Removal of Cd(II) from Water by HPEI Modified Humin
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
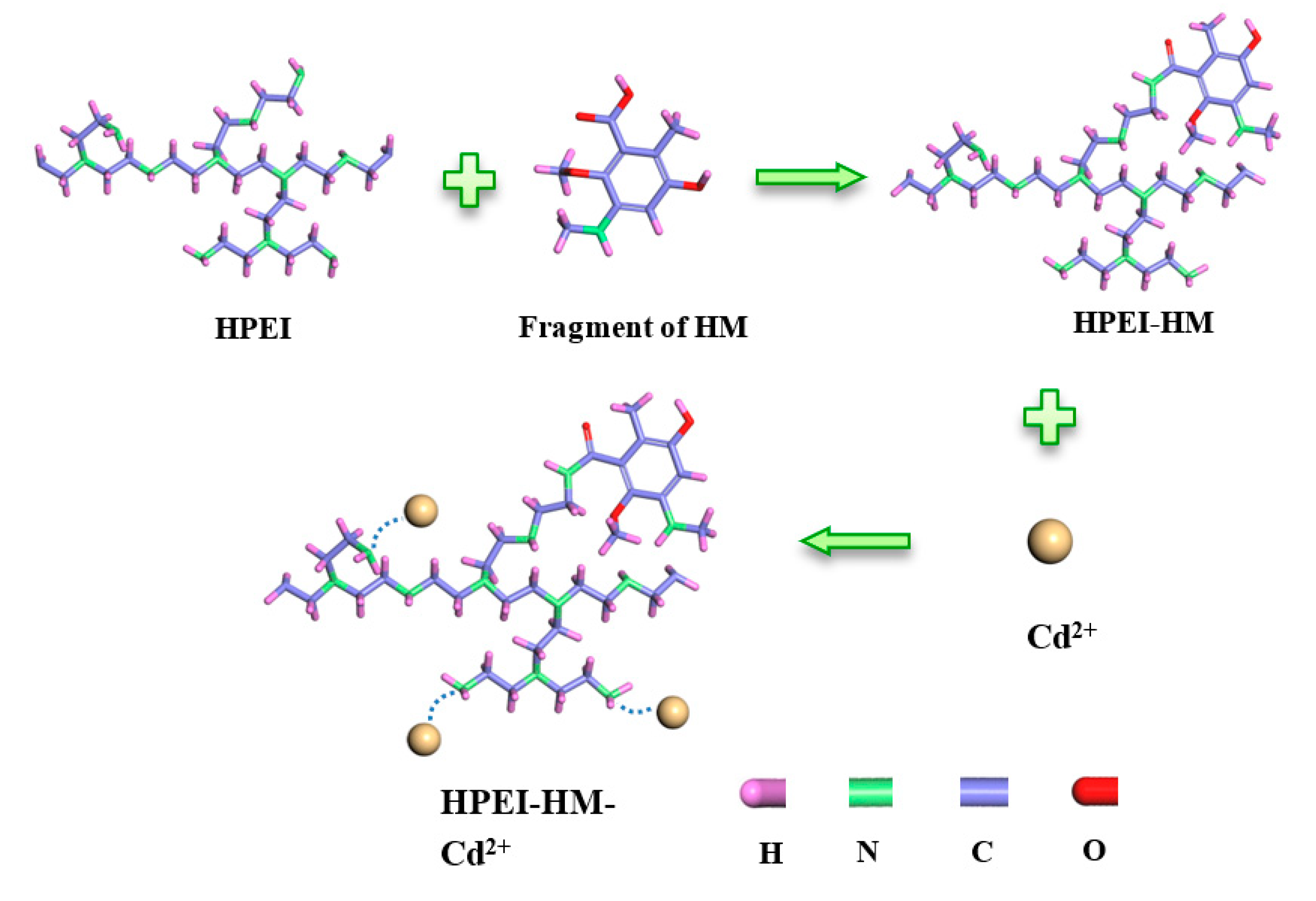
2.2. Preparation of Adsorption Materials
2.3. Characterizations
2.4. Adsorption Experiments
3. Results
3.1. Adsorption Performance
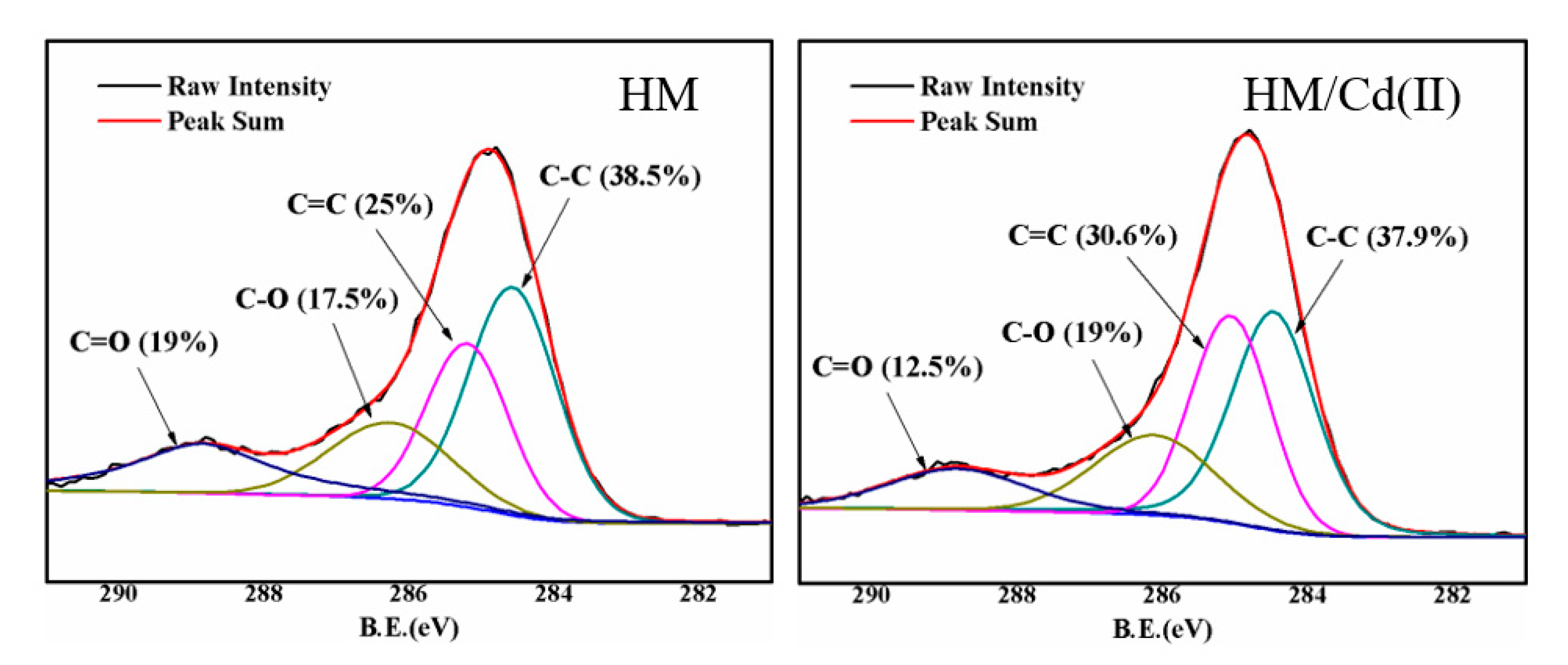
3.2. Adsorbent Characterization
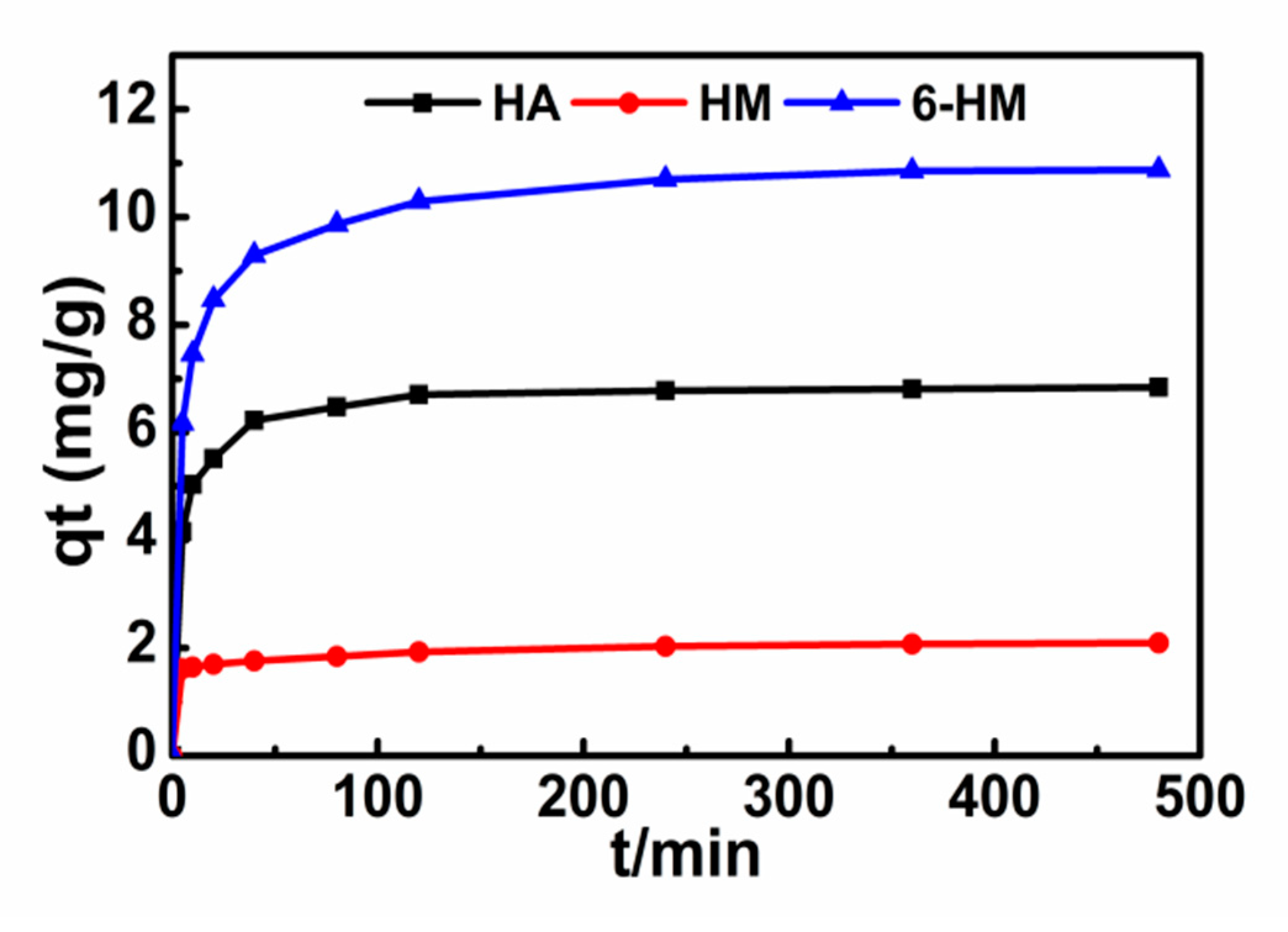
3.3. Adsorption Kinetics
3.4. Adsorption Isotherm
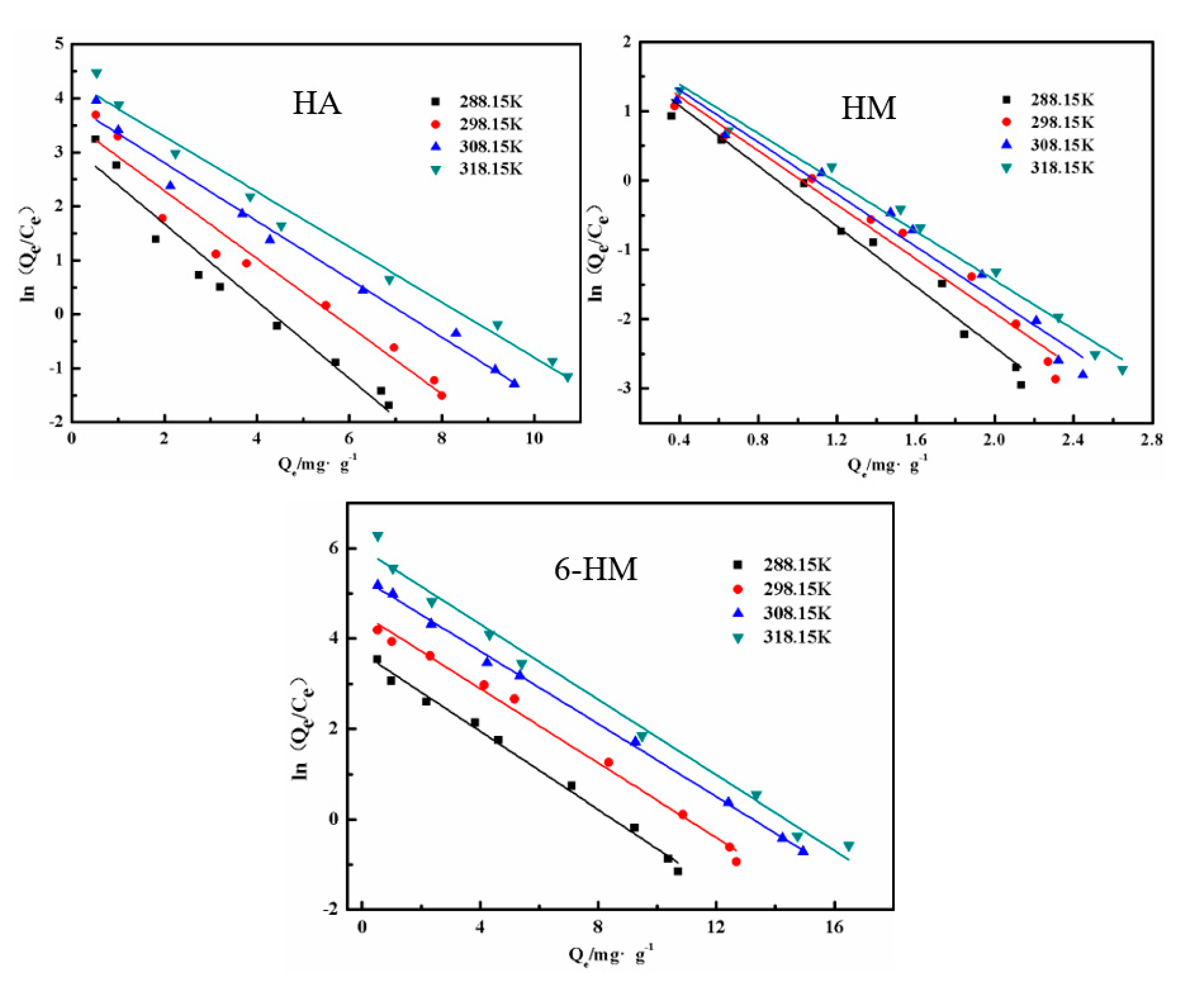
3.5. Thermodynamic Properties
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Liu, K.; Li, F.; Cui, J.; Yang, S.; Fang, L. Simultaneous removal of Cd(II) and As(III) by graphene-like biochar-supported zero-valent iron from irrigation waters under aerobic conditions: Synergistic effects and mechanisms. J. Hazard. Mater. 2020, 395, 122623. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Wang, M.; Zhao, Z.; Ma, C.; Chen, S. Adsorption and desorption of Cd by soil amendment: Mechanisms and environmental implications in field-soil remediation. Sustainability 2018, 10, 2337. [Google Scholar] [CrossRef]
- Shan, C.; Ma, Z.; Tong, M. Efficient removal of free and nitrilotriacetic acid complexed Cd(II) from water by poly(1-vinylimidazole)-grafted Fe3O4@SiO2 magnetic nanoparticles. J. Hazard. Mater. 2015, 299, 479–485. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Huang, Z.; Feng, L.; Luo, X.; Wu, P.; Cui, L.; Mao, X. Modified cellulose by polyethyleneimine and ethylenediamine with induced Cu(II) and Pb(II) adsorption potentialities. Carbohydr. Polym. 2018, 202, 470–478. [Google Scholar] [CrossRef] [PubMed]
- Ding, L.; Lv, W.; Yao, K.; Li, L.; Wang, M.; Liu, G. Remediation of Cd(II)-contaminated soil via humin-enhanced electrokinetic technology. Environ. Sci. Pollut. Res. Int. 2017, 24, 3430–3436. [Google Scholar] [CrossRef]
- Schwieger, A.-C.; Gebauer, K.; Ohle, A.; Beckmann, M. Determination of mercury binding forms in humic substances of lignite. Fuel 2020, 274, 117800. [Google Scholar] [CrossRef]
- Wang, L.; Li, X.; Tsang, D.C.W.; Jin, F.; Hou, D. Green remediation of Cd and Hg contaminated soil using humic acid modified montmorillonite: Immobilization performance under accelerated ageing conditions. J. Hazard. Mater. 2020, 387, 122005. [Google Scholar] [CrossRef]
- Javier, P.E.; Consuelo, E.; Inés, S.; Alberto, M.; Ana, M. Effects of pH conditions and application rates of commercial humic substances on Cu and Zn mobility in anthropogenic mine soils. Sustainability 2019, 11, 4844. [Google Scholar]
- Xu, J.; Dai, Y.; Shi, Y.; Zhao, S.; Tian, H.; Zhu, K.; Jia, H. Mechanism of Cr(VI) reduction by humin: Role of environmentally persistent free radicals and reactive oxygen species. Sci. Total Environ. 2020, 725, 138413. [Google Scholar] [CrossRef]
- Han, D.; Jiang, L.; Zhong, M.; Zhang, R.; Fu, Q.; Zhang, D.; Xia, T. Desorption and bioaccessibility of high-molecular-weight PAHs in aged field soil and humin-like fraction from a coke plant. Environ. Earth Sci. 2020, 79, 1–11. [Google Scholar] [CrossRef]
- Varghese, A.G.; Paul, S.A.; Latha, M.S. Remediation of heavy metals and dyes from wastewater using cellulose-based adsorbents. Environ. Chem. Lett. 2018, 17, 867–877. [Google Scholar] [CrossRef]
- Chou, P.; Ng, D.; Li, I.; Lin, Y. Effects of dissolved oxygen, pH, salinity and humic acid on the release of metal ions from PbS, CuS and ZnS during a simulated storm event. Sci. Total Environ. 2018, 624, 1401–1410. [Google Scholar] [CrossRef] [PubMed]
- Klučáková, M. Adsorption of nitrate on humic acids studied by flow-through coulometry. Environ. Chem. Lett. 2009, 8, 145–148. [Google Scholar] [CrossRef]
- Bonolo, E.; Nonhlangabezo, M.; Soraya, P. Thin film composite membranes consisting of hyperbranched polyethylenimine (HPEI)-cysteamine layer for cadmium removal in water. J. Water Process Eng. 2019, 30, 100686. [Google Scholar]
- Zhang, J.; Wang, S.; Wang, Q.; Wang, N.; Li, C.; Wang, L. First determination of Cu adsorption on soil humin. Environ. Chem. Lett. 2012, 11, 41–46. [Google Scholar] [CrossRef]
- Tofighy, M.A.; Mohammadi, T. Divalent heavy metal ions removal from contaminated water using positively charged membrane prepared from a new carbon nanomaterial and HPEI. Chem. Eng. J. 2020, 388, 124192. [Google Scholar] [CrossRef]
- Liu, Y.; Xu, L.; Liu, J.; Liu, X.; Chen, C.; Li, G.; Meng, Y. Graphene oxides cross-linked with hyperbranched polyethylenimines: Preparation, characterization and their potential as recyclable and highly efficient adsorption materials for lead(II) ions. Chem. Eng. J. 2016, 285, 698–708. [Google Scholar] [CrossRef]
- Zhou, X.; Wang, L.; Liu, X.; Xu, M.; Liu, X. Organic/inorganic hybrid consisting of supportive poly(arylene ether nitrile) microspheres and photocatalytic titanium dioxide nanoparticles for the adsorption and photocatalysis of methylene blue. Compos. Part B 2019, 177, 107414. [Google Scholar] [CrossRef]
- Wang, B.; Yang, K.; Cheng, H.; Ye, T.; Wang, C. A hydrophobic conductive strip with outstanding one-dimensional stretchability for wearable heater and strain sensor. Chem. Eng. J. 2021, 404, 126393. [Google Scholar] [CrossRef]
- Hua, D.; Chung, T. Universal surface modification by aldehydes on polymeric membranes for isopropanol dehydration via pervaporation. J. Membr. Sci. 2015, 492, 197–208. [Google Scholar] [CrossRef]
- Qi, L.; Teng, F.; Deng, X.; Zhang, Y.; Zhong, X. Experimental study on adsorption of Hg(II) with microwave-assisted alkali-modified fly ash. Powder Technol. 2019, 351, 153–158. [Google Scholar] [CrossRef]
- Wang, L.; Liu, R.; Hu, Y.; Sun, W. Adsorption of mixed DDA/NaOL surfactants at the air/water interface by molecular dynamics simulations. Chem. Eng. Sci. 2016, 155, 167–174. [Google Scholar] [CrossRef]
- Zhang, Y.; Lu, X.; Song, D.; Liu, S. The adsorption of a single water molecule on low-index C3S surfaces: A DFT approach. Appl. Surf. Sci. 2019, 471, 658–663. [Google Scholar] [CrossRef]
- Su, Q.; Yi, X.; Miao, J.; Chen, Y.; Chen, J.; Wang, J. A Comparative Study in Vanadium and Tungsten Leaching from Various Sources of SCR Catalysts with Local Difference. Sustainability 2020, 12, 1499. [Google Scholar] [CrossRef]





| Samples | qe,exp/(mg/g) | Pseudo-First-Order Kinetic | Pseudo-Second-Order Kinetic | ||||
|---|---|---|---|---|---|---|---|
| qe,1/(mg/g) | k1/(min−1) | R2 | qe,2/(mg/g) | k2/(g·mg−1·min−1) | R2 | ||
| HA | 6.838 | 1.674 | 0.016 | 0.877 | 6.897 | 0.035 | 0.999 |
| HM | 2.101 | 0.489 | 0.006 | 0.989 | 2.114 | 0.071 | 0.999 |
| 6-HM | 10.875 | 3.421 | 0.013 | 0.950 | 11.013 | 0.014 | 0.999 |
| Samples | Langmuir Equation | Freundlich Equation | ||||
|---|---|---|---|---|---|---|
| Qm (mg/g) | KL (L/mg) | R2 | KF (L/mg)1/n | R2 | ||
| HA | 8.095 | 0.480 | 0.967 | 3.284 | 0.374 | 0.9471 |
| HM | 2.290 | 0.054 | 0.944 | 0.4383 | 0.665 | 0.9735 |
| 6-HM | 11.975 | 0.380 | 0.9804 | 4.380 | 0.474 | 0.9938 |
| Samples | T/(K) | Kd | R2 | ∆G/kJ·mol−1 | ∆S /J·mol−1 | ∆H/kJ·mol−1 |
|---|---|---|---|---|---|---|
| HA | 288.15 | 3.109 | 0.956 | −2.727 | 37.99 | 8.221 |
| 298.15 | 3.546 | 0.958 | −3.107 | |||
| 308.15 | 3.883 | 0.986 | −3.487 | |||
| 318.15 | 4.32 | 0.984 | −3.867 | |||
| HM | 288.15 | 1.952 | 0.974 | −1.595 | 11.97 | 1.855 |
| 298.15 | 1.993 | 0.972 | −1.715 | |||
| 308.15 | 2.059 | 0.971 | −1.835 | |||
| 318.15 | 2.094 | 0.986 | −1.954 | |||
| 6-HM | 288.15 | 3.675 | 0.992 | −3.156 | 54.12 | 12.44 |
| 298.15 | 4.544 | 0.992 | −3.698 | |||
| 308.15 | 5.336 | 0.999 | −4.239 | |||
| 318.15 | 5.991 | 0.988 | −4.78 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, S.; Wu, M.; Lu, L.; Zhu, J. Removal of Cd(II) from Water by HPEI Modified Humin. Sustainability 2020, 12, 7931. https://doi.org/10.3390/su12197931
Li S, Wu M, Lu L, Zhu J. Removal of Cd(II) from Water by HPEI Modified Humin. Sustainability. 2020; 12(19):7931. https://doi.org/10.3390/su12197931
Chicago/Turabian StyleLi, Sanmei, Mingda Wu, Linghong Lu, and Jiabao Zhu. 2020. "Removal of Cd(II) from Water by HPEI Modified Humin" Sustainability 12, no. 19: 7931. https://doi.org/10.3390/su12197931
APA StyleLi, S., Wu, M., Lu, L., & Zhu, J. (2020). Removal of Cd(II) from Water by HPEI Modified Humin. Sustainability, 12(19), 7931. https://doi.org/10.3390/su12197931




