Effects of Tensile Stress and Soil Burial on Mechanical and Chemical Degradation Potential of Agricultural Plastic Films
Abstract
1. Introduction
2. Materials and Methods
2.1. Burial Site Descriptions
2.2. Experimental Design
2.3. Measurements
2.3.1. Film Weight Loss
2.3.2. Film Mechanical Properties
2.3.3. Film Hydrophobicity
2.3.4. Film FTIR Spectroscopy
2.4. Statistical Analysis
3. Results
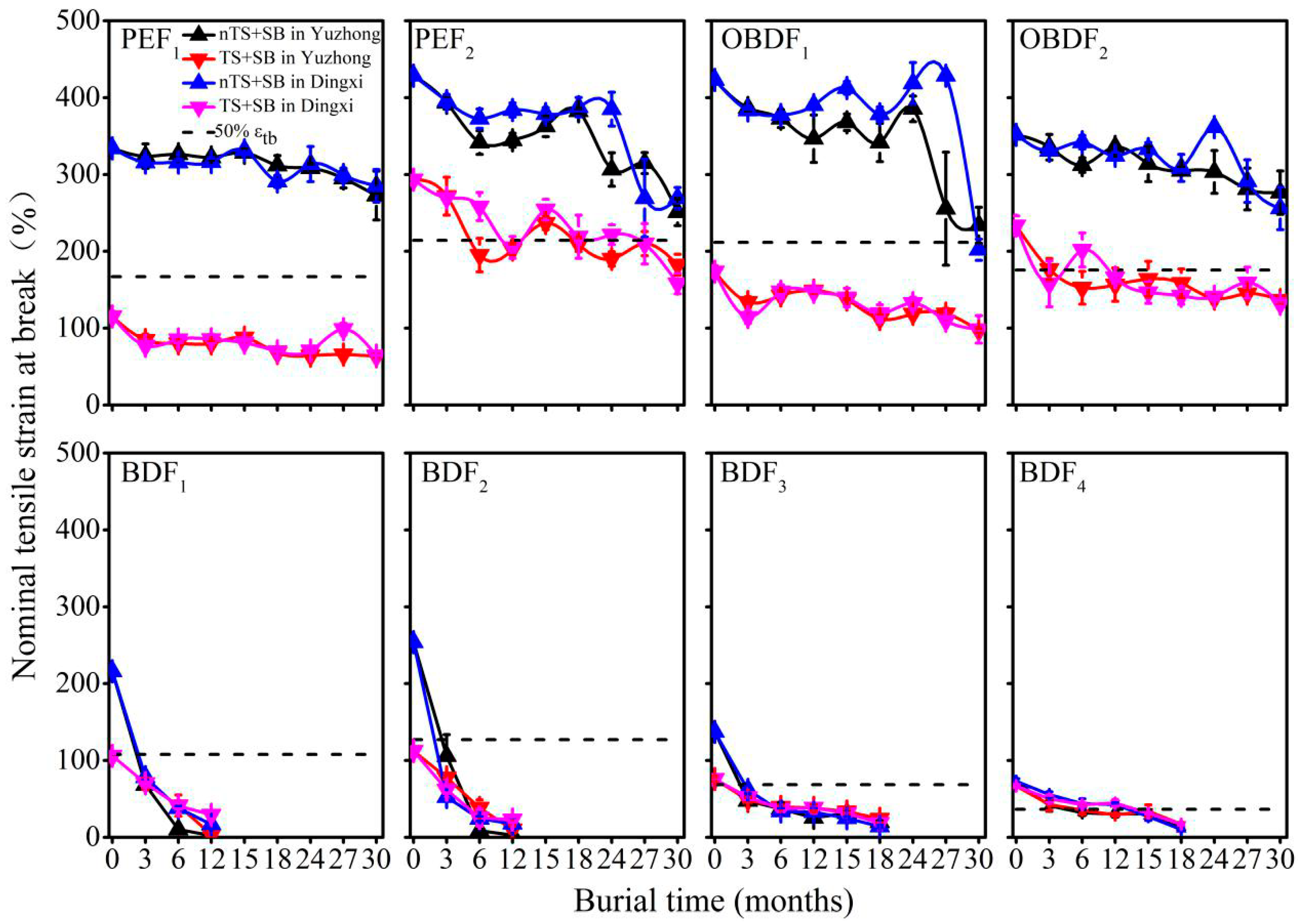
3.1. Changes in Mechanical Properties
3.2. Decreases in Hydrophobicity
3.3. The Formation of Functional Groups
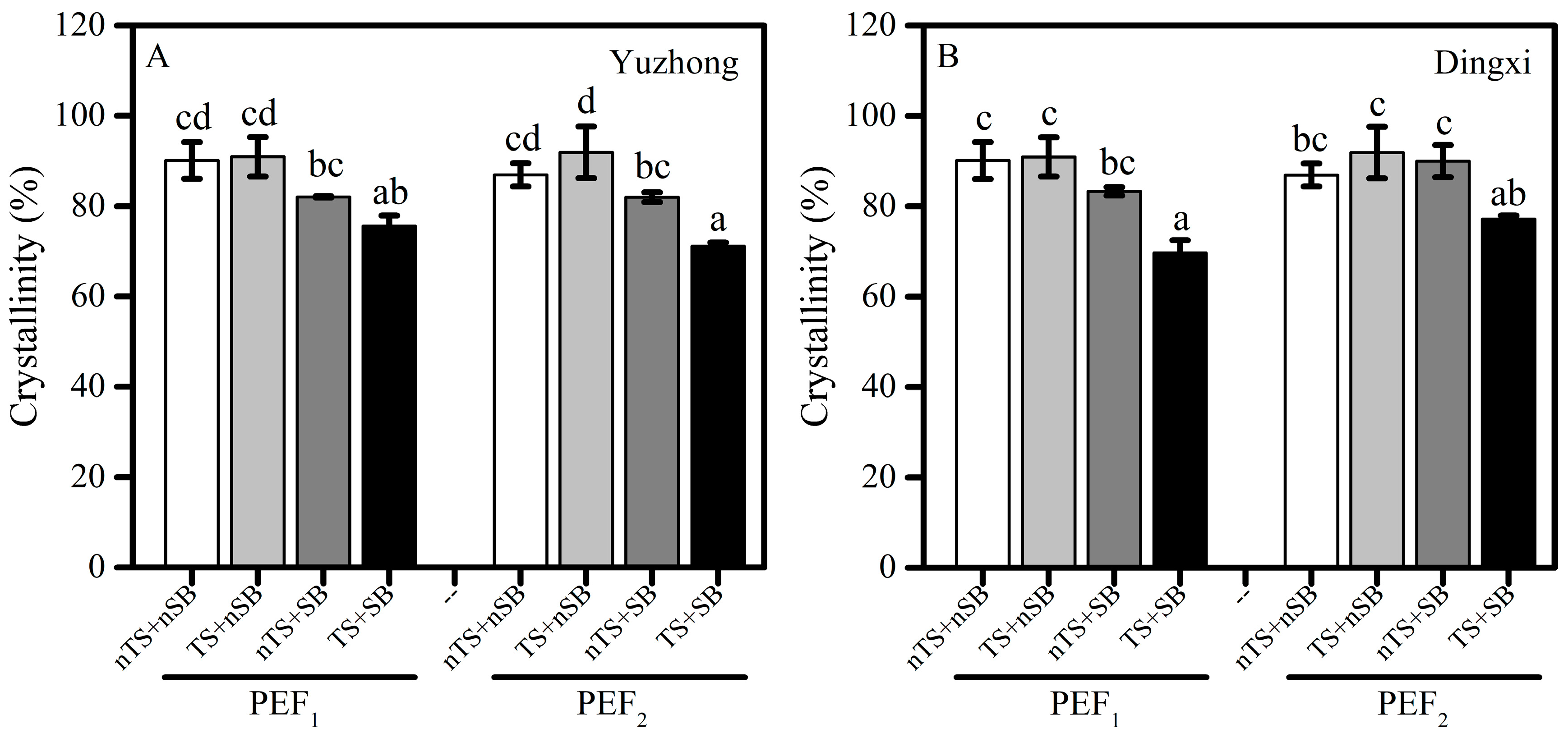
3.4. Decreases in Crystallinity
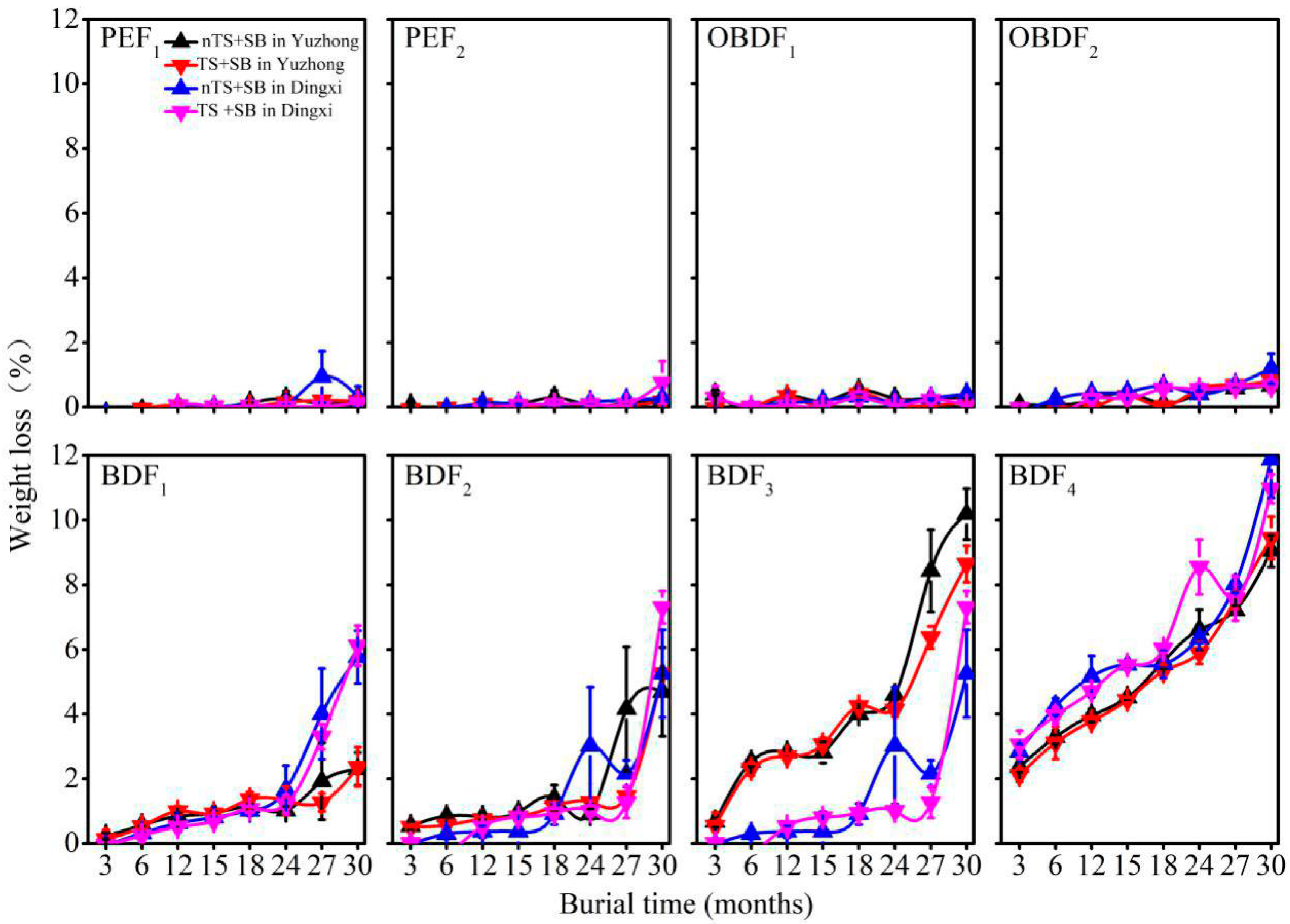
3.5. Weight Loss of Plastic Film
4. Discussion
5. Conclusions
- We tested the effects of soil burial (SB) after tensile stress (TS) on film degradation.
- SB after TS significantly weakened the mechanical properties of plastic film.
- SB after TS increased the presence of functional groups on thin polyethylene film (PEF).
- SB after TS increased the hydrophilicity of plastic film but decreased PEF crystallinity.
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zhou, L.M.; Li, F.M.; Jin, S.L.; Song, Y. How two ridges and the furrow mulched with plastic film affect soil water, soil temperature and yield of maize on the semiarid Loess Plateau of China. Field Crops Res. 2009, 113, 41–47. [Google Scholar] [CrossRef]
- Sintim, H.Y.; Flury, M. Is biodegradable plastic mulch the solution to agriculture’s plastic problem? Environ. Sci. Technol. 2017, 51, 1068–1069. [Google Scholar] [CrossRef] [PubMed]
- Hayes, D.G.; Anunciado, M.B.; DeBruyn, J.M.; Bandopadhyay, S.; Schaeffer, S.; English, M.; Ghimire, S.; Miles, C.; Flury, M.; Sintim, H.Y. Biodegradable plastic mulch films for sustainable specialty crop production. In Polymers for Agri-Food Applications; Gutiérrez, T.J., Ed.; Springer International Publishing: Basel, Switzerland, 2019; pp. 183–213. [Google Scholar]
- Briassoulis, D.; Giannoulis, A. Evaluation of the functionality of bio-based plastic mulching films. Polym. Test. 2018, 67, 99–109. [Google Scholar] [CrossRef]
- Wang, Y.P.; Li, X.G.; Fu, T.; Wang, L.; Turner, N.C.; Siddique, K.H.M.; Li, F.M. Multi-site assessment of the effects of plastic-film mulch on the soil organic carbon balance in semiarid areas of China. Agr. Forest Meteorol. 2016, 228, 42–51. [Google Scholar] [CrossRef]
- Kader, M.A.; Senge, M.; Mojid, M.A.; Ito, K. Recent advances in mulching materials and methods for modifying soil environment. Soil Till. Res. 2017, 168, 155–166. [Google Scholar] [CrossRef]
- Yoon, M.G.; Jeon, H.J.; Kim, M.N. Biodegradation of polyethylene by a soil bacterium and AlkB cloned recombinant cell. Bioremed. Biodegrad. 2012, 3, 1–8. [Google Scholar]
- Qi, Y.; Yang, X.; Pelaez, A.M.; Lwanga, E.H.; Beriot, N.; Gertsen, H.; Grabeva, P.; Geissen, V. Macro- and micro-plastics in soil-plant system: Effects of plastic mulch film residues on wheat (Triticum aestivum) growth. Sci. Total Environ. 2018, 645, 1048–1056. [Google Scholar] [CrossRef]
- Sintim, H.Y.; Bandopadhyay, S.; English, M.E.; Bary, A.I.; DeBruyn, J.M.; Schaeffer, S.M.; Miles, C.A.; Reganold, J.P.; Flury, M. Impacts of biodegradable plastic mulches on soil health. Agr. Ecosyst. Environ. 2019, 273, 36–49. [Google Scholar] [CrossRef]
- Yin, M.; Li, Y.; Fang, H.; Chen, P. Biodegradable mulching film with an optimum degradation rate improves soil environment and enhances maize growth. Agr. Water Manag. 2019, 216, 127–137. [Google Scholar] [CrossRef]
- Astner, A.F.; Hayes, D.G.; O’Neil, H.; Evans, B.R.; Pingali, S.V.; Urban, V.S.; Young, T.M. Mechanical formation of micro- and nano-plastic materials for environmental studies in agricultural ecosystems. Sci. Total. Environ. 2019, 685, 1097–1106. [Google Scholar] [CrossRef]
- Bandopadhyay, S.; Martin-Closas, L.; Pelacho, A.M.; DeBruyn, J.M. Biodegradable plastic mulch films: Impacts on soil microbial communities and ecosystem functions. Front. Microbiol. 2018, 9, 819. [Google Scholar] [CrossRef] [PubMed]
- Gautam, R.; Bassi, A.S.; Yanful, E.K. A review of biodegradation of synthetic plastic and foams. Appl. Biochem. Biotech. 2007, 141, 85–108. [Google Scholar] [CrossRef] [PubMed]
- Esmaeili, A.; Pourbabaee, A.A.; Alikhani, H.A.; Shabani, F.; Esmaeili, E. Biodegradation of low-density polyethylene (LDPE) by mixed culture of Lysinibacillus xylanilyticus and Aspergillus niger in soil. PLoS ONE 2013, 8, e71720. [Google Scholar] [CrossRef] [PubMed]
- Gilan, I.; Hadar, Y.; Sivan, A. Colonization and biofilm formation and biodegradation of polyethylene by a strain of Rhodococcus rubber. Appl. Microbiol. Biotechnol. 2004, 65, 97–104. [Google Scholar]
- Kyrikou, I.; Briassoulis, D. Biodegradation of agricultural plastic films: A critical review. J. Polym. Environ. 2007, 15, 125–150. [Google Scholar] [CrossRef]
- Dilara, P.A.; Briassoulis, D. Degradation and stabilization of low-density polyethylene films used as greenhouse covering materials. J. Agric. Eng. Res. 2000, 76, 309–321. [Google Scholar] [CrossRef]
- Tyler, D.R. Mechanistic aspects of the effects of stress on the rates of photochemical degradation reactions in polymers. J. Macromol. Sci. Part C Polym. Rev. 2004, 44, 351–388. [Google Scholar] [CrossRef]
- Singh, B.; Sharma, N. Mechanistic implications of plastic degradation. Polym. Degrad. Stabil. 2008, 93, 561–584. [Google Scholar] [CrossRef]
- Czerný, J. Thermo-oxidative and photo-oxidative aging of polypropylene under simultaneous tensile stress. J. Appl. Polym. Sci. 1972, 16, 2623–2632. [Google Scholar] [CrossRef]
- Jin, C.; Christensen, P.A.; Egerton, T.A.; White, J.R. Effect of anisotropy on photo-mechanical oxidation of polyethylene. Polymer 2003, 44, 5969–5981. [Google Scholar] [CrossRef]
- Albertsson, A.C.; Barenstedt, C.; Karlsson, S.; Lindberg, T. Degradation product pattern and morphology changes as means to differentiate abiotically and biotically aged degradable polyethylene. Polymer 1995, 36, 3075–3083. [Google Scholar] [CrossRef]
- Tribedi, P.; Sil, A.K. Low-density polyethylene degradation by Pseudomonas sp. AKS2 biofilm. Environ. Sci. Pollut. Res. 2013, 20, 4146–4153. [Google Scholar] [CrossRef] [PubMed]
- Skariyachan, S.; Manjunath, M.; Shankar, A.; Bachappanavar, N.; Patil, A.A. Application of novel microbial consortia for environmental site remediation and hazardous waste management toward low- and high-density polyethylene and prioritizing the cost-effective, eco-friendly, and sustainable biotechnological intervention. In Handbook of Environmental Materials Management; Hussain, C.M., Ed.; Springer International Publishing: Chan, Switzerland, 2018; pp. 1–48. [Google Scholar]
- Emadian, S.M.; Onay, T.T.; Demirel, B. Biodegradation of bioplastics in natural environments. Waste Manag. 2017, 59, 526–536. [Google Scholar] [CrossRef] [PubMed]
- Otake, Y.; Kobayashi, T.; Asabe, H.; Murakami, N.; Ono, K. Biodegradation of low-density polyethylene, polystyrene, polyvinyl chloride, and urea formaldehyde resin buried under soil for over 32 years. J. Appl. Polym. Sci. 1995, 56, 1789–1796. [Google Scholar] [CrossRef]
- Briassoulis, D.; Hiskakis, M.; Tserotas, P. Combined effect of UVA radiation and agrochemicals on the durability of agricultural multilayer films. Polym. Degrad. Stabil. 2018, 154, 261–275. [Google Scholar] [CrossRef]
- Al-Salem, S.M.; Al-Hazza’a, A.; Karam, H.J.; Al-Wadi, M.H.; Al-Dhafeeri, A.T.; Al-Rowaih, A.A. Insights into the evaluation of the abiotic and biotic degradation rate of commercial pro-oxidant filled polyethylene (PE) thin films. J. Environ. Manag. 2019, 250, 109475. [Google Scholar] [CrossRef]
- Briassoulis, D.; Babou, E.; Hiskakis, M.; Kyrikou, I. Analysis of long-term degradation behavior of polyethylene mulching films with pro-oxidants under real cultivation and soil burial conditions. Environ. Sci. Pollut. Res. 2015, 22, 2584–2598. [Google Scholar] [CrossRef]
- Restrepo-Flórez, J.M.; Bassi, A.; Thompson, M.R. Microbial degradation and deterioration of polyethylene—A review. Int. Biodeter. Biodegr. 2014, 88, 83–90. [Google Scholar] [CrossRef]
- Chen, Z.; Zhao, W.; Xing, R.; Xie, S.; Yang, X.; Cui, P.; Lü, J.; Liao, H.; Yu, Z.; Wang, S.; et al. Enhanced in situ, biodegradation of microplastics in sewage sludge using hyperthermophilic composting technology. J. Hazard. Mater. 2020, 384, 121271. [Google Scholar] [CrossRef]
- Albertsson, A.C.; Andersson, S.O.; Karlsson, S. The mechanism of biodegradation of polyethylene. Polym. Degrad. Stabil. 1987, 18, 73–87. [Google Scholar] [CrossRef]
- Balasubramanian, V.; Natarajan, K.; Hemambika, B.; Ramesh, N.; Sumathi, C.S.; Kottaimuthu, R.; Kannan, V.R. High-density polyethylene (HDPE)-degrading potential bacteria from marine ecosystem of Gulf of Mannar, India. Lett. Appl. Microbiol. 2010, 51, 205–211. [Google Scholar] [CrossRef] [PubMed]
- Zerbi, G.; Gallino, G.; Fanti, N.D.; Baini, L. Structural depth profiling in polyethylene films by multiple internal reflection infra-red spectroscopy. Polymer 1989, 30, 2324–2327. [Google Scholar] [CrossRef]
- Albertsson, A.C.; Karlsson, S. The influence of biotic and abiotic environments on the degradation of polyethylene. Prog. Polym. Sci. 1990, 15, 177–192. [Google Scholar] [CrossRef]
- Briassoulis, D. The effects of tensile stress and the agrochemical vapam on the ageing of low density polyethylene (LDPE) agricultural films, part I. mechanical behavior. Polym. Degrad. Stabil. 2005, 88, 489–503. [Google Scholar] [CrossRef]
- Alshabanat, M. Morphological, thermal, and biodegradation properties of LLDPE/treated date palm waste composite buried in a soil environment. J. Saudi Chem. Soc. 2019, 23, 355–364. [Google Scholar] [CrossRef]
- Datta, D.; Halder, G. Enhancing degradability of plastic waste by dispersing starch into low density polyethylene matrix. Process Saf. Environ. 2018, 114, 143–152. [Google Scholar] [CrossRef]
- Huang, Z.; Li, Y.; Ren, X. Comparing cracking time and structure changes of different high-density polyethylenes during stress and photo-oxidative aging. J. Appl. Polym. Sci. 2014, 131, 40904. [Google Scholar] [CrossRef]
- Bonhomme, S.; Cuer, A.; Delort, A.M.; Lemaire, J.; Sancelme, M.; Scott, G. Environmental biodegradation of polyethylene. Polym. Degrad. Stabil. 2003, 81, 441–452. [Google Scholar] [CrossRef]
- Kang, T.K.; Ha, C.S. Effect of processing variables on the crosslinking of HDPE by peroxide. Polym. Test. 2000, 19, 773–783. [Google Scholar] [CrossRef]
- Barragán, D.H.; Pelacho, A.M.; Martin-Closas, L. Degradation of agricultural biodegradable plastics in the soil under laboratory conditions. Soil Res. 2016, 54, 216–224. [Google Scholar] [CrossRef]
- Busfield, W.K.; Taba, P. Photo-oxidative degradation of mechanically stressed polyolefins. Polym. Degrad. Stabil. 1996, 51, 185–196. [Google Scholar] [CrossRef]
- Hu, W.; Liu, W.; Ren, X. The study on aging behaviors and critical stress of cross-linked high-density polyethylene during stress and photo-oxidative aging. J. Polym. Res. 2019, 26, 114. [Google Scholar]
- Tai, N.L.; Adhikari, R.; Shanks, R.; Adhikari, B. Aerobic biodegradation of starch-polyurethane flexible films under soil burial condition: Changes in physical structure and chemical composition. Int. Biodeter. Biodegr. 2019, 145, 104793. [Google Scholar] [CrossRef]
- Kaur, I.; Bhalla, T.C.; Deepika, N.; Gautam, N. Study of the biodegradation behavior of soy protein-grafted polyethylene by the soil burial method. J. Appl. Polym. Sci. 2009, 111, 2460–2467. [Google Scholar] [CrossRef]

| Type | Code | Thickness (μm) a | Color | Main Composition | Company |
|---|---|---|---|---|---|
| PEF | PEF1 | 5.7 | transparent | LLDPE: LDPE: FM = 47.5:1:1 (weight ratio) | Lanzhou lvyuan |
| PEF2 | 10.18 | transparent | LLDPE: LDPE: FM = 47.5:1:1 (weight ratio) | Lanzhou Jintudi | |
| OBDF | OBDF1 | 9.28 | black | PE, EPB b degradation masterbatch (proprietary formula) | Shandong Tianzhuang |
| OBDF2 | 7.93 | transparent | PE, degradation masterbatch (proprietary formula) | Gansu | |
| Dahua | |||||
| BDF | BDF1 | 7.23 | transparent | PE, split agent (proprietary formula) | Lanzhou Xinyinhuan |
| BDF2 | 7.33 | transparent | PE, split agent (proprietary formula) | Lanzhou Xinyinhuan | |
| BDF3 | 9.61 | black | PBAT | Qingdao Hongda | |
| BDF4 | 12.98 | transparent | PBAT | Lanzhou Jintudi |
| Functional Groups | Sample | nTS + nSB | TS + nSB | nTS + SB (YZ) | TS + SB (YZ) | nTS + SB (DX) | TS + SB (DX) |
|---|---|---|---|---|---|---|---|
| KCBI | PEF1 | 0.08 ± 0.02 | 0.03 ± 0.02 | 0.02 ± 0.01 | 0.25 ± 0.06 * | 0.07 ± 0.06 | 0.25 ± 0.01 * |
| PEF2 | 0.07 ± 0.01 | 0.04 ± 0.01 | 0.05 ± 0.01 | 0.06 ± 0.06 | 0.07 ± 0.01 | 0.07 ± 0.03 | |
| ECBI | PEF1 | 0.09 ± 0.01 | 0.03 ± 0.03 | 0.03 ± 0.02 | 0.27 ± 0.06 * | 0.08 ± 0.05 | 0.26 ± 0.02 * |
| PEF2 | 0.07 ± 0.01 | 0.06 ± 0.01 | 0.06 ± 0.01 | 0.05 ± 0.03 | 0.08 ± 0.01 | 0.07 ± 0.02 | |
| VBI | PEF1 | 0.07 ± 0.05 | 0.06 ± 0.02 | 0.02 ± 0.01 | 0.22 ± 0.07 * | 0.07 ± 0.05 | 0.20 ± 0.02 * |
| PEF2 | 0.07 ± 0.03 | 0.02 ± 0.01 | 0.02 ± 0.02 | 0.08 ± 0.00 | 0.03 ± 0.02 | 0.05 ± 0.02 | |
| IDBI | PEF1 | 0.07 ± 0.03 | 0.04 ± 0.03 | 0.09 ± 0.06 | 0.17 ± 0.04 * | 0.11 ± 0.06 | 0.20 ± 0.02 * |
| PEF2 | 0.08 ± 0.03 | 0.09 ± 0.01 | 0.06 ± 0.02 | 0.13 ± 0.01 | 0.04 ± 0.02 | 0.07 ± 0.03 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, Y.; Wei, M.; Shi, X.; Wang, D.; Zhang, X.; Zhao, Y.; Kong, M.; Song, X.; Xie, Z.; Li, F. Effects of Tensile Stress and Soil Burial on Mechanical and Chemical Degradation Potential of Agricultural Plastic Films. Sustainability 2020, 12, 7985. https://doi.org/10.3390/su12197985
Han Y, Wei M, Shi X, Wang D, Zhang X, Zhao Y, Kong M, Song X, Xie Z, Li F. Effects of Tensile Stress and Soil Burial on Mechanical and Chemical Degradation Potential of Agricultural Plastic Films. Sustainability. 2020; 12(19):7985. https://doi.org/10.3390/su12197985
Chicago/Turabian StyleHan, Yanan, Min Wei, Xiaoyan Shi, Dong Wang, Xulong Zhang, Yangyang Zhao, Meng Kong, Xin Song, Zhongkui Xie, and Fengmin Li. 2020. "Effects of Tensile Stress and Soil Burial on Mechanical and Chemical Degradation Potential of Agricultural Plastic Films" Sustainability 12, no. 19: 7985. https://doi.org/10.3390/su12197985
APA StyleHan, Y., Wei, M., Shi, X., Wang, D., Zhang, X., Zhao, Y., Kong, M., Song, X., Xie, Z., & Li, F. (2020). Effects of Tensile Stress and Soil Burial on Mechanical and Chemical Degradation Potential of Agricultural Plastic Films. Sustainability, 12(19), 7985. https://doi.org/10.3390/su12197985








