1. Introduction
In recent years, the number of sewage treatment plants has increased. The construction and operation of a large number of sewage treatment plants enable the effective control of municipal sewage and industrial wastewater. However, a large amount of sludge produced by sewage treatment plants is not properly treated, which causes serious environmental problems [
1]. Sludge from sewage treatment plants is rich in microbial organic matter, thereby increasing the number of microbial organisms [
2] and pollutants in sewage. It contains a large amount of not only organic-inorganic carbon, nitrogen, phosphorus, potassium and other nutrients but also organic matter, parasite eggs [
3], heavy metals [
4] and other toxic and harmful substances. The main method of municipal solid waste treatment is separation in the process of waste collection, and the goal is to reuse materials [
5]. As an organic-inorganic complex, it can utilize its high content of carbon to carry out an oxygen-free pyrolysis treatment, thereby effectively retaining the carbon in it [
6], improving the production rate of biochar, preparing biochar with a high added value, and recycling the mixed combustible gas and other by-products produced in the production process. The process of high temperature dry distillation and pyrolysis of sludge refers to the process method of coal pyrolysis of sludge after drying and drying to produce combustible gas, tar, phenol, acetone, methanol and other chemical raw materials.
Hazelnut shells and sludge were selected for co-pyrolysis. The cellulose and hemicellulose contents in hazelnut shells are higher than those in sawdust, and the lignin content is also higher than that in sawdust. This property can increase the specific surface area of biochar, enhance the porosity, and improve the adsorption capacity of biochar [
7]. As biomass, hazelnut shells are more than 40% cellulose [
8]. This cellulose contributes to providing a high specific surface area and optimizes the porous structure of biochar during co-pyrolysis [
9]. Compared with hazelnut shells, the regeneration of wood resources is slow, time consuming and relatively expensive [
10]. In contrast, hazelnut shells are inexpensive and easy to obtain as a kind of waste [
11].
In recent years, pyrolysis and gasification technology has emerged to increase energy output and reduce environmental impact [
12]. Pyrolysis technology has been used in industrial production for a long time [
13]. It was first used for the carbonization of coal and other materials and was then gradually applied to the petroleum cracking process. A previous study indicated that heavy metals in municipal sewage sludge (MSS) can be transformed from a weakly bound state to a stable state via the pyrolysis process to ensure their security during applications [
14]. Pyrolysis technology offers a practical and alternative method for the stabilization and resource utilization of sewage sludge [
15]. Sludge pyrolysis technology refers to the thermal decomposition of sludge in an inert atmosphere at atmospheric pressure, which transforms the contained organic matter into hydrocarbons [
16]. The final products are tar, pyrolysis gas and carbon [
17]. Part of the products can be burned as a heat source for pre-drying and pyrolysis, and the remaining energy can be recovered in the form of oil. Additionally, the biochar produced by pyrolysis can be used as an effective and multipurpose biomass material, for example, as adsorbents [
18,
19], a potential fertilizer [
20], or a feedstock for energy production [
21]. Sludge gasification technology refers to the use of air, oxygen or steam as gasification agent to convert organic components in sludge into clean combustible gases containing H
2, CH
4, CO and CO
2. Joan et al. [
22] studied the effects of gas residence time and air equivalence ratio on the distribution of sludge gasification products and gas composition by using a bubbling fluidized bed experimental platform. Petersen et al. [
23] developed a circulating fluidized bed sludge gasification device, and studied the effects of gasification agent ratio (CO
2/N
2), gasification temperature, material level height and fluidization speed on gasification products. Gross et al. [
24], a group of German scholars, proposed ETVS and NTVSprocesses for the overall treatment and utilization of sludge, with fluidized bed gasification technology as the core, including several units such as sludge dewatering, drying, gasification, combustion and secondary energy generation. High pressure dewatering and solar energy utilization technologies are respectively adopted in sludge dewatering and drying. The two processes can process and transform the wet sludge in different units, and finally output the available electric energy and heat energy. Finally, only residual mineral ash is treated as waste. The economy of the process is evaluated. In addition to meeting its own heat demand, the surplus heat and electricity can be output.
Wet air oxidation technology refers to a chemical process in which pure oxygen or air is used as oxidant at a certain temperature and pressure to make the organic components in the sludge undergo thermal degradation, hydrolysis and oxidation in the aqueous phase, and finally transform into CO, H2O and N2. There are many factors affecting the wet air oxidation process, such as reaction temperature, reaction pressure, reaction time, raw material properties and catalyst use, among which the reaction temperature is the key factor. The whole process of wet oxidation can be realized under two different conditions, one is the subcritical condition lower than 374 °C and 10 MPa pressure; the other is the supercritical condition higher than 374 °C and 21.8 MPA.
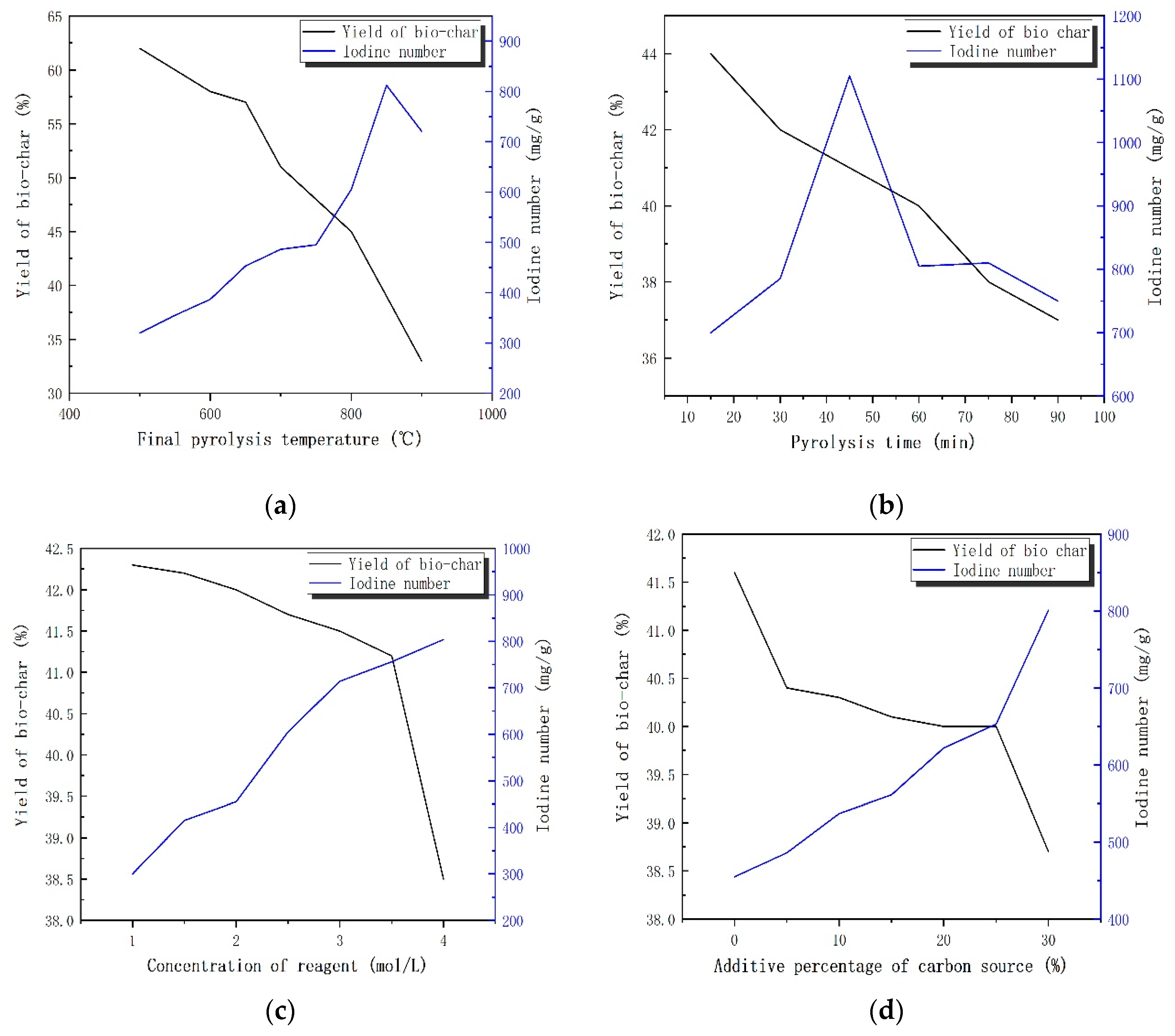
Biochar is widely used in ecological restoration and agriculture because of its large specific surface area, high stability and strong adsorption capacity. The ability of biochar to absorb pollutants depends on its physical and chemical properties. The properties of biochar produced with different raw materials and under various cracking conditions are quite diverse. Due to the preparation of biochar from the direct pyrolysis of sludge, the specific surface area of the carbon material is not ideal, and the porosity is not sufficiently developed. Therefore, sludge is usually chemically activated before pyrolysis [
25]. Okman et al. [
26] used grape seed with K
2CO
3 and KOH to produce activated carbon. The sludge-based carbon materials were prepared by a chemically activated pyrolysis. First, the raw sludge materials were mixed evenly with various chemicals, and then pyrolysis was carried out in an inert atmosphere to obtain biochar with a certain porosity. Through the oxidation and dehydration of chemical activators, hydrogen, oxygen and a small amount of the carbon in raw sludge materials could escape in the form of water vapor and other gases, which formed pores when passing through the carbon layer structure of polycondensated carbon.
Semiconductor photocatalysts have the advantages of a low energy consumption, a lack of secondary pollution, and an ability to convert most organic compounds into carbon dioxide, water and inorganic salt molecules [
27]. However, in the actual process, there are the following shortcomings: First, most of the semiconductors have a low utilization of sunlight and second, the photogenerated electron hole pairs of semiconductors easily recombine, which reduces the quantum yield of light, thus affecting the photocatalytic efficiency. In the process of semiconductor photocatalysis, photogenerated electrons and holes are produced by semiconductor materials under the excitation of light, and then an oxidation-reduction reaction occurs with the groups on the surface of the semiconductor to produce active species or intermediate chemical substances that release heat, while the active species or intermediate chemical products with strong oxidation abilities can oxidize and degrade organic pollutants [
28]. A large number of studies show that semiconductor photocatalysis technology can not only degrade various organic aromatic compounds in water [
29] but also oxidize or decompose toxic precious metal ions in water; these precious metals can be converted into non-toxic or slightly toxic forms to realize the recovery and utilization of precious metals [
30]. Photocatalysts used in the photocatalytic degradation of organic wastewater are inexpensive, non-toxic, stable, recyclable, and do not produce secondary pollution; furthermore, some photocatalytic materials can also directly use sunlight. Therefore, semiconductor photocatalysis technology has become an ideal technology for environmental governance. In this experiment, zinc chloride, sodium hydroxide and biochar were used as raw materials to prepare a composite biochar with different proportions of nano-zinc oxide by a hydrothermal synthesis. The composition, structure and morphology of the composite were characterized by X-ray diffraction (XRD), scanning electron microscopy (SEM) and Fourier transform infrared (FTIR) spectroscopy; additionally, Cu(II) adsorption tests were carried out.
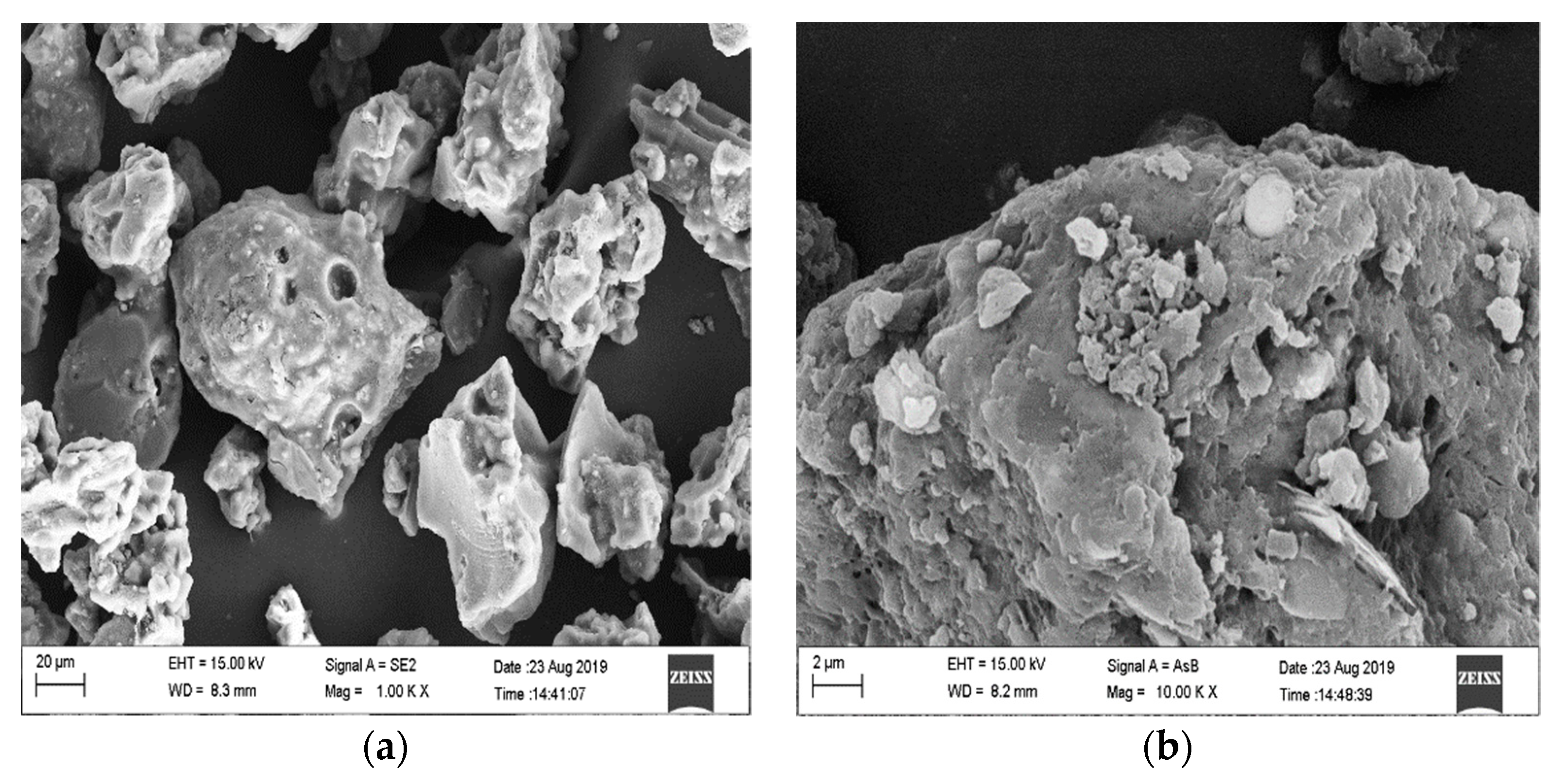
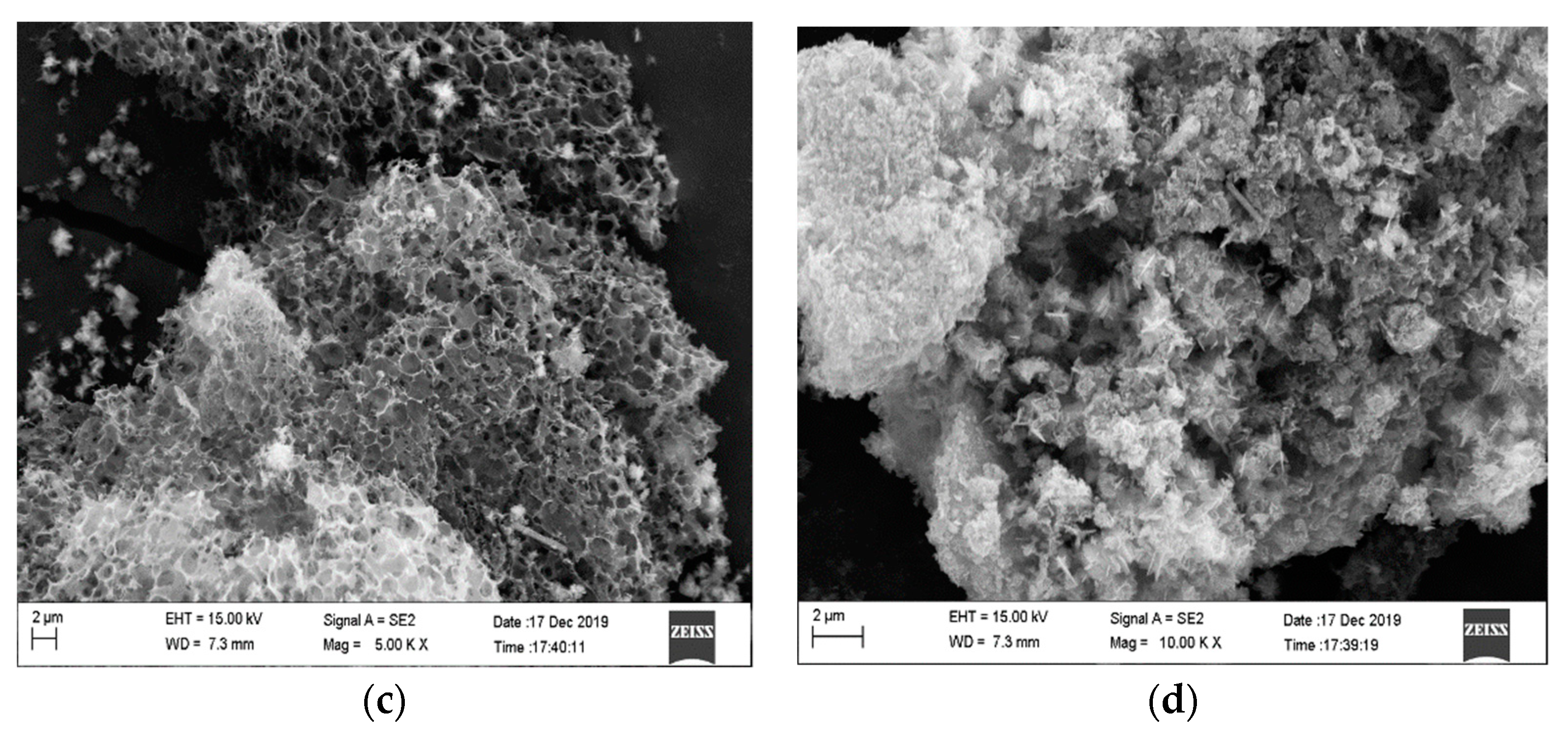
In this experiment, SEM, FTIR, XRD and other modern analytical methods were used to characterize the surface features, surface functional groups and internal crystal structure of biochar, respectively, when prepared under different pyrolysis conditions; additionally, the actual adsorption of Cu(II) by biochar and adsorption kinetic models were determined.