Intensity of the Process Gas Emission from the Thermal Treatment of the 60–340 mm MSW Fraction under Steam
Abstract
1. Introduction
The Aim of the Investigation—Replacement of Incineration by Steam Gasification
2. Materials and Methods
2.1. Residual from Mechanical Treatment of Municipal Solid Waste (RMT-MSW)
2.2. Reactor
2.3. Gasification Procedure
2.4. Analytical Methods
2.4.1. Gas Chromatography—Mass Spectrometry (GC-MS)
2.4.2. Inductively Coupled Plasma Optical Emission Spectrometry (ICP-OES)
2.4.3. Analysis of Cl− and NH4+
3. Results and Discussion
3.1. Intensity of the Gas Emission
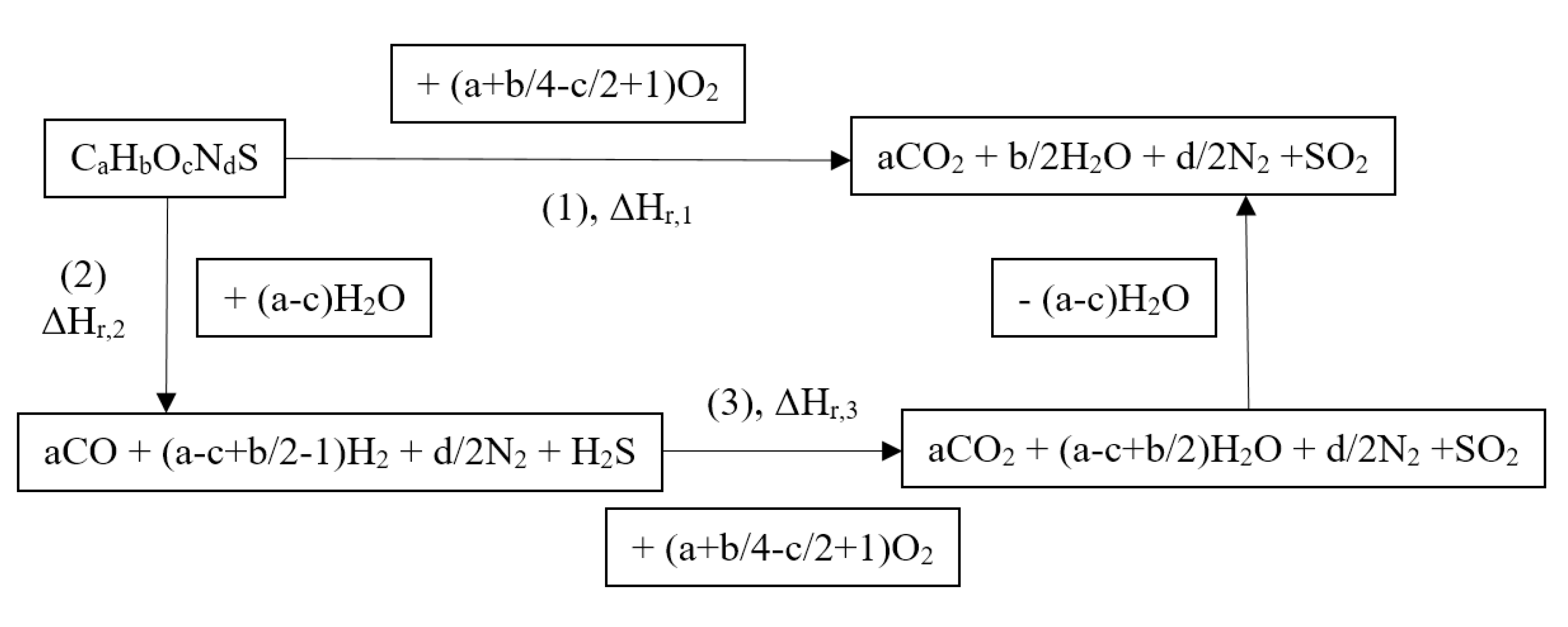
3.2. Thermodynamic Assessment
3.3. Non-Gaseous Substances from Cooling Line
3.3.1. Organic Compounds in Condensate
3.3.2. Metals, Chlorides and Ammonium in the Aqueous Filtrate (AF)
3.3.3. Metals in the Solid Deposit in the Reactor Outlet
3.4. Ashes
4. Summary
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Arena, U. Process and technological aspects of municipal solid waste gasification. A review. Waste Manag. 2012, 32, 625–639. [Google Scholar] [CrossRef]
- Šuhaj, P.; Husár, J.; Haydary, J. Modelling of syngas production from municipal solid waste (MSW) for methanol synthesis. Acta Chim. Slov. 2017, 10, 107–114. [Google Scholar] [CrossRef]
- Chen, D.; Yin, L.; Wang, H.; He, P. Pyrolysis technologies for municipal solid waste: A review. Waste Manag. 2014, 34, 2466–2486. [Google Scholar] [CrossRef] [PubMed]
- Braekman-Danheux, C.; D’haeyere, A.; Fontana, A.; Laurent, P. Upgrading of waste derived solid fuel by steam gasification. Fuel 1998, 77, 55–59. [Google Scholar] [CrossRef]
- Morris, M.; Waldheim, L. Energy recovery from solid waste fuels using advanced gasification technology. Waste Manag. 1998, 18, 557–564. [Google Scholar] [CrossRef]
- Malkow, T. Novel and innovative pyrolysis and gasification technologies for energy efficient and environmentally sound MSW disposal. Waste Manag. 2004, 24, 53–79. [Google Scholar] [CrossRef]
- Guan, Y.; Luo, S.; Liu, S.; Xiao, B.; Cai, L. Steam catalytic gasification of municipal solid waste for producing tar-free fuel gas. Int. J. Hydrogen Energy 2009, 34, 9341–9346. [Google Scholar] [CrossRef]
- Luo, L.; Zhou, Y.; Yi, C. Syngas production by catalytic steam gasification of municipal solid waste in fixed-bed reactor. Energy 2012, 44, 391–395. [Google Scholar] [CrossRef]
- He, M.; Hu, Z.; Xiao, B.; Li, J.; Guo, X.; Luo, S.; Yang, F.; Feng, Y.; Yang, G.; Liu, S. Hydrogen-rich gas from catalytic steam gasification of municipal solid waste (MSW): Influence of catalyst and temperature on yield and product composition. Int. J. Hydrogen Energy 2009, 34, 195–203. [Google Scholar] [CrossRef]
- He, M.; Xiao, B.; Liu, S.; Guo, X.; Luo, S.; Xu, Z.; Feng, Y.; Hu, Z. Hydrogen-rich gas from catalytic steam gasification of municipal solid waste (MSW): Influence of steam to MSW ratios and weight hourly space velocity on gas production and composition. Int. J. Hydrogen Energy 2009, 34, 2174–2183. [Google Scholar] [CrossRef]
- He, M.; Xiao, B.; Liu, S.; Hu, Z.; Guo, X.; Luo, S.; Yang, F. Syngas production from pyrolysis of municipal solid waste (MSW) with dolomite as downstream catalysts. J. Anal. Appl. Pyrol. 2010, 87, 181–187. [Google Scholar] [CrossRef]
- Wang, J.; Cheng, G.; You, Y.; Xiao, B.; Liu, S.; He, P.; Guo, D.; Guo, X.; Zhang, G. Hydrogen-rich gas production by steam gasification of municipal solid waste (MSW) using NiO supported on modified dolomite. Int. J. Hydrogen Energy 2012, 37, 6503–6510. [Google Scholar] [CrossRef]
- Wu, W.; Zhou, X.; Zhang, P.; Liu, W.; Danzeng, D.; Wang, S.; Wang, Y. Study on Characteristics of Synthesis Gas Generation during Catalytic Gasification of Municipal Solid Waste. Procedia Environ. Sci. 2016, 31, 505–513. [Google Scholar] [CrossRef]
- Galvagno, S.; Casciaro, G.; Casu, S.; Martino, M.; Mingazzini, C.; Russo, A.; Portofino, S. Steam gasification of tyre waste, poplar, and refuse-derived fuel: A comparative analysis. Waste Manag. 2009, 29, 678–689. [Google Scholar] [CrossRef]
- Lee, U.; Chung, J.N.; Ingley, H.A. High-Temperature Steam Gasification of Municipal Solid Waste, Rubber, Plastic and Wood. Energy Fuels 2014, 28, 4573–4587. [Google Scholar] [CrossRef]
- Vaish, B.; Sharma, B.; Srivastava, V.; Singh, P.; Ibrahim, M.H.; Singh, R.P. Energy recovery potential and environmental impact of gasification for municipal solid waste. Biofuels 2019, 10, 87–100. [Google Scholar] [CrossRef]
- Xu, P.; Jin, Y.; Cheng, Y. Thermodynamic Analysis of the Gasification of Municipal Solid Waste. Engineering 2017, 3, 416–422. [Google Scholar] [CrossRef]
- Couto, N.; Silva, V.; Monteiro, E.; Rouboa, A. Exergy analysis of Portuguese municipal solid waste treatment via steam gasification. Energy Convers. Manag. 2017, 134, 235–246. [Google Scholar] [CrossRef]
- Lv, L.; Zhang, Z.; Li, H. SNG-electricity cogeneration through MSW gasification integrated with a dual chemical looping process. Chem. Eng. Process. 2019, 145, 107665. [Google Scholar] [CrossRef]
- Hu, M.; Guo, D.; Ma, C.; Hu, Z.; Zhang, B.; Xiao, B.; Luo, S.; Wang, J. Hydrogen-rich gas production by the gasification of wet MSW (municipal solid waste) coupled with carbon dioxide capture. Energy 2015, 90, 857–863. [Google Scholar] [CrossRef]
- Gao, W.; Farahani, M.R.; Rezaei, M.; Hosamani, S.M.; Jamil, M.K.; Imran, M.; Baig, A.Q. Experimental study of steam-gasification of municipal solid wastes (MSW) using Ni-Cu/γ-Al2O3 nano catalysts. Energy Source. Part A 2017, 39, 693–697. [Google Scholar] [CrossRef]
- Xiang, L.Y.; Lin, Q.; Cai, L.; Guan, Y.; Lu, J.; Liu, W. Study of the effect mechanism of municipal solid waste gasification conditions on the production of H2 and CO using modelling technique. J. Environ. Manag. 2019, 230, 301–310. [Google Scholar] [CrossRef] [PubMed]
- Niu, M.; Huang, Y.; Jin, B.; Wang, X. Simulation of syngas production from municipal solid waste gasification in a bubbling fluidized bed using Aspen plus. Ind. Eng. Chem. Res. 2013, 52, 14768–14775. [Google Scholar] [CrossRef]
- Onel, O.; Niziolek, A.M.; Hasan, M.M.F.; Floudas, C.A. Municipal solid waste to liquid transportation fuels e part I: Mathematical modeling of a municipal solid waste gasifier. Comput. Chem. Eng. 2014, 71, 636–647. [Google Scholar] [CrossRef]
- Couto, N.; Silva, V.; Monteiro, E.; Rouboa, A. Assessment of Municipal Solid Wastes Gasification in a Semi-Industrial Gasifier Using Syngas Quality Indices. Energy 2015, 93, 864–873. [Google Scholar] [CrossRef]
- Couto, N.; Monteiro, E.; Silva, V.; Rouboa, A. Hydrogen-rich gas from gasification of Portuguese municipal solid wastes. Int. J. Hydrogen Energy 2016, 41, 10619–10630. [Google Scholar] [CrossRef]
- Zabłocka-Malicka, M.; Szczepaniak, W.; Zielińska, A.; Rutkowski, P. Steam gasification of oat with conversion of tars on clay catalyst and gas cleaning by condensation of steam. Ecol. Chem. Eng. S 2016, 23, 33–48. [Google Scholar] [CrossRef][Green Version]
- Bonazzi, F.A.; Cividino, S.R.S.; Zambon, I.; Mosconi, E.M.; Poponi, S. Building Energy Opportunity with a Supply Chain Based on the Local Fuel-Producing Capacity. Sustainability 2018, 10, 2140. [Google Scholar] [CrossRef]
- Siciliano, A.; Limonti, C.; Mehariya, S.; Molino, A.; Calabrò, V. Biofuel Production and Phosphorus Recovery through an Integrated Treatment of Agro-Industrial Waste. Sustainability 2019, 11, 52. [Google Scholar] [CrossRef]
- Vaskalis, I.; Skoulou, V.; Stavropoulos, G.; Zabaniotou, A. Towards Circular Economy Solutions for The Management of Rice Processing Residues to Bioenergy via Gasification. Sustainability 2019, 11, 6433. [Google Scholar] [CrossRef]
- Zabłocka-Malicka, M.; Rutkowski, P.; Szczepaniak, W. Recovery of copper from PVC multiwire cable waste by steam gasification. Waste Manag. 2015, 46, 488–496. [Google Scholar] [CrossRef]
- Zabłocka-Malicka, M.; Szczepaniak, W.; Rutkowski, P.; Ochromowicz, K.; Leśniewicz, A.; Chęcmanowski, J. Decomposition of the ISA-card under steam for valorized polymetallic raw material. J. Anal. Appl. Pyrol. 2018, 130, 256–268. [Google Scholar] [CrossRef]
- Gurgul, A.; Szczepaniak, W.; Zabłocka-Malicka, M. Incineration and pyrolysis vs. steam gasification of electronic waste. Sci. Total Environ. 2018, 624, 1119–1124. [Google Scholar] [CrossRef] [PubMed]
- Zabłocka-Malicka, M.; Szczepaniak, W.; Szymczycha-Madeja, A. Comparison of leaching of metals from ground ashes prepared by steam gasification and incineration of the 60–340mm MSW fraction. J. Environ. Chem. Eng. 2020, 8, 104029. [Google Scholar] [CrossRef]
- Szczepaniak, W.; Zabłocka-Malicka, M. Equilibrium Thermochemical Cycle for Replacing the Waste Incineration by Steam Gasification. Pers. Manuscr. 2020. Available online: https://www.researchgate.net/publication/342420341 (accessed on 24 June 2020).
- EC-European Commission. 2014/955/EU: Commission Decision of 18 December 2014 amending Decision 2000/532/EC on the list of waste pursuant to Directive 2008/98/EC of the European Parliament and of the Council. 2014. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A32014D0955 (accessed on 25 September 2020).
- Safwat, H.; Motasem, S.; Salam, Al-Zu’bi; Mahmoud, I.; Abdallah, N.; Michael, N. Potential Utilization of RDF as an Alternative Fuel to be Used in Cement Industry in Jordan. Sustainability 2019, 11, 5819. [Google Scholar] [CrossRef]
- Ranieri, E.; Ionescu, G.; Fedele, A.; Palmieri, E.; Ranieri, A.C.; Campanaro, V. Sampling, characterisation and processing of solid recovered fuel production from municipal solid waste: An Italian plant case study. Waste Manag. Res. 2017, 35, 890–898. [Google Scholar] [CrossRef] [PubMed]
- EC Directive. Directive 2008/98/EC Of The European Parliament And Of The Council of 19 November 2008 on waste and repealing certain Directives. 2008. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=celex%3A32008L0098 (accessed on 25 September 2020).
- Ionescu, G.; Rada, E.C.; Ragazzi, M.; Mărculescu, C.; Badea, A.; Apostol, T. Integrated municipal solid waste scenario model using advanced pretreatment and waste to energy processes. Energy Convers. Manag. 2013, 76, 1083–1092. [Google Scholar] [CrossRef]
- Rada, E.C.; Cioca, L. Optimizing the Methodology of Characterization of Municipal Solid Waste in EU under a Circular Economy Perspective. Energy Procedia 2017, 119, 72–85. [Google Scholar] [CrossRef]
- Moré, J.J. The Levenberg-Marquardt algorithm: Implementation and theory. Numer. Anal. 1978, 630, 105–116. [Google Scholar]
- Zhang, J.; Chen, T.; Wu, J.; Wu, J. TG-MS analysis and kinetic study for thermal decomposition of six representative components of municipal solid waste under steam atmosphere. Waste Manag. 2015, 43, 152–161. [Google Scholar] [CrossRef]
- Lai, Z.; Ma, X.; Tang, Y.; Lin, H. Thermogravimetric analysis of the thermal decomposition of MSW in N2, CO2 and CO2/N2 atmospheres. Fuel Process. Technol. 2012, 102, 18–23. [Google Scholar] [CrossRef]
- Chen, S.; Meng, A.; Long, Y.; Zhou, H.; Li, Q.; Zhang, Y. TGA pyrolysis and gasification of combustible municipal solid waste. J. Energy Inst. 2015, 88, 332–343. [Google Scholar] [CrossRef]
- Meng, A.; Chen, S.; Long, Y.; Zhou, H.; Zhang, Y.; Li, Q. Pyrolysis and gasification of typical components in wastes with macro-TGA. Waste Manag. 2015, 46, 247–256. [Google Scholar] [CrossRef]
- Meng, A.; Chen, S.; Zhou, H.; Long, Y.; Zhang, Y.; Li, Q. Pyrolysis and simulation of typical components in wastes with macro-TGA. Fuel 2015, 157, 1–8. [Google Scholar] [CrossRef]
- Tang, Y.; Ma, X.; Wang, Z.; Wu, Z.; Yu, Q. A study of the thermal degradation of six typical municipal waste components in CO2 and N2 atmospheres using TGA-FTIR. Thermochim. Acta 2017, 657, 12–19. [Google Scholar] [CrossRef]
- HSC Chemistry®, Version 6.12; Finland Information Service; Outotec Research Center: Pori, Finland, 2007.
- Yang, Y.; Heaven, S.; Venetsaneas, N.; Banks, C.J.; Bridgwater, A.V. Slow pyrolysis of organic fraction of municipal solid waste (OFMSW): Characterisation of products and screening of the aqueous liquid product for anaerobic digestion. Appl. Energ. 2018, 213, 158–168. [Google Scholar] [CrossRef]
- Komilis., D.; Evangelou, A.; Giannakis, G.; Lymperis, C. Revisiting the elemental composition and the calorific value of the organic fraction of municipal solid wastes. Waste Manag. 2012, 32, 372–381. [Google Scholar] [CrossRef] [PubMed]
- Boumanchar, I.; Chhiti, Y.; M’hamdi Alaoui, F.E.; Sahibed-dine, A.; Bentiss, F.; Jama, C.; Bensitel, M. Municipal solid waste higher heating value prediction from ultimate analysis using multiple regression and genetic programming techniques. Waste Manag. Res. 2019, 37, 578–589. [Google Scholar] [CrossRef]
- Bagheri, M.; Esfilar, R.; Golchi, M.S.; Kennedy, C.A. Towards a circular economy: A comprehensive study of higher heat values and emission potential of various municipal solid wastes. Waste Manag. 2020, 101, 210–221. [Google Scholar] [CrossRef]
- Phua, Z.; Giannis, A.; Dong, Z-L.; Liska, G.; Ng, W.J. Characteristics of incineration ash for sustainable treatment and reutilization. Environ. Sci. Pollut. Res. 2019, 26, 16974–16997. [Google Scholar] [CrossRef]
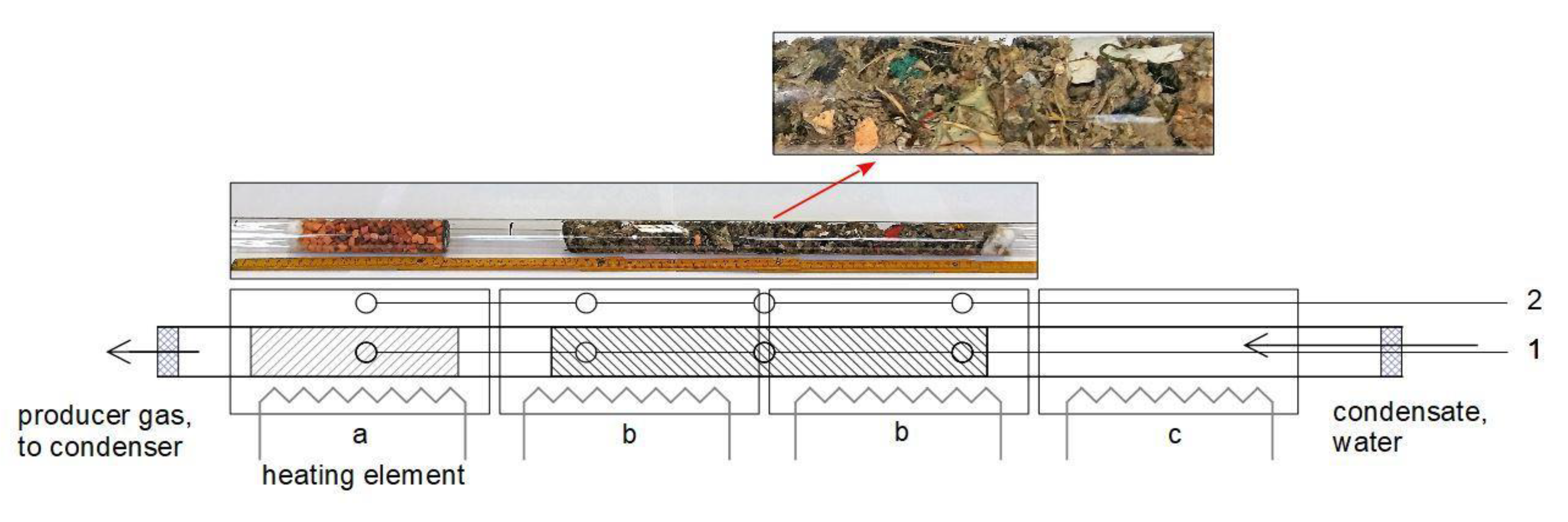



| Experiment | G1 | G2 | G3 | G4 | G5 | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Peak | 1 | 2 | 1 | 2 | 1 | 2 | 1 | 2 | 1 | 2 |
| Time, h:min | 2:18 | 3:56 | 2:00 | 4:25 | 2:14 | 4:29 | 2:32 | 4:30 | 2:19 | 4:27 |
| Ts, °C | 472 | 713 | 454 | 798 | 649 | 851 | 532 | 804 | 631 | 814 |
| Tm, °C | 391#) | 741#) | 247 | 754 | 254 | 708 | 255 | 757 | 230 | 727 |
| Tc, °C | 463 | 750 | 441 | 789 | 455 | 772 | 467 | 779 | 508 | 784 |
| Experiment | G1 | G2 | G3 | G4 | G5 | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| peak | 1 | 2 | 1 | 2 | 1 | 2 | 1 | 2 | 1 | 2 |
| Area (AR), arbitrary unit | 0.171 | 1.85 | 1.33 | 0.976 | 1.07 | 1.29 | 1.25 | 0.960 | 1.20 | 0.725 |
| % of emission (area) | 8 | 92 | 58 | 42 | 45 | 55 | 56 | 44 | 62 | 38 |
| expected gas volume, standard dm3/AR | 48.8 | 46.9 | 40.1 | 47.2 | 61.8 | |||||
| RT | Name | CAS | SL | SF | AF | AS |
|---|---|---|---|---|---|---|
| mg | ||||||
| 4.44 | Ethenylbenzene | 000100-42-5 | 0.06 | 0.17 | ||
| 5.17 | Prop-1-en-2-ylbenzene | 000098-83-9 | 0.02 | |||
| 5.32 | [(E)-prop-1-enyl]benzene | 000637-50-3 | 0.03 | |||
| 5.83 | 1H-indene | 000095-13-6 | 0.06 | 0.24 | ||
| 6.30 | 1,2-bis(ethenyl)benzene | 001321-74-0 | 0.04 | |||
| 6.39 | 1,3-bis(ethenyl)benzene | 000108-57-6 | 0.02 | 0.02 | ||
| 6.96 | Naphthalene | 000091-20-3 | 2.87 | 8.44 | 0.68 | |
| 7.05 | 1-benzothiophene | 000095-15-8 | 0.06 | 0.33 | 0.04 | |
| 7.59 | 2-Methylnaphthalene | 000091-57-6 | 0.15 | 0.87 | 0.01 | |
| 7.71 | 1-Methylnaphthalene | 000090-12-0 | 0.13 | 0.58 | 0.01 | |
| 8.06 | 2-Ethenylnaphthalene | 000827-54-3 | 0.93 | 5.31 | 0.06 | |
| 8.19 | 2,6-dimethylnaphthalene | 000581-42-0 | 0.01 | 0.06 | ||
| 8.28 | 1,8-Dimethylnaphthalene | 000569-41-5 | 0.04 | 0.22 | 0.04 | |
| 8.40 | 1-ethylnaphthalene | 001127-76-0 | 0.08 | 0.52 | ||
| 8.51 | 2,6-ditert-butyl-4-methylphenol | 000128-37-0 | 0.03 | 0.06 | 0.06 | 0.04 |
| 8.58 | 2,6-dimethylnaphthalene | 000644-08-6 | 0.04 | 0.18 | ||
| 8.63 | Acenaphthylene | 000208-96-8 | 0.25 | 1.57 | 0.02 | |
| 8.70 | 1,2-dihydroacenaphthylene | 000083-32-9 | 0.05 | 0.01 | ||
| 8.81 | 1-cyclopenta-2,4-dien-1-ylideneethylbenzene | 002320-32-3 | 0.04 | |||
| 8.90 | Dibenzofuran | 000132-64-9 | 0.06 | 0.42 | ||
| 9.04 | 1-methyl-3-phenylbenzene | 000643-93-6 | 0.04 | |||
| 9.13 | Naphthalene-2-carbonitrile | 000613-46-7 | 0.04 | |||
| 9.18 | 1H-phenalene | 000203-80-5 | 0.01 | 0.06 | ||
| 9.23 | Fluorene | 000086-73-7 | 0.04 | 0.21 | 0.01 | |
| 9.46 | 4-methyldibenzofuran | 007320-53-8 | 0.06 | |||
| 9.73 | 1,2-dihydrophenanthrene | 056179-83-0 | 0.05 | |||
| 9.80 | 1,2-Diphenylethylene | 000588-59-0 | 0.02 | 0.16 | ||
| 10.16 | Dibenzothiophene | 000132-65-0 | 0.11 | 1.04 | ||
| 10.26 | Fluoren-9-one | 000486-25-9 | 0.01 | |||
| 10.32 | Phenanthrene | 000085-01-8 | 1.21 | 8.21 | 0.05 | 0.03 |
| 10.36 | Anthracene | 000120-12-7 | 0.28 | 1.20 | ||
| 10.45 | 9-ethenylanthracene | 002444-68-0 | 0.07 | 0.56 | ||
| 10.59 | 1-phenyl-1H-indene | 001961-96-2 | 0.07 | |||
| 10.67 | Benzo[f]quinoline | 000085-02-9 | 0.06 | |||
| 10.83 | 2-Methylanthracene | 000613-12-7 | 0.02 | 0.12 | ||
| 10.86 | 2-methylphenanthrene | 002531-84-2 | 0.03 | 0.16 | ||
| 10.91 | 3-methylphenanthrene | 000832-71-3 | 0.08 | |||
| 10.97 | 1-methylanthracene | 000610-48-0 | 0.03 | 0.15 | ||
| 11.00 | 1-methylphenanthrene | 000832-69-9 | 0.03 | 0.12 | 0.02 | |
| 11.17 | 2-Phenylnaphthalene | 000612-94-2 | 0.42 | 2.92 | 0.02 | |
| 11.54 | Anthracene-9,10-dione | 000084-65-1 | 0.11 | |||
| 11.71 | 2-phenylnaphthalene | 035465-71-5 | 0.03 | 0.14 | ||
| 11.82 | 1-benzylnaphthalene | 000611-45-0 | 0.07 | |||
| 12.00 | Fluoranthene | 000206-44-0 | 0.30 | 1.79 | ||
| 12.08 | 1,3-diphenylbenzene | 000092-06-8 | 0.08 | 0.34 | ||
| 12.41 | Pyrene | 000129-00-0 | 0.27 | 1.19 | ||
| 12.56 | Naphtho[2,3-b][1]benzofuran | 000243-42-5 | 0.06 | |||
| 12.73 | 2-benzyl-4-chlorophenol | 000120-32-1 | 0.09 | |||
| 12.84 | 1-methylpyrene | 002381-21-7 | 0.04 | |||
| 13.07 | 11H-benzo[b]fluorene | 000243-17-4 | 1.53 | |||
| 13.33 | Chrysene | 000218-01-9 | 0.10 | |||
| 14.99 | Naphtho[1,2-b][1]benzothiole | 000239-35-0 | 0.07 | 0.17 | ||
| 15.08 | 1,3,5-triphenylbenzene | 000612-71-5 | 0.08 | 0.05 | ||
| 15.32 | Benzo[ghi]fluoranthene | 000203-12-3 | 0.04 | 0.10 | ||
| 15.75 | Naphtho[2,3-b][1]benzothiole | 000243-46-9 | 0.06 | |||
| 16.12 | Benzo[a]anthracene | 000056-55-3 | 0.15 | 0.27 | ||
| 16.28 | Triphenylene | 000217-59-4 | 0.22 | 0.40 | ||
| sum: | 8.17 | 40.72 | 1.40 | 0.12 | ||
| mg | K | Na | Ca | Mg | B | Ba | Ti |
| 58.3 ± 0.8 | 18.5 ± 0.2 | 0.59 ± 0.01 | 0.13 ± 0.01 | (9.5 ± 0.1)·10−2 | (3.9 ± 0.1)·10−2 | (3.0 ± 0.1)·10−2 | |
| Cu | Li | Fe | Al | Sr | Cr | Mn | |
| (1.8 ± 0.1)·10−2 | (1.6 ± 0.1)·10−2 | (1.2 ± 0.1)·10−2 | (9.0 ± 0.1)·10−3 | (3.9 ± 0.1)·10−3 | (3.6 ± 0.1)·10−3 | (2.2 ± 0.1)·10−3 |
| wt.% | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| K | Ca | Pb | Mg | Al | Na | |||||||
| 1.14 ± 0.01 | 0.542 ± 0.002 | 0.463 ± 0.006 | 0.310 ± 0.002 | 0.181 ± 0.001 | 0.155 ± 0.001 | |||||||
| ppm | ||||||||||||
| Cd | Zn | Bi | Cu | Fe | Mn | Sn | Ag | Sr | B | |||
| 388 ± 4 | 325 ± 2 | 323 ± 2 | 203 ± 2 | 184 ± 2 | 17.5 ± 0.1 | 9.33 ± 0.09 | 9.16 ± 0.08 | 5.51 ± 0.02 | 4.41 ± 0.03 | |||
| Ti | In | Ba | Sb | Tl | Se | Li | Cr | Hg | V | |||
| 4.38 ± 0.04 | 3.51 ± 0.03 | 3.15 ± 0.01 | 2.62 ± 0.11 | 2.16 ± 0.02 | 1.53 ± 0.05 | 0.82 ± 0.01 | 0.52 ± 0.00 | 0.34 ± 0.01 | 0.27 ± 0.01 | |||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szczepaniak, W.; Zabłocka-Malicka, M.; Wysokiński, R.; Rutkowski, P. Intensity of the Process Gas Emission from the Thermal Treatment of the 60–340 mm MSW Fraction under Steam. Sustainability 2020, 12, 7980. https://doi.org/10.3390/su12197980
Szczepaniak W, Zabłocka-Malicka M, Wysokiński R, Rutkowski P. Intensity of the Process Gas Emission from the Thermal Treatment of the 60–340 mm MSW Fraction under Steam. Sustainability. 2020; 12(19):7980. https://doi.org/10.3390/su12197980
Chicago/Turabian StyleSzczepaniak, Włodzimierz, Monika Zabłocka-Malicka, Rafał Wysokiński, and Piotr Rutkowski. 2020. "Intensity of the Process Gas Emission from the Thermal Treatment of the 60–340 mm MSW Fraction under Steam" Sustainability 12, no. 19: 7980. https://doi.org/10.3390/su12197980
APA StyleSzczepaniak, W., Zabłocka-Malicka, M., Wysokiński, R., & Rutkowski, P. (2020). Intensity of the Process Gas Emission from the Thermal Treatment of the 60–340 mm MSW Fraction under Steam. Sustainability, 12(19), 7980. https://doi.org/10.3390/su12197980






