Comparison of Combustion and Pyrolysis Behavior of the Peanut Shells in Air and N2: Kinetics, Thermodynamics and Gas Emissions
Abstract
1. Introduction
2. Material and Methods
2.1. Sample Preparation
2.2. Thermogravimetric Experiments
2.3. TG-FTIR Analyses
2.4. Pyrolysis Performance Indices
2.5. Kinetic Analyses
3. Results and Discussion
3.1. Chemical Properties and Basic Physical of the PSH
3.2. The Analysis of Thermogravimetric
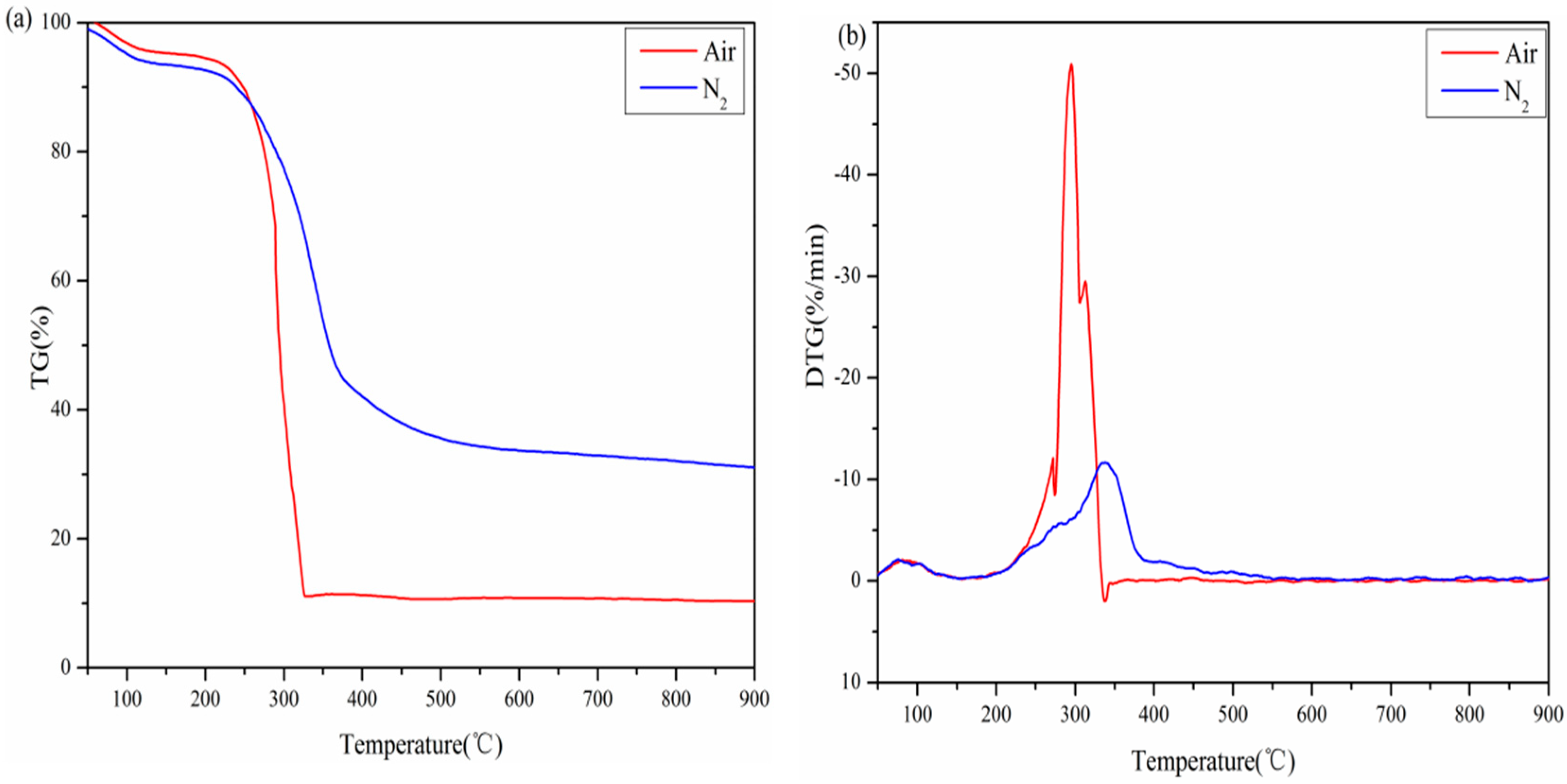
3.2.1. Decomposition Behavior at 20 K/min
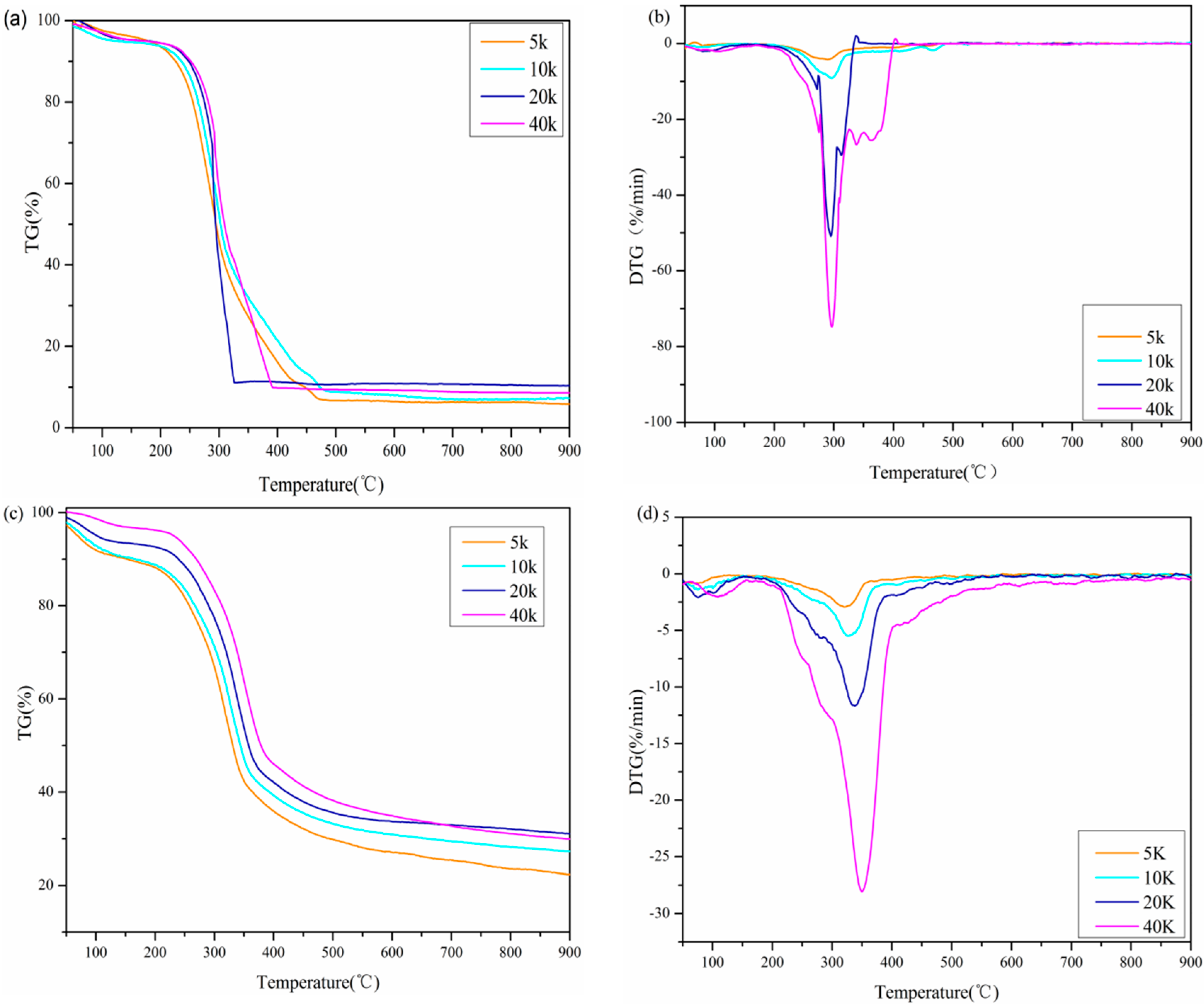
3.2.2. Influence of Different Heating Rate
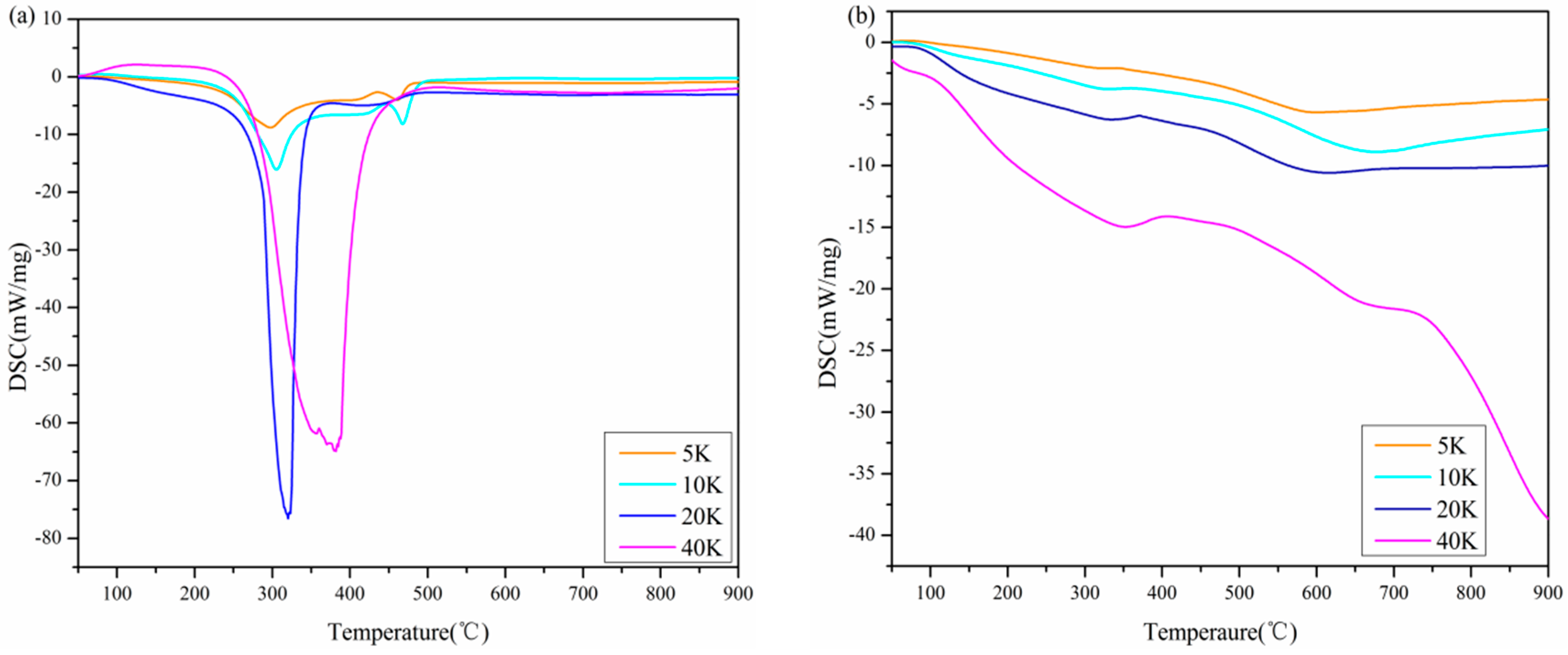
3.3. DSC Analyses
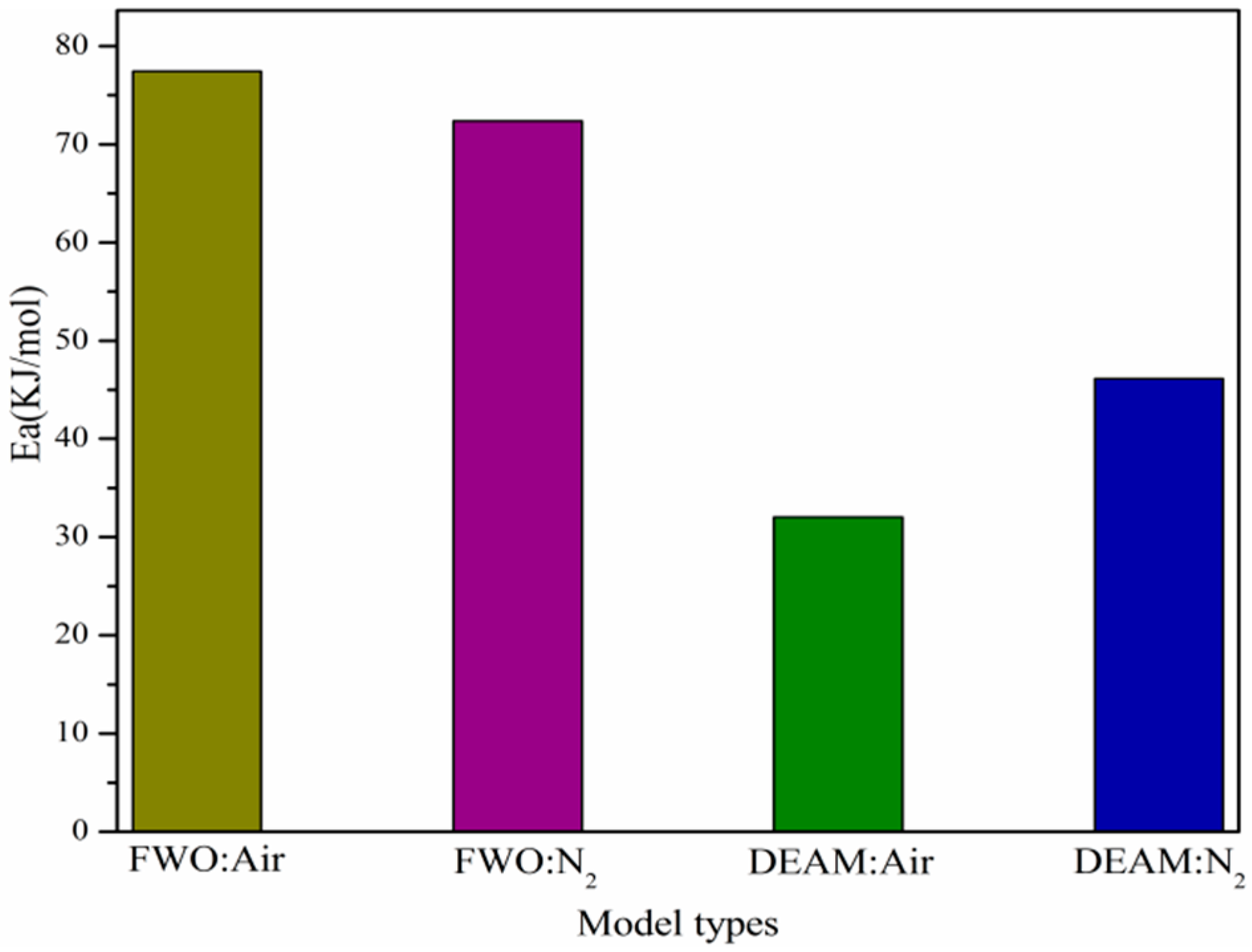
3.4. Kinetic Analyses
3.5. Thermodynamic Analyses
3.6. The Analyses of TG-FTIR
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Saidur, R.; Abdelaziz, E.A.; Demirbas, A.; Hossain, M.S.; Mekhilef, S. A review on biomass as a fuel for boilers. Renew. Sustain. Energy Rev. 2011, 15, 2262–2289. [Google Scholar] [CrossRef]
- Frandsen, F.J. Utilizing biomass and waste for power productionda decade of contributing to the understanding, interpretation and analysis of deposits and corrosion products. Fuels 2005, 84, 1277–1294. [Google Scholar] [CrossRef]
- Xie, W.H.; Huang, J.L.; Liu, J.Y.; Zhao, Y.J.; Chang, K.L.; Kuo, J.H.; He, Y.; Sun, J.; Zheng, L.; Xie, W.M.; et al. Assessing thermal behaviors and kinetics of (co-)combustion of textile dyeing sludge and sugarcane bagasse. Appl. Therm. Eng. 2018, 131, 874–883. [Google Scholar] [CrossRef]
- Klajny, T.; Krzywanski, J.; Nowak, W. Mechanism and Kinetics of Coal Combustion in Oxygen Enhanced Conditions. In Proceedings of the 6th International Symposium on Coal Combustion, Wuhan, China, 1–4 December 2007; pp. 148–153. [Google Scholar]
- Krzywański, J.; Nowak, W. Neurocomputing approach for the prediction of NOx emissions from CFBC in air-fired and oxygen-enriched atmospheres. J. Electr. Eng. Technol. 2017, 97, 75–84. [Google Scholar]
- Muskała, W.; Krzywański, J.; Czakiert, T.; Nowak, W. The Research of CFB boiler operation for oxygen-enhanced dried lignite combustion. Rynek Energii 2011, 92, 172–176. [Google Scholar]
- Huang, J.L.; Liu, J.Y.; Chen, J.C.; Xie, W.H.; Kuo, J.H.; Lu, X.W.; Chang, K.L.; Wen, S.T.; Sun, G.; Cai, H.M.; et al. Combustion behaviors of spent mushroom substrate using TG-MS and TG-FTIR: Thermal conversion, kinetic, thermodynamic and emission analyses. Bioresour. Technol. 2018, 266, 389–397. [Google Scholar] [CrossRef]
- Chandrasekaran, A.; Ramachandran, S.; Subbiah, S. Determination of kinetic parameters in the pyrolysis operation and thermal behavior of Prosopis juliflora using thermogravimetric analysis. Bioresour. Technol. 2017, 233, 413–422. [Google Scholar] [CrossRef]
- Huang, L.M.; Xie, C.D.; Liu, J.Y.; Zhang, X.C.; Chang, K.L.; Kuo, J.H.; Sun, J.; Xie, W.M.; Zheng, L.; Sun, S.Y.; et al. Influence of catalysts on co-combustion of sewage sludge and water hyacinth blends as determined by TG-MS analysis. Bioresour. Technol. 2018, 247, 217–225. [Google Scholar] [CrossRef]
- Soysa, R.; Choi, Y.S.; Kim, S.J.; Choi, S.K. Fast pyrolysis characteristics and kinetic study of Ceylon tea waste. Int. J. Hydrogen Energy 2016, 41, 16436–16443. [Google Scholar] [CrossRef]
- Chen, J.C.; Liu, J.Y.; He, Y.; Huang, L.M.; Sun, S.Y.; Sun, J.; Chang, K.L.; Kuo, J.H.; Huang, S.S.; Ning, X. Investigation of co-combustion characteristics of sewage sludge and coffee grounds mixtures using thermogravimetric analysis coupled to artificial neural networks modeling. Bioresour. Technol. 2017, 225, 234–245. [Google Scholar] [CrossRef]
- Wang, Z.Y.; Xu, Z.H.; Zheng, H.; Zong, H.Y.; Li, L. Effect of peanut Hull biochar on amelioration of typical orchard acidic soil in Northern China. Period. Ocean Univ. China 2013, 43, 86–91. [Google Scholar]
- Collard, F.X.; Blin, J. A review on pyrolysis of biomass constituents: Mechanisms and composition of the products obtained from the conversion of cellulose, hemicelluloses and lignin. Renew. Sustain. Energy Rev. 2014, 38, 594–608. [Google Scholar] [CrossRef]
- Fermoso, J.; Mašek, O. Thermochemical decomposition of coffee ground residues by TG-MS: A kinetic study. J. Anal. Appl. Pyrolysis 2018, 130, 358–367. [Google Scholar] [CrossRef]
- Kayahan, U.; Ozdogan, S. Oxygen enriched combustion and co-combustion of lignites and biomass in a 30 kWth circulating fluidized bed. Energy 2016, 116, 317–328. [Google Scholar] [CrossRef]
- Meng, F.R.; Yu, J.L.; Tahmasebi, A.; Huang, Y. Pyrolysis and combustion behavior of coal gangue in O2/CO2 and O2/N2 mixtures using thermogravimetric analysis and a drop tube furnace. Energy Fuels 2013, 27, 2923–2932. [Google Scholar] [CrossRef]
- Li, J.; Qiao, Y.Y.; Zong, P.J.; Wang, C.B.; Tian, Y.Y. Fast pyrolysis characteristics of two typical coastal zone biomass fuels by thermal gravimetric analyzer and down tube reactor. Bioresour. Technol. 2019, 283, 96–105. [Google Scholar] [CrossRef]
- Huang, X.; Cao, J.P.; Zhao, X.Y.; Wang, J.X.; Fan, X.; Zhao, Y.P.; Wei, X.Y. Pyrolysis kinetics of soybean straw using thermogravimetric analysis. Fuels 2016, 169, 93–98. [Google Scholar] [CrossRef]
- Xu, S.N.; Uzoejinwa, B.B.; Wang, S.; Hu, Y.M.; Qian, L.L.; Liu, L.; Li, B.; He, Z.X.; Wang, Q.; Abomohracb, A.E.; et al. Study on co-pyrolysis synergistic mechanism of seaweed and rice husk by investigation of the characteristics of char/coke. Renew. Energy 2019, 132, 527–542. [Google Scholar] [CrossRef]
- Chen, J.B.; Mu, L.; Cai, J.C.; Yao, P.K.; Song, X.G.; Yin, H.C.; Li, A.M. Pyrolysis and oxy-fuel combustion characteristics and kinetics of petrochemical wastewater sludge using thermogravimetric analysis. Bioresour. Technol. 2015, 198, 115–123. [Google Scholar] [CrossRef]
- Abbasi-Atibeh, E.; Yozgatligil, A. A study on the effects of catalysts on pyrolysis and combustion characteristics of Turkish lignite in oxy-fuel conditions. Fuels 2014, 115, 841–849. [Google Scholar] [CrossRef]
- Maia, A.A.D.; de Morais, L.C. Kinetic parameters of red pepper waste as biomass to solid biofuel. Bioresour. Technol. 2016, 204, 157–163. [Google Scholar] [CrossRef] [PubMed]
- Xie, W.H.; Wen, S.T.; Liu, J.Y.; Xie, W.M.; Kuo, J.H.; Lu, X.W.; Sun, S.Y.; Chang, K.L.; Buyukada, M.; Evrendilek, F. Comparative thermogravimetric analyses of co-combustion of textile dyeing sludge and sugarcane bagasse in carbon dioxide/oxygen and nitrogen/oxygen atmospheres: Thermal conversion characteristics, kinetics, and thermodynamics. Bioresour. Technol. 2018, 255, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.L.; Lian, W.H.; Li, P.; Zhang, Z.L.; Yang, J.X.; Hao, X.G.; Huang, W.; Guan, G.Q. Simulation of pyrolysis in low rank coal particle by using DAEM kinetics model: Reaction behavior and heat transfer. Fuels 2017, 207, 126–135. [Google Scholar] [CrossRef]
- Ozawa, T. A new method of analyzing thermogravimetric data. Bull. Chem. Soc. Jpn. 1965, 38, 1881–1886. [Google Scholar] [CrossRef]
- Ozawa, T. Kinetic analysis by repeated temperature scanning. Part 1. Theory and methods. Thermochim. Acta 2000, 356, 173–180. [Google Scholar] [CrossRef]
- Flynn, J.; Wall, L. Aquick, direct method for the determination of activation energy from thermogravimetric data. J. Polym. Sci. Part B Polym. Lett. 1966, 4, 323–328. [Google Scholar] [CrossRef]
- Wang, X.; Zhai, M.; Wang, Z.; Dong, P.; Lv, W.; Liu, R. Carbonization and combustion characteristics of palm fiber. Fuels 2018, 227, 21–26. [Google Scholar] [CrossRef]
- Zhang, G.Z.; Liu, H.; Wang, J.; Wu, B.J. Catalytic gasification characteristics of rice husk with calcined dolomite. Energy 2018, 165, 1173–1177. [Google Scholar] [CrossRef]
- Tian, L.H.; Shen, B.X.; Xu, H.; Li, F.K.; Wang, Y.; Singh, S. Thermal behavior of waste tea pyrolysis by TG-FTIR analysis. Energy 2016, 103, 533–542. [Google Scholar] [CrossRef]
- Jayaraman, K.; Kok, M.V.; Gokalp, I. Thermogravimetric and mass spectrometric (TG-MS) analysis and kinetics of coal-biomass blends. Renew. Energy 2017, 101, 293–300. [Google Scholar] [CrossRef]
- Ahmad, M.S.; Mehmood, M.A.; Liu, C.G.; Tawab, A.; Bai, F.W.; Sakdaronnarong, C.; Xu, J.R.; Rahimuddin, S.A.; Gull, M. Bioenergy potential of Wolffia arrhiza appraised through pyrolysis, kinetics, thermodynamics parameters and TG-FTIR-MS study of the evolved gases. Bioresour. Technol. 2018, 253, 297–303. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Chen, Z.H.; Dai, M.Q.; Fang, S.W.; Fang, S.; Liao, Y.F.; Yu, Z.S.; Ma, X.Q. Co-pyrolysis kinetics of sewage sludge and bagasse using multiple normal distributed activation energy model (M-DAEM). Bioresour. Technol. 2018, 259, 173–180. [Google Scholar] [CrossRef] [PubMed]
- Zhuo, Z.X.; Liu, J.Y.; Sun, S.Y.; Sun, J.; Kuo, J.H.; Chang, K.L.; Fu, J.W.; Wang, Y.J. Thermogravimetric characteristics of textile dyeing sludge, coal and their blend in N2/O2 and CO2/O2 atmospheres. Appl. Therm. Eng. 2017, 111, 87–94. [Google Scholar] [CrossRef]
- Rodilla, I.; Contreras, M.L.; Bahillo, A. Thermogravimetric and mass spectrometric (TG-MS) analysis of sub-bituminous coal-energy crops blends in N2, air and CO2/O2 atmospheres. Fuels 2018, 215, 506–514. [Google Scholar] [CrossRef]
- Mureddu, M.; Dessì, F.; Orsini, A.; Ferrara, F.; Pettinau, A. Air- and oxygen-blown characterization of coal and biomass by thermogravimetric analysis. Fuels 2018, 212, 626–637. [Google Scholar] [CrossRef]
- Goyal, H.B.; Seal, D.; Saxena, R.C. Bio-fuels from thermochemical conversion of renewable resources: A review. Renew. Sustain. Energy Rev. 2008, 12, 504–517. [Google Scholar] [CrossRef]
- Li, Z.F.; Zhang, Y.L.; Jing, X.X.; Zhang, Y.L.; Chang, L.P. Insight into the intrinsic reaction of brown coal oxidation at low temperature: Differential scanning calorimetry study. Fuel Process. Technol. 2016, 147, 64–70. [Google Scholar] [CrossRef]
- Chen, D.Y.; Zhou, J.B.; Zhang, Q.S. Effects of heating rate on slow pyrolysis behavior, kinetic parameters and products properties of moso bamboo. Bioresour. Technol. 2014, 169, 313–319. [Google Scholar] [CrossRef]
- Galina, N.R.; Romero Luna, C.M.; Arce, G.L.A.F.; Ávilaa, I. Comparative study on combustion and oxy-fuel combustion environments using mixtures of coal with sugarcane bagasse and biomass sorghum bagasse by the thermogravimetric analysis. J. Energy Ins. 2019, 92, 741–754. [Google Scholar] [CrossRef]
- Da Silva, D.R.; Crespi, M.S.; Crnkovic, P.C.G.M.; Ribeiro, C.A. Pyrolysis, combustion and oxy-combustion studies of sugarcane industry wastes and its blends. J. Therm. Anal. Calorim. 2015, 121, 309–318. [Google Scholar] [CrossRef]
- Mehmood, M.A.; Ye, G.B.; Luo, H.B.; Liu, C.G.; Malik, S.; Afzal, I.; Xu, J.R.; Ahmad, M.S. Pyrolysis and kinetic analyses of Camel grass (Cymbopogon schoenanthus) for bioenergy. Bioresour. Technol. 2017, 228, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, M.S.; Mehmood, M.A.; Taqvi, S.T.H.; Elkamel, A.; Liu, C.G.; Xu, J.R.; Rahimuddin, S.A.; Gull, M. Pyrolysis, kinetics analysis, thermodynamics parameters and reaction mechanism of Typha latifolia to evaluate its bioenergy potential. Bioresour. Technol. 2017, 245, 491–501. [Google Scholar] [CrossRef] [PubMed]
- Müsellim, E.; Tahir, M.H.; Ahmad, M.S.; Ceylan, S. Thermokinetic and TG/DSC-FTIR study of pea waste biomass pyrolysis. Appl. Therm. Eng. 2018, 137, 54–61. [Google Scholar] [CrossRef]
- Dhyani, V.; Kumar, J.; Bhaskar, T. Thermal decomposition kinetics of sorghum strawvia thermogravimetric analysis. Bioresour. Technol. 2017, 245, 1122–1129. [Google Scholar] [CrossRef]
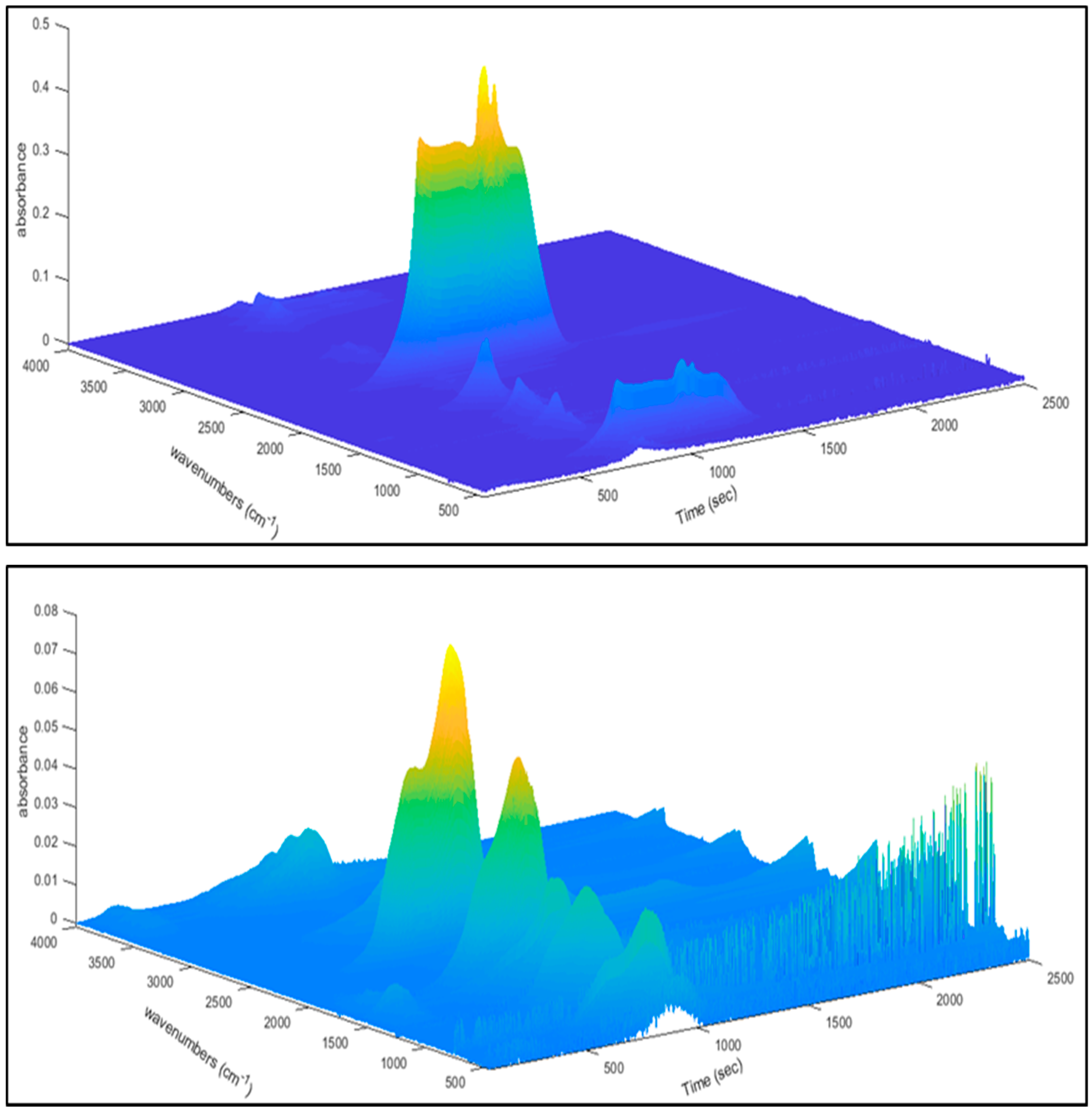
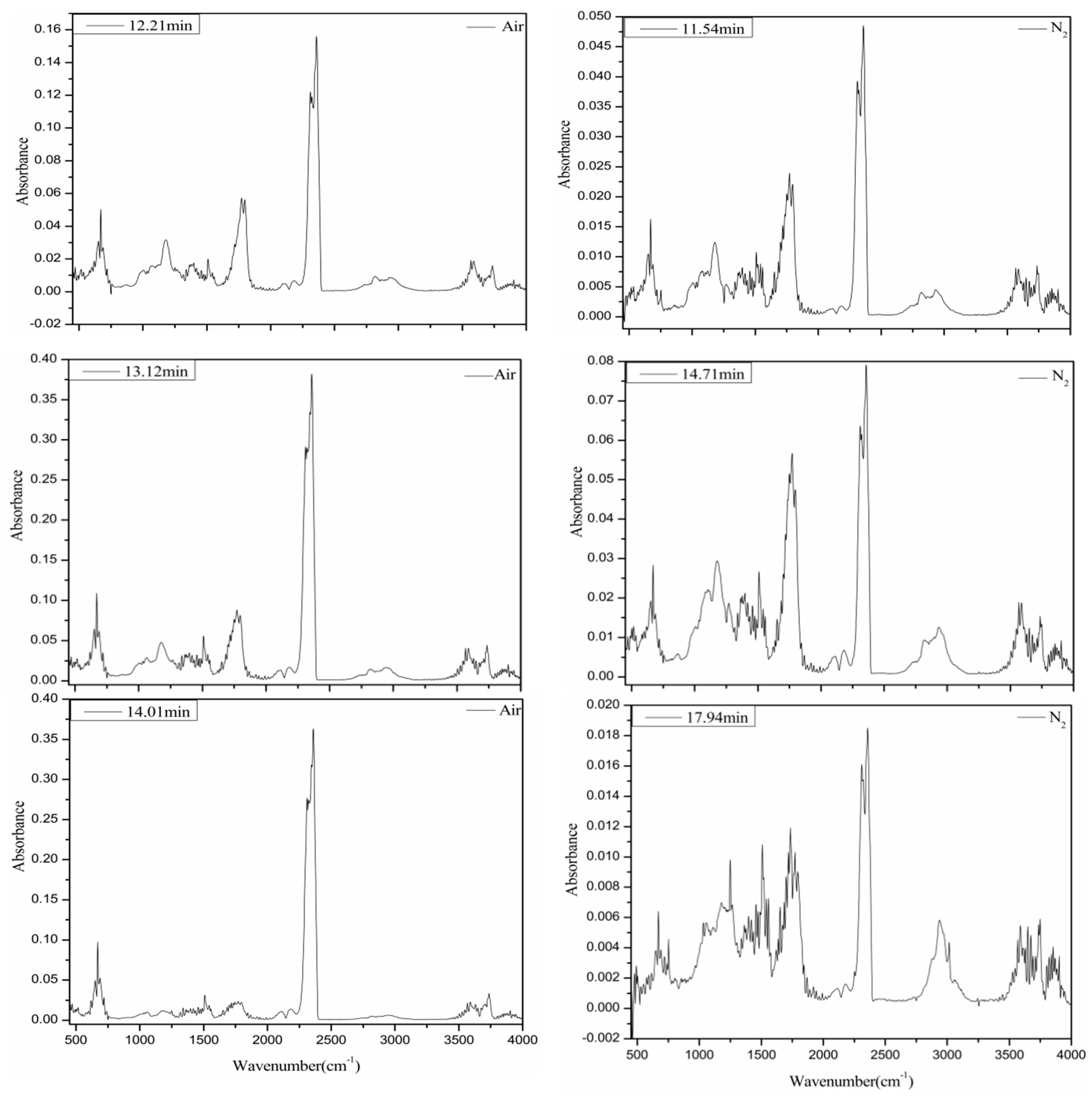
| Biomass | Ultimate Analysis (wt.%) | Proximate Analysis (wt.%) | HHV (MJ/kg) | H/C | O/C | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C | O | H | S | N | M | FC | A | VM | ||||
| Peanut | 43.55 | 34.54 | 5.4 | 0.06 | 1.52 | 7.19 | 17.80 | 7.83 | 67.18 | 19.17 | 0.12 | 0.79 |
| Biomass | β | Temperature (°C) | −RV | −RP | Time (Min) | Pyrolysis Parameters | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ti | Tp | Tb | tp | tb | S | C | Di | Db | ||||
| Air | 5 | 236 | 289 | 346 | 4.21 | 0.55 | 46.70 | 59.43 | 1.201 | 7.559 | 0.242 | 0.011 |
| 10 | 252 | 293 | 348 | 9.09 | 1.07 | 25.34 | 31.64 | 4.401 | 14.314 | 1.644 | 0.204 | |
| 20 | 269 | 295 | 314 | 50.89 | 2.52 | 13.12 | 14.01 | 56.442 | 70.328 | 31.767 | 18.335 | |
| 40 | 270 | 297 | 338 | 74.73 | 5.55 | 7.11 | 7.63 | 168.323 | 102.51 | 164.742 | 270.104 | |
| N2 | 5 | 242 | 316 | 384 | 2.93 | 0.46 | 55.38 | 68.99 | 0.594 | 5.003 | 0.130 | 0.005 |
| 10 | 256 | 331 | 390 | 5.49 | 0.84 | 29.18 | 35.12 | 1.804 | 8.377 | 0.872 | 0.078 | |
| 20 | 268 | 336 | 400 | 11.68 | 1.69 | 14.71 | 17.94 | 6.871 | 16.262 | 6.881 | 0.790 | |
| 40 | 281 | 350 | 406 | 28.05 | 3.91 | 8.39 | 9.58 | 34.211 | 35.524 | 47.355 | 24.235 | |
| FWO | DAEM | |||||||
|---|---|---|---|---|---|---|---|---|
| Air | N2 | Air | N2 | |||||
| a | Ea | R2 | Ea | R2 | Ea | R2 | Ea | R2 |
| 0.10 | 36.34 | 0.9966 | 2.54 | 0.9911 | 32.06 | 0.9703 | 0.24 | 0.9970 |
| 0.15 | 42.81 | 0.9966 | 11.89 | 0.9911 | 38.61 | 0.9818 | 2.70 | 0.9816 |
| 0.20 | 44.35 | 0.997 | 20.72 | 0.9902 | 41.67 | 0.9949 | 9.10 | 0.9978 |
| 0.25 | 48.42 | 0.9933 | 25.88 | 0.9741 | 45.47 | 0.9903 | 20.71 | 0.8392 |
| 0.30 | 53.58 | 0.9993 | 27.00 | 0.9308 | 50.37 | 0.9983 | 23.40 | 0.9996 |
| 0.35 | 70.07 | 0.9861 | 32.63 | 0.9392 | 62.97 | 0.9698 | 32.77 | 0.9995 |
| 0.40 | 85.6 | 0.9990 | 38.02 | 0.9503 | 81.26 | 0.9513 | 42.67 | 0.9998 |
| 0.45 | 97.99 | 0.9205 | 41.53 | 0.9601 | 92.43 | 0.9178 | 51.27 | 1.0000 |
| 0.50 | 117.74 | 0.9142 | 44.67 | 0.9556 | 111.04 | 0.9114 | 60.10 | 0.9998 |
| 0.55 | 117.03 | 0.9752 | 46.88 | 0.9568 | 110.46 | 0.9737 | 68.33 | 0.9999 |
| 0.60 | 131.31 | 0.9600 | 48.11 | 0.9455 | 123.90 | 0.9581 | 76.81 | 0.9986 |
| 0.65 | 116.95 | 0.9211 | 48.91 | 0.9696 | 110.30 | 0.9184 | 85.61 | 0.9919 |
| 0.70 | 151.72 | 0.8566 | 36.51 | 0.93 | 143.02 | 0.8531 | 19.87 | 0.8988 |
| 0.75 | 35.98 | 0.9824 | 25.86 | 0.8588 | 33.09 | 0.9807 | 90.07 | 0.9668 |
| 0.80 | 43.37 | 0.9992 | 34.08 | 0.8296 | 39.94 | 0.9996 | 68.06 | 0.7864 |
| 0.85 | 54.25 | 0.9775 | 32.09 | 0.777 | 50.00 | 0.9194 | 72.67 | 0.6422 |
| 0.90 | 69.22 | 0.9109 | 26.95 | 0.7158 | 63.86 | 0.9146 | 60.23 | 0.7626 |
| Average | 77.45 | 0.9639 | 32.01 | 0.9215 | 72.38 | 0.9531 | 46.15 | 0.9330 |
| a | A (s−1) | ΔH (kJ/mol) | ΔG (kJ/mol) | ΔS (J/mol) | A (s−1) | ΔH (kJ/mol) | ΔG (kJ/mol) | ΔS (J/mol) |
|---|---|---|---|---|---|---|---|---|
| 0.10 | 2.73 × 106 | 34.33 | 72.21 | −128.42 | 1.34 × 10−1 | 1.04 | 90.78 | −226.39 |
| 0.15 | 4.51 × 107 | 40.64 | 71.81 | −105.65 | 1.79 × 101 | 9.88 | 86.46 | −197.04 |
| 0.20 | 8.76 × 107 | 42.10 | 71.72 | −100.42 | 7.34 × 102 | 18.51 | 84.91 | −180.40 |
| 0.25 | 5.01 × 108 | 46.10 | 71.51 | −86.13 | 5.82 × 103 | 23.55 | 84.29 | −177.06 |
| 0.30 | 4.55 × 109 | 51.22 | 71.26 | −67.94 | 9.08 × 103 | 24.57 | 84.17 | −159.07 |
| 0.35 | 4.94 × 1012 | 67.66 | 70.60 | −9.96 | 8.22 × 104 | 30.09 | 83.64 | −142.05 |
| 0.40 | 3.40 × 1015 | 83.19 | 70.11 | 44.33 | 6.60 × 105 | 35.40 | 83.21 | −131.12 |
| 0.45 | 6.09 × 1017 | 95.57 | 69.78 | 87.44 | 2.53 × 106 | 38.84 | 82.97 | −121.37 |
| 0.50 | 2.29 × 1021 | 115.31 | 69.33 | 155.87 | 8.37 × 106 | 41.92 | 82.76 | −114.56 |
| 0.55 | 1.71 × 1021 | 114.59 | 69.34 | 153.37 | 1.94 × 107 | 44.09 | 82.63 | −110.84 |
| 0.60 | 6.48 × 1023 | 128.85 | 69.06 | 202.67 | 3.09 × 107 | 45.26 | 82.56 | −108.46 |
| 0.65 | 1.65 × 1021 | 114.46 | 69.35 | 152.94 | 4.19 × 107 | 46.02 | 82.51 | −147.95 |
| 0.70 | 3.07 × 1027 | 148.70 | 68.71 | 271.16 | 3.69 × 105 | 33.56 | 83.33 | −182.69 |
| 0.75 | 2.33 × 106 | 32.81 | 72.24 | −133.66 | 5.77 × 103 | 22.84 | 84.29 | −156.11 |
| 0.80 | 5.73 × 107 | 40.08 | 71.78 | −107.46 | 1.45 × 105 | 30.94 | 83.52 | −162.92 |
| 0.85 | 6.06 × 109 | 50.83 | 71.23 | −69.16 | 6.66 × 104 | 28.68 | 83.69 | −180.45 |
| 0.90 | 3.45 × 1012 | 65.65 | 70.63 | −16.89 | 8.90 × 103 | 23.16 | 84.17 | −181.59 |
| Average | 1.81 × 1026 | 74.83 | 70.63 | 14.24 | 6.14 × 106 | 29.31 | 84.11 | −157.65 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, Z.; Xiao, X.; Fang, P.; Ye, L.; Huang, J.; Wu, H.; Tang, Z.; Chen, D. Comparison of Combustion and Pyrolysis Behavior of the Peanut Shells in Air and N2: Kinetics, Thermodynamics and Gas Emissions. Sustainability 2020, 12, 464. https://doi.org/10.3390/su12020464
Xu Z, Xiao X, Fang P, Ye L, Huang J, Wu H, Tang Z, Chen D. Comparison of Combustion and Pyrolysis Behavior of the Peanut Shells in Air and N2: Kinetics, Thermodynamics and Gas Emissions. Sustainability. 2020; 12(2):464. https://doi.org/10.3390/su12020464
Chicago/Turabian StyleXu, Zhenghui, Xiang Xiao, Ping Fang, Lyumeng Ye, Jianhang Huang, Haiwen Wu, Zijun Tang, and Dongyao Chen. 2020. "Comparison of Combustion and Pyrolysis Behavior of the Peanut Shells in Air and N2: Kinetics, Thermodynamics and Gas Emissions" Sustainability 12, no. 2: 464. https://doi.org/10.3390/su12020464
APA StyleXu, Z., Xiao, X., Fang, P., Ye, L., Huang, J., Wu, H., Tang, Z., & Chen, D. (2020). Comparison of Combustion and Pyrolysis Behavior of the Peanut Shells in Air and N2: Kinetics, Thermodynamics and Gas Emissions. Sustainability, 12(2), 464. https://doi.org/10.3390/su12020464






