Clays, Limestone and Biochar Affect the Bioavailability and Geochemical Fractions of Cadmium and Zinc from Zn-Smelter Polluted Soils
Abstract
1. Introduction
2. Materials and Methods
2.1. Collection of Contaminated Soils
Immobilizing Agents
2.2. Experimental Design
2.3. Sample Analyses
2.3.1. Soil Sample Analysis
2.3.2. Extraction of PTEs Using DTPA/TEA
2.3.3. Plant Analysis
2.3.4. Chlorophyll Assessment
2.4. Quality Control and Statistical Analysis
3. Results and Discussion
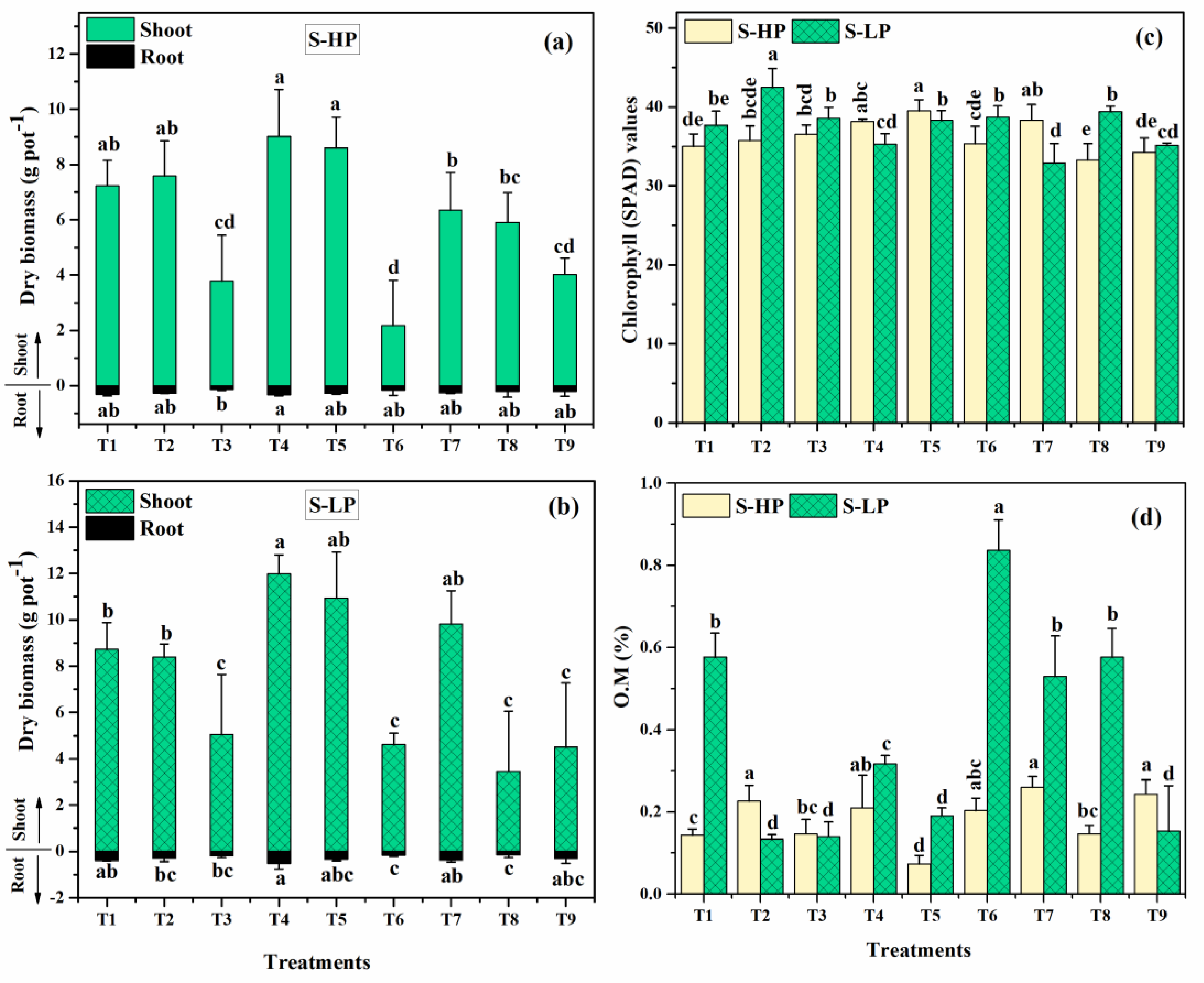
3.1. Plant Growth
3.1.1. Chlorophyll Content
3.1.2. Soil Organic Matter
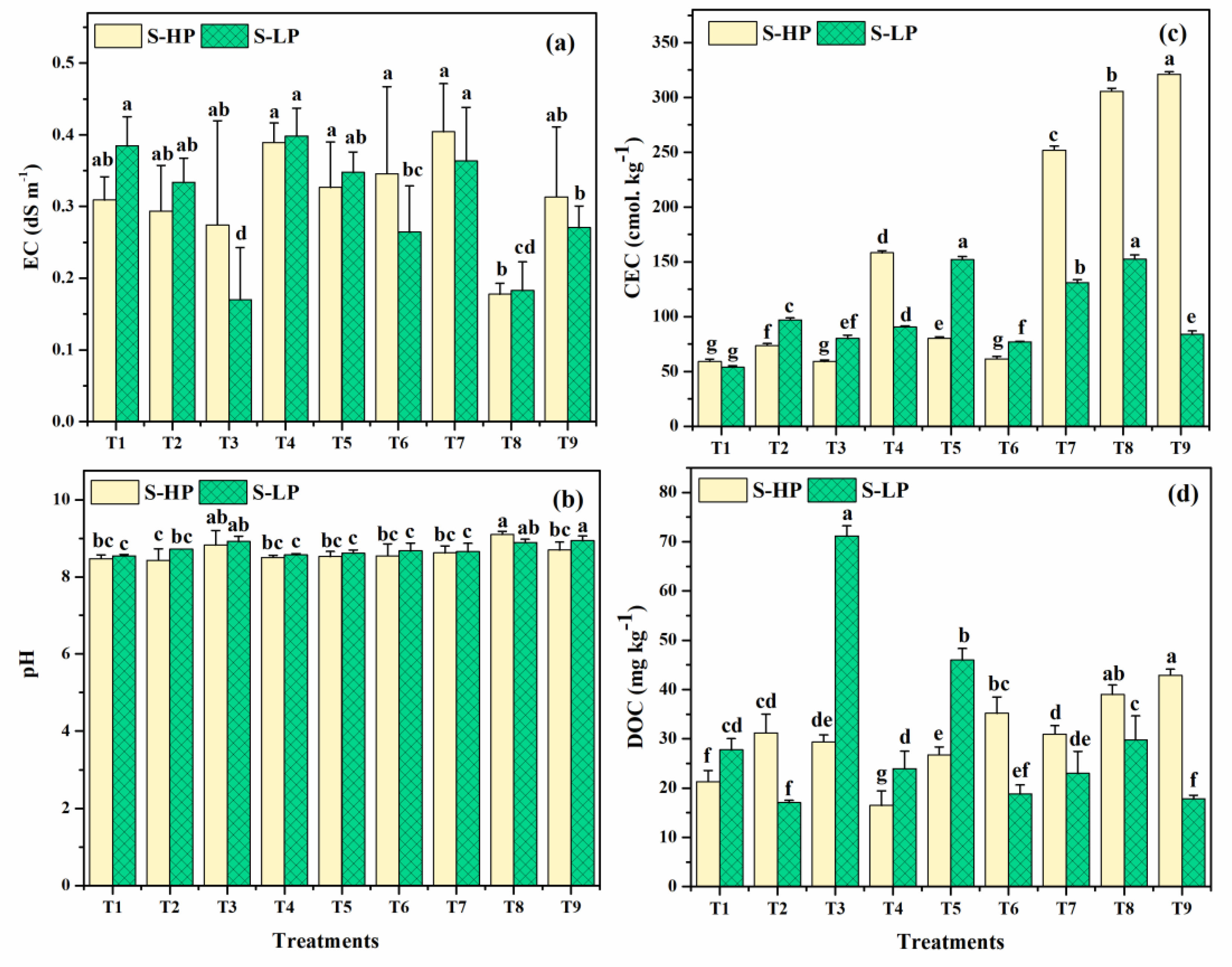
3.1.3. Soil Electrical Conductivity
3.1.4. Soil pH
3.1.5. Soil Cation Exchange Capacity
3.1.6. Soil Dissolved Organic Carbon
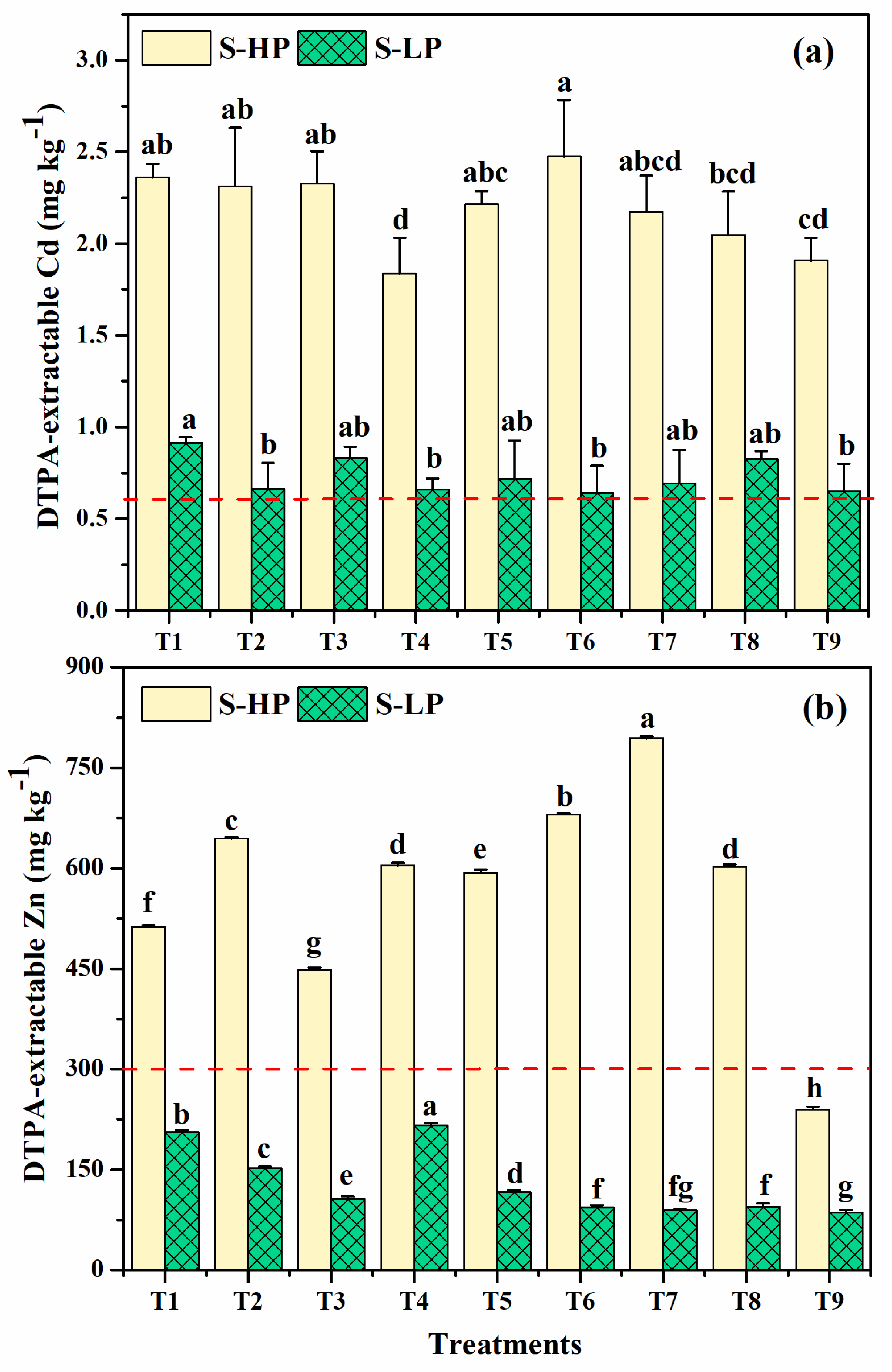
3.1.7. Soil DTPA-Extracted Cd and Zn
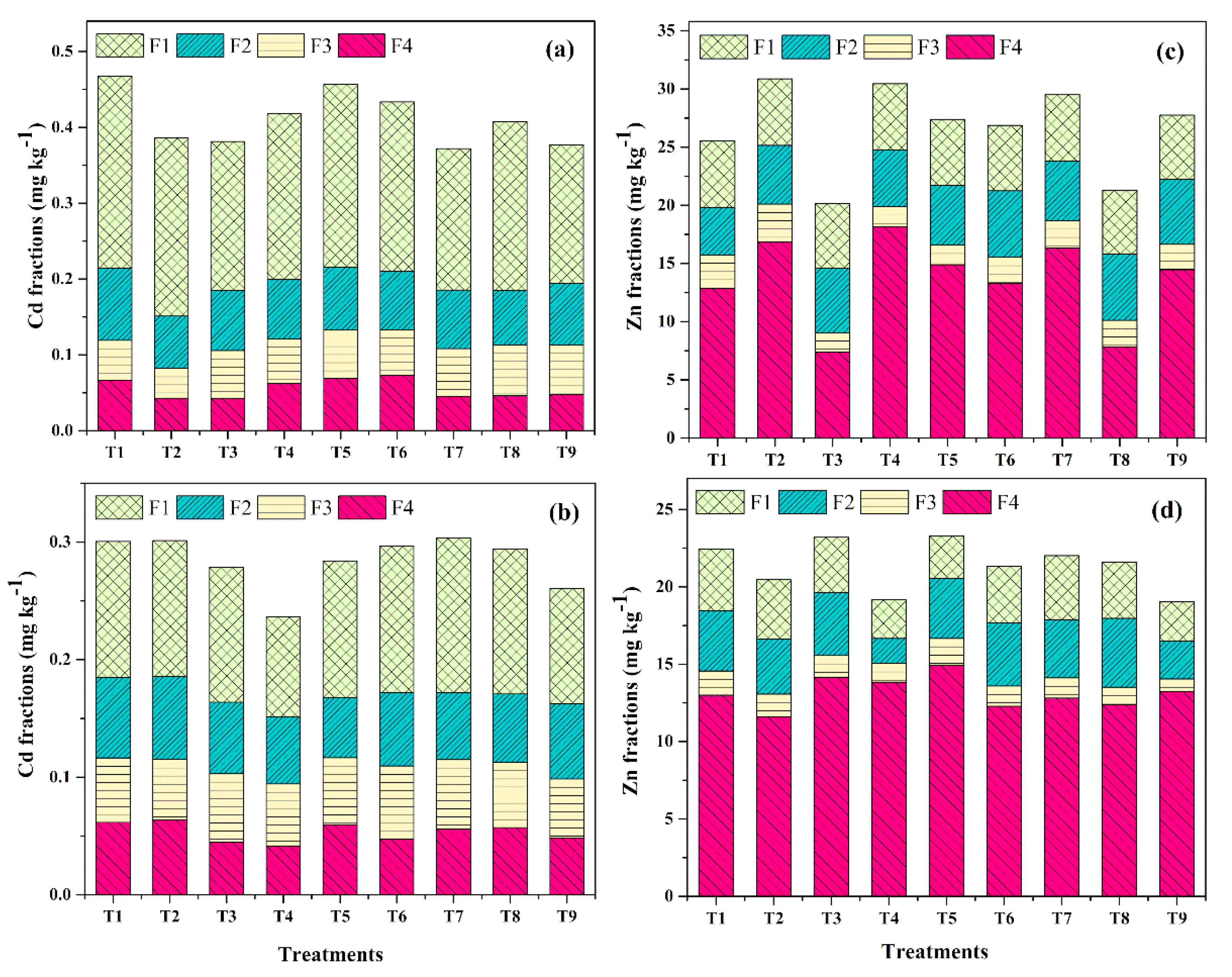
3.1.8. Geochemical forms of Cd and Zn
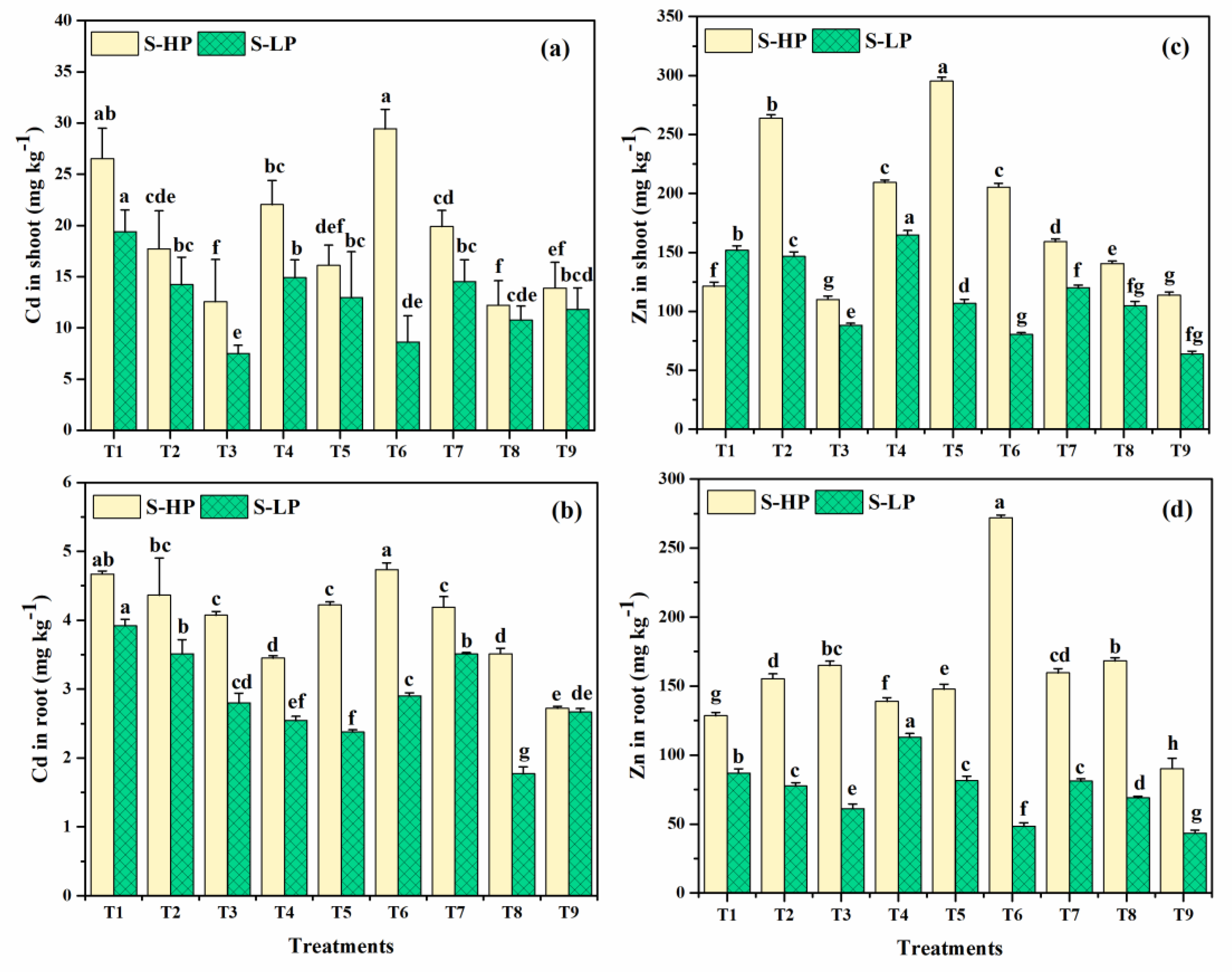
3.1.9. Cd Uptake in Shoots and Roots
3.1.10. Zn Uptake in Shoot and Root
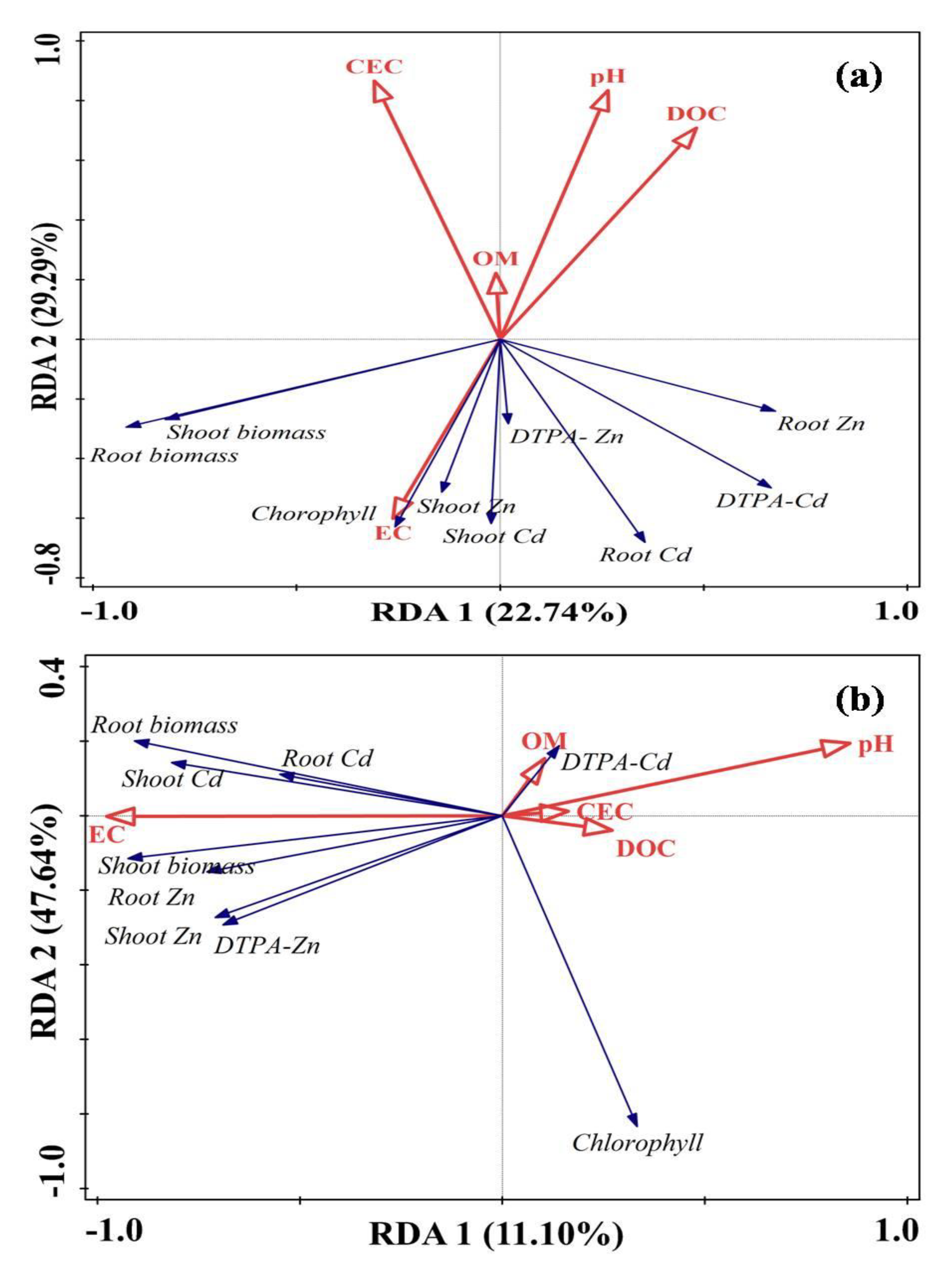
3.2. Redundancy Analysis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Hamid, Y.; Tang, L.; Hussain, B.; Usman, M.; Hashmi, M.L.R.; Khan, M.B.; Yang, X.; He, Z.L. Immobilization and Sorption of Cd and Pb in Contaminated Stagnicanthrosols as Amended with Biochar and Manure Combined With Inorganic Additives. J. Environ. Manag. 2020, 257, 109999. [Google Scholar] [CrossRef]
- De Souza, E.S.; Dias, Y.N.; Da Costa, H.S.C.; Pinto, D.A.; De Oliveira, D.M.; Falção, N.P.D.S.; Teixeira, R.A.; Fernandes, A.R. Organic Residues and Biochar to Immobilize Potentially Toxic Elements in Soil From a Gold Mine in the Amazon. Ecotoxicol. Environ. Saf. 2019, 169, 425–434. [Google Scholar] [CrossRef]
- Roberts, T.L. Cadmium and Phosphorous Fertilizers: The Issues and the Science. Procedia Eng. 2014, 83, 52–59. [Google Scholar] [CrossRef]
- Lahori, A.H.; Gue, Z.Y.; Zhang, Z.Q.; Li, R.H.; Mahar, A.; Awasthi, M.K.; Shen, F.; Sial, T.A.; Kumbhar, F.; Wang, P.; et al. Use of Biochar as Amendment for Soil Heavy Metals Immobilization: Prospects and Challenges. Pedosphere 2017, 27, 991–1014. [Google Scholar] [CrossRef]
- Chipasa, K.B. Accumulation and Fate of Selected Heavy Metals in a Biological Wastewater Treatment System. Waste Manag. 2003, 23, 135–143. [Google Scholar] [CrossRef]
- Kulikowska, D.; Gusiatin, Z.M.; Bułkowska, K.; Kierklo, K. Humic Substances from Sewage Sludge Compost as Washing Agent Effectively Remove Cu and Cd from Soil. Chemosphere 2015, 136, 42–49. [Google Scholar] [CrossRef]
- Guo, G.; Zhou, Q.; Ma, L.Q. Availability and Assessment of Fixing Additives for The In Situ Remediation of Heavy Metal Contaminated Soils: A Review. Environ. Monit. Assess. 2006, 116, 513–528. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, M.; Rajapaksha, A.U.; Lim, J.E.; Zhang, M.; Bolan, N.; Mohan, D.; Vithanage, M.; Lee, S.; Ok, Y.S. Biochar as a Sorbent for Contaminant Management in Soil and Water: A Review. Chemosphere 2014, 99, 19–33. [Google Scholar] [CrossRef]
- Chen, Y.; Xu, J.; Lv, Z.; Xie, R.; Huang, L.; Jiang, J. Impacts of Biochar and Oyster Shells Waste on the Immobilization of Arsenic in Highly Contaminated Soils. J. Environ. Manag. 2018, 217, 646–653. [Google Scholar] [CrossRef]
- Sun, Y.B.; Sun, G.H.; Xu, Y.M.; Wang, L.; Liang, X.F.; Lin, D.S.; Hu, F.Z. Assessment of Natural Sepiolite on Cadmium Stabilization, Microbial Communities and Enzyme Activities in an Acidic Soil. Environ. Sci. Pollut. Res. 2013, 20, 3290–3299. [Google Scholar] [CrossRef]
- Lahori, A.H.; Zhang, Z.; Guo, Z.; Mahar, A.; Li, R.; Awasthi, M.K.; Sial, T.A.; Kumbhar, F.; Wang, P.; Shen, F.; et al. Potential Use of Lime Combined With Additives on (im)Mobilization and Phytoavailability of Heavy Metals From Pb/Zn Smelter Contaminated Soils. Ecotoxicol. Environ. Saf. 2017, 145, 313–323. [Google Scholar] [CrossRef] [PubMed]
- Abbaspour, A.; Golchin, A. Immobilization of Heavy Metals in a Contaminated Soil in Iran Using Di-Ammonium Phosphate, Vermicompost and Zeolite. Environ. Earth Sci. 2010, 63, 935–943. [Google Scholar] [CrossRef]
- Tandy, S.; Healey, J.R.; Nason, M.; Williamson, J.; Jones, D. Heavy Metal Fractionation During the Co-Composting of Biosolids, Deinking Paper Fibre and Green Waste. Bioresour. Technol. 2009, 100, 4220–4226. [Google Scholar] [CrossRef] [PubMed]
- Bolan, N.S.; Adriano, D.C.; Mani, P.A.; Duraisamy, A. Immobilization and Phytoavailability of Cadmium in Variable Charge Soils. II. Effect of Lime Addition. Plant Soil 2003, 251, 187–198. [Google Scholar] [CrossRef]
- Garau, G.; Castaldi, P.; Santona, L.; Deiana, P.; Melis, P. Influence of Red Mud, Zeolite and Lime on Heavy Metal Immobilization, Culturable Heterotrophic Microbial Populations and Enzyme Activities in a Contaminated soil. Geoderma 2007, 142, 47–57. [Google Scholar] [CrossRef]
- Chaves, L.H.G.; Tito, G.A. Cadmium and Copper Adsorption on Bentonite: Effects of Ph and Particle Size. Rev. Cienc. Agron. 2011, 42, 278–284. [Google Scholar] [CrossRef]
- Xu, Y.; Liang, X.; Xu, Y.; Qin, X.; Huang, Q.; Wang, L.; Sun, Y. Remediation of Heavy Metal-Polluted Agricultural Soils Using Clay Minerals: A Review. Pedosphere 2017, 27, 193–204. [Google Scholar] [CrossRef]
- Mohamed, I.; Zhang, G.-S.; Li, Z.-G.; Liu, Y.; Chen, F.; Dai, K. Ecological Restoration of an Acidic Cd Contaminated Soil Using Bamboo Biochar Application. Ecol. Eng. 2015, 84, 67–76. [Google Scholar] [CrossRef]
- Guo, F.; Ding, C.; Zhou, Z.; Huang, G.; Wang, X. Effects of Combined Amendments on Crop Yield and Cadmium Uptake in Two Cadmium Contaminated Soils Under Rice-Wheat Rotation. Ecotoxicol. Environ. Saf. 2018, 148, 303–310. [Google Scholar] [CrossRef]
- Paz-Ferreiro, J.; Lu, H.; Fu, S.; Méndez, A.; Gascó, G. Use of Phytoremediation and Biochar to Remediate Heavy Metal Polluted Soils: A Review. Solid Earth 2014, 5, 65–75. [Google Scholar] [CrossRef]
- Gascó, G.; Álvarez, M.; Paz-Ferreiro, J.; Méndez, A. Combining Phytoextraction by Brassica Napus and Biochar Amendment for the Remediation of a Mining Soil in Riotinto (Spain). Chemosphere 2019, 231, 562–570. [Google Scholar] [CrossRef]
- Patel, S.; Kundu, S.; Halder, P.; Veluswamy, G.; Pramanik, B.; Paz-Ferreiro, J.; Surapaneni, A.; Shah, K. Slow Pyrolysis of Biosolids in a Bubbling Fluidised Bed Reactor Using Biochar, Activated Char and Lime. J. Anal. Appl. Pyrolysis 2019, 144, 104697. [Google Scholar] [CrossRef]
- Álvarez, M.L.; Méndez, A.-M.; Paz-Ferreiro, J.; Gascó, G. Effects of Manure Waste Biochars in Mining Soils. Appl. Sci. 2020, 10, 3393. [Google Scholar] [CrossRef]
- Cárdenas-Aguiar, E.; Suárez, G.; Paz-Ferreiro, J.; Askeland, M.; Méndez, A.; Gascó, G. Remediation of Mining Soils by Combining Brassica Napus Growth and Amendment With Chars From Manure Waste. Chemosphere 2020, 261, 127798. [Google Scholar] [CrossRef] [PubMed]
- USEPA. Method 9045D. Soil and Waste pH; USEPA: Washington, DC, USA, 2004.
- ASTM. ASTM 1125 Standard Test Methods for Electrical Conductivity and Resistivity of Water; ASTM International: West Conshohocken, PA, USA, 2014. [Google Scholar]
- USEPA. Test Methods for Evaluating Solid Waste. Laboratory Manual Physical/Chemical Methods; US Governmental Printing Office: Washington, DC, USA, 1986; Volume 1A.
- Park, J.H.; Choppala, G.K.; Bolan, N.; Chung, J.W.; Chuasavathi, T. Biochar Reduces the Bioavailability and Phytotoxicity of Heavy Metals. Plant Soil 2011, 348, 439–451. [Google Scholar] [CrossRef]
- Abdel-Kader, N.H.; Shahin, R.R.; Khater, H.A. Assessment of Heavy Metals Immobilization in Artificially Contaminated Soils Using Some Local Amendments. Open J. Met. 2013, 3, 68–76. [Google Scholar] [CrossRef]
- ISO. Soil Quality—Determination of Organic Carbon by Sulfochromic Oxidation; 14235; International Organization for Standardization: Genève, Switzerland, 1998. [Google Scholar]
- Sochan, A.; Bieganowski, A.; Ryzak, M.; Dobrowolski, R.; Bartmiñski, P. Comparison of Soil Texture Determined by Two Dispersion Units of Master Sizer 2000. Int. Agrophys. 2012, 26, 99–102. [Google Scholar] [CrossRef]
- USEPA. Method 9080. Cation-Exchange Capacity of Soils (Ammonium Acetate); USEPA: Washington, DC, USA, 1986. [Google Scholar]
- Lo, I.M.-C.; Yang, X.-Y. Removal and Redistribution of Metals from Contaminated Soils by a Sequential Extraction Method. Waste Manag. 1998, 18, 1–7. [Google Scholar] [CrossRef]
- ISO. Soil Quality—Extraction of Trace Elements by Buffered DTPA Solution; 14870; International Organization for Standardization: Genève, Switzerland, 2001. [Google Scholar]
- Mahar, A.; Wang, P.; Ali, A.; Guo, Z.; Awasthi, M.K.; Lahori, A.H.; Wang, Q.; Shen, F.; Li, R.; Zhang, Z. Impact of CaO, Fly Ash, Sulfur and Na2S on the (Im)Mobilization and Phytoavailability of Cd, Cu and Pb in Contaminated Soil. Ecotoxicol. Environ. Saf. 2016, 134, 116–123. [Google Scholar] [CrossRef]
- Cheng, S.-F.; Hseu, Z.-Y. In-Situ Immobilization of Cadmium and Lead by Different Amendments in Two Contaminated Soils. Water Air Soil Pollut. 2002, 140, 73–84. [Google Scholar] [CrossRef]
- Yi, N.; Wu, Y.; Fan, L.; Hu, S. Remediating Cd-Contaminated Soils Using Natural and Chitosan-Introduced Zeolite, Bentonite, and Activated Carbon. Pol. J. Environ. Stud. 2019, 28, 1461–1468. [Google Scholar] [CrossRef]
- Wang, B.; Wang, C.; Li, J.; Sun, H.; Xu, Z. Remediation of Alkaline Soil with Heavy Metal Contamination Using Tourmaline as a Novel Amendment. J. Environ. Chem. Eng. 2014, 2, 1281–1286. [Google Scholar] [CrossRef]
- Wang, Q.; Awasthi, M.K.; Ren, X.; Zhao, J.; Li, R.; Shen, F.; Zhang, Z. Effect of Calcium Bentonite on Zn and Cu Mobility and Their Accumulation in Vegetable Growth in Soil Amended with Compost During Consecutive Planting. Environ. Sci. Pollut. Res. 2017, 24, 15645–15654. [Google Scholar] [CrossRef] [PubMed]
- Abdelhafez, A.A.; Li, J.; Abbas, M.H. Feasibility of Biochar Manufactured From Organic Wastes on the Stabilization of Heavy Metals in a Metal Smelter Contaminated Soil. Chemosphere 2014, 117, 66–71. [Google Scholar] [CrossRef]
- Zhang, R.-H.; Li, Z.-G.; Liu, X.-D.; Wang, B.-C.; Zhou, G.-L.; Huang, X.-X.; Lin, C.-F.; Wang, A.-H.; Brooks, M. Immobilization and Bioavailability of Heavy Metals in Greenhouse Soils Amended With Rice Straw-Derived Biochar. Ecol. Eng. 2017, 98, 183–188. [Google Scholar] [CrossRef]
- Shuman, L.M. Organic Waste Amendments Effect on Zinc Fractions of Two Soils. J. Environ. Qual. 1999, 28, 1442–1447. [Google Scholar] [CrossRef]
- Gadepalle, V.P.; Ouki, S.; Van Herwijnen, R.; Hutchings, T. Immobilization of Heavy Metals in Soil Using Natural and Waste Materials for Vegetation Establishment on Contaminated Sites. Soil Sediment Contam. Int. J. 2007, 16, 233–251. [Google Scholar] [CrossRef]
- Van Herwijnen, R.; Laverye, T.; Poole, E.J.; Hodson, M.E.; Hutchings, T. The Effect of Organic Materials on the Mobility and Toxicity of Metals in Contaminated Soils. Appl. Geochem. 2007, 22, 2422–2434. [Google Scholar] [CrossRef]
- Van Herwijnen, R.; Hutchings, T.R.; Al-Tabbaa, A.; Moffat, A.J.; Johns, M.L.; Ouki, S. Remediation of Metal Contaminated Soil with Mineral-Amended Composts. Environ. Pollut. 2007, 150, 347–354. [Google Scholar] [CrossRef]
- Meng, J.; Tao, M.; Wang, L.; Liu, X.; Xu, J. Changes in Heavy Metal Bioavailability and Speciation from a Pb-Zn Mining Soil Amended with Biochars From Co-Pyrolysis of Rice Straw and Swine Manure. Sci. Total Environ. 2018, 633, 300–307. [Google Scholar] [CrossRef]
- Li, H.; Shi, W.-Y.; Shao, H.-B.; Shao, M. The Remediation of the Lead-Polluted Garden Soil by Natural Zeolite. J. Hazard. Mater. 2009, 169, 1106–1111. [Google Scholar] [CrossRef]
- Wu, Y.-J.; Zhou, H.; Zou, Z.-J.; Zhu, W.; Yang, W.-T.; Peng, P.-Q.; Zeng, M.; Liao, B.-H. A Three-Year in-Situ Study on the Persistence of a Combined Amendment (Limestone+Sepiolite) for Remedying Paddy Soil Polluted With Heavy Metals. Ecotoxicol. Environ. Saf. 2016, 130, 163–170. [Google Scholar] [CrossRef] [PubMed]
- Boostani, H.R.; Hardie, A.G.; Najafi-Ghiri, M.; Khalili, D. Investigation of Cadmium Immobilization in a Contaminated Calcareous Soil as Influenced by Biochars and Natural Zeolite Application. Int. J. Environ. Sci. Technol. 2017, 15, 2433–2446. [Google Scholar] [CrossRef]
- Liu, C.; Wang, H.; Tang, X.-Y.; Guan, Z.; Reid, B.J.; Rajapaksha, A.U.; Ok, Y.S.; Sun, H. Biochar Increased Water Holding Capacity but Accelerated Organic Carbon Leaching From a Sloping Farmland Soil in China. Environ. Sci. Pollut. Res. 2015, 23, 995–1006. [Google Scholar] [CrossRef]
- Gao, J.; Lv, J.; Wu, H.; Dai, Y.; Nasir, M. Impacts of Wheat Straw Addition on Dissolved Organic Matter Characteristics in Cadmium-Contaminated Soils: Insights from Fluorescence Spectroscopy and Environmental Implications. Chemosphere 2018, 193, 1027–1035. [Google Scholar] [CrossRef] [PubMed]
- Usman, A.R.A.; Kuzyakov, Y.; Stahr, K. Effect of Clay Minerals on Extractability of Heavy Metals and Sewage Sludge Mineralization in Soil. Chem. Ecol. 2004, 20, 123–135. [Google Scholar] [CrossRef]
- Zhou, H.; Zhou, X.; Zeng, M.; Liao, B.-H.; Liu, L.; Yang, W.-T.; Wu, Y.-M.; Qiu, Q.-Y.; Wang, Y.-J. Effects of Combined Amendments on Heavy Metal Accumulation in Rice (Oryza Sativa L.) Planted on Contaminated Paddy Soil. Ecotoxicol. Environ. Saf. 2014, 101, 226–232. [Google Scholar] [CrossRef]
- Li, Q.; Cai, S.; Mo, C.; Chu, B.; Peng, L.; Yang, F. Toxic Effects of Heavy Metals and Their Accumulation in Vegetables Grown in a Saline Soil. Ecotoxicol. Environ. Saf. 2010, 73, 84–88. [Google Scholar] [CrossRef]
- Cui, H.; Zhou, J.; Si, Y.; Mao, J.; Zhao, Q.; Fang, G.; Liang, J. Immobilization of Cu and Cd in a Contaminated Soil: One-and Four-Year Field Effects. J. Soils Sediments 2014, 14, 1397–1406. [Google Scholar] [CrossRef]
- Khan, M.J.; Jones, D.L. Chemical and Organic Immobilization Treatments for Reducing Phytoavailability of Heavy Metals in Copper-Mine Tailings. J. Plant Nutr. Soil Sci. 2008, 171, 908–916. [Google Scholar] [CrossRef]
- Zhang, M.; Pu, J. Mineral Materials as Feasible Amendments to Stabilize Heavy Metals in Polluted Urban Soils. J. Environ. Sci. 2011, 23, 607–615. [Google Scholar] [CrossRef]
- Almås, Å.R.; McBride, M.B.; Singh, B.R. Solubility and Lability of Cadmium and Zinc in Two Soils Treated with Organic Matter. Soil Sci. 2000, 165, 250–259. [Google Scholar] [CrossRef]
- Zhao, B.; Xu, R.; Ma, F.; Li, Y.; Wang, L. Effects of Biochars Derived From Chicken Manure and Rape Straw on Speciation and Phytoavailability of Cd to Maize in Artificially Contaminated Loess Soil. J. Environ. Manag. 2016, 184, 569–574. [Google Scholar] [CrossRef] [PubMed]
- Bashir, S.; Rizwan, M.S.; Salam, A.; Fu, Q.; Zhu, J.; Shaaban, M.; Hu, H. Cadmium Immobilization Potential of Rice Straw-Derived Biochar, Zeolite and Rock Phosphate: Extraction Techniques and Adsorption Mechanism. Bull. Environ. Contam. Toxicol. 2018, 100, 727–732. [Google Scholar] [CrossRef]
- Castaldi, P.; Santona, L.; Melis, P. Heavy Metal Immobilization by Chemical Amendments in a Polluted Soil and Influence on White Lupin Growth. Chemosphere 2005, 60, 365–371. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, M.; Usman, A.R.A.; Al-Faraj, A.S.; Ahmad, M.; Sallam, A.; Al-Wabel, M.I. Phosphorus-Loaded Biochar Changes Soil Heavy Metals Availability and Uptake Potential of Maize (Zea mays L.) Plants. Chemosphere 2018, 194, 327–339. [Google Scholar] [CrossRef]
- Bogusz, A.; Oleszczuk, P. Sequential Extraction of Nickel and Zinc in Sewage Sludge-or Biochar/Sewage Sludge-Amended Soil. Sci. Total Environ. 2018, 636, 927–935. [Google Scholar] [CrossRef]
- Sun, Y.; Li, Y.; Xu, Y.; Liang, X.; Wang, L. In Situ Stabilization Remediation of Cadmium (Cd) and Lead (Pb) Co-Contaminated Paddy Soil Using Bentonite. Appl. Clay Sci. 2015, 105, 200–206. [Google Scholar] [CrossRef]
- Bashir, S.; Zhu, J.; Fu, Q.; Hu, H. Cadmium Mobility, Uptake and Anti-Oxidative Response of Water Spinach (Ipomoea Aquatic) Under Rice Straw Biochar, Zeolite and Rock Phosphate as Amendments. Chemosphere 2018, 194, 579–587. [Google Scholar] [CrossRef]
- Wen, J.; Peng, Z.; Liu, Y.; Fang, Y.; Zeng, G.; Zhang, S. A Case Study of Evaluating Zeolite, CaCO3, and MnO2 for Cd-contaminated Sediment Reuse in Soil. J. Soils Sediments 2017, 18, 323–332. [Google Scholar] [CrossRef]
- Kumararaja, P.; Manjaiah, K.M.; Datta, S.; Sarkar, B. Remediation of Metal Contaminated Soil by Aluminium Pillared Bentonite: Synthesis, Characterisation, Equilibrium Study and Plant Growth Experiment. Appl. Clay Sci. 2017, 137, 115–122. [Google Scholar] [CrossRef]
| Parameters | S-HP | S-LP | CB | L | TB | Z |
|---|---|---|---|---|---|---|
| Textural class | Sandy loam | Sandy loam | - | - | - | - |
| EC (1:5) (dS m−1) | 0.3 | 0.2 | 0.1 | 6.4 | 11.8 | 0.1 |
| pH (1:5) | 8.6 | 8.8 | 8.2 | 11.7 | 10.5 | 7.9 |
| Organic matter (%) | 0.17 | 0.6 | - | - | 63.0 | - |
| Cation exchange capacity (cmol kg−1) | 21.4 | 29.2 | 51.0 | 0.1 | 49.1 | 6.1 |
| Dissolved organic carbon (mg kg−1) | 20.8 | 26.1 | 70.1 | 8.4 | 21.4 | 79.1 |
| Total Cd (mg kg−1) | 19.1 | 10.3 | 0.9 | 1.4 | 5.2 | 2.6 |
| Total Zn (mg kg−1) | 519.2 | 234.3 | 65.2 | 6.2 | 29.4 | 17.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lahori, A.H.; Mierzwa-Hersztek, M.; Demiraj, E.; Idir, R.; Bui, T.T.X.; Vu, D.D.; Channa, A.; Samoon, N.A.; Zhang, Z. Clays, Limestone and Biochar Affect the Bioavailability and Geochemical Fractions of Cadmium and Zinc from Zn-Smelter Polluted Soils. Sustainability 2020, 12, 8606. https://doi.org/10.3390/su12208606
Lahori AH, Mierzwa-Hersztek M, Demiraj E, Idir R, Bui TTX, Vu DD, Channa A, Samoon NA, Zhang Z. Clays, Limestone and Biochar Affect the Bioavailability and Geochemical Fractions of Cadmium and Zinc from Zn-Smelter Polluted Soils. Sustainability. 2020; 12(20):8606. https://doi.org/10.3390/su12208606
Chicago/Turabian StyleLahori, Altaf Hussain, Monika Mierzwa-Hersztek, Erdona Demiraj, Rachida Idir, Thi Tuyet Xuan Bui, Dinh Duy Vu, Amanullah Channa, Naeem Akhtar Samoon, and Zengqiang Zhang. 2020. "Clays, Limestone and Biochar Affect the Bioavailability and Geochemical Fractions of Cadmium and Zinc from Zn-Smelter Polluted Soils" Sustainability 12, no. 20: 8606. https://doi.org/10.3390/su12208606
APA StyleLahori, A. H., Mierzwa-Hersztek, M., Demiraj, E., Idir, R., Bui, T. T. X., Vu, D. D., Channa, A., Samoon, N. A., & Zhang, Z. (2020). Clays, Limestone and Biochar Affect the Bioavailability and Geochemical Fractions of Cadmium and Zinc from Zn-Smelter Polluted Soils. Sustainability, 12(20), 8606. https://doi.org/10.3390/su12208606








