX of USD 654.96 million were calculated for the production process. The lactic acid production unit was found to account for the highest part of the operating cost contribution. The sensitivity analysis revealed that each of the cost items was significantly influenced by the natural gas flow rate and lactic acid productivity. Additionally, the economic analysis of methane bio-conversion to various chemical products in a biorefinery concept has been reported by Cantera et al. [60]. The OPEX of the methane bioconversion was estimated as USD 1021/h and the total bioproduct values of USD 10,000/h. The reactor energy consumption accounted for a large proportion of the OPEX. Based on an extensive literature search, there are myriads of studies on economic analysis of various thermochemical processes used for natural gas conversion to value-added products. However, there is a dearth of literature on the economic analysis of natural gas conversion by biochemical and photocatalytic conversion. The lack of studies on economic analysis of the natural gas conversion by biochemical and photocatalytic could impede a comparative analysis of the whole process for economic viability.
3.2. Well Established Technologies
Steam Methane Reforming
Steam methane reforming is a well-established process used for commercial production of hydrogen. However, due to constraints such as emissions of CO2 and high operating cost due to catalyst deactivation, research focus had centered on designing highly stable catalysts as well as incorporating carbon capture facilities to curb CO2 emission. Hence, various studies have investigated if several design modifications to the existing steam methane reforming process are economically viable. Recently, sorption enhances steam reforming with carbon capture has been reported as one of the strategies to overcome the challenges with the conventional steam methane reforming. To this effect, Diglio et al. [61] investigated the economic implications of hydrogen production by sorption-enhanced steam methane reforming with carbon capture. The cost of hydrogen production by the sorption-enhanced steam methane reforming was calculated to be 1.6 EUR per kg methane which is lower compared to that of steam methane reforming estimated as 117 EUR/MWh [51]. Unlike the work of Keipi et al. [51], the reaction network (which consists of eight packed bed reactors) of the sorption-enhanced steam methane reforming was found to account for about 70% of the total capital cost. Moreover, based on sensitivity analysis, the specific cost of the natural gas was found to significantly influenced the economic performance of the sorption-enhanced steam methane reforming which is consistent with the work of Keipi et al. [51]. The integration of the sorption-enhanced steam methane reforming with solid oxide fuel cells for power generation resulted in a better economic performance with a high daily revenue. The economic analysis of the co-utilization of carbon dioxide and methane in the steam reforming process for methanol synthesis has been reported by Zhang et al. [62]. Contrary to the work of Keipi et al. [51] and Diglio et al. [61], the authors employed economic evaluation indicators such as net present value (NPV), internal rate of return (IRR), and discounted payback period (DPBP) for measuring the economic viability of the methanol production process. Based on the NPV, IRR, and the DPBP values obtained for the process, the co-utilization of the carbon dioxide and methane for methanol production by steam reforming was found to be economically competitive than a scenario whereby only natural gas was utilized. However, the economic analysis of Zhang et al. [63] did not put into consideration the cost associated with the catalytic network which is a vital component of the production process as reported by Diglio et al. [62]. Similar to the work of Keipi et al. [51], Mondal and Ramesh Chandran [53] evaluated the economic performance of hydrogen production from steam methane reforming in comparison with thermal decomposition of methane. Based on the economic analysis, the CAPEX and the hydrogen production cost from the steam methane reforming were calculated as USD 291 million and 899/MT, respectively. While the CAPEX and the hydrogen production cost by the thermal decomposition of methane were calculated as USD 301 million and 948/MT, respectively. The higher cost of production calculated for the hydrogen by thermal decomposition of methane can be attributed to the high cost of electricity compared to the steam reforming process.
The integration of various hydrogen production process could have a synergistic effect on the overall economic performance of the process. This has been demonstrated in the work of Gangadharan et al. [63] who performed an economic analysis of hydrogen production by combined carbon dioxide and steam methane reforming. The total capital investment cost of the combined dry methane reforming and steam methane reforming was found to be higher compared to the standalone steam methane reforming process which can be attributed to the cost of additional process equipment integrated with the steam methane reforming process. However, the study did not consider the other economic evaluation indicators such as the hydrogen production cost, and the operating costs. Moreover, the estimation of the payback period or return on investment could be advantageous in determining whether the combined process is viable compared to the standalone process. The choice of onsite hydrogen production by steam methane reforming for utilization by hydrogen fuel cell vehicle has been reported by Luk et al. [64]. The study revealed that onsite hydrogen production offers the benefits of a shorter payback period and a high rate of return on investment compared to the conventional means of hydrogen production. The cost associated with transporting and storing hydrogen production by the conventional steam methane reforming are eliminated using onsite production for direct utilization. The comparative analysis of the cost components of steam methane reforming, auto thermal methane reforming, syngas chemical looping and chemical looping reforming with or without carbon capture are depicted in Figure 3. The total capital cost for each of the reforming processes is strongly dependent on whether carbon capture facilities are incorporated into the design. The reforming processes without carbon capture facilities are found to be less capital intensive compared to those with carbon capture facilities [56]. The total capital costs for the steam methane reforming, auto-thermal reforming, syngas chemical looping and the chemical looping reforming with carbon capture were estimated as USD 831 million, 628 million, 885 million, and 638 million, respectively. The high capital cost of the syngas chemical looping could be attributed to the incorporation of the chemical looping gasification reactor. The total capital costs of the reforming processes without carbon were found to be less capital intensive. The total capital cost for steam methane reforming, auto-thermal reforming, syngas chemical looping and the chemical looping reforming without carbon capture was estimated as USD 241 million, 326 million, 813 million, and 570 million, respectively.
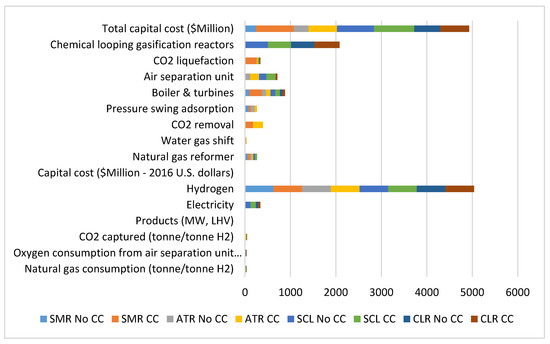
Figure 3.
Cost distributions of hydrogen production from various reforming process with or without carbon capture (CC) (adapted from [57]).
Obviously, the economic analysis of the various technological pathways for natural gas conversion revealed that the estimated costs for each process differs. Technically, each of the natural gas conversion process has a unique configuration from the processing of the feedstocks to finished products. For instance, in the well-established steam reforming of natural gas, the reforming reaction occurs in the reformer, hence most of the CAPEX and OPEX costs revolve round the reformer. However, as a result of advances in research and development, the design modifications to the steam methane reforming whereby carbon capture, storage and utilization facilities in incorporated have been proposed to mitigate the effects of CO2 emissions. Although these additional facilities have the potential of reducing the CO2 emissions, nevertheless additional costs are inbuilt into the CAPEX and OPEX of the overall process. Besides, the analysis of each the process was based on different scenarios. For example, Keipi et al. [52] performed economic analysis of thermal decomposition of methane based on four scenarios namely the scenario with energy from partial combustion of methane, energy from electricity, energy from carrier catalyst and using a regenerative heat exchanger reactor. The detailed analysis of these scenarios revealed that the economic performance of each of the processes differs. Additionally, Mondal et al. [53] reported that the economic performance of the thermo-catalytic methane decomposition varies depending on the conversion rate of the process. The economic analysis of various technologies used for natural gas conversion to valuable products has been reported in the literature, as summarized in Table 1.

Table 1.
Summary of literature on the economic analysis and environmental impact of various natural gas conversion technologies.
4. Environmental Impact of Natural Gas Conversion Technologies
The various types of natural gas conversion technologies have been adjudged to have great potentials of producing important energy carriers such as hydrogen and other value-added chemicals and chemical intermediates. However, these emerging technologies employed for natural gas conversion have been associated with various types of environmental impact based on the life cycle assessment studies. The environmental impact and global warming potential of combined dry methane reforming and steam methane reforming has been reported by Gangadharan et al. [63]. The authors employed the waste reduction algorithm to estimate the potential environmental impact and global warming potential. The analysis revealed that the combined dry and steam methane reforming has a lower global warming potential compared to the standalone steam methane reforming. This can be attributed to the utilization of carbon dioxide as a feedstock for the combined process whereas, in the standalone steam methane reforming process, carbon dioxide is emitted. However, the total potential environmental impact of the combined conversion process which is a measure of the human toxicity, ecological toxicity, regional atmospheric, global warming potential, and ozone depletion potential was found to be higher compared to the standalone steam methane reforming process.
The environmental feasibility and greenhouse gas emissions reduction of thermal and autocatalytic decomposition of methane in comparison with steam methane reforming with or without carbon capture have been investigated by Dufour et al. [67]. Using the life cycle assessment approach, the authors analyzed the environmental impact of the various technological scenarios. The analysis revealed that the steam methane reforming without carbon capture has the highest CO2 emissions whereas the autocatalytic methane conversion with 100% conversion recorded the lowest CO2 emission. However, the steam methane reforming with carbon captured was found to significantly reduced the CO2 emissions by over 60%. This is an indication that appropriate technological modification to an existing conversion process with high CO2 emissions can significantly reduce the global warming potential of the process. Moreover, for a catalytic conversion process, an optimized catalytic design can also mitigate the amount of CO2 emission from the process. This was demonstrated in the work of Dufour et al. [68] who employed investigated the environmental impact of methane thermal decomposition using a carbonaceous catalyst. The analysis revealed that the methane decomposition with 96% conversion using the carbonaceous catalyst have the lowest CO2 emissions compared to steam methane reforming with carbon capture and other scenarios. With further modifications on the thermal decomposition of methane, the CO2 emissions from the process can be reduced [69]. Dufour et al. [66] reported the substitution of direct heating using natural gas as fuel for thermal decomposition of methane with a solar concentrator and found that there was over 25% reduction in CO2 emissions. Comparing the greenhouse gas emissions of hydrogen production by thermal methane decompositions with auto thermal methane reforming and steam methane reforming, Dufour et al. [65] found that the hydrogen production by auto-thermal methane reforming has the lowest CO2 emissions. However, with design modifications such as integrating the steam methane reforming with carbon capture and methane auto-maintained decomposition, the CO2 emissions were significantly reduced compared to the unmodified hydrogen production processes. Kothari et al. [70] compared the environmental impact of hydrogen production by steam methane reforming with CO conversion and partial oxidation reforming and found that a lower CO2 emission was obtained from the steam methane reforming with CO conversion compared to the partial oxidation reforming.
The environmental impact of bioconversion of natural gas to lactic acid using methanotrophic bacteria has been reported by Fei et al. [59]. Based on the life cycle assessment, the natural gas bioconversion process was found to have some potential environmental impacts. The global warming potential was calculated as 5.83 kgCO2eq with negligible effects of acidification, ozone depletion carcinogenic and respiratory effects. In a similar study, the environmental impact of methane bioconversion to various chemical products was reported by Cantera et al. [60]. The bioconversion process was reported to have 4807 kgCO2/h with CO2 emitted from energy consumption accounted for 3157 kg/h. Despite there being several studies on the environmental impact of various thermochemical conversion processes used for natural gas, there is a dearth of literature on the environmental impact of photocatalytic and bioconversion of natural gas. Therefore, more research attention should be focused on the environmental impact of the photocatalytic and bioconversion of natural gas for a comparative analysis with that of thermochemical conversion processes.
5. Effect of Operating Parameters on the CO2 Emissions and Process Economics
Study has shown that process parameters such as the flowrate of the reactants, and the reaction temperature usually influence the amount of CO2 emissions and the process economics [71]. A sensitivity analysis reported by Hamid et al. [71] revealed that the increase in the methane flowrate during steam methane reforming resulted in the increase in the CAPEX, OPEX and the CO2 emissions as shown in Figure 4. The increase in the methane flowrates results in a corresponding increase in the conversion of the methane to gaseous products such as hydrogen, CO, and CO2. As the natural gas conversion progresses with time on stream, there could be challenges of catalysts deactivation by methane cracking, CO2 disproportionation and sintering thereby resulting in the periodic replacements of the catalysts. The periodic replacement of catalysts during the natural gas conversion will certainly influence the CAPEX and OPEX of the process. Additionally, in the same study by Hamid et al. [71], increasing the water contents during steam reforming of natural gas, was reported to have a significant influence on the OPEX per hydrogen production as shown in Figure 5. Since water is needed to produce steam needed for the reforming process, the amount of steam produced is depended on the amount of water available. As the water contents increases, more steam is generated for the natural gas conversion process, thereby driving the reaction towards more products. As more products are formed the life span of the reforming catalysts are shortened with eventuality of replacements.
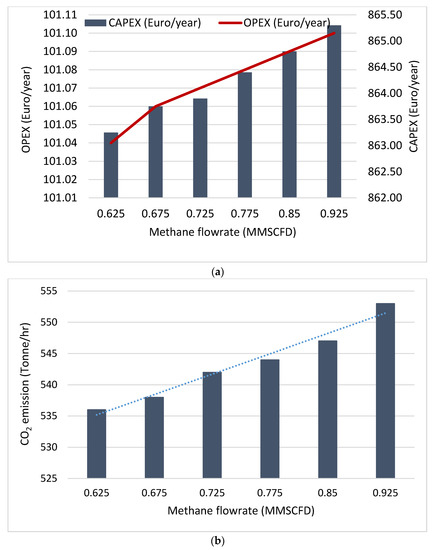
Figure 4.
Effect of methane flowrate on the (a) economics of natural gas conversion by steam reforming and (b) CO2 emission from natural gas conversion by steam reforming.
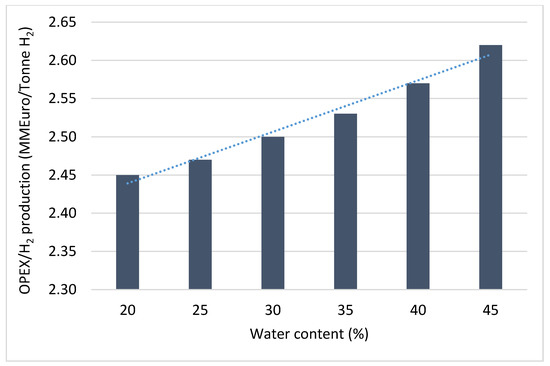
Figure 5.
Effect of water content on the OPEX of natural gas conversion process by steam reforming.
6. Conclusions and Research Outlook
Natural gas as a fossil fuel resource can be applied directly in electricity generation, domestic heating, and as starting materials to produce valuable chemicals and chemical intermediates. Besides being used directly for various applications, natural gas can be converted to various valuable chemical products and chemical intermediates. Steam reforming is a well-established thermochemical process used for converting natural gas to hydrogen and syngas. The steam reforming process is however constraint with challenges such as catalyst deactivation, the high thermal energy required for steam generation and to initiate the reaction and the emissions of CO2. Research attention has focused on other emerging thermochemical processes such as the CO2 reforming of methane, partial oxidation reforming, auto-thermal reforming, and photocatalytic reforming to overcome the challenges associated with the steam reforming. Although efforts have been made to tackle some of these challenges, these emerging processes are yet to be fully developed into commercial-scale production. Besides the thermochemical process, other processes such as the biochemical and the photocatalytic processes are currently receiving research attention. To ascertain the economic viability and environmental impact of these emerging technologies in comparison with the well-established steam reforming process, several researchers have conducted an economic analysis and environmental impacts on various natural gas conversion technologies. Based on several studies on the economic analysis, there is a consensus that the emerging technologies are yet to be economically competitive with the well-established steam reforming. However, there is a huge environmental benefit of these emerging technologies in terms of CO2 emissions and the total environmental index. The study revealed that there is a wide research gap on the economic analysis and environmental impact of the conversion of natural gas by the biochemical and photocatalytic processes. Therefore, the economic feasibilities and the environmental impacts of the biochemical and photocatalytic processes cannot be compared with the steam reforming process. The study also revealed that technical modifications to the original design of the thermochemical processes significantly reduced CO2 emissions. More research attention needs to be focused on comparative analysis of the thermochemical, biochemical, and photocatalytic conversions process to determine their economic feasibility and environmental impact.
Author Contributions
F.O.A. and B.V.A. conceptualized the idea and wrote the draft manuscript. S.I.M. and N.M. reviewed the draft manuscript for technicality. S.I.M. acquired the funding for the research. All authors have read and agreed to the published version of the manuscript.
Funding
The research was financially supported by Universiti Tenaga Nasional through BOLD2025 Grant (No. 10436494/B/2019008).
Acknowledgments
B.V.A., S.I.M., and S.M. acknowledge the financial support of Universiti Tenaga Nasional, Malaysia.
Conflicts of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Abbreviations/Symbols
| ATR | Auto-thermal methane reforming |
| CAPEX | Capital Expenditure |
| CCS | Carbon Capture and Storage |
| CLR | Chemical Looping reforming |
| CO2 | Carbon dioxide |
| DPBP | Discounted payback period |
| OPEX | Operating Expenditure |
| H2 | Hydrogen |
| IRR | Internal rate of return |
| Kg | Kilogram |
| SMR | Steam methane reforming |
| SCL | Syngas chemical looping |
| NPV | Net profit value |
| MMSCFD | Million standard cubic feet per day |
| TcF | Trillion cubic feet |
| EUR | Euro |
| USD | US dollar |
References
- Leung, G.C.K. Natural Gas as a Clean Fuel. In Handbook Clean Energy Systems; American Cancer Society: Atlanta, GA, USA, 2015; pp. 1–15. [Google Scholar] [CrossRef]
- Steele, B.C.H. Running on natural gas. Nature 1999, 400, 619–621. [Google Scholar] [CrossRef]
- Faramawy, S.; Zaki, T.; Sakr, A.A.-E. Natural gas origin, composition, and processing: A review. J. Nat. Gas. Sci. Eng. 2016, 34, 34–54. [Google Scholar] [CrossRef]
- Fadiran, G.; Adebusuyi, A.T.; Fadiran, D. Natural gas consumption and economic growth: Evidence from selected natural gas vehicle markets in Europe. Energy 2019, 169, 467–477. [Google Scholar] [CrossRef]
- Powell, J.B. Natural gas utilization: Current status and opportunities. Catal. Today 2020. [CrossRef]
- Khan, M.I. Policy options for the sustainable development of natural gas as transportation fuel. Energy Policy 2017, 110, 126–136. [Google Scholar] [CrossRef]
- Ogden, J.; Jaffe, A.M.; Scheitrum, D.; McDonald, Z.; Miller, M. Natural gas as a bridge to hydrogen transportation fuel: Insights from the literature. Energy Policy 2018, 115, 317–329. [Google Scholar] [CrossRef]
- Walker, S.B.; Lanen, D.; van Mukherjee, U.; Fowler, M. Greenhouse gas emissions reductions from applications of Power-to-Gas in power generation. Sustain. Energy Technol. Assess. 2017, 20, 25–32. [Google Scholar] [CrossRef]
- Thiruvengadam, A.; Besch, M.; Padmanaban, V.; Pradhan, S.; Demirgok, B. Natural gas vehicles in heavy-duty transportation-A review. Energy Policy 2018, 122, 253–259. [Google Scholar] [CrossRef]
- Gilbert, A.Q.; Sovacool, B.K. Benchmarking natural gas and coal-fired electricity generation in the United States. Energy 2017, 134, 622–628. [Google Scholar] [CrossRef]
- International Energy Agency. World Energy Outlook 2016; International Energy Agency: Paris, France, 2016. [Google Scholar] [CrossRef]
- International Energy Agency. World Energy Outlook 2019; International Energy Agency: Paris, France, 2019. [Google Scholar] [CrossRef]
- Carmona, M.; Feria, J.; Golpe, A.A.; Iglesias, J. Energy consumption in the US reconsidered. Evidence across sources and economic sectors. Renew. Sustain. Energy Rev. 2017, 77, 1055–1068. [Google Scholar] [CrossRef]
- Ayodele, B.V.; Hossain, S.S.; Lam, S.S.; Osazuwa, O.U.; Khan, M.R.; Cheng, C.K. Syngas production from CO2 reforming of methane over neodymium sesquioxide supported cobalt catalyst. J. Nat. Gas Sci. Eng. 2016, 34, 873–885. [Google Scholar] [CrossRef]
- Ayodele, B.V.; Bin Mohd Yassin, M.Y.; Naim, R.; Abdullah, S. Hydrogen production by thermo-catalytic conversion of methane over lanthanum strontium cobalt ferrite (LSCF) and αAl2O3 supported Ni catalysts. J. Energy Inst. 2019, 92, 892–903. [Google Scholar] [CrossRef]
- Ayodele, B.V.; Khan, M.R.; Cheng, C.K. Greenhouse gases mitigation by CO2 reforming of methane to hydrogen-rich syngas using praseodymium oxide supported cobalt catalyst. Clean Technol. Environ. Policy 2016, 1–13. [Google Scholar] [CrossRef]
- LeValley, T.L.; Richard, A.R.; Fan, M. The progress in water gas shift and steam reforming hydrogen production technologies—A review. Int. J. Hydrogen Energy 2014, 39, 16983–17000. [Google Scholar] [CrossRef]
- Ayodele, B.V.; Khan, M.R.; Lam, S.S.; Cheng, C.K. Production of CO-rich hydrogen from methane dry reforming over lanthania-supported cobalt catalyst: Kinetic and mechanistic studies. Int. J. Hydrogen Energy 2016, 41, 4603–4615. [Google Scholar] [CrossRef]
- Dagle, R.A.; Dagle, V.; Bearden, M.D.; Holladay, J.D.; Krause, T.R.; Ahmed, S. An Overview of Natural Gas Conversion Technologies for Co-Production of Hydrogen and Value-Added Solid Carbon Products; (No PNNL-26726, ANL-17/11); Pacific Northwest Natl Lab(PNNL): Richland, WA USA; Argonne Natl Lab(ANL): DuPage, IL, USA, 2017; Volume 65. [CrossRef]
- Gharibi, M.; Zangeneh, F.T.; Yaripour, F.; Sahebdelfar, S. Nanocatalysts for conversion of natural gas to liquid fuels and petrochemical feedstocks. Appl. Catal. A Gen. 2012, 443, 8–26. [Google Scholar] [CrossRef]
- Gür, T.M. Comprehensive review of methane conversion in solid oxide fuel cells: Prospects for efficient electricity generation from natural gas. Prog. Energy Combust. Sci. 2016, 54, 1–64. [Google Scholar] [CrossRef]
- Pirker, G.; Wimmer, A. Sustainable power generation with large gas engines. Energy Convers. Manag. 2017, 149, 1048–1065. [Google Scholar] [CrossRef]
- Alipour-Dehkordi, A.; Khademi, M.H. Use of a micro-porous membrane multi-tubular fixed-bed reactor for tri-reforming of methane to syngas: CO2, H2O or O2 side-feeding. Int. J. Hydrogen Energy 2019, 44, 32066–32079. [Google Scholar] [CrossRef]
- Kathe, M.; Fryer, C.; Sandvik, P.; Kong, F.; Zhang, Y.; Empfield, A.; Fan, L.S. Modularization strategy for syngas generation in chemical looping methane reforming systems with CO2 as feedstock. AIChE J. 2017, 63, 3343–3360. [Google Scholar] [CrossRef]
- Kim, S.; Crandall, B.S.; Lance, M.J.; Cordonnier, N.; Lauterbach, J.; Sasmaz, E. Activity and stability of NiCe@SiO2 multi–yolk–shell nanotube catalyst for tri-reforming of methane. Appl. Catal. B Environ. 2019, 259, 118037. [Google Scholar] [CrossRef]
- Tahir, M.; Tahir, B.; Zakaria, Z.Y.; Muhammad, A. Enhanced photocatalytic carbon dioxide reforming of methane to fuels over nickel and montmorillonite supported TiO2 nanocomposite under UV-light using monolith photoreactor. J. Clean. Prod. 2019, 213, 451–461. [Google Scholar] [CrossRef]
- Hafizi, A.; Rahimpour, M.R.; Hassanajili, S. Calcium promoted Fe/Al2O3 oxygen carrier for hydrogen production via cyclic chemical looping steam methane reforming process. Int. J. Hydrogen Energy 2015, 40, 16159–16168. [Google Scholar] [CrossRef]
- Sastre, D.; Serrano, D.P.; Pizarro, P.; Coronado, J.M. Chemical insights on the activity of La1-xSrxFeO3 perovskites for chemical looping reforming of methane coupled with CO2-splitting. J. CO2 Util. 2019, 31, 16–26. [Google Scholar] [CrossRef]
- Aboosadi, Z.A.; Yadecoury, M.F. Thermally Intensification of Steam Reforming Process by Use of Methane Tri-Reforming: A Review. Int. J. Chem. React. Eng. 2019, 17. [Google Scholar] [CrossRef]
- Angeli, S.D.; Monteleone, G.; Giaconia, A.; Lemonidou, A.A. State-of-the-art catalysts for CH4 steam reforming at low temperature. Int. J. Hydrogen Energy 2014, 39, 1979–1997. [Google Scholar] [CrossRef]
- Nikolaidis, P.; Poullikkas, A. A comparative overview of hydrogen production processes. Renew. Sustain. Energy Rev. 2017, 67, 597–611. [Google Scholar] [CrossRef]
- Sidik, S.M.; Triwahyono, S.; Jalil, A.A.; Majid, Z.A.; Salamun, N.; Talib, N.B.; Abdullah, T.A. CO2 reforming of CH4 over Ni-Co/MSN for syngas production: Role of Co as a binder and optimization using RSM. Chem. Eng. J. 2016, 295, 1–10. [Google Scholar] [CrossRef]
- Aman, D.; Radwan, D.; Ebaid, M.; Mikhail, S.; van Steen, E. Comparing nickel and cobalt perovskites for steam reforming of glycerol. Mol. Catal. 2018, 452, 60–67. [Google Scholar] [CrossRef]
- Sadykov, V.A.; Gubanova, E.L.; Sazonova, N.N.; Pokrovskaya, S.A.; Chumakova, N.A.; Mezentseva, N.V.; Bobin, A.S.; Gulyaev, R.V.; Ishchenko, A.V.; Krieger, T.A.; et al. Dry reforming of methane over Pt/PrCeZrO catalyst: Kinetic and mechanistic features by transient studies and their modeling. Catal. Today 2011, 171, 140–149. [Google Scholar] [CrossRef]
- Gokon, N.; Yamawaki, Y.; Nakazawa, D.; Kodama, T. Kinetics of methane reforming over Ru/γ-Al2O3-catalyzed metallic foam at 650–900 °C for solar receiver-absorbers. Int. J. Hydrogen Energy 2011, 36, 203–215. [Google Scholar] [CrossRef]
- Abdullah, B.; Abd Ghani, N.A.; Vo, D.V.N. Recent advances in dry reforming of methane over Ni-based catalysts. J. Clean. Prod. 2017, 162, 170–185. [Google Scholar] [CrossRef]
- Abdulrasheed, A.; Jalil, A.A.; Gambo, Y.; Ibrahim, M.; Hambali, H.U.; Shahul Hamid, M.Y. A review on catalyst development for dry reforming of methane to syngas: Recent advances. Renew. Sustain. Energy Rev. 2019, 108, 175–193. [Google Scholar] [CrossRef]
- Rategarpanah, A.; Meshkani, F.; Wang, Y.; Arandiyan, H.; Rezaei, M. Thermocatalytic conversion of methane to highly pure hydrogen over Ni–Cu/MgO·Al2O3 catalysts: Influence of noble metals (Pt and Pd) on the catalytic activity and stability. Energy Convers. Manag. 2018, 166, 268–280. [Google Scholar] [CrossRef]
- Kumar, R.; Kumar, K.; Choudary, N.V.; Pant, K.K. Effect of support materials on the performance of Ni-based catalysts in tri-reforming of methane. Fuel Process. Technol. 2019, 186, 40–52. [Google Scholar] [CrossRef]
- Kang, D.; Lim, H.S.; Lee, J.W. Mesoporous Fe2O3–CeO2–Al2O3 Oxygen Carrier for Chemical Looping Dry Reforming with Subsequent Water Splitting. Ind. Eng. Chem. Res. 2020, 59, 15912–15920. [Google Scholar] [CrossRef]
- Chuayboon, S.; Abanades, S.; Rodat, S. Solar chemical looping reforming of methane combined with isothermal H2O/CO2 splitting using ceria oxygen carrier for syngas production. J. Energy Chem. 2020, 41, 60–72. [Google Scholar] [CrossRef]
- Wang, Y.; Zheng, Y.; Wang, Y.; Li, K.; Wang, Y.; Jiang, L.; Zhu, X.; Wei, Y.; Wang, H. Syngas production modified by oxygen vacancies over CeO2-ZrO2-CuO oxygen carrier via chemical looping reforming of methane. Appl. Surf. Sci. 2019, 481, 151–160. [Google Scholar] [CrossRef]
- Henard, C.A.; Smith, H.; Dowe, N.; Kalyuzhnaya, M.G.; Pienkos, P.T.; Guarnieri, M.T. Bioconversion of methane to lactate by an obligate methanotrophic bacterium. Sci. Rep. 2016, 6, 21585. [Google Scholar] [CrossRef]
- Shoji, S.; Peng, X.; Yamaguchi, A.; Watanabe, R.; Fukuhara, C.; Cho, Y.; Yamamoto, T.; Matsumura, S.; Yu, M.W.; Ishii, S.; et al. Photocatalytic uphill conversion of natural gas beyond the limitation of thermal reaction systems. Nat. Catal. 2020, 3, 148–153. [Google Scholar] [CrossRef]
- Aznar-Sánchez, J.A.; Belmonte-Ureña, L.J.; Velasco-Muñoz, J.F.; Manzano-Agugliaro, F. Economic analysis of sustainable water use: A review of worldwide research. J. Clean. Prod. 2018, 198, 1120–1132. [Google Scholar] [CrossRef]
- Jouny, M.; Luc, W.; Jiao, F. General Techno-Economic Analysis of CO2 Electrolysis Systems. Ind. Eng. Chem. Res. 2018, 57, 2165–2177. [Google Scholar] [CrossRef]
- Towler, G.; Sinnott, R.A.Y. Chemical Engineering Design: Principles, Practice and Economics of Plant and Process Design; Elsevier: Amsterdam, The Netherlands, 2013. [Google Scholar] [CrossRef]
- Nikookaran, N.; Karakostas, G.; Todd, T.D. Combining Capital and Operating Expenditure Costs in Vehicular Roadside Unit Placement. IEEE Trans. Veh. Technol. 2017, 66, 7317–7331. [Google Scholar] [CrossRef]
- Qian, J.X.; Chen, T.W.; Enakonda, L.R.; Liu, D.B.; Basset, J.M.; Zhou, L. Methane decomposition to pure hydrogen and carbon nano materials: State-of-the-art and future perspectives. Int. J. Hydrogen Energy 2020, 45, 15721–15743. [Google Scholar] [CrossRef]
- Mueller-Langer, F.; Tzimas, E.; Kaltschmitt, M.; Peteves, S. Techno-economic assessment of hydrogen production processes for the hydrogen economy for the short and medium term. Int. J. Hydrogen Energy 2007, 32, 3797–3810. [Google Scholar] [CrossRef]
- Keipi, T.; Tolvanen, H.; Konttinen, J. Economic analysis of hydrogen production by methane thermal decomposition: Comparison to competing technologies. Energy Convers. Manag. 2018, 159, 264–273. [Google Scholar] [CrossRef]
- Keipi, T.; Hankalin, V.; Nummelin, J.; Raiko, R. Techno-economic analysis of four concepts for thermal decomposition of methane: Reduction of CO2 emissions in natural gas combustion. Energy Convers. Manag. 2016, 110, 1–12. [Google Scholar] [CrossRef]
- Mondal, K.C.; Ramesh Chandran, S. Evaluation of the economic impact of hydrogen production by methane decomposition with steam reforming of methane process. Int. J. Hydrogen Energy 2014, 39, 9670–9674. [Google Scholar] [CrossRef]
- Timmerberg, S.; Kaltschmitt, M.; Finkbeiner, M. Hydrogen and hydrogen-derived fuels through methane decomposition of natural gas—GHG emissions and costs. Energy Convers. Manag. X 2020, 7, 100043. [Google Scholar] [CrossRef]
- Labanca, A.; Feugeas, J.; Miranda, P. Economic Analysis of CO2-Free Hydrogen Production from Natural Gas by Plasma Pyrolysis; Rio Oil & Gas Expo and Conference: Rio de Janeiro, Brazil, 2006; pp. 1–19. [Google Scholar]
- Gaudernack, B.; Lynum, S. Hydrogen from natural gas without release of CO2 to the atmosphere. Int. J. Hydrogen Energy 1998, 23, 1087–1093. [Google Scholar] [CrossRef]
- Khojasteh Salkuyeh, Y.; Saville, B.A.; MacLean, H.L. Techno-economic analysis and life cycle assessment of hydrogen production from natural gas using current and emerging technologies. Int. J. Hydrogen Energy 2017, 42, 18894–18909. [Google Scholar] [CrossRef]
- Godini, H.R.; Xiao, S.; Jašo, S.; Stünkel, S.; Salerno, D.; Son, N.X.; Song, S.; Wozny, G. Techno-economic analysis of integrating the methane oxidative coupling and methane reforming processes. Fuel Process Technol. 2013, 106, 684–694. [Google Scholar] [CrossRef]
- Fei, Q.; Liang, B.; Tao, L.; Tan, E.C.; Gonzalez, R.; Henard, C.; Guarnieri, M. Biological valorization of natural gas for the production of lactic acid: Techno-economic analysis and life cycle assessment. Biochem. Eng. J. 2020, 158, 107500. [Google Scholar] [CrossRef]
- Cantera, S.; Muñoz, R.; Lebrero, R.; López, J.C.; Rodríguez, Y.; García-Encina, P.A. Technologies for the bioconversion of methane into more valuable products. Curr. Opin. Biotechnol. 2018, 50, 128–135. [Google Scholar] [CrossRef]
- Diglio, G.; Hanak, D.P.; Bareschino, P.; Mancusi, E.; Pepe, F.; Montagnaro, F.; Manovic, V. Techno-economic analysis of sorption-enhanced steam methane reforming in a fixed bed reactor network integrated with fuel cell. J. Power Sources 2017, 364, 41–51. [Google Scholar] [CrossRef]
- Zhang, C.; Jun, K.W.; Gao, R.; Kwak, G.; Park, H.G. Carbon dioxide utilization in a gas-to-methanol process combined with CO2/Steam-mixed reforming: Techno-economic analysis. Fuel 2017, 190, 303–311. [Google Scholar] [CrossRef]
- Gangadharan, P.; Kanchi, K.C.; Lou, H.H. Evaluation of the economic and environmental impact of combining dry reforming with steam reforming of methane. Chem. Eng. Res. Des. 2012, 90, 1956–1968. [Google Scholar] [CrossRef]
- Luk, H.T.; Lei, H.M.; Ng, W.Y.; Ju, Y.; Lam, K.F. Techno-economic analysis of distributed hydrogen production from natural gas. Chin. J. Chem. Eng. 2012, 20, 489–496. [Google Scholar] [CrossRef]
- Dufour, J.; Serrano, D.P.; Gálvez, J.L.; Moreno, J.; González, A. Hydrogen production from fossil fuels: Life cycle assessment of technologies with low greenhouse gas emissions. Energy Fuels 2011, 25, 2194–2202. [Google Scholar] [CrossRef]
- Dufour, J.; Serrano, D.P.; Gálvez, J.L.; González, A.; Soria, E.; Fierro, J.L.G. Life cycle assessment of alternatives for hydrogen production from renewable and fossil sources. Int. J. Hydrogen Energy 2012, 37, 1173–1183. [Google Scholar] [CrossRef]
- Dufour, J.; Serrano, D.P.; Gálvez, J.L.; Moreno, J.; García, C. Life cycle assessment of processes for hydrogen production. Environmental feasibility and reduction of greenhouse gases emissions. Int. J. Hydrogen Energy 2009, 34, 1370–1376. [Google Scholar] [CrossRef]
- Dufour, J.; Gálvez, J.L.; Serrano, D.P.; Moreno, J.; Martínez, G. Life cycle assessment of hydrogen production by methane decomposition using carbonaceous catalysts. Int. J. Hydrogen Energy 2010, 35, 1205–1212. [Google Scholar] [CrossRef]
- Srilatha, K.; Bhagawan, D.; Kumar, S.S.; Himabindu, V. Sustainable fuel production by thermocatalytic decomposition of methane—A review. S. Afr. J. Chem. Eng. 2017, 24, 156–167. [Google Scholar] [CrossRef]
- Kothari, R.; Buddhi, D.; Sawhney, R.L. Comparison of environmental and economic aspects of various hydrogen production methods. Renew. Sustain. Energy 2008, 12, 553–563. [Google Scholar] [CrossRef]
- Hamid, U.; Rauf, A.; Ahmed, U.; Selim Arif Sher Shah, M.; Ahmad, N. Techno-economic assessment of process integration models for boosting hydrogen production potential from coal and natural gas feedstocks. Fuel 2020, 266, 117111. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).