3.1. Baseline 15-Year-Old Adolescents
Table 4 reports the regression outcomes based on the model given by Equation (3). Columns (1) and (2) report the estimation results based on the simple OLS procedure, and columns (3) and (4)—based on median regression. The latter procedure has the advantage that the median is unaffected by outliers (e.g., [
26], p. 445). While columns (1) and (3) report the estimation results where the dependent variable
, columns (2) and (4) report the estimation results where the dependent variable
. The latter dependent variable provides a percent approximation between projected BMI of Hebrew speakers and that of the twelve languages other than Hebrew. The precise percent difference is given by the formula
, where the circumflex denotes the estimated parameter (e.g., [
26], pp. 42–44) (Johnston and Dinardo [
26] discuss the following constant growth rate model of a compounded interest:
where g is the growth rate and t is the time variable. Taking the natural logarithm and estimation of this model gives:
, where the circumflex denotes estimated coefficients,
and
. The
is known as “continuous growth rate”, which provides good approximation to
(the discrete growth rate) in the case that
is small. Taking an exponent for both sides yields:
. Subtracting one from both sides yields:
).
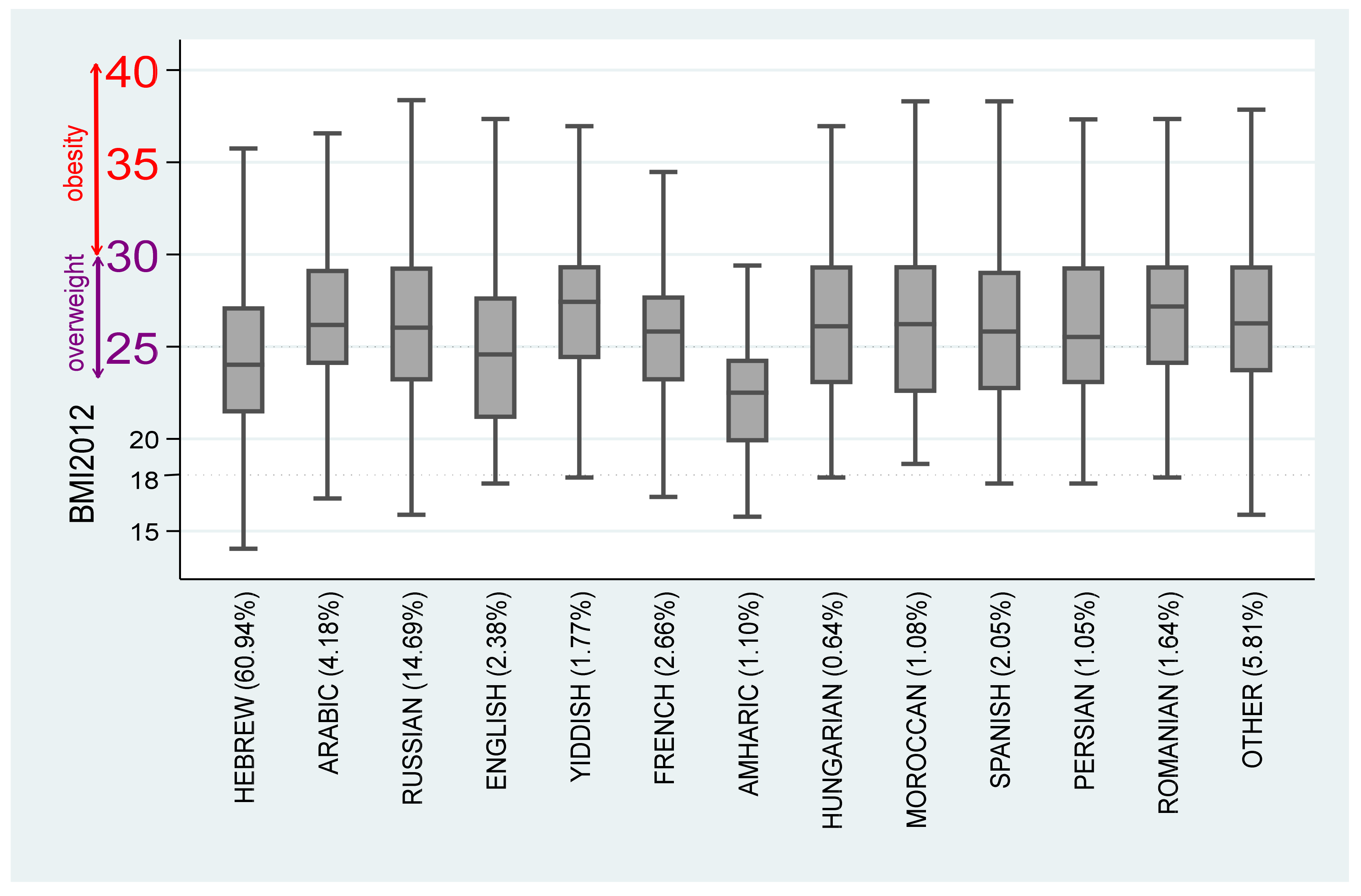
As can be seen from the table, among the baseline 15-year-old Hebrew speakers, and based on both the average and the median, there are no overweight problems. The 95% confidence interval of projected BMI among this cohort is 22.11–22.58 and 21.52–22.05. Consequently, the null hypothesis of overweight is rejected.
Comparing the projected BMI differences between the 15-year-old baseline Hebrew speakers demonstrates that the projected BMI of 15-year-old Arabic language speakers is higher by approximately 22.4% ()–26.2% (); Russian speakers—by 3.32% ()–4.08% (); Yiddish speakers—by 21.1% ()–23.7% (); French speakers—by 7.98% ()–10.5% (); Hungarian speakers—by 25.5% ()–36.4% (); Persian speakers—by 20.4% ()–21.6% (); Romanian speakers—by 15.0 ()–11.9% (); and speakers of other languages—by 9.91% ()–9.79% () (The discrete growth rates of selected cases are: Arabic: ; ; Russian: ; ; Yiddish: ; . Note that the continous and discrete growth rates are similar).
While compared to Hebrew speakers, almost all the immigrants in the 15-year-old cohort exhibit higher or equal projected BMI, with the exception of Ethiopian immigrants. Compared to the 15-year-old baseline Hebrew speakers, the projected BMI of Amharic speakers is 7.71% ()–7.72% () lower, even when the age variable is controlled.
At the bottom of
Table 4, the projected BMI and 95% confidence intervals for the 15-year-old cohort stratified by native language are reported based on the regression outcomes. The two most left columns give the native language and the minimal age for each group. For the semi-logarithmic model (columns (2) and (4)),
. Based on this table, we note that while 15-year-old Hebrew, Russian, English, and Amharic speakers do not suffer from either overweight or obesity (rejection of the null hypothesis BMI = 25 or BMI = 30 where the upper bound of the 95% CI is below the overweight benchmark, namely proj(BMI) < 25, French, Spanish, and Romanian speakers suffer from overweight (the null hypothesis BMI = 25 cannot be rejected at the 5% significance level) and Arabic, Yiddish, Hungarian, Moroccan, and Persian speakers suffer from obesity (the null hypothesis BMI = 30 cannot be rejected at the 5% significance level). Note also, that the minimal age among Arabic speakers is 23 years (8 years above the minimal 15-year-old age); Hungarian speakers—27 years (12 years above the minimal 15-year-old age); Moroccan speakers—42 years (27 years above the minimal 15-year-old age); Spanish speakers—22 years (7 years above the minimal 15-year-old age); and Persian speakers—25 years (10 years above the minimal 15-year-old age).
A possible interpretation of these outcomes is that native Israeli adolescents are more influenced by Western values and norms regarding a slim body image of women. Other examples of this interpretation are given in [
15,
16].
Other potential reasons to obesity are related to genetics [
17], nutrition, lack of physical activity [
18] and relationship to urban environment, and lack of vitamin D absorption [
13] (Foss [
13] suggests that: “Larger body size confers a survival advantage in the cold ambient temperatures and food scarcity of the winter climate by reducing surface area to volume ratio and by providing an energy store in the form of fat mass. In addition, it is suggested that the phenotypic metabolic and physiological changes observed as the metabolic syndrome, including hypertension and insulin resistance, could result from a winter metabolism which increases thermogenic capacity. Common obesity and the metabolic syndrome may therefore result from an anomalous adaptive winter response. The stimulus for the winter response is proposed to be a fall in vitamin D.” (page 314—the abstract). “{...}Common obesity is closely associated with the urban-industrial environment (82). In this environment, the two determinants of vitamin D, i.e., exposure of the skin to UV-B radiation and dietary vitamin D, are reduced. With regard to the first determinant, UV-B irradiance in urban areas is reduced by several factors. Cities often develop in low altitude, sheltered regions and some urban construction is underground. Incident solar UV-B radiation is absorbed and scattered by buildings and concrete ground cover in the built environment. Emissions from industry and transport alter the atmospheric composition and pollutants such as sulphur dioxide, nitrogen dioxide and ozone (SO
2, NO
2 and O
3) absorb in the UV-B spectrum, while black carbon and PM10 can also reduce UV-B irradiance (83,84). The interiors of buildings are designed to reproduce infra-red radiation (heat) and visible radiation (light), but not ultraviolet radiation. In particular, large public buildings such as hospitals, office buildings and factories allow little, if any, penetration of UV radiation. Urbanisation is essentially a trend towards a sheltered, UV-deprived habitat. At a personal level, clothing acts as an effective sunscreen (85) and standard styles of outdoor clothing cover an extensive proportion of the body surface. The most significant cause of vitamin D deficiency in urban developments may be the reduction in time that individuals spend exposed to sunlight, as work and leisure activities are mostly conducted indoors. With regard to the second determinant of Vitamin D status, dietary trends associated with the urban-industrial environment have reduced the quantity of vitamin D obtained from the diet. These include a reduction in the consumption of fish and sea mammals, animal fat, eggs, and dairy fat. In addition, food production methods such as indoor housing of layer hens and increased milk yield per cow may have contributed to lower vitamin D intake. Milk and dairy fat intake have been inversely associated with obesity and with the metabolic syndrome (86,87). The decrease in total milk consumption (88), replacement of whole milk with reduced-fat milks and phasing out of school milk after the 1970s may have contributed to a critical fall in dietary vitamin D intake in the past three decades.” (pp. 319)). In fact, based on twin studies, Stunkard, Foch, and Hrubec [
25] found that the heritability of weight was 0.78–0.81. Heritability is defined as “the proportion of phenotypic variation in a population that is attributable to genetic factors and is only an estimate at best” ([
17], p. 14). By comparing siblings who migrated to urban regions and those who remained in rural areas, Kinra et al. [
18] found that increased energy intake and reduced energy expenditures contributed equally to greater adiposity among urban migrants in India. Sallis et al. [
1] suggest that the design of urban environments has the potential to contribute substantially to physical activity. Similar findings across cities suggest the potential of urban planning, transportation, and open space planning in efforts to reduce the health burden of the global physical inactivity pandemic. (For a similar line of research, see, for example, [
20,
21]) (In a recent conference held in Leeds, Moore and Boesch [
24] stated that: “While fundamentally obesity is a disorder of energy balance, several decades of research has demonstrated that maintaining energy balance is much more complex than the ‘energy in equals energy out’ equation that was once touted. The purpose of the 2018 Nutrition Society Summer Conference, ‘Getting energy balance right’ was to provide insight into the numerous factors influencing energy balance, considering varying needs across the lifespan, while highlighting advances and gaps in knowledge. Papers presented in this issue illustrate the wide range of factors involved in maintaining energy balance, including: Epigenetics, the gut microbiome, physical activity and dietary factors including sugar.” (page 259—the abstract)).
3.2. Results
Having demonstrated the cultural projected BMI differences among the 15-year-old cohort, the objective of the current section is: (1) to measure the cohort effect on the projected BMI of selected groups; (2) compare this cohort effect across groups; and (3) compare this cohort effect across gender for females and males who share the same native language.
Consider the following model, which extends the model given by Equation (2) and is applied only to Hebrew and Russian speakers (the largest group of immigrants):
Once again, and following Ramanathan [
20], the model is reshaped, so as to capture the baseline of the 15-year-old cohort (AGE = 15) by the constant terms
(projected BMI of 15-year-old Hebrew speakers) and
(projected BMI of 15-year-old Russian speakers). Here, we permit the model to vary with the age variable by adding the quadratic element
, where the age with the maximum BMI is
for Hebrew speakers if
and
for Russian speakers if
(This may be demonstrated as follows. For Hebrew speakers (RUSSIAN=0), the model becomes:
. The first derivative yields:
. To find the maximum projected BMI (in the case that
), the first derivative is equated to zero:
. Division by
and changing sides yield:
. Finally, adding 15 to both sides yields the requested outcome:
. For Russian speakers (RUSSIAN = 1), the model becomes:
. By rearranging terms:
. The first derivative yields:
. To find the maximum projected BMI (in the case that
), the first derivative is equated to zero:
. Division by
and changing sides yield:
. Finally, adding 15 to both sides yields the requested outcome:
).
The estimation results of this model are given in
Table 5 for the 2012 and 2016 samples. At the bottom of
Table 5, we report the 95% confidence intervals based on the estimation outcomes for the minimal age (= 15 years), the age that Russian speakers cross the BMI = 25 benchmark (=35 years) and the age that yields the maximum projected BMI (
years for Russian speakers and
years for Hebrew speakers). The outcomes show that both coefficients of (AGE-15) and (AGE-15)
2 are statistically significant. Consequently, the null hypothesis that compared to the linear model, the quadratic model better fits the data, cannot be rejected empirically.
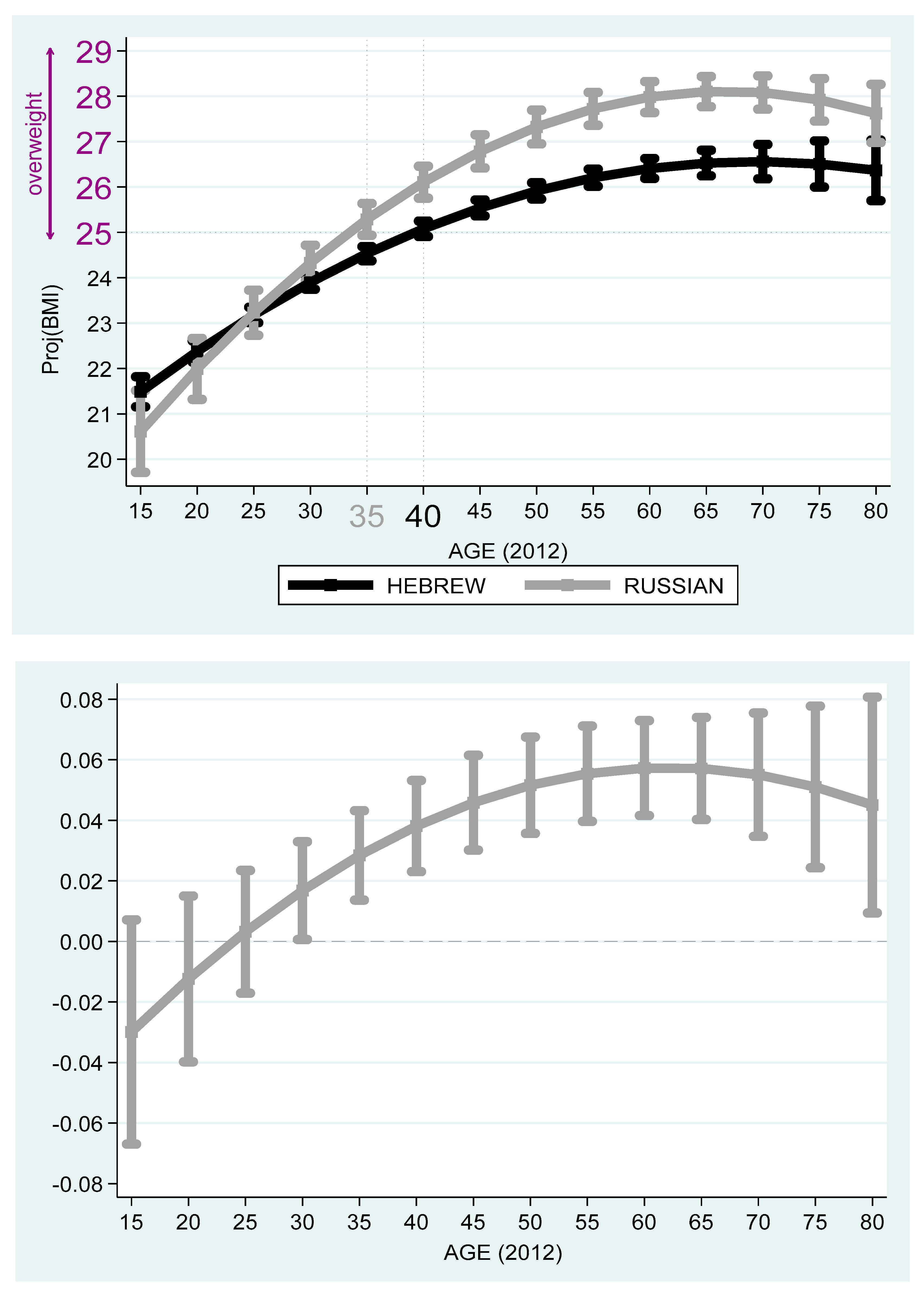
Based on the projected values obtained from
Table 4,
Figure 3 and
Figure 4 compare between Russian and Hebrew speakers by age in 2012 and 2016. The top 2012 graph gives the projected BMI and 95% confidence intervals of individuals whose native language is Hebrew (4166 persons) and Russian (1004 persons), respectively, where the age variable is controlled. The bottom graph gives approximated Russian–Hebrew projected BMI differences in percent and 95% confidence intervals. In both graphs, and in projected BMI terms, the Russian speakers’ 15-year-old benchmark is lower than the Hebrew speakers’. Yet, the growth of projected BMI with age is steeper among Russian speakers. Starting from 30 years in 2012 and 35 years in 2016, the null hypothesis of equality of projected BMI among Russian and Hebrew speakers is rejected at the 5% significance level. While the Russian speakers cross the benchmark of overweight at age 35, the Hebrew speakers cross this benchmark five year later at around 40. Finally, both Hebrew and Russian speakers reach the peak of projected BMI around age 67–70.
Next, consider the following two models applied separately to Hebrew speakers and Russian speakers:
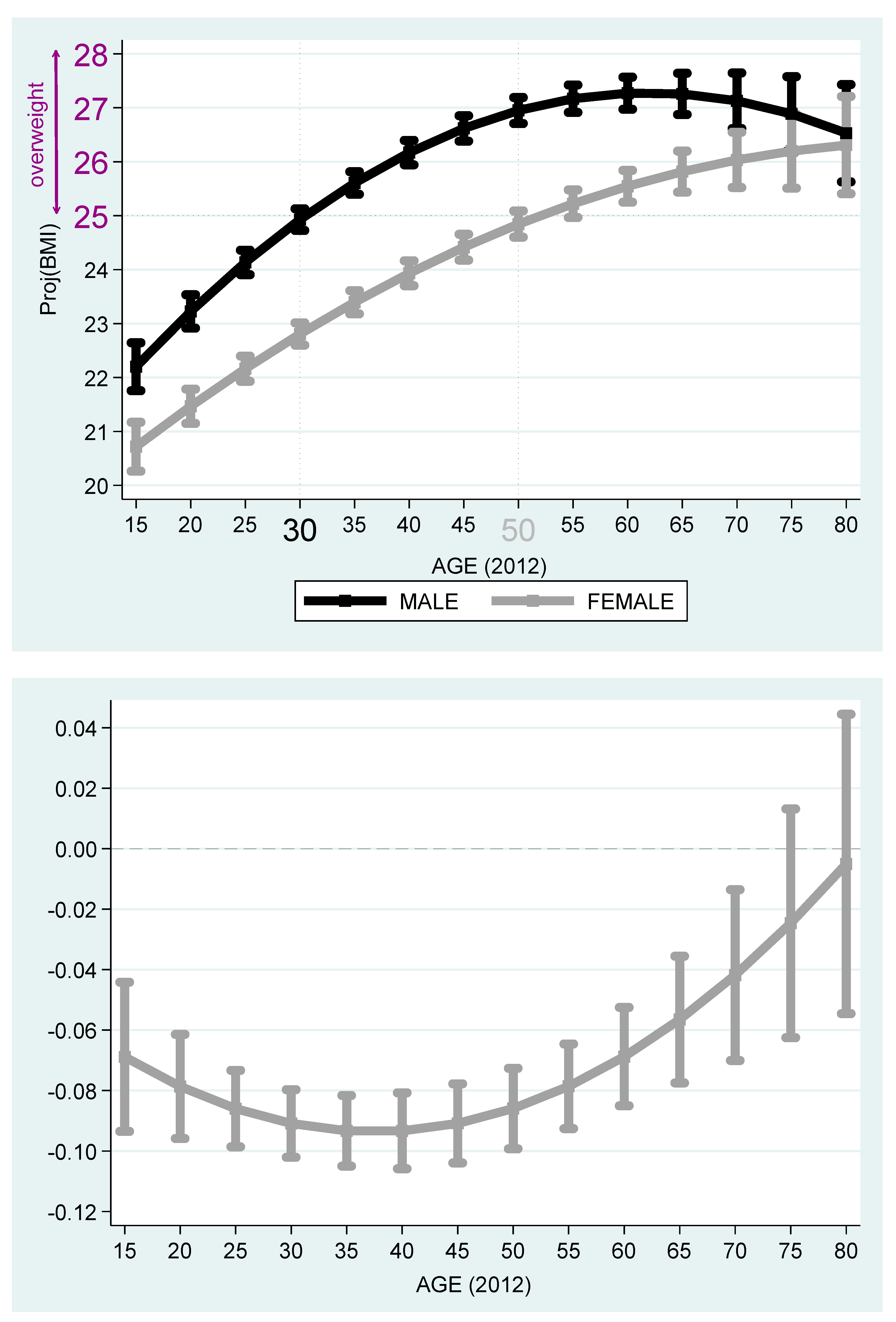
Table 6 reports the estimation outcomes for Hebrew and Russian speakers. Based on the outcomes reported in
Table 6,
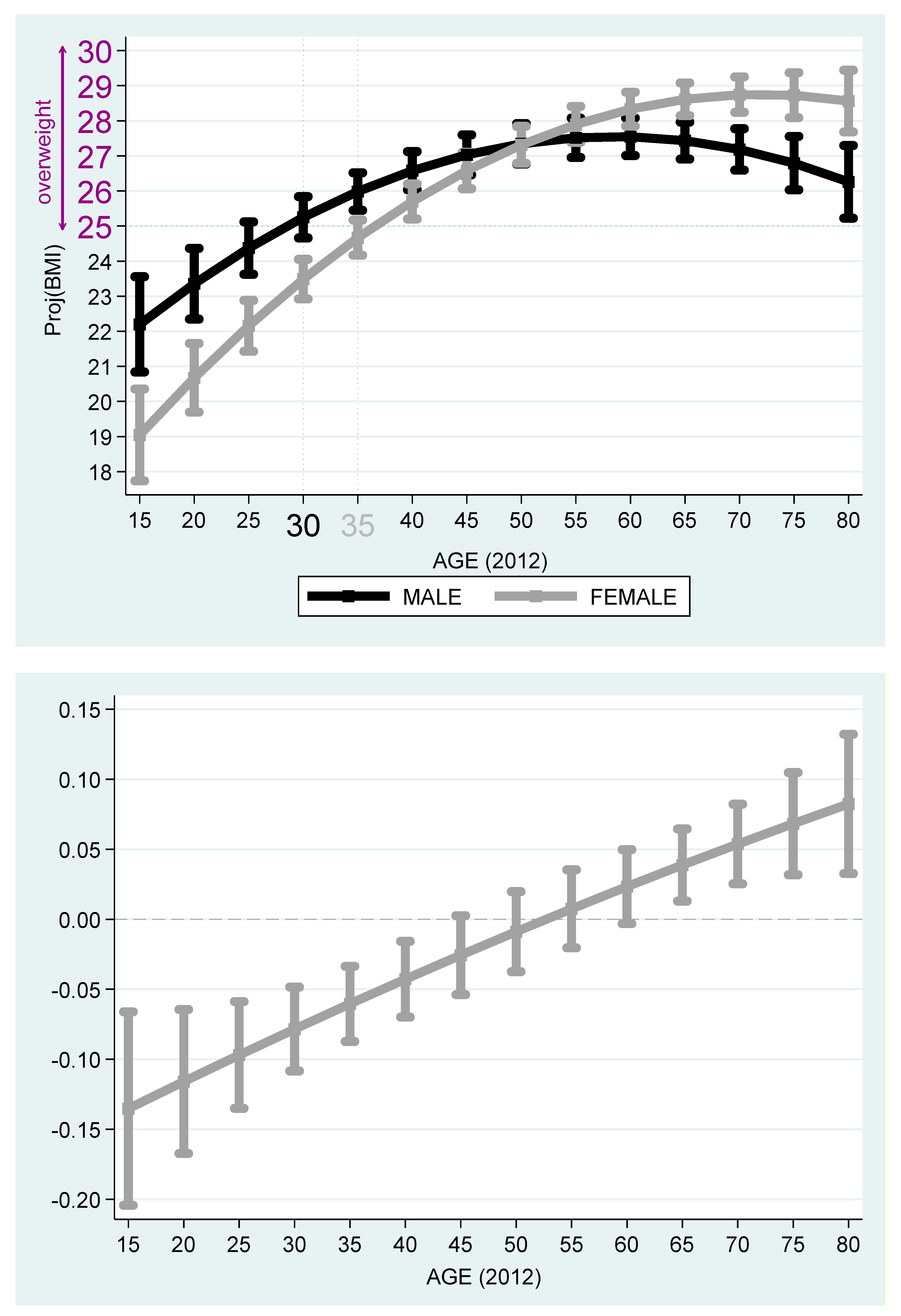
Figure 5 and
Figure 6 describe the projected BMI by age and stratified by females and males. It is evident from the figures that among Jewish Native Israelis, the projected BMI of the males is higher than that of the females, even after adjustment for the age cohort. While 30-year-old native Israeli males cross the overweight benchmark at age 30, 50-year-old native Israeli females cross this benchmark, so that the age gap of crossing this benchmark is 20 years in favor of women. The maximum projected BMI is obtained at
years old for native Israeli males and is equal to
(below the obesity benchmark of BMI ≥ 30). The same phenomenon exists among Russian speakers. Yet, the gap of crossing this overweight benchmark reduces to only five years in favor of females (age 30 for males and 35 for females). The maximum projected BMI is obtained at
years old for native Israeli males and is equal to
(below the obesity benchmark of BMI ≥ 30).
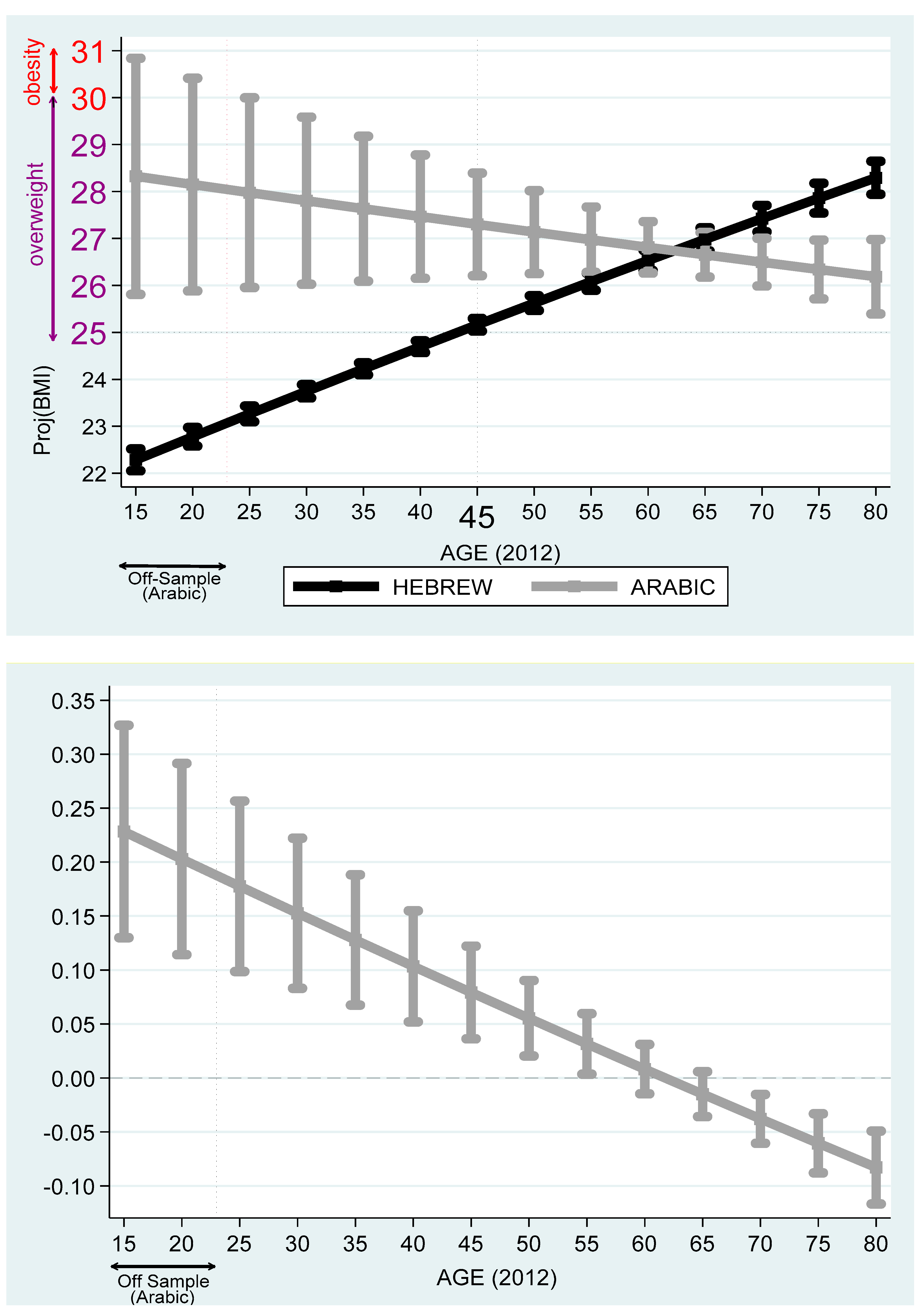
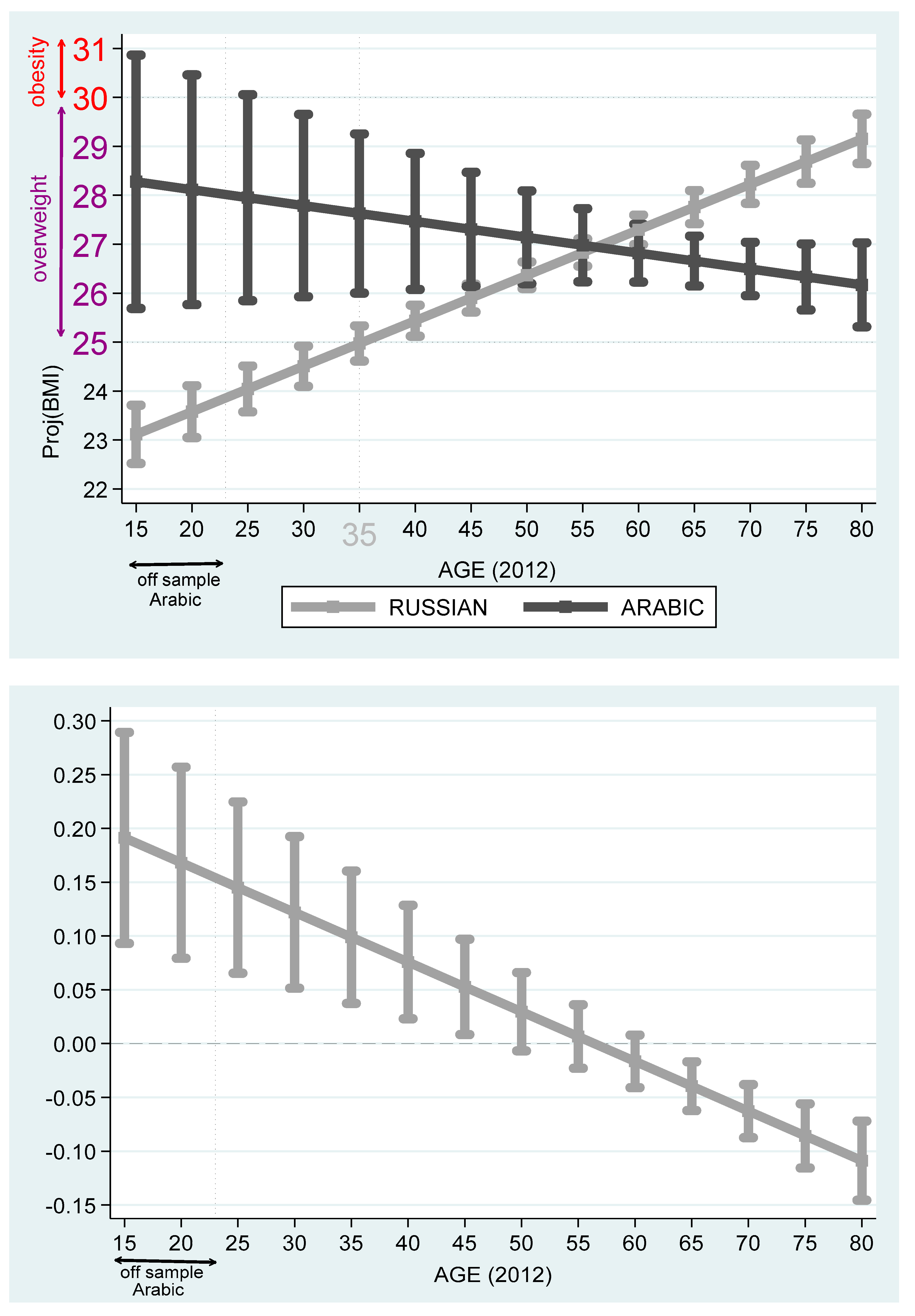
Table 7 reports the projected BMI differences among Hebrew, Arabic, Russian, and Amharic speakers. Based on these outcomes,
Figure 7 compares Arabic and Hebrew speakers and
Figure 8, Russian and Arabic speakers. It is evident from the figures that Arabic speakers suffer from overweight and obesity problems, particularly for the early age cohorts. Unlike Hebrew or Russian speakers, among Arabic speakers, on the one hand, the projected BMI drops with the age variable. On the other hand, there is a wide spread of projected BMI at the young age cohort.