Characterizing Humic Substances from Native Halophyte Soils by Fluorescence Spectroscopy Combined with Parallel Factor Analysis and Canonical Correlation Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area and Sampling Strategy
2.2. Physico-Chemical Analyses
2.3. Isolation of FA and HA Fractions
2.4. EEM Spectroscopy Detection and PARAFAC
2.5. Multivariable Analyses
3. Results and Discussion
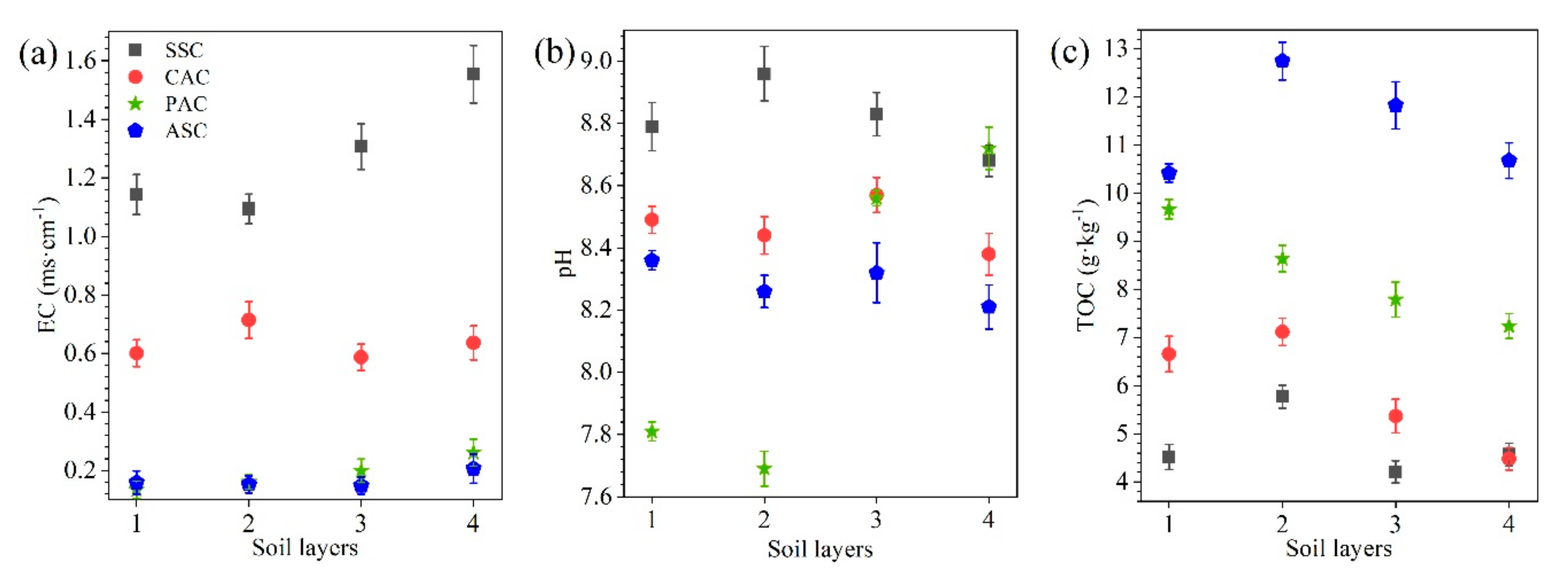
3.1. Physico-Chemical Characteristics
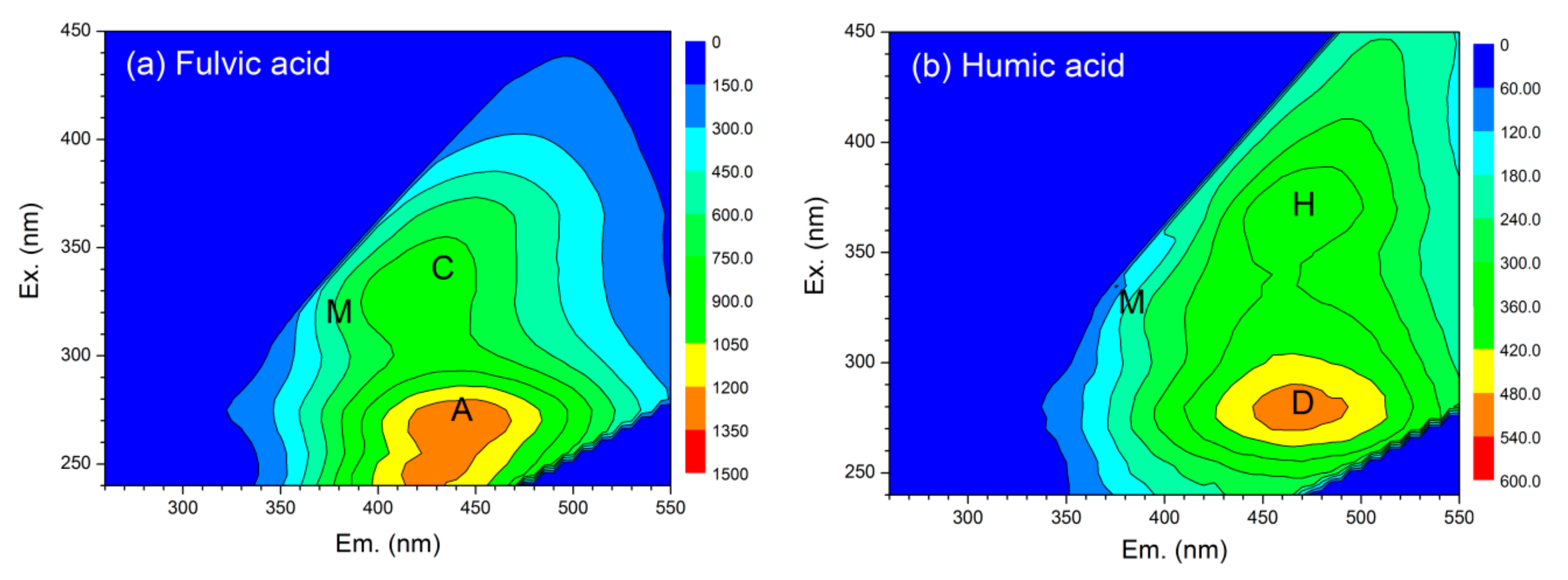
3.2. EEM Spectroscopy Characteristics
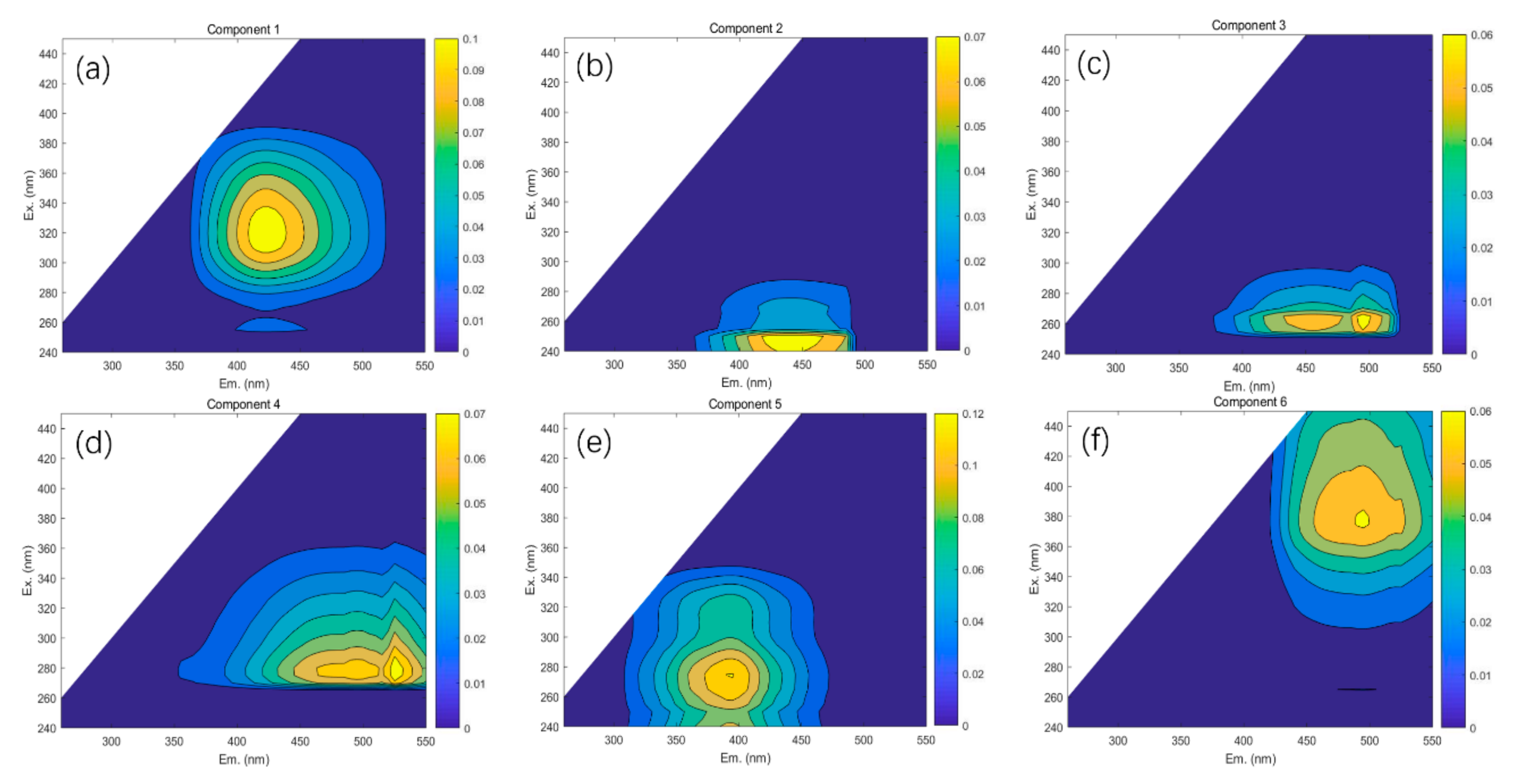
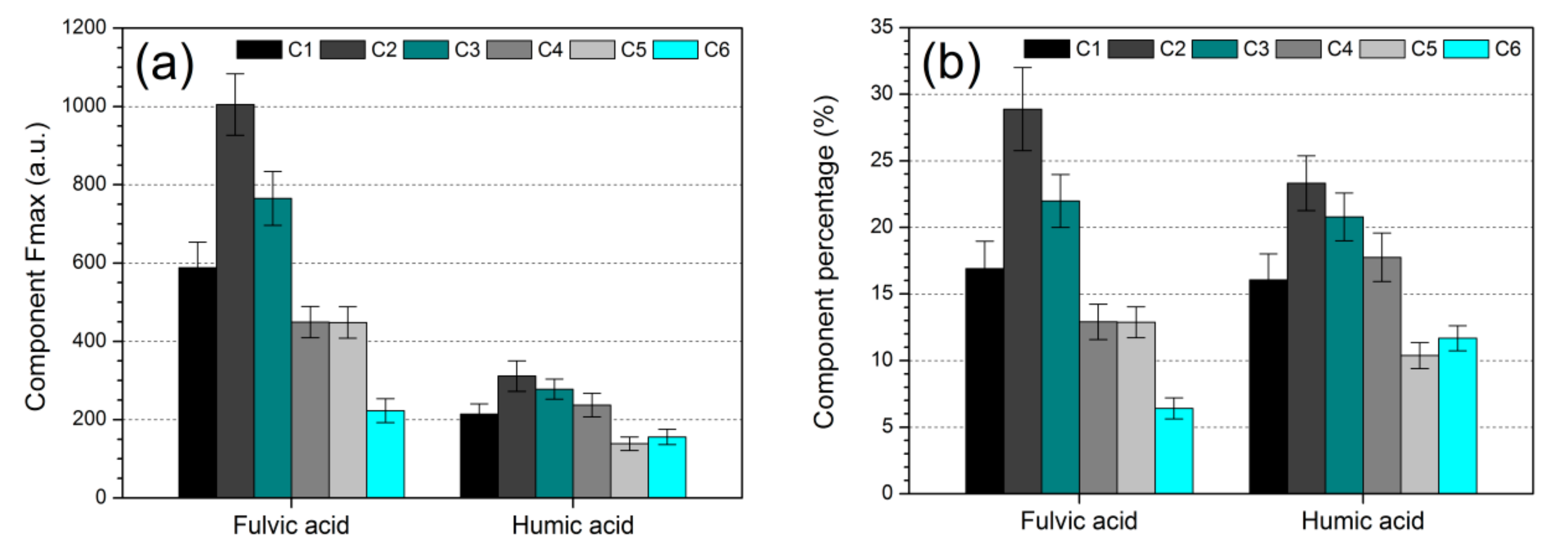
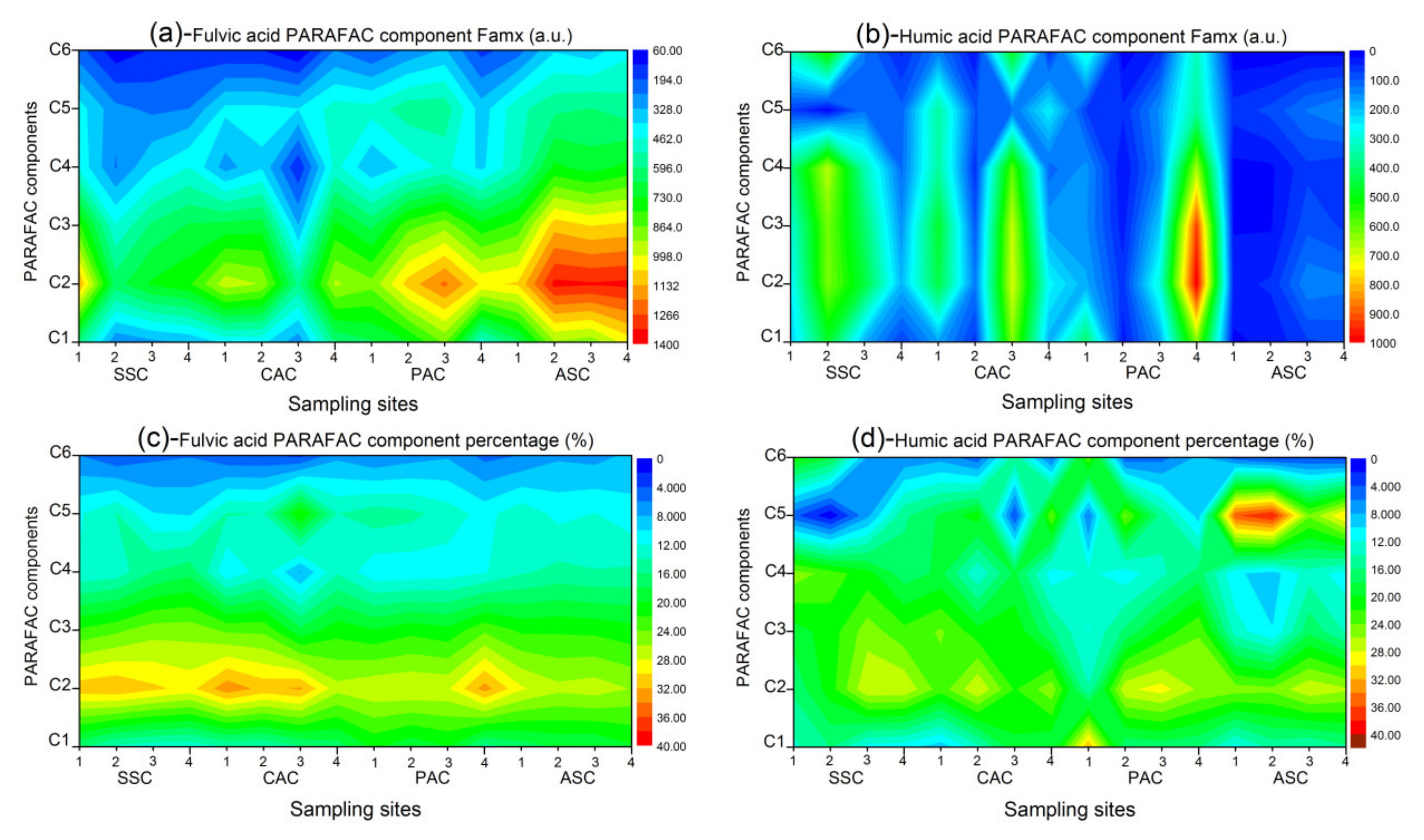
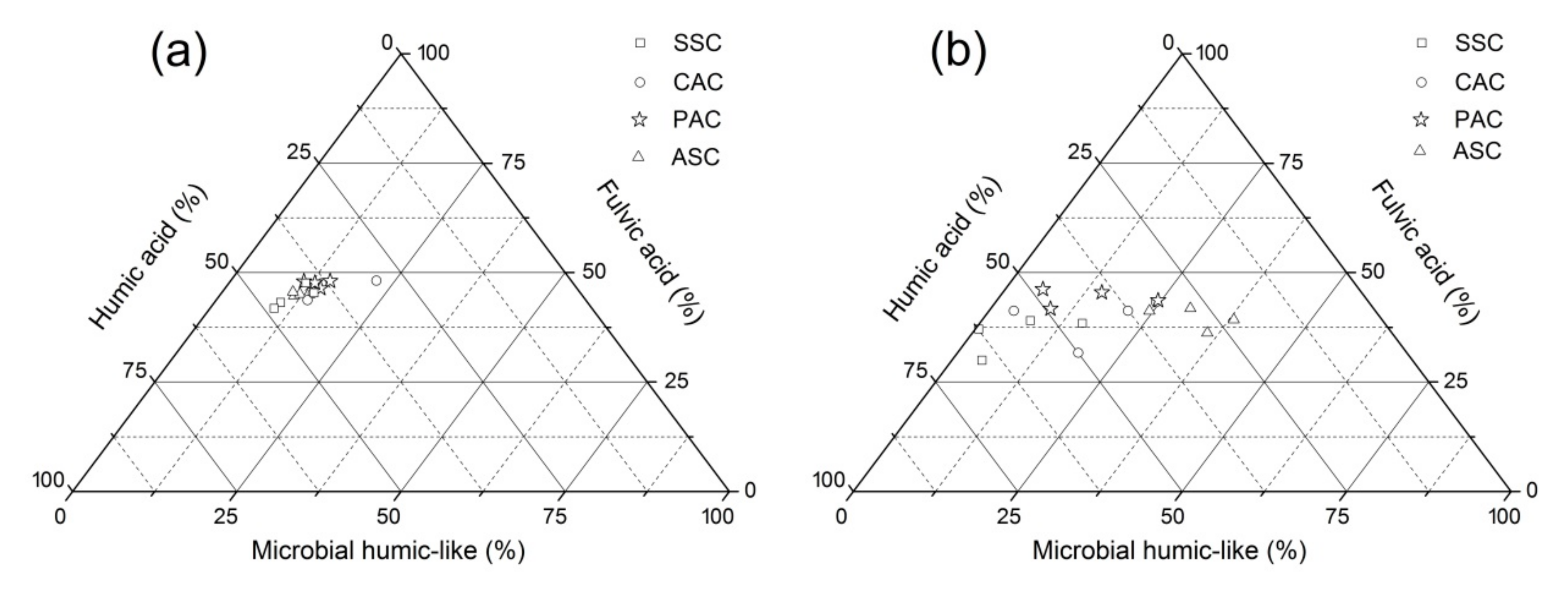
3.3. Assignments and Variations of Fluorescent Components
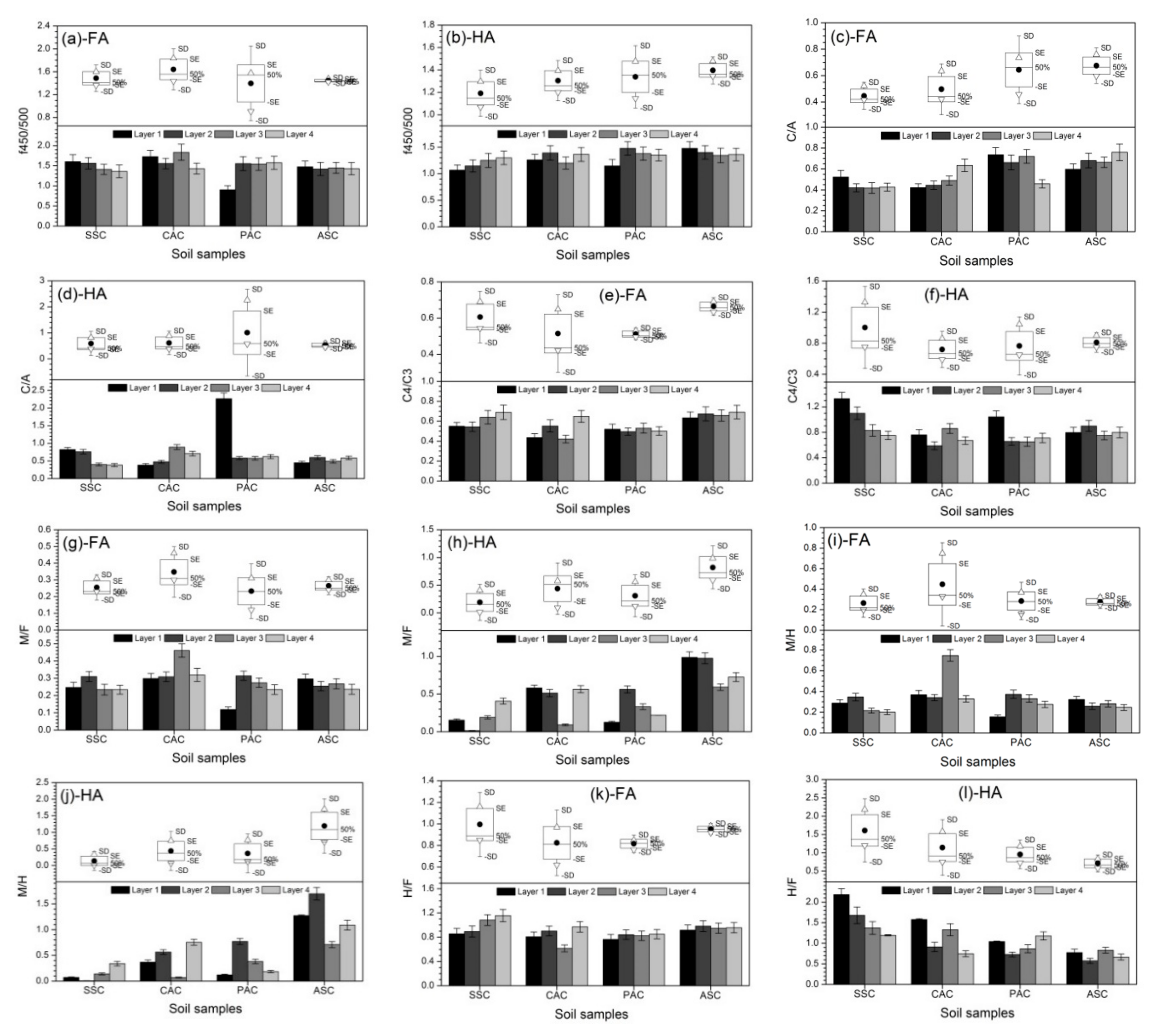
3.4. Deduction of Fluorescence Indices
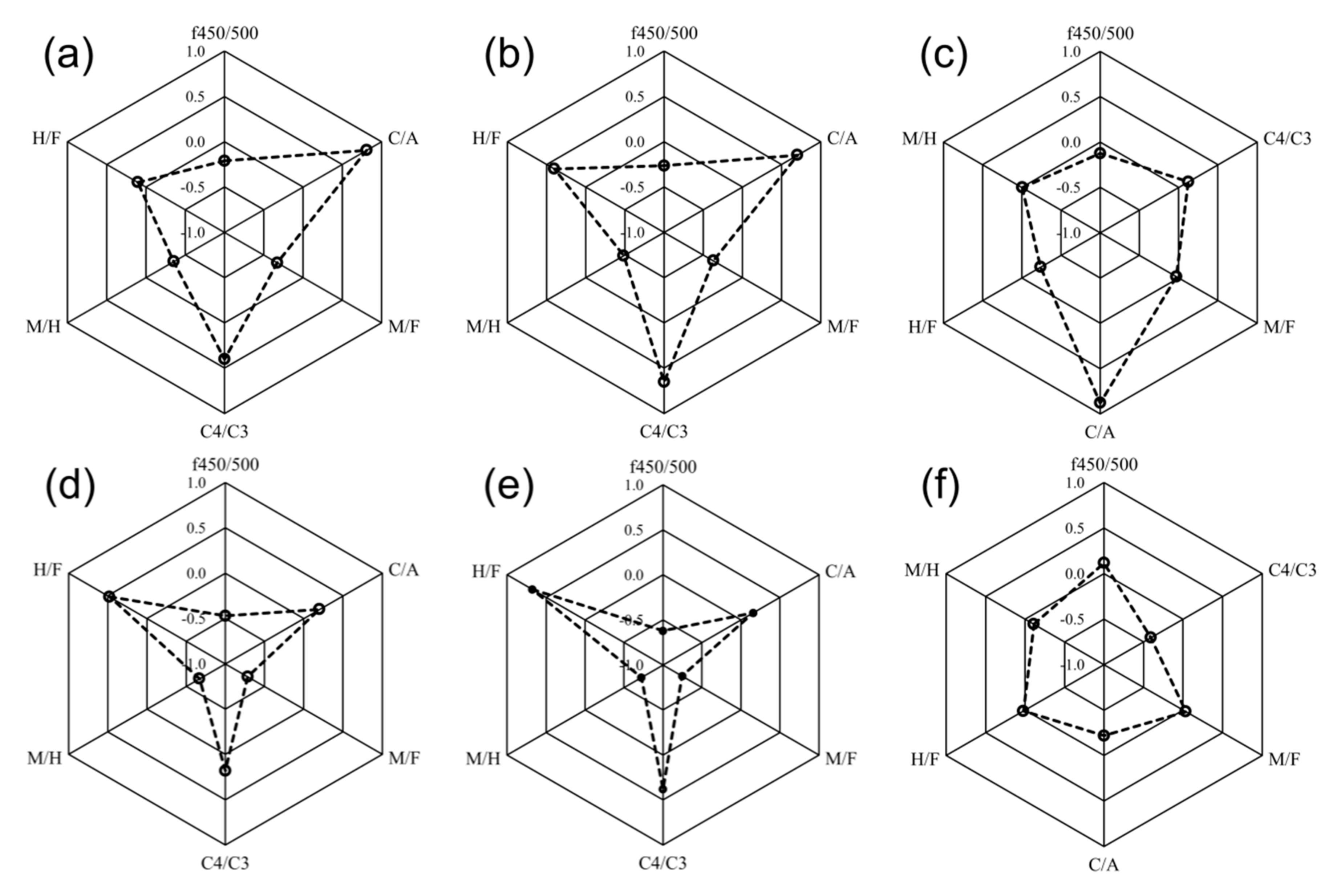
3.5. Correlations between Fluorescent Components and Fluorescence Indices
3.5.1. nMDS Analyses
3.5.2. Correlation Analyses
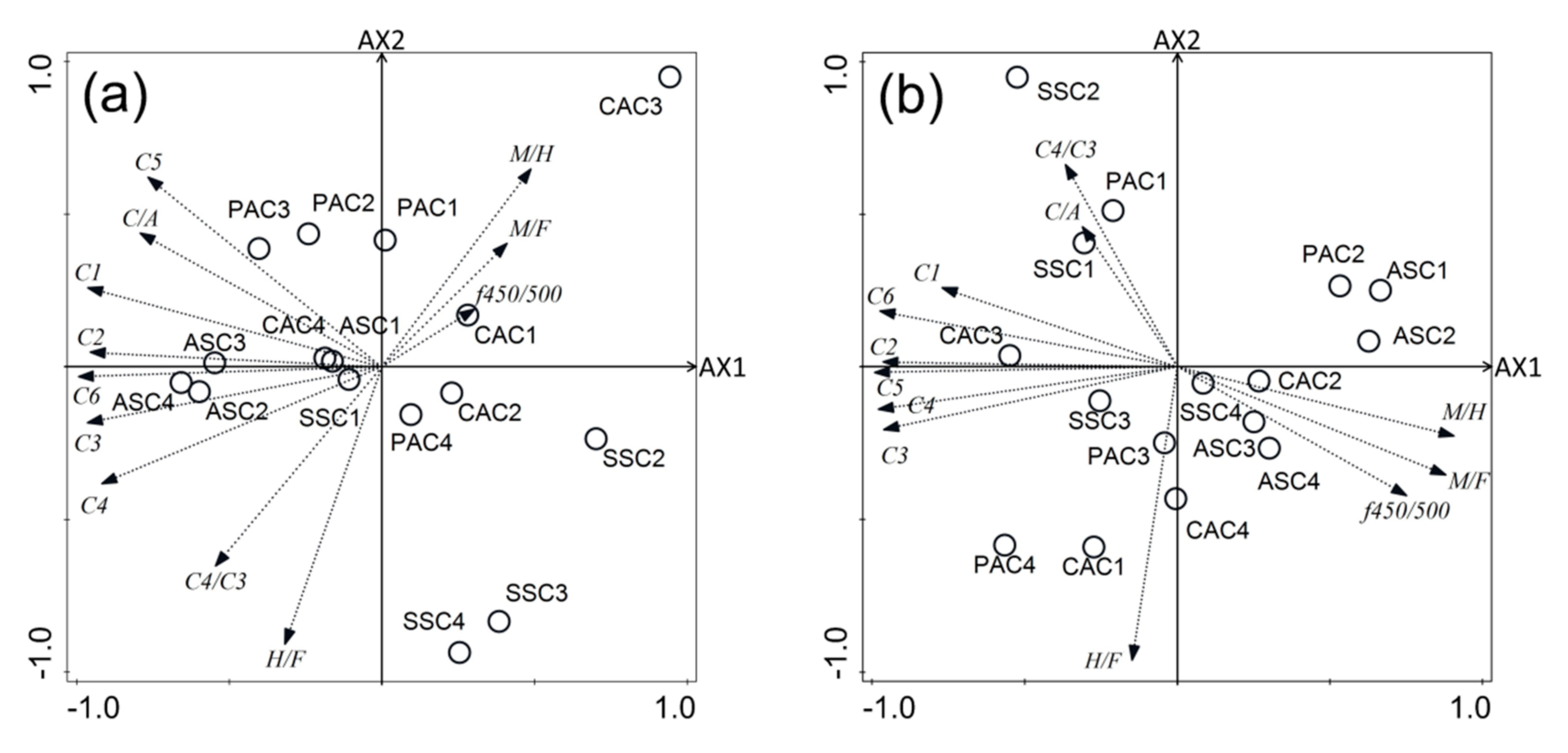
3.5.3. CCA Analyses
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Santín, C.; Yamashita, Y.; Otero, X.L.; Alvarez, M.A.; Jaffe, R. Characterizing humic substances from estuarine soils and sediments by excitation-emission matrix spectroscopy and parallel factor analysis. Biogeochemistry 2009, 96, 131–147. [Google Scholar] [CrossRef]
- Aristilde, L.; Sposito, G. Complexes of the antimicrobial ciprofloxacin with soil, peat, and aquatic humic substances. Environ. Toxicol. Chem. 2013, 32, 1467–1478. [Google Scholar] [CrossRef] [PubMed]
- Shultz, M.; Pellerin, B.A.; Aiken, G.R.; Martin, J.; Raymond, P.A. High Frequency Data Exposes Nonlinear Seasonal Controls on Dissolved Organic Matter in a Large Watershed. Environ. Sci. Technol. 2018, 52, 5644–5652. [Google Scholar] [CrossRef]
- Hayes, M.H.B.; Clapp, C.E. Humic substances: Considerations of compositions, aspects of structure, and environmental influences. Soil Sci. 2001, 166, 723–737. [Google Scholar] [CrossRef]
- Sutton, R.; Sposito, G. Molecular structure in soil humic substances: The new view. Environ. Sci. Technol. 2005, 39, 9009–9015. [Google Scholar] [CrossRef] [PubMed]
- Bianco, A.; Minella, M.; De Laurentiis, E.; Maurino, V.; Minero, C.; Vione, D. Photochemical generation of photoactive compounds with fulvic-like and humic-like fluorescence in aqueous solution. Chemosphere 2014, 111, 529–536. [Google Scholar] [CrossRef]
- Oliveira, J.L.; Boroski, M.; Azevedo, J.C.R.; Nozaki, J. Spectroscopic investigation of humic substances in a tropical lake during a complete hydrological cycle. Acta Hydrochim. Hydrobiol. 2006, 34, 608–617. [Google Scholar] [CrossRef]
- Sinsabaugh, R.L. Phenol oxidase, peroxidase and organic matter dynamics of soil. Soil Biol. Biochem. 2010, 42, 391–404. [Google Scholar] [CrossRef]
- Gabor, R.S.; Eilers, K.G.; Mcknight, D.M.; Fierer, N.; Anderson, S.P. From the litter layer to the saprolite: Chemical changes in water-soluble soil organic matter and their correlation to microbial community composition. Soil Biol. Biochem. 2014, 68, 166–176. [Google Scholar] [CrossRef]
- Perdrial, J.; Perdrial, N.; Harpold, A.A.; Gao, X.; Gabor, R.S.; Lasharr, K.; Chorover, J. Impacts of Sampling Dissolved Organic Matter with Passive Capillary Wicks Versus Aqueous Soil Extraction. Soil Sci. Soc. Am. J. 2012, 76, 2019–2030. [Google Scholar] [CrossRef]
- Gabor, R.S.; Burns, M.A.; Lee, R.H.; Elg, J.B.; Kemper, C.J.; Barnard, H.R.; Mcknight, D.M. Influence of Leaching Solution and Catchment Location on the Fluorescence of Water-Soluble Organic Matter. Environ. Sci. Technol. 2015, 49, 4425–4432. [Google Scholar] [CrossRef] [PubMed]
- Canellas, L.P.; Olivares, F.L.; Aguiar, N.O.; Jones, D.L.; Nebbioso, A.; Mazzei, P.; Piccolo, A. Humic and fulvic acids as biostimulants in horticulture. Sci. Hortic. 2015, 196, 15–27. [Google Scholar] [CrossRef]
- Kulikova, N.A.; Filippova, O.I.; Volikov, A.B.; Perminova, I.V. Slow nitrogen release from humic substances modified with aminoorganosilanes. J. Soils Sediments 2018, 18, 1–9. [Google Scholar] [CrossRef]
- Aiken, G.; McKnight, D.; Harnish, R.; Wershaw, R. Geochemistry of aquatic humic substances in the Lake Fryxell basin, Antarctica. Biogeochemistry 1996, 34, 157–188. [Google Scholar] [CrossRef]
- Haitzer, M.; Aiken, G.R.; Ryan, J.N. Binding of mercury (ii) to aquatic humic substances: Influence of pH and source of humic substances. Environ. Sci. Technol. 2003, 37, 2436–2441. [Google Scholar] [CrossRef] [PubMed]
- Huber, S.A.; Balz, A.; Abert, M.; Pronk, W. Characterisation of aquatic humic and non-humic matter with size-exclusion chromatography—Organic carbon detection—organic nitrogen detection (LC–OCD–OND). Water Res. 2011, 45, 879–885. [Google Scholar] [CrossRef]
- Nardi, S.; Pizzeghello, D.; Muscolo, A.; Vianello, A. Physiological effects of humic substances on higher plants. Soil Biol. Biochem. 2002, 34, 1527–1536. [Google Scholar] [CrossRef]
- Fellman, J.B.; Hood, E.; Spencer, R.G.M. Fluorescence spectroscopy opens new windows into dissolved organic matter dynamics in freshwater ecosystems: A review. Limnol. Oceanogr. 2010, 55, 2452–2462. [Google Scholar] [CrossRef]
- Watras, C.J.; Hanson, P.C.; Stacy, T.L.; Morrison, K.M.; Mather, J.; Hu, Y.H.; Milewski, P. A temperature compensation method for CDOM fluorescence sensors in freshwater. Limnol. Oceanogr. Methods 2011, 9, 296–301. [Google Scholar] [CrossRef]
- Murphy, K.R.; Stedmon, C.A.; Wenig, P.; Bro, R. Open Fluor an online spectral library of auto-fluorescence by organic compounds in the environment. Anal. Methods 2014, 6, 658–661. [Google Scholar] [CrossRef]
- Helms, J.R.; Stubbins, A.; Perdue, E.M.; Green, N.W.; Chen, H.; Mopper, K. Photochemical bleaching of oceanic dissolved organic matter and its effect on absorption spectral slope and fluorescence. Mar. Chem. 2013, 115, 81–91. [Google Scholar] [CrossRef]
- Stedmon, C.A.; Bro, R. Characterizing dissolved organic matter fluorescence with parallel factor analysis: A tutorial. Limnol. Oceanogr. Methods 2008, 6, 572–579. [Google Scholar] [CrossRef]
- Korak, J.A.; Dotson, A.; Summers, R.S.; Rosario-Ortiz, F.L. Critical analysis of commonly used fluorescence metrics to characterize dissolved organic matter. Water Res. 2014, 49, 327–338. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Song, Y.; Du, E.; Yang, N.; Peng, J.; Liu, R. Comparison of PARAFAC components of fluorescent dissolved and particular organic matter from two urbanized rivers. Environ. Sci. Pollut. Res. 2016, 23, 10644–10655. [Google Scholar] [CrossRef]
- Pan, H.; Yu, H.; Song, Y.; Zhu, L.; Liu, R.; Du, E. Tracking fluorescent components of dissolved organic matter from soils in large-scale irrigated area. Environ. Sci. Pollut. Res. 2017, 24, 6563–6571. [Google Scholar] [CrossRef] [PubMed]
- Wei, H.; Yu, H.; Pan, H.; Gao, H. Application of UV-visible absorption spectroscopy combined with two-dimensional correlation for insight into DOM fractions from native halophyte soils in a larger estuarine delta. Environ. Sci. Pollut. Res. 2018, 25, 14197–14205. [Google Scholar] [CrossRef]
- Xu, J.; Liu, Y.; Zhu, C.; Jia, H.; Tian, C.; Ma, H.; Lv, G. NaCl Improves Suaeda salsa Aniline Tolerance in Wastewater. Sustainability 2020, 12, 7457. [Google Scholar] [CrossRef]
- Vizetto-Duarte, C.; Figueiredo, F.; Rodrigues, M.J.; Polo, C.; Rešek, E.; Custódio, L. Sustainable Valorization of Halophytes from the Mediterranean Area: A Comprehensive Evaluation of Their Fatty Acid Profile and Implications for Human and Animal Nutrition. Sustainability 2019, 11, 2197. [Google Scholar] [CrossRef]
- Feng, W.; Yang, F.; Cen, R.; Liu, J.; Qu, Z. Effects of straw biochar application on soil temperature, available nitrogen and growth of corn. J. Environ. Manag. 2020, 277, 111331. [Google Scholar] [CrossRef]
- Dodla, S.K.; Wang, J.J.; DeLaune, R.D.; Cook, R.L. Denitrification potential and its relation to organic carbon quality in three coastal wetland soils. Sci. Total. Environ. 2008, 407, 471–480. [Google Scholar] [CrossRef]
- Ma, X.; Green, S.A. Fractionation and spectroscopic properties of fulvic acid and its extract. Chemosphere 2008, 72, 1425–1434. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Song, Y.; Xi, B.; Zhang, M.; He, X. Application of derivative synchronous fluorescence spectroscopy (DSFS) to indicate salinisation processes of saline soils in semi-arid region. Ecol. Indic. 2012, 18, 532–539. [Google Scholar] [CrossRef]
- Osburn, C.L.; Handsel, L.T.; Mikan, M.P.; Paerl, H.W.; Montgomery, M.T. Fluorescence tracking of dissolved and particulate organic matter quality in a river-dominated estuary. Environ. Sci. Technol. 2012, 46, 8628–8636. [Google Scholar] [CrossRef] [PubMed]
- Ohno, T. Fluorescence inner-filtering correction for determining the humification index of dissolved organic matter. Environ. Sci. Technol. 2002, 36, 742–746. [Google Scholar] [CrossRef] [PubMed]
- Coble, P.G. Characterization of marine and terrestrial DOM in seawater using excitation-emission matrix spectroscopy. Mar. Chem. 1996, 51, 325–346. [Google Scholar] [CrossRef]
- Galapate, R.P.; Baes, A.U.; Ito, K.; Mukai, T.; Shoto, E.; Okada, M. Detection of domestic wastes in Kurose river using synchronous fluorescence spectroscopy. Water Res. 1998, 32, 2232–2239. [Google Scholar] [CrossRef]
- Sierra, M.M.D.; Giovanela, M.; Parlanti, E.; Soriano-Sierra, E.J. Fluorescence fingerprint of fulvic and humic acids from varied origins as viewed by single-scan and excitation/emission matrix techniques. Chemosphere 2005, 58, 715–733. [Google Scholar] [CrossRef]
- He, Z.; Ohno, T.; Cade-Menum, B.J.; Erich, M.S.; Honeycutt, C.W. Spectral and chemical characterization of phosphates associated with humic substances. Soil Sci. Soc. Am. J. 2006, 70, 1741–1751. [Google Scholar] [CrossRef]
- Coelho, C.; Guyot, G.; ter Halle, A.; Cavani, L.; Ciavatta, C.; Richard, C. Photoreactivity of humic substances: Relationship between fluorescence and singlet oxygen production. Environ. Chem. Lett. 2011, 9, 447–451. [Google Scholar] [CrossRef]
- Alberts, J.J.; Takács, M. Comparison of the natural fluorescence distribution among size fractions of terrestrial fulvic and humic acids and aquatic natural organic matter. Org. Geochem. 2004, 35, 1141–1149. [Google Scholar] [CrossRef]
- Huguet, A.; Vacher, L.; Relexans, S.; Saubusse, S.; Froidefond, J.M.; Parlanti, E. Properties of fluorescent dissolved organic matter in the Gironde Estuary. Org. Geochem. 2009, 40, 706–719. [Google Scholar] [CrossRef]
- Jiang, T.; Skyllberg, U.; Bjorn, E.; Green, N.W.; Tang, J.; Wang, D.; Gao, J.; Li, C. Characteristics of dissolved organic matter (DOM) and relationship with dissolved mercury in Xiaoqing River-Laizhou Bay estuary, Bohai Sea, China. Environ. Pollut. 2017, 223, 19–30. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.; Chen, Y.; Nie, D.; Liu, Y.; Ge, X. Light-absorbing and fluorescent properties of atmospheric brown carbon: A case study in Nanjing, China. Chemosphere 2020, 251, 126350. [Google Scholar] [CrossRef] [PubMed]
- Francisco, J.; Vidal, R.; García-Valverde, M.; Ortega-Azabache, B. Characterization of urban and industrial wastewaters using excitation-emission matrix (EEM) fluorescence: Searching for specific fingerprints. J. Environ. Manag. 2020, 263, 110396. [Google Scholar]
- Bilal, M.; Jaffrezic, A.; Dudal, Y.; Le Guillou, C.; Menasseri, S.; Walter, C. Discrimination of farm waste contamination by fluorescence spectroscopy coupled with multivariate analysis during a biodegradation study. J. Agric. Food Chem. 2010, 58, 3093–3100. [Google Scholar] [CrossRef]
- He, W.; Lee, J.; Hur, J. Anthropogenic signature of sediment organic matter probed by UV-Visible and fluorescence spectroscopy and the association with heavy metal enrichment. Chemosphere 2016, 150, 184–193. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, D.; Yu, H.; Yang, F.; Liu, L.; Gao, H.; Cui, B. Characterizing Humic Substances from Native Halophyte Soils by Fluorescence Spectroscopy Combined with Parallel Factor Analysis and Canonical Correlation Analysis. Sustainability 2020, 12, 9787. https://doi.org/10.3390/su12239787
Liu D, Yu H, Yang F, Liu L, Gao H, Cui B. Characterizing Humic Substances from Native Halophyte Soils by Fluorescence Spectroscopy Combined with Parallel Factor Analysis and Canonical Correlation Analysis. Sustainability. 2020; 12(23):9787. https://doi.org/10.3390/su12239787
Chicago/Turabian StyleLiu, Dongping, Huibin Yu, Fang Yang, Li Liu, Hongjie Gao, and Bing Cui. 2020. "Characterizing Humic Substances from Native Halophyte Soils by Fluorescence Spectroscopy Combined with Parallel Factor Analysis and Canonical Correlation Analysis" Sustainability 12, no. 23: 9787. https://doi.org/10.3390/su12239787
APA StyleLiu, D., Yu, H., Yang, F., Liu, L., Gao, H., & Cui, B. (2020). Characterizing Humic Substances from Native Halophyte Soils by Fluorescence Spectroscopy Combined with Parallel Factor Analysis and Canonical Correlation Analysis. Sustainability, 12(23), 9787. https://doi.org/10.3390/su12239787





