Linkages among Soil Properties and Litter Quality in Agroforestry Systems of Southeastern Brazil
Abstract
1. Introduction
2. Materials and Methods
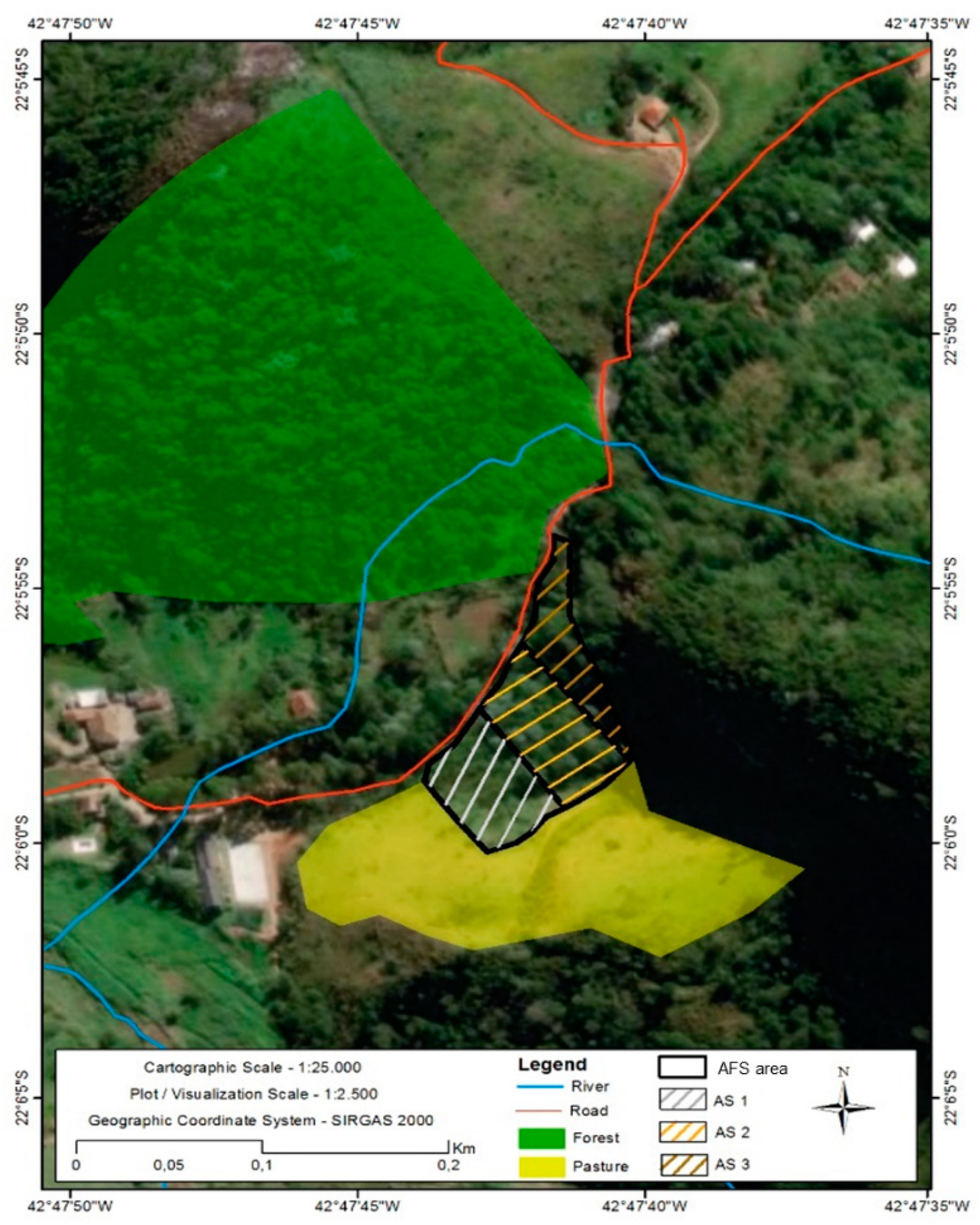
2.1. Site Description and Land Uses
2.2. Soil, Litter and Arthropod Sampling
2.3. Soil Microbial Measurements
2.4. Soil Physicochemical Analyses
2.5. Litter Nutrient Analysis
2.6. Statistical Analyses
3. Results
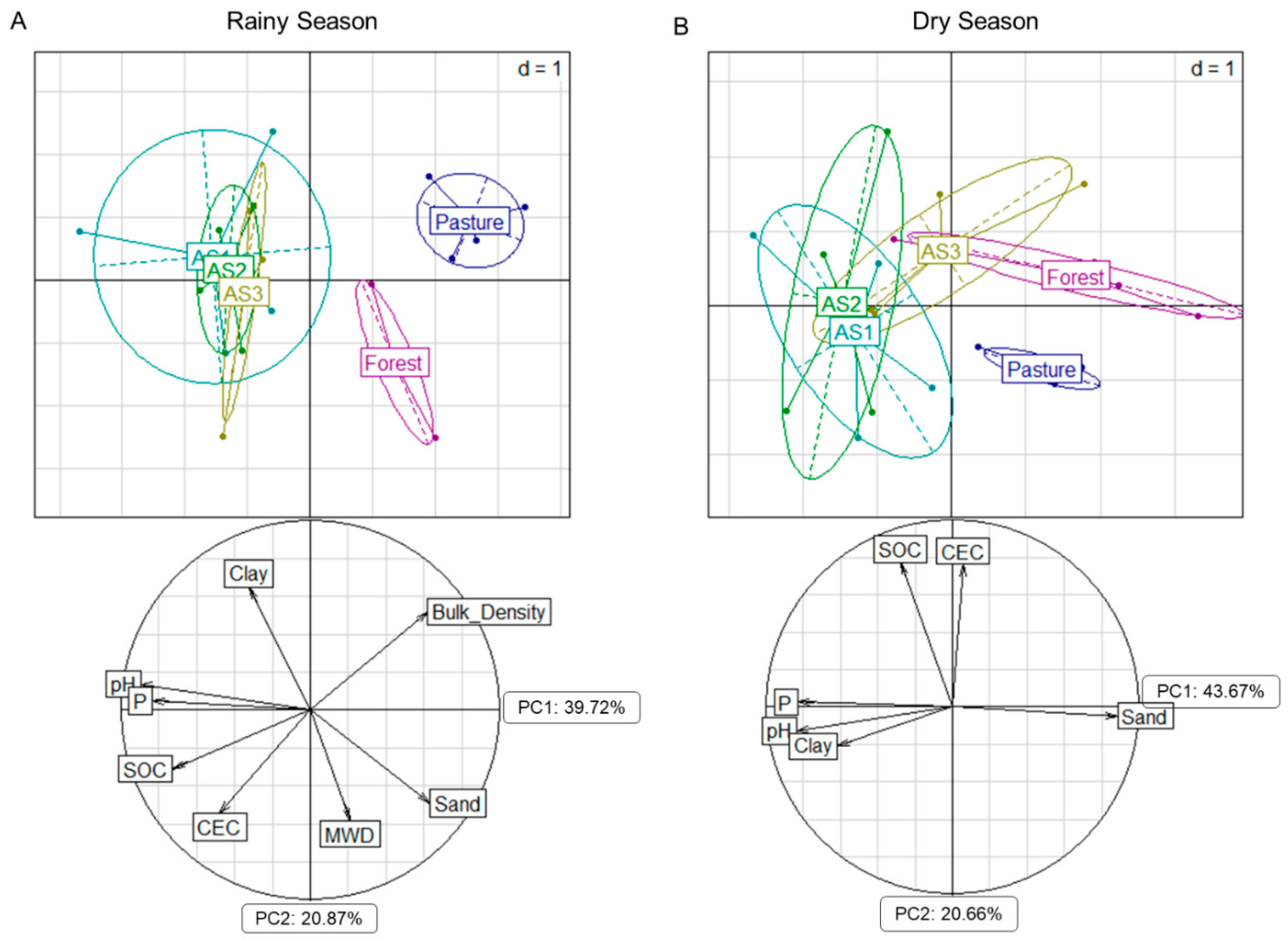
3.1. Soil Chemical and Physical Properties
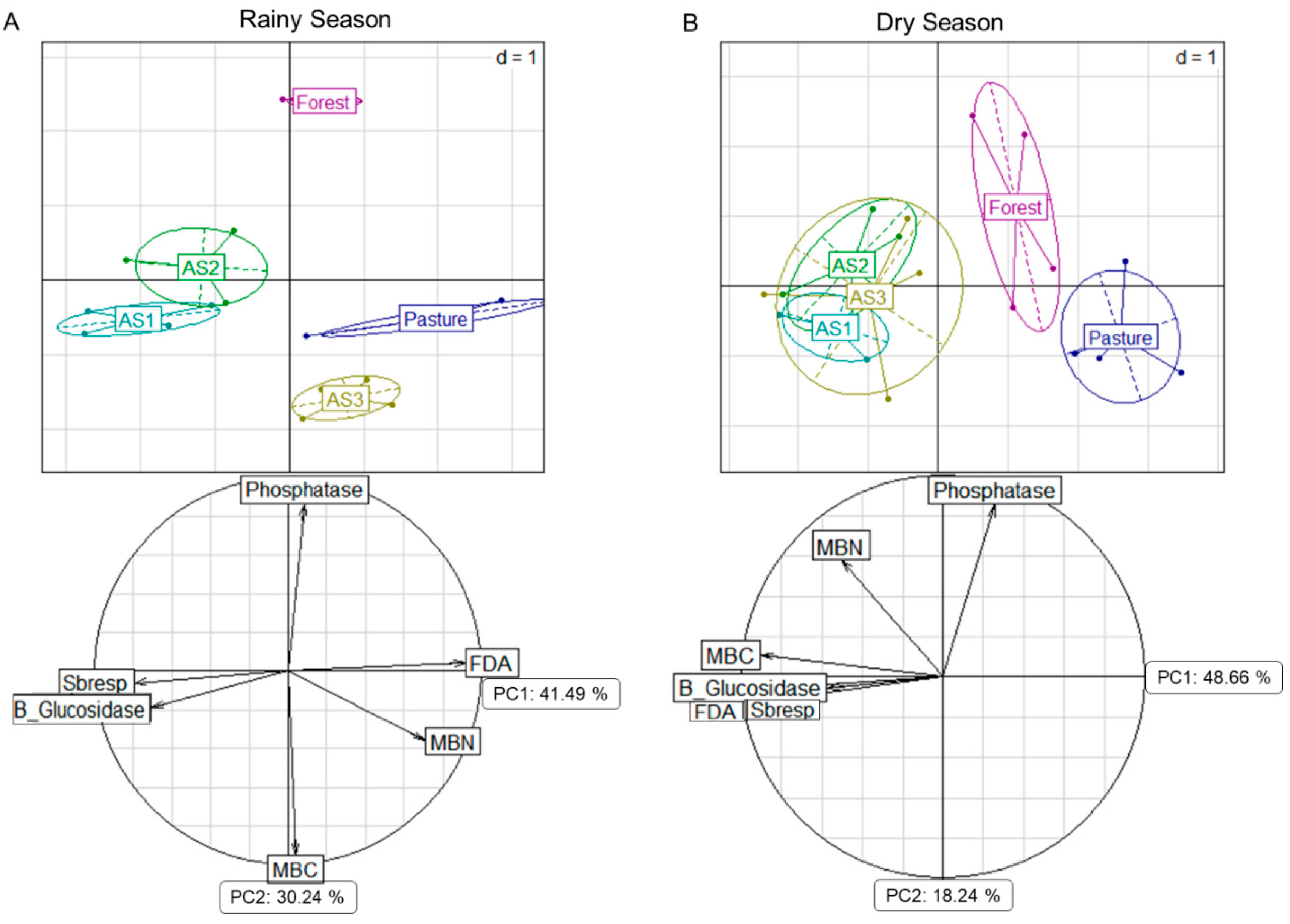
3.2. Microbiological Properties
3.3. Litter and Soil Dwelling Arthropods and Diversity Indices
3.4. Litter Chemical Properties
3.5. Relationships Between Litter Quality, SOM and Key Soil Quality Parameters
4. Discussion
4.1. Soil Chemical and Physical Properties Across Different Land Uses
4.2. Soil Biological Properties
4.3. Linkages Between Litter Quality, SOM and Soil Quality Parameters
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Brancalion, P.H.S.; Niamir, A.; Broadbent, E.; Crouzeilles, R.; Barros, F.S.M.; Zambrano, A.M.A.; Baccini, A.; Aronson, J.; Goetz, S.; Reid, J.L.; et al. Global restoration opportunities in tropical rainforest landscapes. Sci. Adv. 2019, 5, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Gurgel, Â.C.; Costa, C.F. Reversing the Carbon Sign of Brazilian Agriculture—An Estimation of the Mitigation Potential of Technologies of the ABC Plan from 2012 to 2023; ABC Observatório: Santo André, Brazil, 2015. [Google Scholar]
- Dias Filho, M.B. Pasture Diagnosis in Brasil-Embrapa; Embrapa Eastern Amazon: Brasília, Brazil, 2014; pp. 1–36. [Google Scholar]
- Ribeiro, N.G., Jr.; Adriano, A.P.R.; Silva, I.V. Death of pastures syndrome: Tissue changes in Urochloa hybrida cv. Mulato II and Urochloa brizantha cv. Marandu. Braz. J. Biol. 2017, 77, 97–107. [Google Scholar] [CrossRef]
- Food and Agriculture Organization of the United Nations. Healthy Soils are the Basis for Healthy Food Production; FAO: Rome, Italy, 2015. [Google Scholar]
- Schulz, J. Imitating Natural Ecosystems through Successional Agroforestry for the Regeneration of Degraded Lands-a Case Study of Smallholder Agriculture in Northeastern Brazil; Nova Science Publishers: New York, NY, USA, 2011. [Google Scholar]
- Dagar, J.C. Agroforestry: Four decades of research development. Indian J. Agrofor. 2016, 18, 1–32. [Google Scholar]
- Lewis, S.L.; Wheeler, C.E.; Mitchard, E.T.; Koch, A. Regenerate natural forests to store carbon. Nat. Commun. 2020, 568, 25–28. [Google Scholar] [CrossRef] [PubMed]
- Schulz, B.; Becker, B.; Gotsch, E. Indigenous knowledge in a modern sustainable agroforestry system—A case study from eastern Brazil. Agroforest. Syst. 1994, 25, 59–69. [Google Scholar] [CrossRef]
- Moressi, M.; Padovan, M.P.; Pereira, Z.V. Seed bank as indicator of restoration in multistrata agroforestry systems in southwestern of mato grosso do sul, Brazil. Rev. Árvore 2014, 38, 1073–1083. [Google Scholar] [CrossRef]
- Lenz, A.M.; Rosa, H.A.; Mercante, E. Expansion of eucalyptus energy plantations under a Livestock-Forestry Integration scenario for agroindustries in Western Paraná, Brazil. Ecol. Indic. 2019, 98, 39–48. [Google Scholar] [CrossRef]
- United Nations. Transforming Our World: The 2030 Agenda for Sustainable Development—Finalised Text for Adoption; UN: New York, NY, USA, 2015. [Google Scholar]
- Ministério do Meio Ambiente, REBRAF. Brazilian Agroforestry Network, Public Policies and Financing for Agroforestry Development in Brazil; Seminar Report; Ministry of the Environment: Brasília, Brazil, 2005.
- Bini, D.; dos Santos, C.A.; Bouillet, J.P.; Goncalves, J.L.D.; Cardoso, E. Eucalyptus grandis and Acacia mangium in monoculture and intercropped plantations: Evolution of soil and litter microbial and chemical attributes during early stages of plant development. Appl. Soil Ecol. 2013, 63, 57–66. [Google Scholar] [CrossRef]
- Wartenberg, A.C.; Blaser, W.J.; Gattinger, A.; Roshetko, J.M.; Noordwijk, M.V.; Six, J. Does shade tree diversity increase soil fertility in cocoa plantations? Agric. Ecosyst. Environ. 2017, 248, 190–199. [Google Scholar] [CrossRef]
- Chen, C.; Chen, H.Y.H.; Chen, X.; Huang, Z. Meta-analysis shows positive effects of plant diversity on microbial biomass and respiration. Nat. Commun. 2019, 10, 1–10. [Google Scholar] [CrossRef]
- Oliveira, P.H.G.; Gama-Rodrigues, A.C.; Gama-Rodrigues, E.F.; Sales, M.V.S. Litter and soil-related variation in functional group abundances in cacao agroforests using structural equation modeling. Ecol. Indic. 2018, 84, 254–262. [Google Scholar] [CrossRef]
- Hergoualc’h, K.; Blanchart, E.; Skiba, U.; Hénault, C.; Harmand, J.M. Changes in carbon stock and greenhouse gas balance in a coffee (Coffea arabica) monoculture versus an agroforestry system with Inga densiflora, in Costa Rica. Agric. Ecosyst. Environ. 2012, 15, 102–110. [Google Scholar] [CrossRef]
- Tumwebaze, S.B.; Bevilacqua, E.; Briggs, R.; Volk, T. Soil organic carbon under a linear simultaneous agroforestry system in Uganda. Agroforest. Syst. 2012, 80, 1–13. [Google Scholar] [CrossRef]
- Duarte, E.M.G.; Cardoso, I.M.; Stijnen, T.; Mendonça, M.A.F.C.; Coelho, M.S.; Cantarutti, R.B.; Kuyper, T.W.; Villani, E.M.D.A.; Mendonça, E.D.S. Decomposition and nutrient release in leaves of Atlantic Rainforest tree species used in agroforestry systems. Agroforest. Syst. 2013, 87, 835–847. [Google Scholar] [CrossRef]
- Rousseau, L.; Fonte, S.J.; Téllez, O.; van der Hoek, R.; Lavelle, P. Soil macrofauna as indicators of soil quality and land use impacts in smallholder agroecosystems of western Nicaragua. Ecol. Indic. 2013, 27, 71–82. [Google Scholar] [CrossRef]
- Kamau, S.; Barrios, E.; Karanja, N.K.; Ayuke, F.O.; Lehmann, J. Soil macrofauna abundance under dominant tree species increases along a soil degradation gradient. Soil Biol. Biochem. 2017, 112, 35–46. [Google Scholar] [CrossRef]
- Shi, L.; Feng, W.; Xu, J.; Kuzyakov, Y. Agroforestry systems: Meta-analysis of soil carbon stocks, sequestration processes, and potential. Land. Degrad. 2018, 29, 3886–3897. [Google Scholar] [CrossRef]
- Zaro, G.C.; Caramori, P.H.; Yada, G.M., Jr.; Sanquetta, C.R.; Filho, A.A.; Nunes, A.L.P.; Prete, C.E.C.; Voroney, P. Carbon sequestration in an agroforestry system of coffee with rubber trees compared to open-grown coffee in southern Brazil. Agroforest. Syst. 2019, 94, 1–11. [Google Scholar] [CrossRef]
- Kearney, S.P.; Fonte, S.J.; García, E.K.M.; Chan, P. Evaluating ecosystem service trade-offs and synergies from slash-and-mulch agroforestry systems. Ecol. Indic. 2019, 105, 264–278. [Google Scholar] [CrossRef]
- Jian, J.; Du, X.; Stewart, R.D. A database for global soil health assessment. Sci. Data. 2020, 7, 1–8. [Google Scholar] [CrossRef]
- USDA-Natural Resources Conservation Service. Keys to Soil Taxonomy, 12th ed.; NRCS: Washington, DC, USA, 2014.
- IBGE, Instituto Brasileiro de Geografia e Estatística. Technical Manual of the Brazilian Vegetation, 2nd ed.; IBGE, Instituto Brasileiro de Geografia e Estatística: Rio de Janeiro, Brazil, 2012.
- Assunção, S.A.; Pereira, M.G.; Rosset, J.S.; Berbara, R.L.L.; García, A.C. Carbon input and the structural quality of soil organic matter as a function of agricultural management in a tropical climate region of Brazil. Sci. Total Environ. 2019, 658, 901–911. [Google Scholar] [CrossRef] [PubMed]
- Dos Ferreira, C.R.; da Neto, S.E.C.; Pereira, M.G.; do Guedes, J.N.; Rosset, J.S.; dos Anjos, L.H.C. Dynamics of soil aggregation and organic carbon fractions over 23 years of no-till management. Soil Till. Res. 2020, 198, 1–9. [Google Scholar] [CrossRef]
- Francaviglia, R.; Renzi, G.; Ledda, L.; Benedetti, A. Organic carbon pools and soil biological fertility are affected by land use intensity in Mediterranean ecosystems of Sardinia, Italy. Sci. Total Environ. 2017, 599–600, 789–796. [Google Scholar] [CrossRef] [PubMed]
- Lagomarsino, A.; Benedetti, A.; Marinari, S.; Pompili, L.; Moscatelli, M.C.; Roggero, P.P.; Lai, R.; Ledda, L.; Grego, S. Soil organic C variability and microbial functions in a Mediterranean agro-forest ecosystem. Biol. Fertil. Soils 2011, 47, 283–291. [Google Scholar] [CrossRef]
- Teixeira, P.C.; Donagema, G.K.; Fontana, A.; Texeira, W.G.M. Soil Analysis Methods Manual, 3rd ed.; Embrapa: Brasília, Brazil, 2017. [Google Scholar]
- Moldenke, A.R. Arthropods. In Methods of Soil Analysis: Microbiological and Biochemical Properties; Weaver, R.W., Ed.; SSSA: Madison, WI, USA, 1994. [Google Scholar]
- Gallo, D.; Nakano, O.; Silveira Neto, S.; Carvalho, R.P.L.; Batista, G.C.; Filho, B.E.; Parra, J.R.P.; Zucchi, R.A.; Alves, S.B.; Vendramim, J.D. Agricultural Entomology Manual, 2nd ed.; Agronômica Ceres: São Paulo, Brazil, 1988. [Google Scholar]
- Dindal, D. Soil Biology Guide; John Wiley and Sons: New York, NY, USA, 1990; Volume 79, pp. 7–31. [Google Scholar]
- Shannon, C.E.A. Mathematical Theory of Communication. Bell Syst. Tech. J. 1948, 27, 379–423. [Google Scholar] [CrossRef]
- Sanquetta, C.R. Methods for determining forest biomass. In Forests and Carbon; Sanquetta, C.R., Ed.; Imprensa Universitária da UFPR: Curitiba, Brazil, 2002; pp. 119–140. [Google Scholar]
- Vance, E.D.; Brookes, P.; Jenkinson, D.S. An extraction method for measuring soil microbial biomass-C. Soil Biol. Biochem. 1987, 19, 703–707. [Google Scholar] [CrossRef]
- Tate, K.R.; Ross, D.J.; Feltham, C.W. A direct extraction method to estimate soil microbial C: Effects of experimental variables and some different calibration procedures. Soil Biol. Biochem. 1988, 20, 329–335. [Google Scholar] [CrossRef]
- Bartlett, R.J.; Ross, D.S. Colorimetric determination of oxidizable carbon in acid soil solutions. Soil Sci. Soc. Am. J. 1988, 52, 1191–1192. [Google Scholar] [CrossRef]
- Brookes, P.C.; Andrea, L.; Prudent, G.; Jenkinson, D.S. Chloroform fumigation and the release of soil nitrogen: A rapid direct extraction method to measure microbial biomass nitrogen in soil. Soil Biol. Biochem. 1985, 17, 837–842. [Google Scholar] [CrossRef]
- Jenkinson, D.S.; Powlson, D.S. The effects of biocidal treatments on metabolism in soil. V. A method for measuring soil biomass. Soil Biol. Biochem. 1976, 8, 209–213. [Google Scholar] [CrossRef]
- Tabatabai, M.A. Soil enzymes. In Methods of Soil Analysis. Part 2: Microbiological and Biochemical Properties; Weaver, R.W., Angle, S., Bottomley, P., Bezdicek, D., Smith, S., Tabatabai, A., Wollum, A., Eds.; SSSA: Madison, WI, USA, 1994; Volume 5, pp. 775–833. [Google Scholar]
- Schnürer, J.; Rosswall, T. Fluorescein Diacetate Hydrolysis as a Measure of Total Microbial Activity in Soil and Litter. Appl. Environ. Microbiol. 1982, 43, 1256–1261. [Google Scholar] [CrossRef] [PubMed]
- Dick, R.P.; Breakwell, D.P.; Turco, R.F. Soil enzyme activity and biodiversity measurements as integrative microbiological indicators. In Methods for Assessing Soil Quality; Doran, J.W., Jones, A.J., Eds.; SSSA Special Publication: Madison, WI, USA, 1996; Volume 49, pp. 247–271. [Google Scholar]
- Yeomans, J.C.; Bremner, J.M. A rapid and precise method for routine determination of organic carbon in soil. Commun. Soil Sci. Plan. 1988, 19, 1467–1476. [Google Scholar] [CrossRef]
- Weil, R.; Islam, K.; Stine, M.; Gruver, J.; Samson-Liebig, S. Estimating active carbon for soil quality assessment: A simplified method for laboratory and field use. Am. J. Alternative Agric. 2003, 18, 3–17. [Google Scholar] [CrossRef]
- Yoder, R.E. A direct method of aggregate analysis of soil and a study of the physical nature of erosion losses. Agron. J. 1936, 28, 337–351. [Google Scholar] [CrossRef]
- Van Bavel, C.H.M. Mean weight diameter of soil aggregates as a statistical index of aggregation. Soil Sci. Soc. Am. J. 1950, 14, 20–23. [Google Scholar] [CrossRef]
- Ruiz, H.A. Increased accuracy in particle-size analysis by sampling the silt + clay suspensión. Rev. Bras. Cienc. Solo 2005, 29, 297–300. [Google Scholar] [CrossRef]
- U.S. EPA. Method 3051A (SW-846)-Microwave Assisted acid Digestion of Sediments, Sludges, Soils, and Oils, Revision 1; U.S. EPA: Washington, DC, USA, 2007.
- Foundation for Statistical Computing, Development Core Team. A Language and Environment for Statistical Computing; Foundation for Statistical Computing: Vienna, Austria, 2019. [Google Scholar]
- Oksanen, J.; Blanchet, F.G.; Kindt, R.; Legendre, P.; Minchin, P.R.; O’Hara, R.B.; Simpson, G.L.; Solymos, P.; Stevens, M.H.H.; Wagner, H. Vegan: Community Ecology Package. R Package Version 2; CRAN; 2018; pp. 5–22. [Google Scholar]
- Dray, S.; Dufour, A.B.; Chessel, D. The ade4 Package-II: Two-Table and K-Table Methods. R News 2007, 7, 47–52. [Google Scholar]
- SAS Institute. JMP Pro 14,14.0.0.; SAS Institute: Cary, NC, USA, 2018. [Google Scholar]
- Utomo, M. Effect of rock phosphate on soil properties and apparent phosphorus recovery in acid soil of Sumatra. Plant Soil 1995, 171, 199–202. [Google Scholar] [CrossRef]
- Jama, B.; Palm, C.A.; Buresh, R.J.; Niang, A.; Gachengo, C.; Nziguheba, G.; Amadalo, B. Tithonia diversifolia as a green manure for soil fertility improvement in western Kenya: A review. Agroforest. Syst. 2000, 49, 201–221. [Google Scholar] [CrossRef]
- Ayaga, G.; Todd, A.; Brookes, P.C. Enhanced biological cycling of phosphorus increases its availability to crops in low-input subsaharan farming systems. Soil Biol. Biochem. 2006, 38, 81–90. [Google Scholar] [CrossRef]
- Cotrufo, M.F.; Wallenstein, M.D.; Boot, C.M.; Denef, K.; Paul, E. The Microbial Efficiency-Matrix Stabilization (MEMS) framework integrates plant litter decomposition with soil organic matter stabilization: Do labile plant inputs form stable soil organic matter? Glob. Chang. Biol. 2013, 19, 988–995. [Google Scholar] [CrossRef] [PubMed]
- Manzoni, S.; Čapek, P.; Mooshammer, M.; Lindahl, B.D.; Richter, A.; Šantrůčková, H. Optimal metabolic regulation along resource stoichiometry gradients. Ecol. Lett. 2017, 20, 1182–1191. [Google Scholar] [CrossRef] [PubMed]
- Nciizah, A.D.; Wakindiki, I.I.C. Physical indicators of soil erosion; aggregate stability and erodibility. Arch. Agron. Soil Sci. 2015, 61, 827–842. [Google Scholar] [CrossRef]
- Nunes, M.R.; Denardin, J.E.; Pauletto, E.A.; Faganello, A.; Pinto, L.F.S. Mitigation of clayey soil compaction managed under no-tillage. Soil Tillage Res. 2015, 148, 119–126. [Google Scholar] [CrossRef]
- Fonte, S.J.; Hegglin, D.; Nesper, M.; Velásquez, J.E.; Ramirez, B.; Rao, I.M.; Bernasconi, S.; Bünemann, E.K.; Frossard, E.; Oberson, A. Pasture degradation impacts soil phosphorus storage via changes to aggregate-associated soil organic matter in highly weathered tropical soils of Caquetá, Colombia. Soil Biol. Biochem. 2014, 68, 150–157. [Google Scholar] [CrossRef]
- Fonte, S.J.; Schowalter, T.D. Decomposition of greenfall vs. senescent foliage in a tropical forest ecosystem in Puerto Rico. Biotropica 2004, 36, 474–482. [Google Scholar] [CrossRef]
- Olander, L.P.; Vitousek, P.M. Regulation of Soil Phosphatase and Chitinase Activity by N and P Availability. Biogeochemistry 2000, 49, 175–190. [Google Scholar] [CrossRef]
- Sanabria, C.; Dubs, F.; Lavelle, P.; Fonte, S.J.; Barot, S. Influence of regions, land uses and soil properties on termite and ant communities in agricultural landscapes of the Colombian Llanos. Eur. J. Soil Biol. 2016, 74, 81–92. [Google Scholar] [CrossRef]
- Santonja, M.; Fernandez, C.; Proffit, M.; Gers, C.; Gauquelin, T.; Reiter, I.M.; Cramer, W.; Baldy, V. Plant litter mixture partly mitigates the negative effects of extended drought on soil biota and litter decomposition in a Mediterranean oak forest. J. Ecol. 2017, 105, 801–815. [Google Scholar] [CrossRef]
- Work, T.T.; Koivula, M.; Klimaszewski, J.; Langor, D.; Spence, J.; Sweeney, J.; Hébert, C. Evaluation of carabid beetles as indicators of forest change in Canada. Can. Entomol. 2008, 140, 393–414. [Google Scholar] [CrossRef]
- Parton, W.; Silver, W.L.; Burke, I.C.; Grassens, L.; Harmon, M.E.; Currie, W.S.; King, J.Y.; Adair, E.C.; Brandt, L.A.; Hart, S.C.; et al. Global-scale similarities in nitrogen release patterns during long-term decomposition. Science 2007, 315, 361–364. [Google Scholar] [CrossRef] [PubMed]
- Carrillo, Y.; Ball, B.A.; Bradford, M.A.; Jordan, C.F.; Molina, M. Soil fauna alter the effects of litter composition on nitrogen cycling in a mineral soil. Soil Biol. Biochem. 2011, 43, 1440–1449. [Google Scholar] [CrossRef]
- Fujii, S.; Cornelissen, J.H.C.; Berg, M.; Mori, A.S. Tree leaf and root traits mediate soil faunal contribution to litter decomposition across an elevational gradient. Funct. Ecol. 2018, 32, 840–852. [Google Scholar] [CrossRef]
- Six, J.; Bossuyt, H.; Degryze, S.D.; Denef, K. A History of Research on the Link between (Micro) Aggregates, Soil Biota, and Soil Organic Matter Dynamics. Soil Till. Res. 2004, 79, 7–31. [Google Scholar] [CrossRef]
- Hays, Z.; Watson, D. Fungal Ecology, Diversity and Metabolites, 1st ed.; Ed-Tech Press: Waltham Abbey, UK, 2019. [Google Scholar]
- Lehmann, A.; Zheng, W.; Rillig, M.C. Soil biota contributions to soil aggregation. Nat. Ecol. Evol. 2017, 1, 1828–1835. [Google Scholar] [CrossRef] [PubMed]
- Lavelle, P.; Barros, E.; Blanchart, E.; Brown, G.; Desjardins, T.; Mariani, L.; Rossi, J.P. SOM management in the tropics: Why feeding the soil macrofauna? Nutr. Cycl. Agroecosyst. 2001, 61, 53–61. [Google Scholar] [CrossRef]
- Moore, J.C.; Berlow, E.L.; Coleman, D.C.; De Suiter, P.C.; Dong, Q.; Hastings, A.; Johnson, N.C.; McCann, K.S.; Melville, K.; Morin, P.J.; et al. Detritus, trophic dynamics and biodiversity. Ecol. Lett. 2004, 7, 584–600. [Google Scholar] [CrossRef]
- Craswell, E.T.; Lefroy, R.D.B. The role and function of organic matter in tropical soils. Nutr. Cycl. Agroecosys. 2001, 61, 7–18. [Google Scholar] [CrossRef]
- King, A.E.; Ali, G.A.; Gillespie, A.W.; Wagner-Riddle, C. Soil Organic Matter as Catalyst of Crop Resource Capture. Front. Environ. Sci. 2020, 8, 1–8. [Google Scholar] [CrossRef]

| System | Scientific Name | Family | Common Name | Function | Introduction |
|---|---|---|---|---|---|
| AS1 | Musa paradisiaca L. | Musaceae | banana | fruit production | planted |
| Coffea arabica | Rubiaceae | coffee | grain production | planted | |
| Carica papaya L. | Caricaceae | papaya | fruit production | planted | |
| Morus nigra L. | Moraceae | black mulberry | fruit production | planted | |
| Eugenia uniflora L. | Myrtaceae | Brazilian cherry | fruit production | planted | |
| Inga edulis Mart. | Fabaceae | ice-cream bean | fruit production | planted | |
| Myrciaria glazioviana (Kiaersk.) G. M. Barroso ex Sobral | Myrtaceae | “cabeludinha” * | fruit production | planted | |
| Malpighia glabra L. | Malpighiaceae | “acerola” * | fruit production | planted | |
| Mangifera indica L. | Anacardiaceae | mango | fruit production | planted | |
| Psidium guajava L. | Myrtaceae | guava | fruit production | natural/regenerated | |
| Hymenaea courbaril L. | Fabaceae | “jatobá” * | timber production | planted | |
| Tithonia diversifolia (Hemsl.) A. Gray | Arecaceae | mexican-sunflower | biomass production | planted | |
| Solanum mauritianum Scop | Solanaceae | “fumo-bravo” * | shade and biomass production | natural/regenerated | |
| Trema micrantha (L.) Blume | Cannabaceae | “trema” * | shade and biomass production | natural/regenerated | |
| Sapiumglandulatum (Vell.) Pax | Euphorbiaceae | “burra-leiteira” * | shade and biomass production | natural/regenerated | |
| Vernonia polycephala Less. | Asteraceae | “assa-peixe” * | pollination services | natural/regenerated | |
| AS2 | Musa acuminata | Musaceae | banana | fruit production | planted |
| Musa paradisiaca L. | Musaceae | banana | fruit production | planted | |
| Jatropha curcas L. | Euphorbiaceae | physic nut | energy production | planted | |
| Persea Americana Mill. | Lauraceae | avocado | fruit production | planted | |
| Morus nigra L. | Moraceae | black mulberry | fruit and biomass production | planted | |
| Inga edulis | Fabaceae | ice-cream bean | fruit and biomass production | planted | |
| Eriobotrya japonica (Thunb.) Lindl. | Rosaceae | “nêspera” * | fruit production | planted | |
| Carica papaya L. | Caricaceae | papaya | fruit production | planted | |
| Mangifera indica L. | Anacardiaceae | mango | fruit production | planted | |
| Eugenia uniflora | Myrtaceae | cherry | fruit production | planted | |
| Tithonia diversifolia (Hemsl.) A. Gray | Arecaceae | Mexican-sunflower | biomass production | planted | |
| Solanum mauritianum Scop | Solanaceae | “fumo-bravo” * | shade and biomass production | natural/regenerated * | |
| Piper aduncum L. | Piperaceae | “aperta-ruão” * | biomass production | natural/regenerated | |
| Vernonia polycephala Less. | Asteraceae | “assa-peixe” * | pollination services | natural/regenerated | |
| AS3 | Musa acuminata | Musaceae | banana | fruit production | planted |
| Musa paradisiaca L. | Musaceae | banana | fruit production | planted | |
| Mangifera indica L. | Anacardiaceae | mango | fruit production | planted | |
| Artocarpus heterophyllus | Moraceae | jack fruit | fruit production | planted | |
| Citrus sp. | Rutaceae | cravo lemon | fruit production | planted | |
| Plinia trunciflora (O. Berg) Kausel | Myrtaceae | jabuticaba | fruit production | planted | |
| Campomanesia phaea (O. Berg.) Landrum | Myrtaceae | cambuci | fruit production | planted | |
| Solanum mauritianum Scop | Solanaceae | “fumo-bravo” * | shade and biomass production | natural/regenerated | |
| Piper aduncum L. | Piperaceae | “aperta-ruão” * | biomass production | natural/regenerated | |
| Tithonia diversifolia (Hemsl.) A. Gray | Arecaceae | mexican-sunflower | biomass production | planted |
| Rainy Season (April) | Dry Season (September) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Soil Variable | Forest | Pasture | AS1 | AS2 | AS3 | p-Value | Forest | Pasture | AS1 | AS2 | AS3 | p-Value |
| pH | 4.5 c | 4.6 c | 5.7 a | 5.3 ab | 5.2 b | <0.001 | 4.5 a | 4.8 a | 5.1 a | 5.2 a | 4.8 a | |
| 0.1 | 0.1 | 0.3 | 0.2 | 0.1 | 0.4 | 0.1 | 0.2 | 0.4 | 0.4 | |||
| SOC (g kg−1) | 23.5 ab | 23.1 b | 28.0 a | 25.3 ab | 26.4 ab | 0.039 | 21.6 a | 21.4 a | 24.2 a | 22.6 a | 24.5 a | |
| 0.44 | 1.9 | 2.26 | 2.12 | 3.4 | 0.858 | 1.14 | 4.46 | 4.45 | 1.13 | |||
| POXC (mg kg−1) | 653 a | 653 a | 788 a | 1092 a | 826 a | 786 ab | 576 b | 1121 a | 747 ab | 660 b | 0.023 | |
| 333 | 371 | 126 | 112 | 197 | 234 | 101 | 38.7 | 225 | 323 | |||
| Avail.P (mg kg−1) | 26.5 ab | 22.5 b | 29.5 a | 31.5 a | 28.5 a | 0.002 | 20.4 bc | 18.8 c | 25.6 a | 27.7 a | 24.2 ab | <0.001 |
| 0.6 | 0.6 | 4.7 | 3.1 | 1 | 2.4 | 1.03 | 2.3 | 1.6 | 1.1 | |||
| Ca2+(meq 100 mg−1) | 0.8 b | 0.6 b | 3 a | 2.9 a | 3 a | <0.001 | 0.8 c | 0.7 c | 2.4 b | 4.4 a | 3.4 ab | <0.001 |
| 0.2 | 0.1 | 0.4 | 0.7 | 0.4 | 0.3 | 0.2 | 0.3 | 0.9 | 0.6 | |||
| Mg2+(meq 100 mg−1) | 0.7 b | 0.5 b | 2.0 a | 2.0 a | 2.1 a | <0.001 | 0.7b | 0.4 b | 2.2 a | 1.7 a | 1.4 a | <0.001 |
| 0.2 | 0.1 | 0.2 | 0.2 | 0.3 | 0.3 | 0.1 | 0.1 | 0.5 | 0.1 | |||
| K+(meq 100 mg−1) | 0.2 d | 0.1 d | 0.6 b | 0.5 c | 0.7 a | <0.001 | 0.2 c | 0.1 c | 0.6 a | 0.5 a | 0.4 b | <0.001 |
| 0.08 | 0.01 | 0.08 | 0.03 | 0.04 | 0.1 | 0.02 | 0.1 | 0.1 | 0.04 | |||
| Na+(meq 100 mg−1) | 0.1 a | 0.1 a | 0.1 a | 0.1a | 0.1 a | 0.03 b | 0.04 a | 0.04 a | 0.04 a | 0.03 ab | <0.001 | |
| 0 | 0.01 | 0.01 | 0 | 0.01 | 0.01 | 0.04 | 0.01 | 0 | 0.01 | |||
| Al3+(meq 100 mg−1) | 1.5 a | 1.6 a | 0.3 b | 0.3 b | 0.2 b | <0.001 | 1.7 a | 1.6 a | 0.3 c | 0.4 b | 0.4 bc | <0.001 |
| 0.2 | 0.06 | 0.2 | 0.2 | 0.2 | 0.4 | 0.1 | 0.1 | 0.1 | 0.1 | |||
| CEC (cmol kg−1) | 16.9 ab | 15.8 b | 16.7 ab | 18.2 ab | 19.0 a | 0.019 | 16.5 a | 14.2 a | 13.8 a | 16.1 a | 16.1 a | |
| 1 | 0.9 | 2.1 | 1.2 | 0.8 | 1.5 | 0.5 | 1.4 | 1.4 | 1.4 | |||
| BD (g m−3) | 1.5 b | 1.8 a | 1.6 b | 1.5 b | 1.5 b | <0.001 | ||||||
| 0.04 | 0.04 | 0.1 | 0.1 | 0.04 | ||||||||
| Clay (%) | 28.1 a | 30.3 a | 30.5 a | 33.8 a | 31.4 a | |||||||
| 3.3 | 3.3 | 1.1 | 5.1 | 4.7 | ||||||||
| Sand (%) | 55.8 a | 55.7 a | 40.01 a | 46.9 a | 49.6 a | |||||||
| 14.1 | 5.9 | 3.8 | 5 | 8.2 | ||||||||
| MWD (mm) | 4.7 a | 4.5 ab | 4.5 ab | 4.5 ab | 4.3 b | 0.025 | ||||||
| 0.04 | 0.1 | 0.3 | 0.02 | 0.1 | ||||||||
| Rainy Season (April) | Dry Season (September) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Microbiological Variables | Forest | Pasture | AS1 | AS2 | AS3 | p-Value | Forest | Pasture | AS1 | AS2 | AS3 | p-Value |
| MBC (mg microbial C kg−1 soil) | 339 d | 507 b | 530 ab | 429 c | 571 a | <0.001 | 667 b | 580 c | 708 a | 716 a | 727 a | <0.001 |
| 22.5 | 20.6 | 26.6 | 27.4 | 22.1 | 20.2 | 13.5 | 14 | 27.9 | 6.9 | |||
| MBN (mg microbial N kg−1 soil) | 42.9 b | 51.7 ab | 29.6 c | 28.9 c | 63.2 a | <0.001 | 70.6 a | 66.6 a | 72 a | 87.3 a | 75.1 a | |
| 4.1 | 5.9 | 4.3 | 6.1 | 6.9 | 15.6 | 7.8 | 5.2 | 6.3 | 15.2 | |||
| Sbresp (µg C-CO2 g−1 h−1) | 1.9 a | 1.7 a | 2.3 a | 2.1 a | 1.9 a | 2.2 bc | 1.9 c | 3.3 a | 2.8 ab | 2.7 abc | <0.001 | |
| 0.2 | 0.5 | 0.4 | 0.2 | 0.4 | 0.2 | 0.5 | 0.3 | 0.1 | 0.4 | |||
| FDA (µg fluorescein g−1 soil h−1) | 114 b | 128 a | 96.2 c | 102.4 bc | 113 b | <0.001 | 101.2 b | 88.2 b | 130 a | 126 a | 126 a | <0.001 |
| 5.2 | 3.22 | 2.7 | 5.4 | 9.8 | 9.1 | 8.2 | 11.9 | 11.6 | 7.7 | |||
| β-glucosidase (μmol g−1 h−1 p-nitrophenyl) | 6.5 ab | 5.7 b | 8.1 a | 7.1 ab | 7.1 ab | 0.072 | 9.9 a | 8.5 a | 10.5 a | 9.9 a | 10.2 a | |
| 1.02 | 0.8 | 1.3 | 1.1 | 1.02 | 1.02 | 0.8 | 1.3 | 1.1 | 1.02 | |||
| Acidic Phosphatase (μmol g−1 h−1 p-nitrophenyl) | 8.6 a | 5.2 b | 5.6 b | 4.9 b | 5 b | <0.001 | 13.9 a | 5.7 b | 6.1 b | 6.3 b | 6.7 b | 0.008 |
| 0.3 | 0.3 | 0.7 | 0.5 | 0.4 | 4.5 | 2.4 | 2.9 | 2.4 | 2.8 | |||
| Rainy Season (April) | Dry Season (September) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Epigeal Fauna (Ind. trap−1 day−1) | Forest | Pasture | AS1 | AS2 | AS3 | p-Value | Forest | Pasture | AS1 | AS2 | AS3 | p-Value |
| Acari | 0.27 b | 0.53 b | 0.76 b | 0.94 b | 1.96 a | <0.001 | 0.65 c | 0.71 bc | 2.38 ab | 1.89 abc | 2.68 a | <0.01 |
| 0.2 | 0.6 | 0.6 | 0.7 | 0.8 | 0.3 | 0.6 | 1.9 | 0.7 | 1.4 | |||
| Araneae | 1.11 a | 1.68 a | 1.09 a | 0.44 a | 0.63 a | 1.07 a | 2.22 a | 1.75 a | 0.68 a | 1.86 a | ||
| 0.7 | 2.2 | 0.5 | 0.4 | 0.6 | 0.5 | 3.5 | 1.4 | 0.5 | 0.8 | |||
| Coleoptera | 1.22 a | 0.39 b | 1.06 ab | 0.65 ab | 0.57 ab | 0.013 | 2.37 a | 0.33 c | 1.36 b | 0.81 bc | 0.98 bc | <0.001 |
| 1.2 | 0.4 | 0.6 | 0.4 | 0.5 | 0.2 | 0.3 | 0.7 | 0.6 | 0.5 | |||
| Collembola | 4.02 ab | 6.46 a | 3.36 ab | 4.46 ab | 2.25 b | 0.051 | 4.03 a | 7.6 a | 5.57 a | 7.19 a | 5.65 a | |
| 1.2 | 3.6 | 2.8 | 3.5 | 1.3 | 1.5 | 3.9 | 3.6 | 1.9 | 2.2 | |||
| Diptera | 2.83 ab | 1.17 b | 4.14 ab | 1.42 b | 5.76 a | 0.016 | 4.15 a | 1.6 b | 2.42 ab | 2.81 ab | 2.51 ab | 0.073 |
| 1.9 | 0.9 | 2.4 | 1.3 | 5.3 | 1.7 | 1.1 | 2.2 | 1.9 | 0.7 | |||
| Formicidae | 1.1 b | 30.3 a | 2.40 b | 1.89b | 4.9 b | 0.002 | 1.21 b | 17.8 a | 2.39 b | 1.92 b | 1.44 b | <0.001 |
| 0.4 | 32 | 1.8 | 1.5 | 7.9 | 1 | 17.2 | 1.2 | 0.8 | 0.9 | |||
| Others | 1.98 b | 1.56 b | 2.82 ab | 2.01 b | 4.92 a | 0.006 | 1.82 a | 1.38 a | 3.74 a | 2.64 a | 2.44 a | |
| 0.42 | 1.01 | 2.20 | 1.04 | 2.95 | 1.19 | 0.68 | 2.67 | 1.22 | 1.32 | |||
| Total Abundance | 12.5 b | 42.1a | 15.6 b | 11.8 b | 21 ab | 0.005 | 15.3 b | 31.6 a | 19.6 ab | 17.9 ab | 17.6 ab | 0.048 |
| 4.1 | 32.3 | 6.8 | 6.2 | 15.2 | 3.9 | 21.4 | 9.01 | 4.8 | 3.7 | |||
| Richness (S) | 6.86 a | 6.25 a | 6.88 a | 6.62 a | 6.88 a | 6.88 a | 6.43 a | 6.62 a | 6.75 a | 7.00 a | ||
| 0.38 | 1.16 | 0.35 | 0.52 | 0.35 | 0.35 | 0.79 | 1.06 | 0.46 | 0.00 | |||
| Shannon (H) | 1.99 a | 1.2 b | 1.93 a | 1.83 a | 1.96 a | <0.001 | 1.9 a | 1.4 b | 1.99 a | 1.9 a | 2.04 a | <0.001 |
| 0.1 | 0.5 | 0.2 | 0.2 | 0.3 | 0.2 | 0.3 | 0.4 | 0.2 | 0.1 | |||
| Rainy Season (April) | Dry Season (September) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Litter Variables | Forest | AS1 | AS2 | AS3 | p-Value | Forest | AS1 | AS2 | AS3 | p-Value |
| C:N | 28.4 a | 25.4 ab | 21.5 b | 22.9 b | 0.026 | 23.4 a | 18.6 b | 18.3 b | 19.1 b | 0.028 |
| 1.6 | 3.9 | 4.7 | 5.9 | 1.8 | 1.4 | 0.7 | 1.2 | |||
| P (mg kg−1) | 0.05 b | 0.11 a | 0.11 a | 0.09 a | <0.001 | 0.03 a | 0.06 a | 0.06 a | 0.06 a | |
| 0.02 | 0.02 | 0.02 | 0.02 | 0.01 | 0.02 | 0.01 | 0.01 | |||
| Ca2+ (mg kg−1) | 94.65 b | 156.7 a | 148.7 ab | 136.9 a | 0.009 | 99.3 a | 129 a | 101 a | 101 a | |
| 9.7 | 5.2 | 15.4 | 15.6 | 29.1 | 9.7 | 12.7 | 12.4 | |||
| Mg2+ (mg kg−1) | 18.2 b | 26.2 ab | 23.2 ab | 41.3 a | 0.017 | 23.2 a | 28.1 a | 20.0 a | 25.5 a | |
| 3.3 | 8.9 | 2.5 | 7.5 | 7.8 | 2.9 | 7.3 | 7.2 | |||
| K+ (mg kg−1) | 9.99 b | 16.4 a | 10.4 b | 15.3 ab | 0.008 | 17.2 a | 29.9 a | 20.3 a | 39.3 a | |
| 0.7 | 3.1 | 1.9 | 4.5 | 7.7 | 3.6 | 4.2 | 27.6 | |||
| Biomass (kg ha−1) | 1578 a | 1366 b | 1112 c | 1499 ab | <0.001 | 1410 a | 1086 a | 1261 a | 1193 a | |
| 64.8 | 60.6 | 56.3 | 79.1 | 64 | 149 | 208 | 264 | |||
| Soil Response Variable | C:N Ratio | C:N Effect Direction * | Sampling Time | C:N × Time | SOM | SOM Effect Direction * | Sampling Time | SOM × Time |
|---|---|---|---|---|---|---|---|---|
| Abundance | 0.029 | - | ns | ns | ns | ns | ns | |
| Richness | ns | ns | ns | 0.061 | + | ns | ns | |
| Shannon | ns | ns | ns | 0.058 | + | ns | ns | |
| MBC | 0.025 | - | <0.001 | ns | 0.009 | + | <0.001 | ns |
| MBN | ns | <0.001 | ns | ns | <0.001 | ns | ||
| FDA | 0.030 | Apr+, Sep− | ns | <0.001 | ns | Apr−, Sep+ | ns | 0.002 |
| Sbresp | 0.003 | Apr 0, Sep− | ns | 0.010 | 0.034 | + | <0.001 | ns |
| β-glucosidase | ns | <0.001 | ns | <0.001 | + | <0.001 | ns | |
| Phosphatase | 0.003 | + | <0.001 | ns | 0.093 | - | ns | ns |
| K | 0.002 | - | 0.001 | ns | <0.001 | + | ns | ns |
| CEC | ns | 0.017 | 0.010 | 0.035 | + | 0.009 | ns | |
| pH | 0.045 | - | 0.009 | ns | 0.002 | Apr+, Sep 0 | ns | 0.021 |
| P | 0.021 | - | <0.001 | ns | 0.057 | + | 0.023 | ns |
| BD | 0.030 | - | ns | ns | 0.012 | - | ns | ns |
| MWD | 0.022 | + | 0.088 | ns | ns | ns | ns | |
| POXC | ns | ns | ns | ns | ns | ns | ||
| SOM | ns | 0.081 | ns | NA | NA | NA |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matos, P.S.; Fonte, S.J.; Lima, S.S.; Pereira, M.G.; Kelly, C.; Damian, J.M.; Fontes, M.A.; Chaer, G.M.; Brasil, F.C.; Zonta, E. Linkages among Soil Properties and Litter Quality in Agroforestry Systems of Southeastern Brazil. Sustainability 2020, 12, 9752. https://doi.org/10.3390/su12229752
Matos PS, Fonte SJ, Lima SS, Pereira MG, Kelly C, Damian JM, Fontes MA, Chaer GM, Brasil FC, Zonta E. Linkages among Soil Properties and Litter Quality in Agroforestry Systems of Southeastern Brazil. Sustainability. 2020; 12(22):9752. https://doi.org/10.3390/su12229752
Chicago/Turabian StyleMatos, Priscila S., Steven J. Fonte, Sandra S. Lima, Marcos G. Pereira, Courtland Kelly, Júnior M. Damian, Marcelo A. Fontes, Guilherme M. Chaer, Felipe C. Brasil, and Everaldo Zonta. 2020. "Linkages among Soil Properties and Litter Quality in Agroforestry Systems of Southeastern Brazil" Sustainability 12, no. 22: 9752. https://doi.org/10.3390/su12229752
APA StyleMatos, P. S., Fonte, S. J., Lima, S. S., Pereira, M. G., Kelly, C., Damian, J. M., Fontes, M. A., Chaer, G. M., Brasil, F. C., & Zonta, E. (2020). Linkages among Soil Properties and Litter Quality in Agroforestry Systems of Southeastern Brazil. Sustainability, 12(22), 9752. https://doi.org/10.3390/su12229752






