Waste Conversion into a Sweetener—Development of an Innovative Strategy for Erythritol Production by Yarrowia lipolytica
Abstract
1. Introduction
2. Materials and Methods
2.1. Microorganisms
2.2. Substrates
2.3. Media
2.4. Culture Conditions
2.5. Analytical Methods
2.6. Enzyme Assays
2.7. Statystical Analysis
3. Results
3.1. The Effect of Crude Fatty Acid Fraction Application
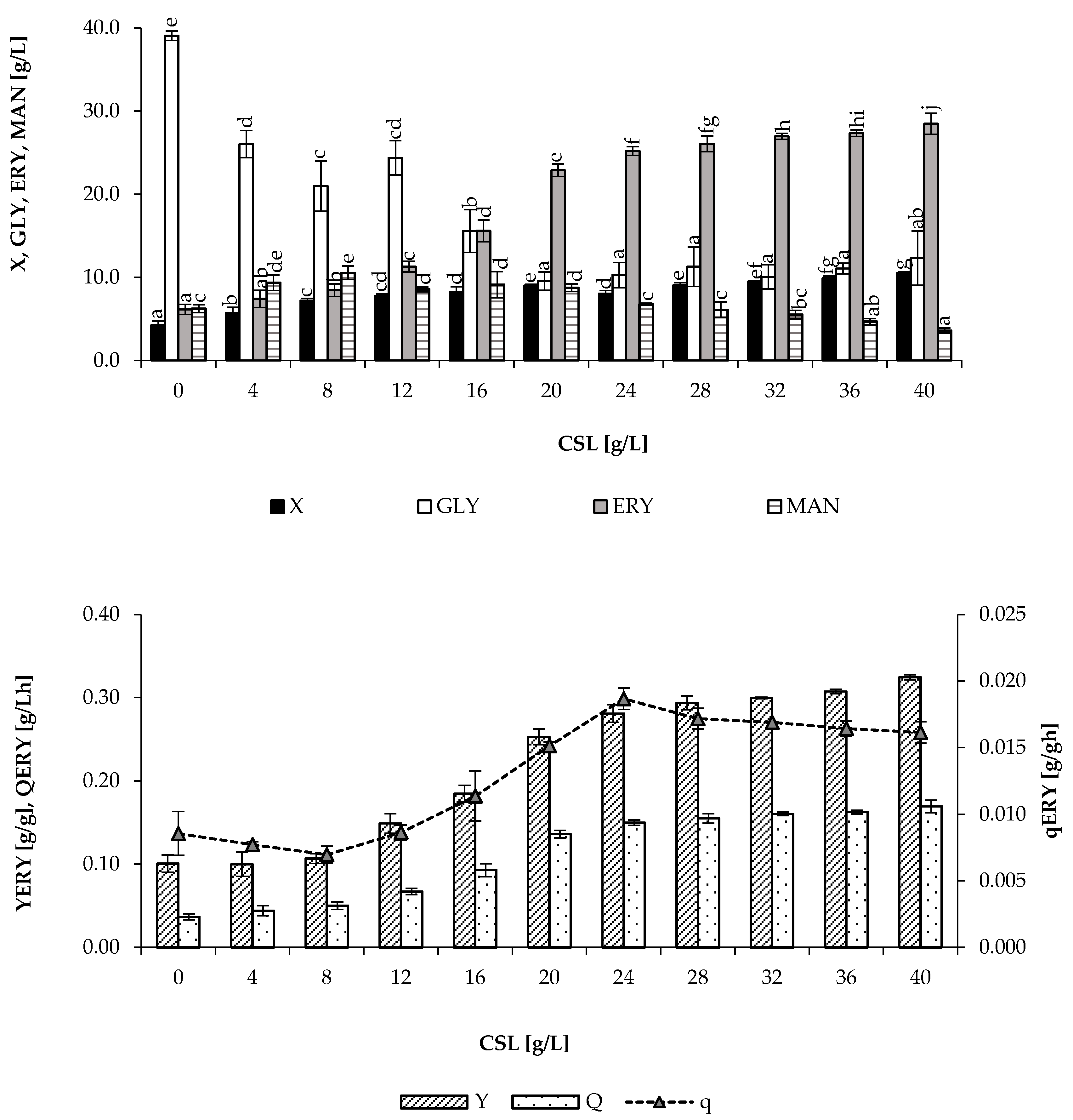
3.2. The Effect of CSL Application
3.3. Comparison of Factors Causing the Increase of the Osmotic Pressure
3.4. Comparison of Mineral and Low-Cost Medium
3.5. Development of Waste-Substrate Based Medium
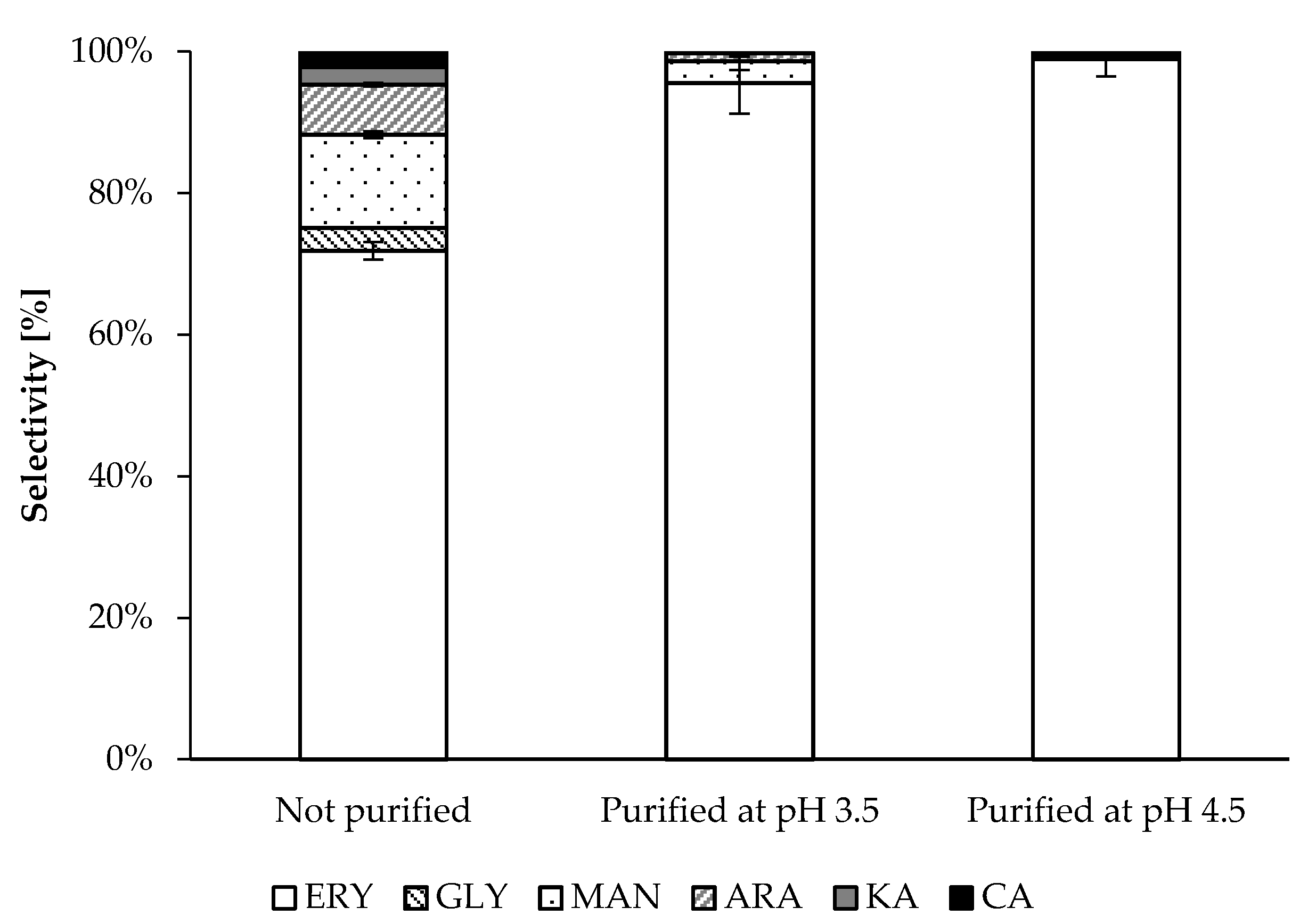
3.6. Natural Purification Process
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
References
- Moon, H.-J.; Jeya, M.; Kim, I.-W.; Lee, J.-K. Biotechnological production of erythritol and its applications. Appl. Microbiol. Biotechnol. 2010, 86, 1017–1025. [Google Scholar] [CrossRef]
- Regnat, K.; Mach, R.L.; Mach-Aigner, A. Erythritol as sweetener—Wherefrom and whereto? Appl. Microbiol. Biotechnol. 2017, 102, 587–595. [Google Scholar] [CrossRef]
- Carly, F.; Gamboa-Melendez, H.; Vandermies, M.; Damblon, C.; Nicaud, J.M.; Fickers, P. Identification and characterization of EYK1, a key gene for erythritol catabolism in Yarrowia lipolytica. Appl. Microbiol. Biotechnol. 2017, 101, 6587–6596. [Google Scholar] [CrossRef]
- Li, L.; Yang, T.; Guo, W.; Ju, X.; Hu, C.; Tang, B.; Fu, J.; Gu, J.; Zhang, H. Construction of an efficient mutant strain of Trichosporonoides oedocephalis with HOG1 gene deletion for production of erythritol. J. Microbiol. Biotechnol. 2016, 26, 700–709. [Google Scholar] [CrossRef]
- Mirończuk, A.M.; Furgała, J.; Rakicka-Pustułka, M.; Rymowicz, W. Enhanced production of erythritol by Yarrowia lipolytica on glycerol in repeated batch cultures. J. Ind. Microbiol. Biotechnol. 2013, 41, 57–64. [Google Scholar] [CrossRef]
- Rakicka-Pustułka, M.; Biegalska, A.; Rymowicz, W.; Dobrowolski, A.; Mirończuk, A.M. Polyol production from waste materials by genetically modified Yarrowia lipolytica. Bioresour. Technol. 2017, 243, 393–399. [Google Scholar] [CrossRef] [PubMed]
- Tomaszewska, L.; Rywińska, A.; Rymowicz, W. High selectivity of erythritol production from glycerol by Yarrowia lipolytica. Biomass-Bioenergy 2014, 64, 309–320. [Google Scholar] [CrossRef]
- Guo, J.; Li, J.; Chen, Y.; Guo, X.; Xiao, D. Improving erythritol production of Aureobasidium pullulans from xylose by mutagenesis and medium optimization. Appl. Biochem. Biotechnol. 2016, 180, 717–727. [Google Scholar] [CrossRef]
- Rywińska, A.; Marcinkiewicz, M.; Cibis, E.; Rymowicz, W. Optimization of medium composition for erythritol production from glycerol by Yarrowia lipolytica using response surface methodology. Prep. Biochem. Biotechnol. 2014, 45, 515–529. [Google Scholar] [CrossRef]
- Yang, L.-B.; Zhan, X.-B.; Zhu, L.; Gao, M.; Lin, C.-C. Optimization of a low-cost hyperosmotic medium and establishing the fermentation kinetics of erythritol production by Yarrowia lipolytica from crude glycerol. Prep. Biochem. Biotechnol. 2015, 46, 376–383. [Google Scholar] [CrossRef] [PubMed]
- Jovanović, B.; Mach, R.L.; Mach-Aigner, A. Erythritol production on wheat straw using Trichoderma reesei. AMB Express 2014, 4, 34. [Google Scholar] [CrossRef]
- Lee, D.-H.; Lee, Y.-J.; Ryu, Y.; Seo, J.-H. Molecular cloning and biochemical characterization of a novel erythrose reductase from Candida magnoliae JH110. Microb. Cell Factories 2010, 9, 43. [Google Scholar] [CrossRef]
- Savergave, L.S.; Gadre, R.V.; Vaidya, B.K.; Narayanan, K. Strain improvement and statistical media optimization for enhanced erythritol production with minimal by-products from Candida magnoliae mutant R23. Biochem. Eng. J. 2011, 55, 92–100. [Google Scholar] [CrossRef]
- Tomaszewska, L.; Rywińska, A.; Gładkowski, W. Production of erythritol and mannitol by Yarrowia lipolytica yeast in media containing glycerol. J. Ind. Microbiol. Biotechnol. 2012, 39, 1333–1343. [Google Scholar] [CrossRef] [PubMed]
- Rzechonek, D.A.; Dobrowolski, A.; Rymowicz, W.; Mirończuk, A.M. Recent advances in biological production of erythritol. Crit. Rev. Biotechnol. 2017, 38, 620–633. [Google Scholar] [CrossRef] [PubMed]
- Tomaszewska, L.; Rakicka-Pustułka, M.; Rymowicz, W.; Rywińska, A. A comparative study on glycerol metabolism to erythritol and citric acid in Yarrowia lipolytica yeast cells. FEMS Yeast Res. 2014, 14, 966–976. [Google Scholar] [CrossRef] [PubMed]
- Rywińska, A.; Juszczyk, P.; Wojtatowicz, M.; Robak, M.; Lazar, Z.; Tomaszewska, L.; Rymowicz, W. Glycerol as a promising substrate for Yarrowia lipolytica biotechnological applications. Biomass-Bioenergy 2013, 48, 148–166. [Google Scholar] [CrossRef]
- Rakicka-Pustułka, M.; Rukowicz, B.; Rywińska, A.; Lazar, Z.; Rymowicz, W. Technology of efficient continuous erythritol production from glycerol. J. Clean. Prod. 2016, 139, 905–913. [Google Scholar] [CrossRef]
- Juszczyk, P.; Tomaszewska, L.; Kita, A.; Rymowicz, W. Biomass production by novel strains of Yarrowia lipolytica using raw glycerol, derived from biodiesel production. Bioresour. Technol. 2013, 137, 124–131. [Google Scholar] [CrossRef]
- Papanikolaou, S.; Blanchard, F.; Rondags, E.; Gardeli, C.; Koutinas, A.; Aggelis, G.; Kampisopoulou, E.; Chevalot, I. Production of secondary metabolites through glycerol fermentation under carbon-excess conditions by the yeasts Yarrowia lipolytica and Rhodosporidium toruloides. Eur. J. Lipid Sci. Technol. 2017, 119, 1600507. [Google Scholar] [CrossRef]
- Xiaoyan, L.; Yu, X.; Lv, J.; Xu, J.; Xia, J.; Wu, Z.; Zhang, T.; Deng, Y. A cost-effective process for the coproduction of erythritol and lipase with Yarrowia lipolytica M53 from waste cooking oil. Food Bioprod. Process. 2017, 103, 86–94. [Google Scholar] [CrossRef]
- Liu, X.; Lv, J.; Xu, J.; Xia, J.; He, A.; Zhang, T.; Li, X.; Xu, J. Effects of osmotic pressure and pH on citric acid and erythritol production from waste cooking oil by Yarrowia lipolytica. Eng. Life Sci. 2018, 18, 344–352. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Yu, X.; Xia, J.; Lv, J.; Xu, J.; Dai, B.; Xu, X.; Xu, J. Erythritol production by Yarrowia lipolytica from okara pretreated with the in-house enzyme pools of fungi. Bioresour. Technol. 2017, 244, 1089–1095. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Yu, X.; Zhang, T.; Wang, Z.; Xu, J.; Xia, J.; He, A.; Yan, Y.; Xu, J.; Xiaoyan, L.; et al. Novel two-stage solid-state fermentation for erythritol production on okara–buckwheat husk medium. Bioresour. Technol. 2018, 266, 439–446. [Google Scholar] [CrossRef]
- Rakicka-Pustułka, M.; Lazar, Z.; Rywińska, A.; Rymowicz, W. Efficient utilization of inulin and glycerol as fermentation substrates in erythritol and citric acid production using Yarrowia lipolytica expressing inulinase. Chem. Pap. 2016, 70, 1452–1459. [Google Scholar] [CrossRef]
- Cybulski, K.; Tomaszewska-Hetman, L.; Rakicka, M.; Juszczyk, P.; Rywińska, A. Production of pyruvic acid from glycerol by Yarrowia lipolytica. Folia Microbiol. 2019, 64, 809–820. [Google Scholar] [CrossRef]
- Makri, A.; Fakas, S.; Aggelis, G. Metabolic activities of biotechnological interest in Yarrowia lipolytica grown on glycerol in repeated batch cultures. Bioresour. Technol. 2010, 101, 2351–2358. [Google Scholar] [CrossRef]
- Tomaszewska-Hetman, L.; Rywińska, A. Erythrose reductase as an enzyme responsible for erytrhitol overproduction in Yarrowia lipolytica yeast grown on glycerol media. Electron. J. Pol. Agric. Univ. 2015, 18. Available online: http://www.ejpau.media.pl/volume18/issue4/art-08.html (accessed on 15 July 2020).
- Kamzolova, S.V.; Morgunov, I.G. Biosynthesis of pyruvic acid from glycerol-containing substrates and its regulation in the yeast Yarrowia lipolytica. Bioresour. Technol. 2018, 266, 125–133. [Google Scholar] [CrossRef]
- Lazar, Z.; Liu, N.; Stephanopoulos, G. Holistic approaches in lipid production by Yarrowia lipolytica. Trends Biotechnol. 2018, 36, 1157–1170. [Google Scholar] [CrossRef]
- Tomaszewska-Hetman, L.; Rywińska, A. Erythritol biosynthesis from glycerol by Yarrowia lipolytica yeast: Effect of osmotic pressure. Chem. Pap. 2016, 70, 272–283. [Google Scholar] [CrossRef]
- Yang, L.-B.; Zhan, X.-B.; Zheng, Z.-Y.; Wu, J.-R.; Gao, M.-J.; Lin, C.-C. A novel osmotic pressure control fed-batch fermentation strategy for improvement of erythritol production by Yarrowia lipolytica from glycerol. Bioresour. Technol. 2014, 151, 120–127. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Wang, X.-F.; Xu, J.; Xia, J.; Lv, J.; Zhang, T.; Wu, Z.; Deng, Y.; He, J. Citric acid production by Yarrowia lipolytica SWJ-1b using corn steep liquor as a source of organic nitrogen and vitamins. Ind. Crop. Prod. 2015, 78, 154–160. [Google Scholar] [CrossRef]
- Xi, Y.-L.; Chen, K.; Dai, W.-Y.; Ma, J.; Zhang, M.; Jiang, M.; Wei, P.; Ouyang, P. Succinic acid production by Actinobacillus succinogenes NJ113 using corn steep liquor powder as nitrogen source. Bioresour. Technol. 2013, 136, 775–779. [Google Scholar] [CrossRef] [PubMed]
- Souza, A.F.; Rodríguez, D.M.; Ribeaux, D.R.; Luna, M.A.C.; Silva, T.A.L.; Andrade, R.F.S.; Gusmão, N.B.; Campos-Takaki, G.M.; E Silva, T.L. Waste soybean oil and corn steep liquor as economic substrates for bioemulsifier and biodiesel production by Candida lipolytica UCP 0998. Int. J. Mol. Sci. 2016, 17, 1608. [Google Scholar] [CrossRef]
- Park, E.-H. Role of osmotic and salt stress in the expression of erythrose reductase in Candida magnoliae. J. Microbiol. Biotechnol. 2011, 21, 1064–1068. [Google Scholar] [CrossRef]
- Kona, R.; Qureshi, N.; Pai, J. Production of glucose oxidase using Aspergillus niger and corn steep liquor. Bioresour. Technol. 2001, 78, 123–126. [Google Scholar] [CrossRef]
- Chatzifragkou, A.; Papanikolaou, S. Effect of impurities in biodiesel-derived waste glycerol on the performance and feasibility of biotechnological processes. Appl. Microbiol. Biotechnol. 2012, 95, 13–27. [Google Scholar] [CrossRef]
- Rzechonek, D.A.; Neuvéglise, C.; Devillers, H.; Rymowicz, W.; Mirończuk, A.M. EUF1—A newly identified gene involved in erythritol utilization in Yarrowia lipolytica. Sci. Rep. 2017, 7, 12507. [Google Scholar] [CrossRef]
- Coelho, M.A.Z.; Amaral, P.F.F.; Belo, I. Yarrowia Lipolytica: An industrial workhorse. In Current Research, Technology and Education Topics in Applied Microbiology and Microbial Biotechnology; Mendez-Vilas, A., Ed.; Formatex Research Center: Badajoz, Spain, 2010; pp. 930–944. [Google Scholar]
| NaCl | Time * | X | ERY | MAN | YERY | QERY ** | qERY ** | Osmotic Pressure *** | |
|---|---|---|---|---|---|---|---|---|---|
| [g/L] | Supplementation Method | [h] | [g/L] | [g/g] | [g/Lh] | [g/gh] | [Osm/kg] | ||
| Y. lipolytica A-3 | |||||||||
| - | - | 125.0 ± 4.1 b,c | 28.8 ± 2.3 c,d | 72.5 ± 3.7 a,b | 21.9 ± 3.9 c | 0.37 | 0.72 | 0.025 | 0.2→2.6 |
| 25 | with glycerol addition | 135.5 ± 5.5 c | 27.3 ± 1.2 c | 89.7 ± 5.2 c | 12.1 ± 4.4 b | 0.42 | 0.81 | 0.030 | 0.2→3.4 |
| 25 | in the initial medium | 115.5 ± 3.3 b | 27.1 ± 2.1 c | 87.1 ± 4.4 c | 5.3 ± 2.9 a | 0.43 | 0.96 | 0.035 | 1.1→3.3 |
| Y. lipolytica A-6 | |||||||||
| - | - | 92.0 ± 4.0 a | 32.3 ± 4.5 d,e | 66.0 ± 4.9 a | 43.0 ± 6.2 d | 0.34 | 0.97 | 0.030 | 0.2→2.3 |
| 25 | with glycerol addition | 125.5 ± 6.0 b,c | 34.3 ± 3.8 e | 75.6 ± 3.5 b | 15.1 ± 1.8 b,c | 0.37 | 0.74 | 0.022 | 0.2→3.2 |
| 25 | in the initial medium | 99.5 ± 2.6 a | 34.4 ± 1.9 e | 89.5 ± 2.4 c | 16.0 ± 3.0 b,c | 0.43 | 1.19 | 0.035 | 1.1→3.1 |
| Y. lipolytica A-311 | |||||||||
| - | - | 169.5 ± 6.8 d | 21.2 ± 1.6 a,b | 86.3 ± 5.1 c | 41.0 ± 5.1 d | 0.43 | 0.59 | 0.028 | 0.2→2.1 |
| 25 | with glycerol addition | 247.0 ± 10.1 f | 25.5 ± 3.5 b,c | 125.3 ± 7.0 e | 21.4 ± 3.3 c | 0.58 | 0.56 | 0.022 | 0.2→3.1 |
| 25 | in the initial medium | 191.0 ± 7.6 e | 20.2 ± 1.5 a | 107.3 ± 4.3 d | 16.4 ± 2.3 b,c | 0.52 | 0.65 | 0.032 | 1.1→3.3 |
| CSL [g/L] | Time | X | ERY | MAN | YERY | QERY | qERY |
|---|---|---|---|---|---|---|---|
| [h] | [g/L] | [g/g] | [g/Lh] | [g/gh] | |||
| 24 | 147 ± 4.7 a | 11.5 ± 1.8 a | 75 ± 3.6 a | 4.2 ± 2.9 a | 0.52 | 0.51 | 0.040 |
| 40 | 77 ± 3.5 b | 25.4 ± 2.6 b | 83 ± 1.7 b | 2.2 ± 1.5 a | 0.56 | 1.08 | 0.042 |
| Medium | Osmotic Pressure | Time | X | ERY | MAN | YERY | QERY | qERY | GK | GPDH | TK | ER |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| [Osm/kg] | [h] | [g/L] | [g/g] | [g/Lh] | [g/gh] | [U/mg] | ||||||
| without NaCl 150 g/L of glycerol | 2.1 | 108 ± 4.8 a | 28.1 ± 3.0 a | 62.2 ± 2.8 a | 22.8 ± 3.1 a | 0.40 | 0.58 | 0.020 | 0.021 ± 0.002 a,b | 0.016 ± 0.001 b | 0.051 ± 0.005 a | 0.060 ± 0.006 a |
| without NaCl 220 g/L of glycerol | 3.1 | 99 ± 3.5 b | 31.0 ± 1.8 a | 93.0 ± 4.6 b | 20.1 ± 1.9 a | 0.42 | 0.95 | 0.030 | 0.024 ± 0.001 b | 0.003 ± 0.000 a | 0.064 ± 0.004 b | 0.075 ± 0.004 b |
| 32.5 g/L NaCl 150 g/L of glycerol | 3.2 | 74 ± 3.3 c | 27.0 ± 1.7 a | 74.4 ± 5.4 c | 2.4 ± 2.1 b | 0.49 | 1.00 | 0.037 | 0.020 ± 0.002 a | 0.017 ± 0.001 b | 0.069 ± 0.001 b | 0.120 ± 0.009 c |
| Medium | Glycerol | Time | X | ERY | MAN | YERY | QERY | qERY |
|---|---|---|---|---|---|---|---|---|
| [h] | [g/L] | [g/g] | [g/Lh] | [g/gh] | ||||
| Mineral (MPM) | Pure | 99 ± 4.3 a | 31.8 ± 3.4 c | 93.0 ± 4.2 a | 20.2 ± 2.6 a | 0.42 | 0.95 | 0.030 |
| Low-cost (LPM) | Pure | 159 ± 5.1 b | 16.4 ± 2.2 a | 94.0 ± 3.5 a | 17.0 ± 2.4 a | 0.40 | 0.59 | 0.036 |
| Mineral (MPM) | Crude | 100 ± 4.1 a | 28.8 ± 1.7 b,c | 111.0 ± 6.3 b | 6.1 ± 3.1 b | 0.50 | 1.11 | 0.039 |
| Low-cost (LPM) | Crude | 104 ± 3.3 a | 25.4 ± 2.3 b | 108.0 ± 2.1 b | 8.1 ± 1.2 b | 0.45 | 1.04 | 0.041 |
| Glycerol | Time * | X | ERY | MAN | YERY | QERY ** | qERY ** |
|---|---|---|---|---|---|---|---|
| [h] | [g/L] | [g/g] | [g/Lh] | [g/gh] | |||
| Pure 200 g/L | 187.0 ± 5.5 b | 28.1 ± 2.7 b | 110.3 ± 6.6 b | 14.8 ± 3.0 a | 0.51 | 0.67 | 0.024 |
| Crude 200 g/L | 208.5 ± 6.1 c | 26.4 ± 2.9 b | 108.1 ± 3.3 b | 10.8 ± 1.9 a | 0.47 | 0.57 | 0.022 |
| Crude 150 g/L | 172.0 ± 4.6 a | 20.3 ± 1.7 a | 63.5 ± 4.4 a | 12.4 ± 1.8 a | 0.42 | 0.42 | 0.021 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tomaszewska-Hetman, L.; Rymowicz, W.; Rywińska, A. Waste Conversion into a Sweetener—Development of an Innovative Strategy for Erythritol Production by Yarrowia lipolytica. Sustainability 2020, 12, 7122. https://doi.org/10.3390/su12177122
Tomaszewska-Hetman L, Rymowicz W, Rywińska A. Waste Conversion into a Sweetener—Development of an Innovative Strategy for Erythritol Production by Yarrowia lipolytica. Sustainability. 2020; 12(17):7122. https://doi.org/10.3390/su12177122
Chicago/Turabian StyleTomaszewska-Hetman, Ludwika, Waldemar Rymowicz, and Anita Rywińska. 2020. "Waste Conversion into a Sweetener—Development of an Innovative Strategy for Erythritol Production by Yarrowia lipolytica" Sustainability 12, no. 17: 7122. https://doi.org/10.3390/su12177122
APA StyleTomaszewska-Hetman, L., Rymowicz, W., & Rywińska, A. (2020). Waste Conversion into a Sweetener—Development of an Innovative Strategy for Erythritol Production by Yarrowia lipolytica. Sustainability, 12(17), 7122. https://doi.org/10.3390/su12177122





