Medium Optimization for Spore Production of a Straw-Cellulose Degrading Actinomyces Strain under Solid-State Fermentation Using Response Surface Method
Abstract
1. Introduction
2. Materials and Methods
2.1. Strain and Chemicals
2.2. Medium and Culture Condition
2.3. Analytical Methods
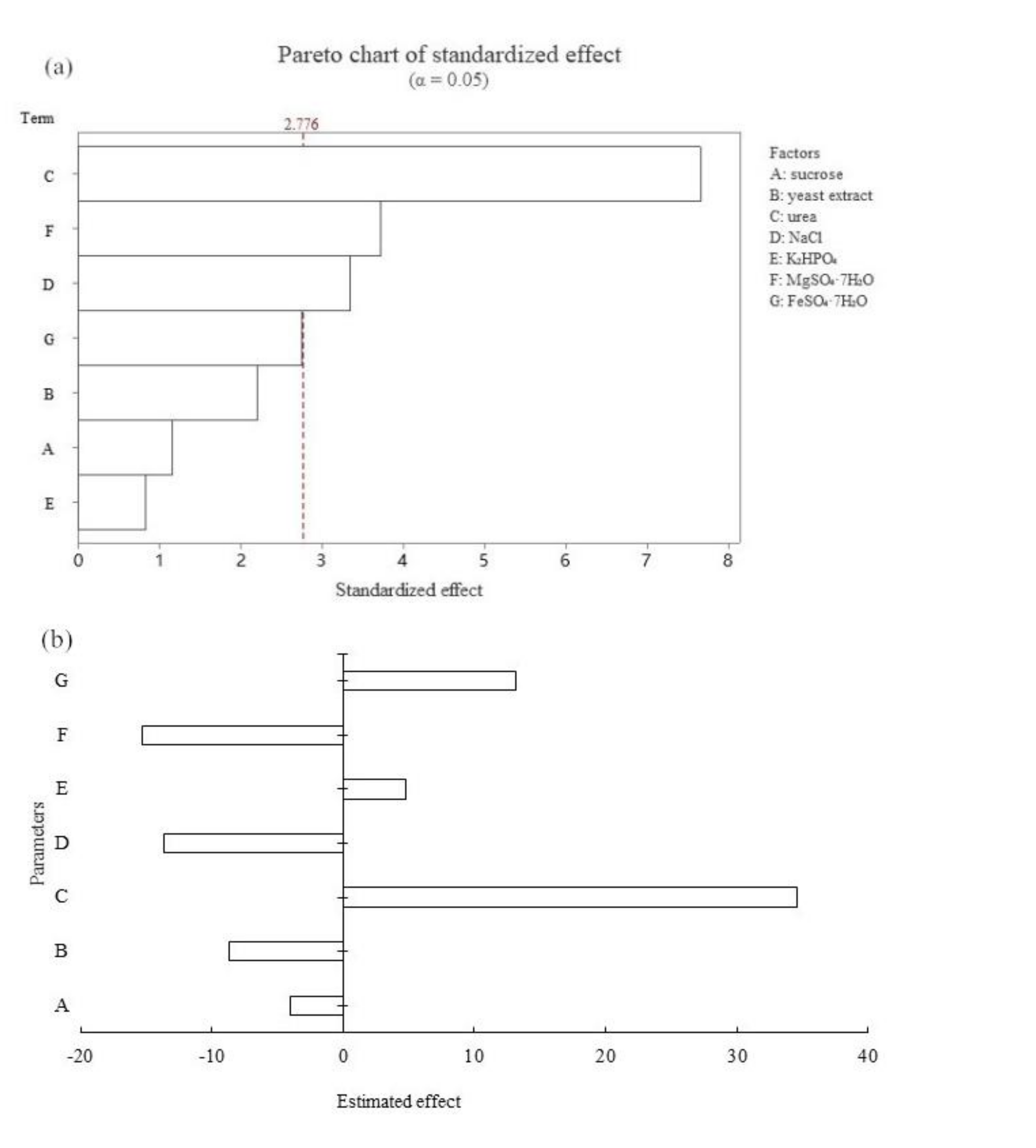
2.4. Plackett–Burman Design (PBD)
2.5. The Path of Steepest Ascent
2.6. Central Composite Design
2.7. Statistical Analysis
3. Results and Discussion
3.1. Effect of Different Carbon and Nitrogen Sources
3.2. Significance Factors for Spore Production
3.3. The Steepest Ascent Path Analysis
3.4. Optimization of the Medium
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Xiong, X.Q.; Liao, H.D.; Ma, J.S.; Liu, X.M.; Zhang, L.Y.; Shi, X.W.; Yang, X.L.; Lu, X.N.; Zhu, Y.H. Isolation of a rice endophytic bacterium, Pantoea sp. Sd-1, with ligninolytic activity and characterization of its rice straw degradation ability. Lett. Appl. Microbiol. 2014, 58, 123–129. [Google Scholar] [CrossRef]
- Ma, Y.; Shen, Y.; Liu, Y. State of the art of straw treatment technology: Challenges and solutions forward. Bioresour. Technol. 2020, 313, 123656. [Google Scholar] [CrossRef]
- Seglah, P.A.; Wang, Y.; Wang, H.; Bi, Y. Estimation and Efficient Utilization of Straw Resources in Ghana. Sustainability 2019, 11, 4172. [Google Scholar] [CrossRef]
- Bassani, A.; Fiorentini, C.; Vadivel, V.; Moncalvo, A.; Spigno, G. Implementation of Auto-Hydrolysis Process for the Recovery of Antioxidants and Cellulose from Wheat Straw. Appl. Sci. 2020, 10, 6112. [Google Scholar] [CrossRef]
- Wu, X.; Huang, C.; Zhai, S.; Liang, C.; Huang, C.; Lai, C.; Yong, Q. Improving enzymatic hydrolysis efficiency of wheat straw through sequential autohydrolysis and alkaline post-extraction. Bioresour. Technol. 2018, 251, 374–380. [Google Scholar] [CrossRef]
- Huang, C.; Zeng, G.; Huang, D.; Lai, C.; Xu, P.; Zhang, C.; Cheng, M.; Wan, J.; Hu, L.; Zhang, Y. Effect of Phanerochaete chrysosporium inoculation on bacterial community and metal stabilization in lead-contaminated agricultural waste composting. Bioresour. Technol. 2017, 243, 294–303. [Google Scholar] [CrossRef]
- Chen, X.; Liu, R.; Hao, J.; Li, D.; Wei, Z.; Teng, R.; Sun, B. Protein and carbohydrate drive microbial responses in diverse ways during different animal manures composting. Bioresour. Technol. 2019, 271, 482–486. [Google Scholar] [CrossRef]
- Wei, Y.; Wu, D.; Wei, D.; Zhao, Y.; Wu, J.; Xie, X.; Zhang, R.; Wei, Z. Improved lignocellulose-degrading performance during straw composting from diverse sources with actinomycetes inoculation by regulating the key enzyme activities. Bioresour. Technol. 2019, 271, 66–74. [Google Scholar] [CrossRef]
- Feng, H.; Zhi, Y.; Shi, W.; Mao, L.; Zhou, P. Isolation, identification and characterization of a straw degrading Streptomyces griseorubens JSD-1. Afr. J. Microbiol. Res. 2013, 7, 2730–2735. [Google Scholar]
- Feng, H.; Mao, L.; Sun, Y.; Zhi, Y.; Zhou, P. Effects of inoculation of Streptomyces Griseorubens on soil physical and chemical properties and microbial community of rice straw composting and returning to the field. J. Shanghai Jiaotong Univ. (Agric. Sci. Ed.) 2015, 33, 25–32. [Google Scholar]
- Zhao, L.; Zhi, Y.; Liu, H.; Zhang, D.; Zhou, P. Preliminary Study on Antibacterial Activity of Actinomycete JSD-1 and Optimization of Fermentation Conditions Affecting the Antibacterial Activity of JSD-1. Modern Food Sci. Technol. 2019, 35, 176–181. [Google Scholar]
- Pandey, A. Solid-state fermentation. Biochem. Eng. J. 2003, 13, 81–84. [Google Scholar] [CrossRef]
- Devi, P.S.V.; Ravinder, T.; Jaidev, C. Cost-effective production of Bacillus thuringiensis by solid-state fermentation. J. Invertebr. Pathol. 2005, 88, 163–168. [Google Scholar] [CrossRef]
- Mejias, L.; Cerda, A.; Barrena, R.; Gea, T.; Sánchez, A. Microbial Strategies for Cellulase and Xylanase Production through Solid-State Fermentation of Digestate from Biowaste. Sustainability 2018, 10, 2433. [Google Scholar] [CrossRef]
- Asensio-Grau, A.; Calvo-Lerma, J.; Heredia, A.; Andrés, A. Enhancing the nutritional profile and digestibility of lentil flour by solid state fermentation with Pleurotus ostreatus. Food Funct. 2020, 11, 7905–7912. [Google Scholar] [CrossRef]
- Majumder, K.; Paul, B.; Sundas, R. An analysis of exo-polygalacturonase bioprocess in submerged and solid-state fermentation by Pleurotus ostreatus using pomelo peel powder as carbon source. Journal of Genet. Eng. Biotechnol. 2020, 18, 47. [Google Scholar] [CrossRef]
- Wang, F.; Xu, L.; Zhao, L.; Ding, Z.; Ma, H.; Terry, N. Fungal Laccase Production from Lignocellulosic Agricultural Wastes by Solid-State Fermentation: A Review. Microorganisms 2019, 7, 665. [Google Scholar] [CrossRef]
- Shen, T.; Wang, C.; Yang, H.; Deng, Z.; Wang, S.; Shen, B.; Shen, Q. Identification, solid-state fermentation and biocontrol effects of Streptomyces hygroscopicus B04 on strawberry root rot. Appl. Soil Ecol. 2016, 103, 36–43. [Google Scholar] [CrossRef]
- Wang, Y. Optimization of Solid Fermentation Conditions of Streptomyces NEAU6 and Its Effect on Growth of four Vegetables; Northeast Agricultural University: Harbin, China, 2019. [Google Scholar]
- Rao, Y.K.; Tsay, K.-J.; Wu, W.-S.; Tzeng, Y.-M. Medium optimization of carbon and nitrogen sources for the production of spores from Bacillus amyloliquefaciens B128 using response surface methodology. Process Biochem. 2007, 42, 535–541. [Google Scholar] [CrossRef]
- Ramírez-López, C.; Chairez, I.; Fernández-Linares, L. A novel culture medium designed for the simultaneous enhancement of biomass and lipid production by Chlorella vulgaris UTEX 26. Bioresour. Technol. 2016, 212, 207–216. [Google Scholar] [CrossRef]
- Kong, Y.; Zou, P.; Miao, L.; Qi, J.; Song, L.; Zhu, L.; Xu, X. Medium optimization for the production of anti-cyanobacterial substances by Streptomyces sp. HJC-D1 using response surface methodology. Environ. Sci. Pollut. Res. 2014, 21, 5983–5990. [Google Scholar] [CrossRef]
- Abdulrasheed, M.; Zulkharnain, A.; Zakaria, N.N.; Roslee, A.F.A.; Abdul Khalil, K.; Napis, S.; Convey, P.; Gomez-Fuentes, C.; Ahmad, S.A. Response Surface Methodology Optimization and Kinetics of Diesel Degradation by a Cold-Adapted Antarctic Bacterium, Arthrobacter sp. Strain AQ5-05. Sustainability 2020, 12, 6966. [Google Scholar] [CrossRef]
- Noman, E.; Al-Gheethi, A.A.; Talip, B.A.; Mohamed, R.; Kassim, A.H. Oxidative enzymes from newly local strain Aspergillus iizukae EAN605 using pumpkin peels as a production substrate: Optimized production, characterization, application and techno-economic analysis. J. Hazard. Mater. 2020, 386, 121954. [Google Scholar] [CrossRef]
- Park, Y.S.; Kang, S.W.; Lee, J.S.; Hong, S.I.; Kim, S.W. Xylanase production in solid state fermentation by Aspergillus niger mutant using statistical experimental designs. Appl. Microbiol. Biotechnol. 2002, 58, 761–766. [Google Scholar]
- Nguyen, H.P.T.; Morançais, M.; Fleurence, J.; Dumay, J. Mastocarpus stellatus as a source of R-phycoerythrin: Optimization of enzyme assisted extraction using response surface methodology. J. Appl. Phycol. 2017, 29, 1563–1570. [Google Scholar] [CrossRef]
- Patil, S.S.; Jena, H.M. Statistical Optimization of Phenol Degradation by Bacillus pumilus OS1 Using Plackett–Burman Design and Response Surface Methodology. Arab. J. Sci. Eng. 2015, 40, 2141–2151. [Google Scholar] [CrossRef]
- Popa Ungureanu, C.; Favier, L.; Bahrim, G.; Amrane, A. Response surface optimization of experimental conditions for carbamazepine biodegradation by Streptomyces MIUG 4.89. New Biotechnol. 2015, 32, 347–357. [Google Scholar] [CrossRef]
- Wang, Z.; Quan, Y.; Zhou, F. Optimization of medium composition for exopolysaccharide production by Phellinus nigricans. Carbohydr. Polym. 2014, 105, 200–206. [Google Scholar] [CrossRef]
- Tang, X.-J.; He, G.-Q.; Chen, Q.-H.; Zhang, X.-Y.; Ali, M.A.M. Medium optimization for the production of thermal stable β-glucanase by Bacillus subtilis ZJF-1A5 using response surface methodology. Bioresour. Technol. 2004, 93, 175–181. [Google Scholar] [CrossRef]
- Elibol, M. Optimization of medium composition for actinorhodin production by Streptomyces coelicolor A3(2) with response surface methodology. Process Biochem. 2004, 39, 1057–1062. [Google Scholar] [CrossRef]
- Ajdari, Z.; Ebrahimpour, A.; Manan, M.A.; Hamid, M.; Mohamad, R.; Ariff, A.B. Nutritional Requirements for the Improvement of Growth and Sporulation of Several Strains of Monascus purpureus on Solid State Cultivation. J. Biomed. Biotechnol. 2011, 2011, 487329. [Google Scholar] [CrossRef]
- Chen, X.; Li, Y.; Du, G.; Chen, J. Application of response surface methodology in medium optimization for spore production of Coniothyrium minitans in solid-state fermentation. World J. Microbiol. Biotechnol. 2005, 21, 593–599. [Google Scholar] [CrossRef]



| Run | Variables | Spore Production(×108 CFU/g) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A | B | C | D | E | F | G | H a | I a | J a | K a | ||
| 1 | −1 | −1 | −1 | −1 | −1 | −1 | −1 | −1 | −1 | −1 | −1 | 12.77 ± 0.62 |
| 2 | 1 | −1 | −1 | −1 | 1 | −1 | 1 | 1 | −1 | 1 | 1 | 8.79 ± 1.48 |
| 3 | −1 | 1 | 1 | −1 | 1 | 1 | 1 | −1 | −1 | −1 | 1 | 13.57 ± 0.86 |
| 4 | 1 | 1 | −1 | −1 | −1 | 1 | −1 | 1 | 1 | −1 | 1 | 1.73 ± 0.11 |
| 5 | −1 | −1 | −1 | 1 | −1 | 1 | 1 | −1 | 1 | 1 | 1 | 5.43 ± 0.66 |
| 6 | 1 | 1 | −1 | 1 | 1 | 1 | −1 | −1 | −1 | 1 | −1 | 1.48 ± 0.26 |
| 7 | −1 | 1 | 1 | 1 | −1 | −1 | −1 | 1 | −1 | 1 | 1 | 9.33 ± 0.54 |
| 8 | −1 | 1 | −1 | 1 | 1 | −1 | 1 | 1 | 1 | −1 | −1 | 7.33 ± 0.24 |
| 9 | −1 | −1 | 1 | −1 | 1 | 1 | −1 | 1 | 1 | 1 | −1 | 13.15 ± 1.24 |
| 10 | 1 | −1 | 1 | 1 | 1 | −1 | −1 | −1 | 1 | −1 | 1 | 12.79 ± 0.78 |
| 11 | 1 | 1 | 1 | −1 | −1 | −1 | 1 | −1 | 1 | 1 | −1 | 16.47 ± 0.60 |
| 12 | 1 | −1 | 1 | 1 | −1 | 1 | 1 | 1 | −1 | −1 | −1 | 10.57 ± 0.42 |
| Run | C. Urea (w/v) | D. NaCl (w/v) | F. MgSO4·7H2O (w/v) | Spore Production (×108 CFU/g) |
|---|---|---|---|---|
| 1 | 2.2% | 0.09% | 0.09% | 11.67 ± 0.59 |
| 2 | 2.4% | 0.08% | 0.08% | 15.70 ± 0.91 |
| 3 | 2.6% | 0.07% | 0.07% | 16.95 ± 0.90 |
| 4 | 2.8% | 0.06% | 0.06% | 15.17 ± 0.57 |
| 5 | 3.0% | 0.05% | 0.05% | 10.57 ± 0.52 |
| 6 | 3.2% | 0.04% | 0.04% | 8.51 ± 0.35 |
| Significant Variables | Levels (w/v) | |||||
|---|---|---|---|---|---|---|
| Code | Terms | −1.68 (−α) | −1 | 0 | +1 | 1.68 (+α) |
| C | Urea | 2.264% | 2.400% | 2.600% | 2.800% | 2.936% |
| D | NaCl | 0.053% | 0.060% | 0.070% | 0.080% | 0.087% |
| F | MgSO4·7H2O | 0.053% | 0.060% | 0.070% | 0.080% | 0.087% |
| Variables | Levels (w/v) | F-value | p-value | Rank | |
|---|---|---|---|---|---|
| Code Terms | Low (−1) | High (+1) | |||
| A. Sucrose | 2% | 4% | 1.35 | 0.3100 | 6 |
| B. Yeast extract | 1% | 2% | 4.89 | 0.0915 | 5 |
| C. Urea | 1% | 2% | 58.64 | 0.0016 | 1 ** |
| D. NaCl | 0.1% | 0.2% | 11.18 | 0.0287 | 3 * |
| E. K2HPO4 | 0.1% | 0.2% | 0.7101 | 0.4468 | 7 |
| F. MgSO4·7H2O | 0.1% | 0.2% | 13.85 | 0.0205 | 2 * |
| G. FeSO4·7H2O | 0.01% | 0.02% | 7.64 | 0.0506 | 4 |
| Sources | Sum of Squares | Degree of Freedom | Mean Square | F-value | p-value |
|---|---|---|---|---|---|
| Model | 878.41 | 9 | 97.57 | 21.01 | <0.0001 * |
| x3 | 20.30 | 1 | 20.30 | 4.38 | 0.0429 * |
| x4 | 1.67 | 1 | 1.67 | 0.36 | 0.5637 |
| x6 | 1.71 | 1 | 1.71 | 0.37 | 0.5581 |
| x3x4 | 19.16 | 1 | 19.16 | 4.13 | 0.0697 |
| x3x6 | 26.72 | 1 | 26.72 | 5.74 | 0.0375 * |
| x4x6 | 58.34 | 1 | 58.34 | 12.55 | 0.0053 * |
| x32 | 68.54 | 1 | 68.54 | 14.74 | 0.0033 * |
| x42 | 377.41 | 1 | 377.41 | 81.30 | <0.0001 * |
| x62 | 422.71 | 1 | 422.71 | 91.03 | <0.0001 * |
| Residual | 46.52 | 10 | 4.65 | ||
| Lack of fit | 8.91 | 5 | 1.78 | 0.2368 | 0.9303 |
| Pure error | 37.61 | 5 | 7.52 | ||
| Cor total | 924.66 | 19 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, H.; Zhang, D.; Zhang, X.; Zhou, C.; Zhou, P.; Zhi, Y. Medium Optimization for Spore Production of a Straw-Cellulose Degrading Actinomyces Strain under Solid-State Fermentation Using Response Surface Method. Sustainability 2020, 12, 8893. https://doi.org/10.3390/su12218893
Liu H, Zhang D, Zhang X, Zhou C, Zhou P, Zhi Y. Medium Optimization for Spore Production of a Straw-Cellulose Degrading Actinomyces Strain under Solid-State Fermentation Using Response Surface Method. Sustainability. 2020; 12(21):8893. https://doi.org/10.3390/su12218893
Chicago/Turabian StyleLiu, Huanran, Dan Zhang, Xia Zhang, Chuanzhi Zhou, Pei Zhou, and Yuee Zhi. 2020. "Medium Optimization for Spore Production of a Straw-Cellulose Degrading Actinomyces Strain under Solid-State Fermentation Using Response Surface Method" Sustainability 12, no. 21: 8893. https://doi.org/10.3390/su12218893
APA StyleLiu, H., Zhang, D., Zhang, X., Zhou, C., Zhou, P., & Zhi, Y. (2020). Medium Optimization for Spore Production of a Straw-Cellulose Degrading Actinomyces Strain under Solid-State Fermentation Using Response Surface Method. Sustainability, 12(21), 8893. https://doi.org/10.3390/su12218893





