Nature-Based Solution for Reducing CO2 Levels in Museum Environments: A Phytoremediation Study for the Leonardo da Vinci’s “Last Supper”
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Experimental Design
2.2. CO2 Fumigation Experiment in Controlled Environmental Conditions
Steady State Gas Exchange Measurements
2.3. Photosynthetic Light Response at Different CO2 Concentrations
2.4. Estimate of Laurus nobilis Capacity to Remove Indoor CO2
2.5. Statistical Analysis
3. Results
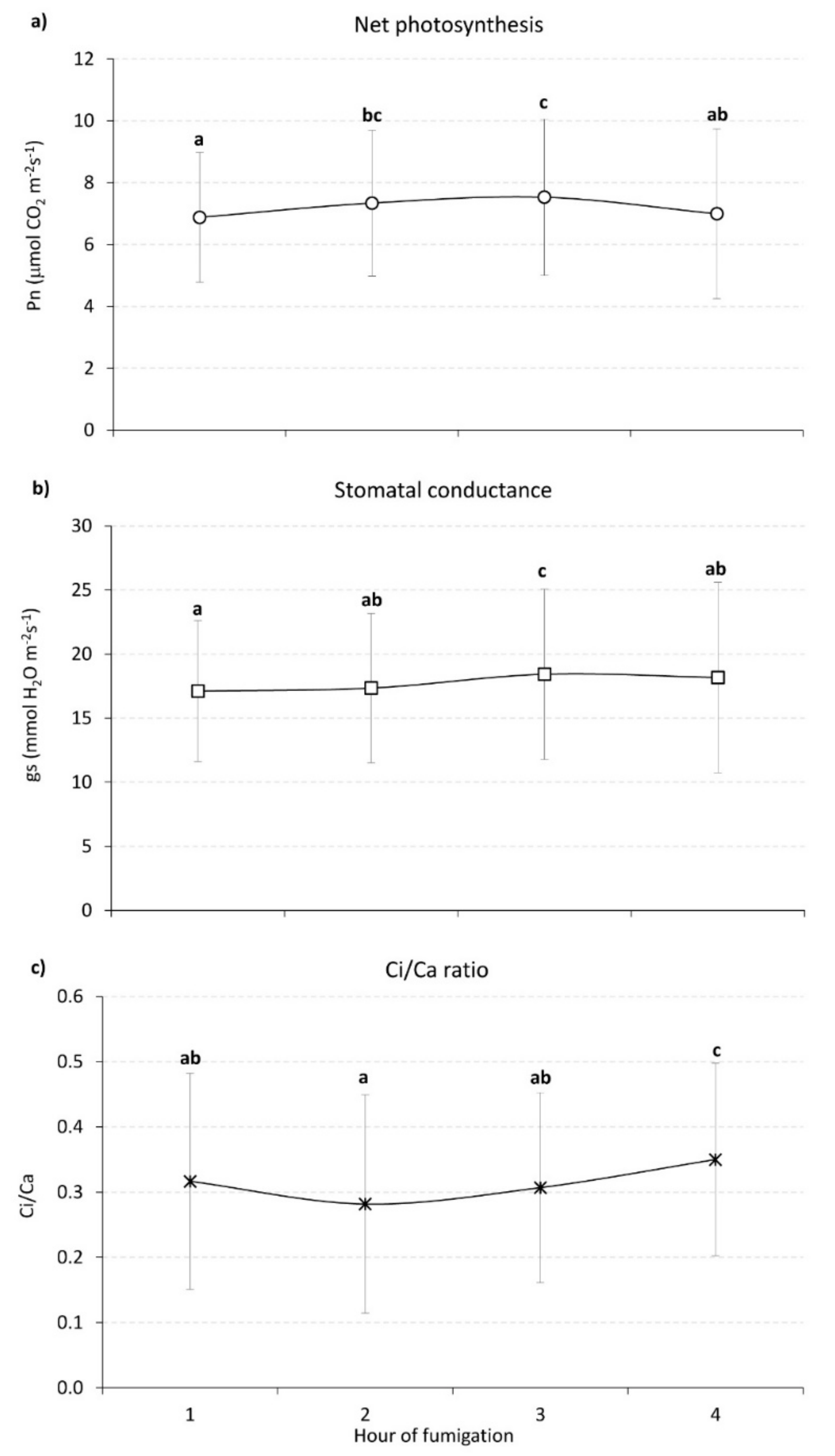
3.1. CO2 Fumigation Experiment
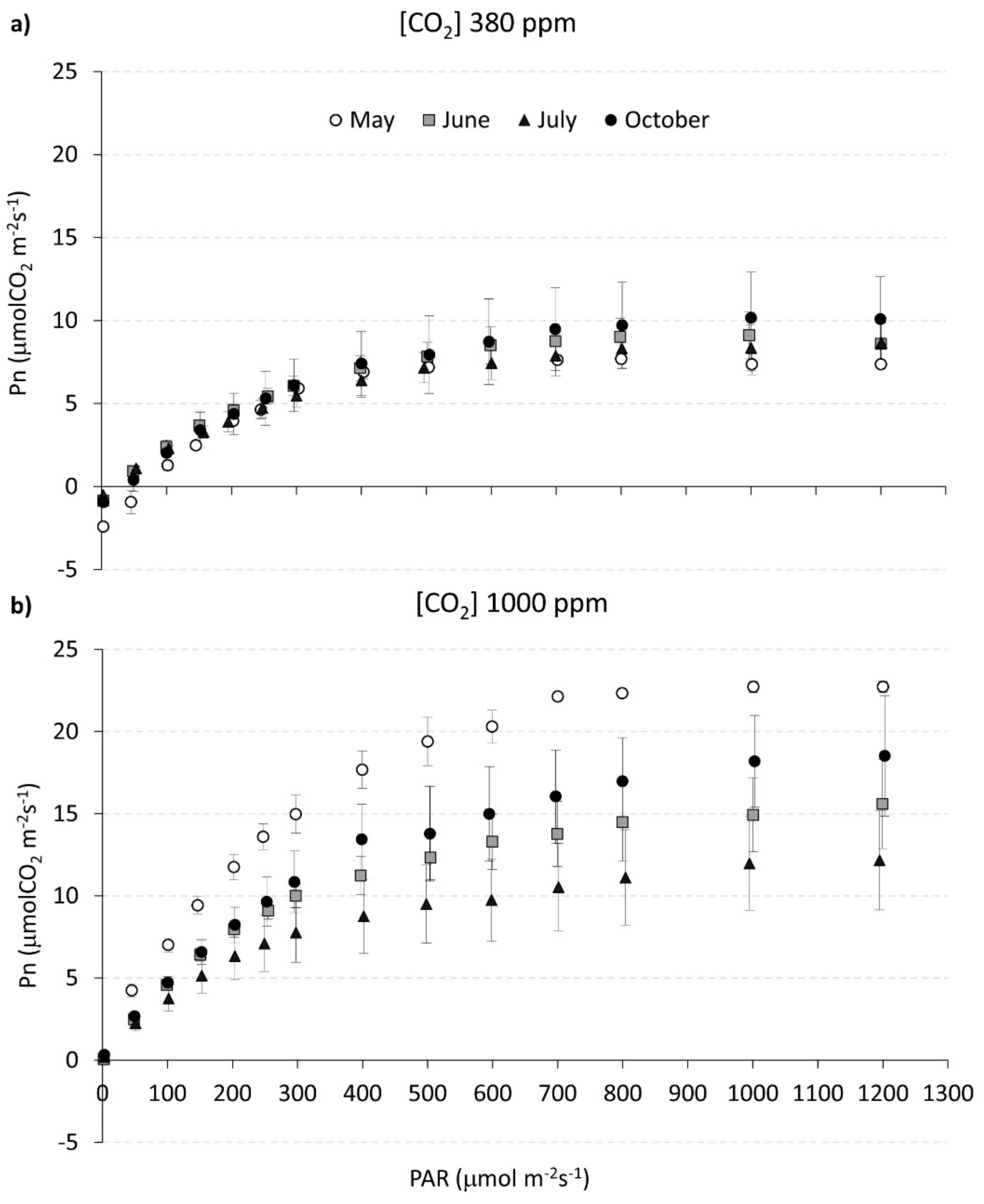
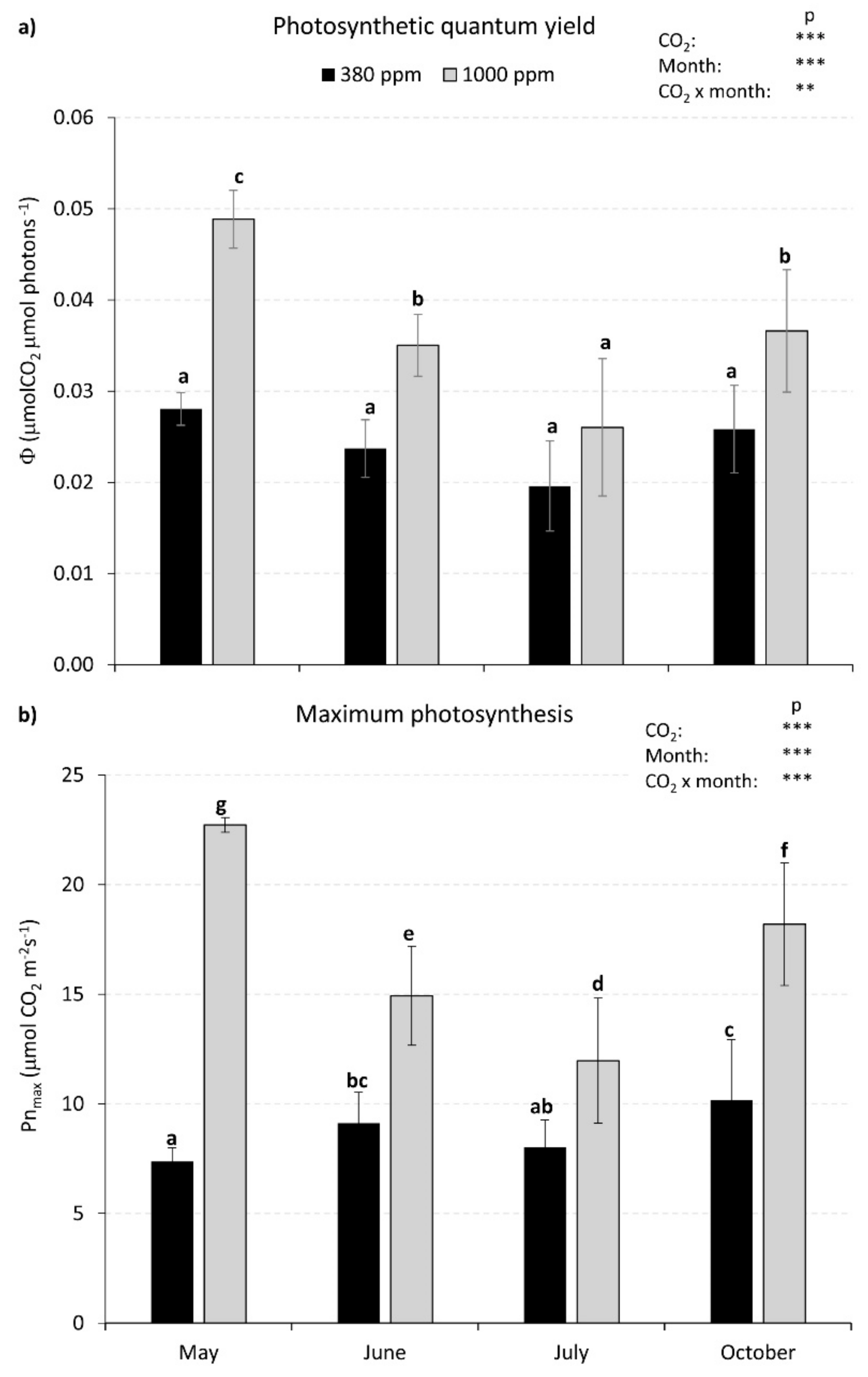
3.2. Photosynthetic Light Response Curves under Different CO2 Concentrations
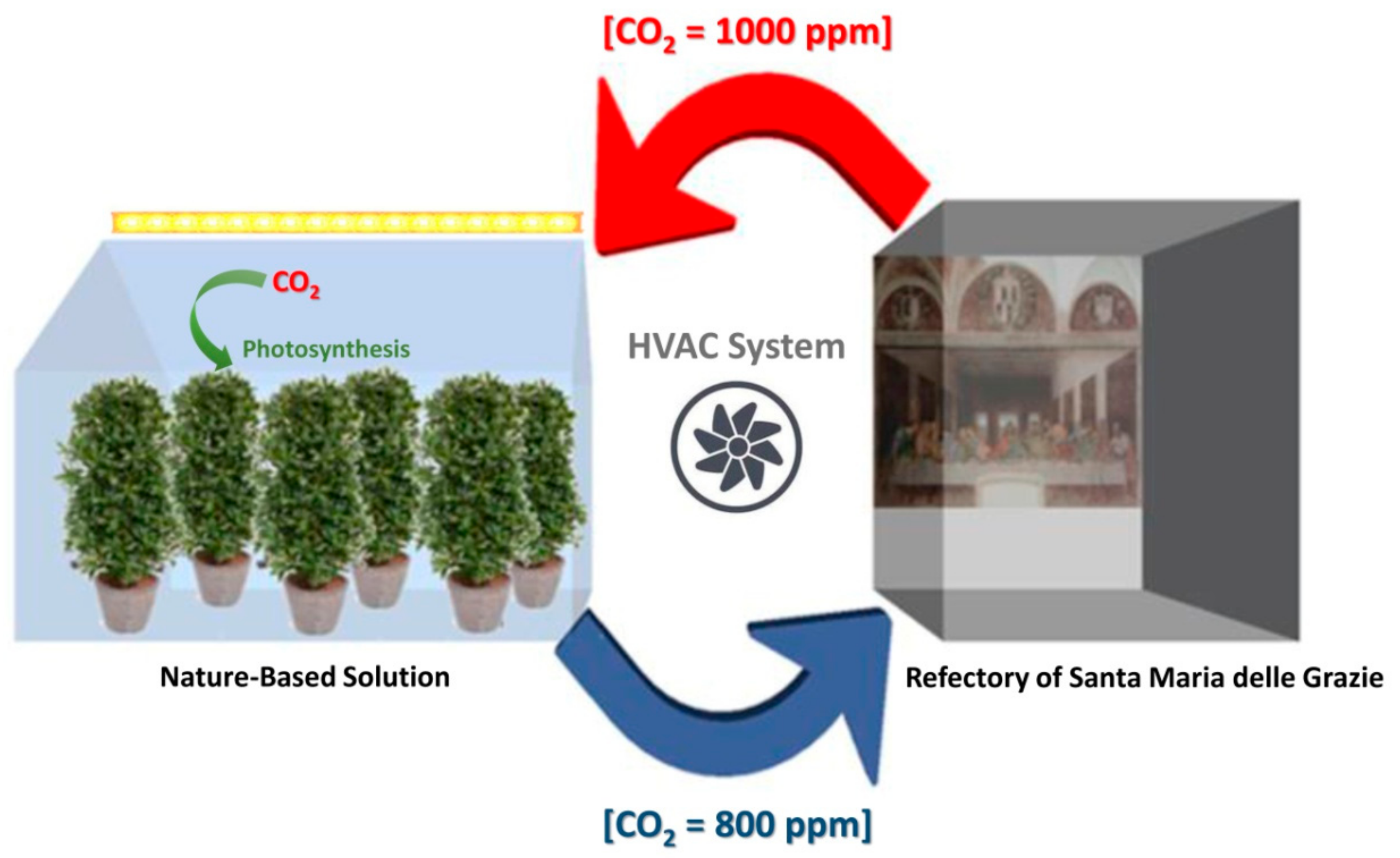
3.3. Estimate of Laurus nobilis Capacity to Remove Indoor CO2 from the Refectory of Santa Maria delle Grazie Church
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
References
- European Commission. Indoor Air Pollution: New EU Research Reveals Higher Risks than Previously Thought. Press Release. Available online: https://europa.eu/rapid/press-release_IP-03-1278_en.htm (accessed on 3 October 2019).
- World Health Organization (WHO). Who Guidelines for Indoor Air Quality: Selected Pollutants; WHO: Copenhagen, Denmark, 2010; ISBN 978-92-890-0213-4. [Google Scholar]
- Al Horr, Y.; Arif, M.; Katafygiotou, M.; Mazroei, A.; Kaushik, A.; Elsarrag, E. Impact of indoor environmental quality on occupant well-being and comfort: A review of the literature. Int. J. Sustain. Built Environ. 2016, 5, 1–11. [Google Scholar] [CrossRef]
- Blunden, J.; Arndt, D.S. State of the Climate in 2018. Bull. Amer. Meteor. Soc. 2019, 100. [Google Scholar] [CrossRef]
- Satish, U.; Mendell, M.J.; Shekhar, K.; Hotchi, T.; Sullivan, D.; Streufert, S.; Fisk, W.J. Is CO2 an Indoor Pollutant? Direct Effects of Low-to-Moderate CO2 Concentrations on Human Decision-Making Performance. Environ. Health Perspect. 2012, 120, 1671–1677. [Google Scholar] [CrossRef] [PubMed]
- Seppänen, O.A.; Fisk, W.J.; Mendell, M.J. Association of Ventilation Rates and CO2 Concentrations with Health and Other Responses in Commercial and Institutional Buildings. Indoor Air 1999, 9, 226–252. [Google Scholar] [CrossRef] [PubMed]
- ASHRAE. ANSI/ASHRAE Standard 62.1-2013: The Standards for Ventilation and Indoor Air Quality; American Society for Heating, Refrigeration and Air Conditioning Engineering: Atlanta, GA, USA, 2013. [Google Scholar]
- Krupińska, B.; Van Grieken, R.; De Wael, K. Air quality monitoring in a museum for preventive conservation: Results of a three-year study in the Plantin-Moretus Museum in Antwerp, Belgium. Microchem. J. 2013, 110, 350–360. [Google Scholar] [CrossRef]
- Camuffo, D. Microclimate for Cultural Heritage: Measurement, Risk Assessment, Conservation, Restoration, and Maintenance of Indoor and Outdoor Monuments; Elsevier: Amsterdam, The Netherlands, 2019; ISBN 978-0-444-64107-6. [Google Scholar]
- UNI 10829. Historical and Cultural Heritage: Environmental Conditions for Preservation; Ente Italiano di Unificazione: Milan, Italy, 1999. [Google Scholar]
- Camuffo, D.; Bernardi, A. The microclimate of Leonardo’s Last Supper. Boll. geofisico. Eur. Cult. Herit. Newsl. Res. 1991, 14, 39–75. [Google Scholar]
- Bellia, L.; Capozzoli, A.; Mazzei, P.; Minichiello, F. A comparison of HVAC systems for artwork conservation. Int. J. Refrig. 2007, 30, 1439–1451. [Google Scholar] [CrossRef]
- Camuffo, D.; Van Grieken, R.; Busse, H.-J.; Sturaro, G.; Valentino, A.; Bernardi, A.; Blades, N.; Shooter, D.; Gysels, K.; Deutsch, F.; et al. Environmental monitoring in four European museums. Atmos. Environ. 2001, 35, S127–S140. [Google Scholar] [CrossRef]
- Lee, K.-M.; Lim, Y.-H.; Park, C.-J.; Jo, Y.-M. Adsorption of Low-Level CO2 Using Modified Zeolites and Activated Carbon. Ind. Eng. Chem. Res. 2012, 51, 1355–1363. [Google Scholar] [CrossRef]
- Hu, S.-C.; Shiue, A.; Chang, S.-M.; Chang, Y.-T.; Tseng, C.-H.; Mao, C.-C.; Hsieh, A.; Chan, A. Removal of carbon dioxide in the indoor environment with sorption-type air filters. Int. J. Low-Carbon Technol. 2017, 12, 330–334. [Google Scholar] [CrossRef]
- Shiue, A.; Hu, S.-C.; Chang, S.-M.; Ko, T.-Y.; Hsieh, A.; Chan, A. Adsorption Kinetics and Breakthrough of Carbon Dioxide for the Chemical Modified Activated Carbon Filter Used in the Building. Sustainability 2017, 9, 1533. [Google Scholar] [CrossRef]
- Rajan, P.E.; Krishnamurthy, A.; Morrison, G.; Rezaei, F. Advanced buffer materials for indoor air CO2 control in commercial buildings. Indoor Air 2017, 27, 1213–1223. [Google Scholar] [CrossRef] [PubMed]
- Soreanu, G.; Dixon, M.; Darlington, A. Botanical biofiltration of indoor gaseous pollutants—A mini-review. Chem. Eng. J. 2013, 229, 585–594. [Google Scholar] [CrossRef]
- United States Environmental Protection Agency (EPA). Guide to Air Cleaners in the Home; EPA 402-F-08-004; United States Environmental Protection Agency (EPA): Washington, DC, USA, 2008.
- European Commission. Towards an EU Research and Innovation Policy Agenda for Nature-Based Solutions & Re-Naturing Cities: Final Report of the Horizon 2020 Expert Group on ’Nature-Based Solutions and Re-Naturing Cities’; European Commission: Brussel, Belgium, 2015; ISBN 978-92-79-46051-7. [Google Scholar]
- Maes, J.; Jacobs, S. Nature-Based Solutions for Europe’s Sustainable Development. Conserv. Lett. 2017, 10, 121–124. [Google Scholar] [CrossRef]
- Manes, F.; Vitale, M.; Maria Fabi, A.; De Santis, F.; Zona, D. Estimates of potential ozone stomatal uptake in mature trees of Quercus ilex in a Mediterranean climate. Env. Exp. Bot. 2007, 59, 235–241. [Google Scholar] [CrossRef]
- Fares, S.; Savi, F.; Fusaro, L.; Conte, A.; Salvatori, E.; Aromolo, R.; Manes, F. Particle deposition in a peri-urban Mediterranean forest. Environ. Pollut. 2016, 218, 1278–1286. [Google Scholar] [CrossRef]
- Janhäll, S. Review on urban vegetation and particle air pollution—Deposition and dispersion. Atmos. Environ. 2015, 105, 130–137. [Google Scholar] [CrossRef]
- Li, Y.; Babcock, R.W. Green roofs against pollution and climate change. A review. Agron. Sustain. Dev. 2014, 34, 695–705. [Google Scholar] [CrossRef]
- Pugh, T.A.M.; MacKenzie, A.R.; Whyatt, J.D.; Hewitt, C.N. Effectiveness of Green Infrastructure for Improvement of Air Quality in Urban Street Canyons. Environ. Sci. Technol. 2012, 46, 7692–7699. [Google Scholar] [CrossRef]
- Manes, F.; Incerti, G.; Salvatori, E.; Vitale, M.; Ricotta, C.; Costanza, R. Urban ecosystem services: Tree diversity and stability of tropospheric ozone removal. Ecol. Appl. 2012, 22, 349–360. [Google Scholar] [CrossRef]
- Manes, F.; Marando, F.; Capotorti, G.; Blasi, C.; Salvatori, E.; Fusaro, L.; Ciancarella, L.; Mircea, M.; Marchetti, M.; Chirici, G.; et al. Regulating Ecosystem Services of forests in ten Italian Metropolitan Cities: Air quality improvement by PM10 and O3 removal. Ecol. Indic. 2016, 67, 425–440. [Google Scholar] [CrossRef]
- Marando, F.; Salvatori, E.; Fusaro, L.; Manes, F. Removal of PM10 by Forests as a Nature-Based Solution for Air Quality Improvement in the Metropolitan City of Rome. Forests 2016, 7, 150. [Google Scholar] [CrossRef]
- Fusaro, L.; Mereu, S.; Salvatori, E.; Agliari, E.; Fares, S.; Manes, F. Modeling ozone uptake by urban and peri-urban forest: A case study in the Metropolitan City of Rome. Environ. Sci. Pollut. Res. 2018, 25, 8190–8205. [Google Scholar] [CrossRef] [PubMed]
- Nowak, D.J.; Hirabayashi, S.; Doyle, M.; McGovern, M.; Pasher, J. Air pollution removal by urban forests in Canada and its effect on air quality and human health. Urban For. Urban 2018, 29, 40–48. [Google Scholar] [CrossRef]
- Oh, G.S.; Jung, G.J.; Seo, M.H.; Im, Y.B. Experimental study on variations Green. of CO2 concentration in the presence of indoor plants and respiration of experimental animals. Hortic. Environ. Biotechnol. 2011, 52, 321–329. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, J.S. Characterization and performance evaluation of a full-scale activated carbon-based dynamic botanical air filtration system for improving indoor air quality. Build. Environ. 2011, 46, 758–768. [Google Scholar] [CrossRef]
- Pennisi, S.V.; van Iersel, M.W. Quantification of Carbon Assimilation of Plants in Simulated and In Situ Interiorscapes. Hort. Sci. 2012, 47, 468–476. [Google Scholar] [CrossRef]
- Dela Cruz, M.; Christensen, J.H.; Thomsen, J.D.; Müller, R. Can ornamental potted plants remove volatile organic compounds from indoor air?—A review. Environ. Sci. Pollut. Res. 2014, 21, 13909–13928. [Google Scholar] [CrossRef]
- Torpy, F.R.; Irga, P.J.; Burchett, M.D. Profiling indoor plants for the amelioration of high CO2 concentrations. Urban For. Urban Green. 2014, 13, 227–233. [Google Scholar] [CrossRef]
- Torpy, F.; Zavattaro, M.; Irga, P. Green wall technology for the phytoremediation of indoor air: A system for the reduction of high CO2 concentrations. Air Qual. Atmos. Health 2017, 10, 575–585. [Google Scholar] [CrossRef]
- Cao, Y.; Li, F.; Wang, Y.; Yu, Y.; Wang, Z.; Liu, X.; Ding, K. Assisted Deposition of PM2.5 from Indoor Air by Ornamental Potted Plants. Sustainability 2019, 11, 2546. [Google Scholar] [CrossRef]
- Brilli, F.; Fares, S.; Ghirardo, A.; de Visser, P.; Calatayud, V.; Muñoz, A.; Annesi-Maesano, I.; Sebastiani, F.; Alivernini, A.; Varriale, V.; et al. Plants for Sustainable Improvement of Indoor Air Quality. Trends Plant Sci. 2018, 23, 507–512. [Google Scholar] [CrossRef] [PubMed]
- Pettit, T.; Irga, P.J.; Torpy, F.R. Towards practical indoor air phytoremediation: A review. Chemosphere 2018, 208, 960–974. [Google Scholar] [CrossRef] [PubMed]
- Irga, P.J.; Pettit, T.J.; Torpy, F.R. The phytoremediation of indoor air pollution: A review on the technology development from the potted plant through to functional green wall biofilters. Rev. Environ. Sci. Biotechnol. 2018, 17, 395–415. [Google Scholar] [CrossRef]
- Gasparini, F.; Christescu, A. Controllo della qualità dell’aria. In Leonardo. L’ultima Cena. Indagini, Ricerche, Restauro; Basile, G., Marabelli, M., Eds.; Istituto Centrale per il Restauro and Nardini: Firenze, Italy, 2007; pp. 107–114. ISBN 978-88-404-4159-7. [Google Scholar]
- Daher, N.; Ruprecht, A.; Invernizzi, G.; De Marco, C.; Miller-Schulze, J.; Heo, J.B.; Shafer, M.M.; Schauer, J.J.; Sioutas, C. Chemical Characterization and Source Apportionment of Fine and Coarse Particulate Matter Inside the Refectory of Santa Maria delle Grazie Church, Home of Leonardo Da Vinci’s “Last Supper”. Environ. Sci. Technol. 2011, 45, 10344–10353. [Google Scholar] [CrossRef]
- Gasparini, F.; Stolfi, G. The Cenacolo Vinciano: Engineering and Microclimate within the Refectory. In Proceedings of the 49th AiCARR Internationa Conference, Rome, Italy, 26–28 February 2014; pp. 93–109. [Google Scholar]
- Caneva, G.; Bohuny, L. Botanic analysis of Livia’s villa painted flora (Prima Porta, Roma). J. Cult. Herit. 2003, 4, 149–155. [Google Scholar] [CrossRef]
- Singsaas, E.L.; Ort, D.R.; DeLucia, E.H. Variation in measured values of photosynthetic quantum yield in ecophysiological studies. Oecologia 2001, 128, 15–23. [Google Scholar] [CrossRef]
- Sharkey, T.D.; Bernacchi, C.J.; Farquhar, G.D.; Singsaas, E.L. Fitting photosynthetic carbon dioxide response curves for C3 leaves. Plant Cell Environ. 2007, 30, 1035–1040. [Google Scholar] [CrossRef]
- Irga, P.J.; Torpy, F.R.; Burchett, M.D. Can hydroculture be used to enhance the performance of indoor plants for the removal of air pollutants? Atmos. Environ. 2013, 77, 267–271. [Google Scholar] [CrossRef]
- Arena, C.; Vitale, L.; Santo, A.V. de Photosynthesis and photoprotective strategies in Laurus nobilis L. and Quercus ilex L. under summer drought and winter cold. Plant Biosyst. 2008, 142, 472–479. [Google Scholar] [CrossRef]
- Manes, F.; Seufert, G.; Vitale, M. Ecophysiological studies of Mediterranean plant species at the Castelporziano state. Atmos. Environ. 1997, 31, 51–60. [Google Scholar] [CrossRef]
- Salvatori, E.; Fusaro, L.; Manes, F. Chlorophyll Fluorescence for Phenotyping Drought-Stressed Trees in a Mixed Deciduous Forest. Ann. Bot. 2016, 6, 39–49. [Google Scholar]
- Maatallah, S.; Ghanem, M.E.; Albouchi, A.; Bizid, E.; Lutts, S. A greenhouse investigation of responses to different water stress regimes of Laurus nobilis trees from two climatic regions. J. Arid Environ. 2010, 74, 327–337. [Google Scholar] [CrossRef]
- Killi, D.; Bussotti, F.; Gottardini, E.; Pollastrini, M.; Mori, J.; Tani, C.; Papini, A.; Ferrini, F.; Fini, A. Photosynthetic and morphological responses of oak species to temperature and [CO2] increased to levels predicted for 2050. Urban For. Urban Green. 2018, 31, 26–37. [Google Scholar] [CrossRef]
- Hättenschwiler, S.; Miglietta, F.; Raschi, A.; Körner, C. Thirty years of in situ tree growth under elevated CO2: A model for future forest responses? Glob. Chang. Biol. 1997, 3, 463–471. [Google Scholar] [CrossRef]
- Ainsworth, E.A.; Long, S.P. What have we learned from 15 years of free-air CO2 enrichment (FACE)? A meta-analytic review of the responses of photosynthesis, canopy properties and plant production to rising CO2. New Phytol. 2005, 165, 351–372. [Google Scholar] [CrossRef]
- Ainsworth, E.A.; Rogers, A. The response of photosynthesis and stomatal conductance to rising [CO2]: Mechanisms and environmental interactions. Plant Cell Environ. 2007, 30, 258–270. [Google Scholar] [CrossRef]
- Manes, F.; Vitale, M.; Donato, E.; Paoletti, E. O3 and O3+CO2 effects on a mediterranean evergreen broadleaf tree, holm oak (Quercus ilex L.). Chemosphere 1998, 36, 801–806. [Google Scholar] [CrossRef]
- Paoletti, E.; Manes, F. Effects of Elevated Carbon Dioxide and Acidic Rain on the Growth of Holm Oak. In Developments in Environmental Science; Air Pollution, Global Change and Forests in the New Millenium; Elsevier: Amsterdam, The Netherlands, 2003; Volume 3, pp. 375–389. [Google Scholar]
- Tognetti, R.; Johnson, J.D.; Michelozzi, M.; Raschi, A. Response of foliar metabolism in mature trees of Quercus pubescens and Quercus ilex to long-term elevated CO2. Environ. Exp. Bot. 1998, 39, 233–245. [Google Scholar] [CrossRef]
- Körner, C.; Miglietta, F. Long term effects of naturally elevated CO2 on mediterranean grassland and forest trees. Oecologia 1994, 99, 343–351. [Google Scholar] [CrossRef]
- Scarascia-Mugnozza, G.; Angelis, P.D.; Matteucci, G.; Valentini, R. Long-term exposure to elevated [CO2] in a natural Quercus ilex L. community: Net photosynthesis and photochemical efficiency of PSII at different levels of water stress. Plant Cell Environ. 1996, 19, 643–654. [Google Scholar] [CrossRef]
- Ceulemans, R.; Taylor, G.; Bosac, C.; Wilkins, D.; Besford, R.T. Photosynthetic acclimation to elevated CO2 in poplar grown in glasshouse cabinets or in open top chambers depends on duration of exposure. J. Exp. Bot. 1997, 48, 1681–1689. [Google Scholar] [CrossRef]
- Zielinska-Dabkowska, K.M.; Hartmann, J.; Sigillo, C. LED Light Sources and Their Complex Set-Up for Visually and Biologically Effective Illumination for Ornamental Indoor Plants. Sustainability 2019, 11, 2642. [Google Scholar] [CrossRef]
- Fusaro, L.; Salvatori, E.; Mereu, S.; Marando, F.; Scassellati, E.; Abbate, G.; Manes, F. Urban and peri-urban forests in the metropolitan area of Rome: Ecophysiological response of Quercus ilex L. in two green infrastructures in an ecosystem services perspective. Urban For. Urban Green. 2015, 14, 1147–1156. [Google Scholar] [CrossRef]
- Sperlich, D.; Chang, C.T.; Peñuelas, J.; Gracia, C.; Sabaté, S. Seasonal variability of foliar photosynthetic and morphological traits and drought impacts in a Mediterranean mixed forest. Tree Physiol. 2015, 35, 501–520. [Google Scholar] [CrossRef]
- Quentin, A.G.; Crous, K.Y.; Barton, C.V.M.; Ellsworth, D.S. Photosynthetic enhancement by elevated CO2 depends on seasonal temperatures for warmed and non-warmed Eucalyptus globulus trees. Tree Physiol. 2015, 35, 1249–1263. [Google Scholar]
- Gubb, C.; Blanusa, T.; Griffiths, A.; Pfrang, C. Can houseplants improve indoor air quality by removing CO2 and increasing relative humidity? Air Qual. Atmos. Health 2018, 11, 1191–1201. [Google Scholar] [CrossRef]

| Month | CO2 Concentration (ppm) | |
|---|---|---|
| Minimum | Maximum | |
| January | 433.55 | 996.18 |
| February | 421.31 | 925.83 |
| March | 397.87 | 886.77 |
| April | 335.83 | 840.38 |
| May | 314.97 | 767.8 |
| June | 308.51 | 752.44 |
| July | 312.44 | 729.88 |
| August | 300.24 | 705.17 |
| September | 300.24 | 748.65 |
| October | 328.81 | 819.41 |
| November | 338.74 | 885.08 |
| December | 341.47 | 923.75 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salvatori, E.; Gentile, C.; Altieri, A.; Aramini, F.; Manes, F. Nature-Based Solution for Reducing CO2 Levels in Museum Environments: A Phytoremediation Study for the Leonardo da Vinci’s “Last Supper”. Sustainability 2020, 12, 565. https://doi.org/10.3390/su12020565
Salvatori E, Gentile C, Altieri A, Aramini F, Manes F. Nature-Based Solution for Reducing CO2 Levels in Museum Environments: A Phytoremediation Study for the Leonardo da Vinci’s “Last Supper”. Sustainability. 2020; 12(2):565. https://doi.org/10.3390/su12020565
Chicago/Turabian StyleSalvatori, Elisabetta, Chiara Gentile, Antonella Altieri, Fabio Aramini, and Fausto Manes. 2020. "Nature-Based Solution for Reducing CO2 Levels in Museum Environments: A Phytoremediation Study for the Leonardo da Vinci’s “Last Supper”" Sustainability 12, no. 2: 565. https://doi.org/10.3390/su12020565
APA StyleSalvatori, E., Gentile, C., Altieri, A., Aramini, F., & Manes, F. (2020). Nature-Based Solution for Reducing CO2 Levels in Museum Environments: A Phytoremediation Study for the Leonardo da Vinci’s “Last Supper”. Sustainability, 12(2), 565. https://doi.org/10.3390/su12020565







