Ophiolite Chromite Deposits as a New Source for the Production of Refractory Chromite Sands
Abstract
:1. Introduction
2. Materials and Methods
2.1. Grain Size Distribution
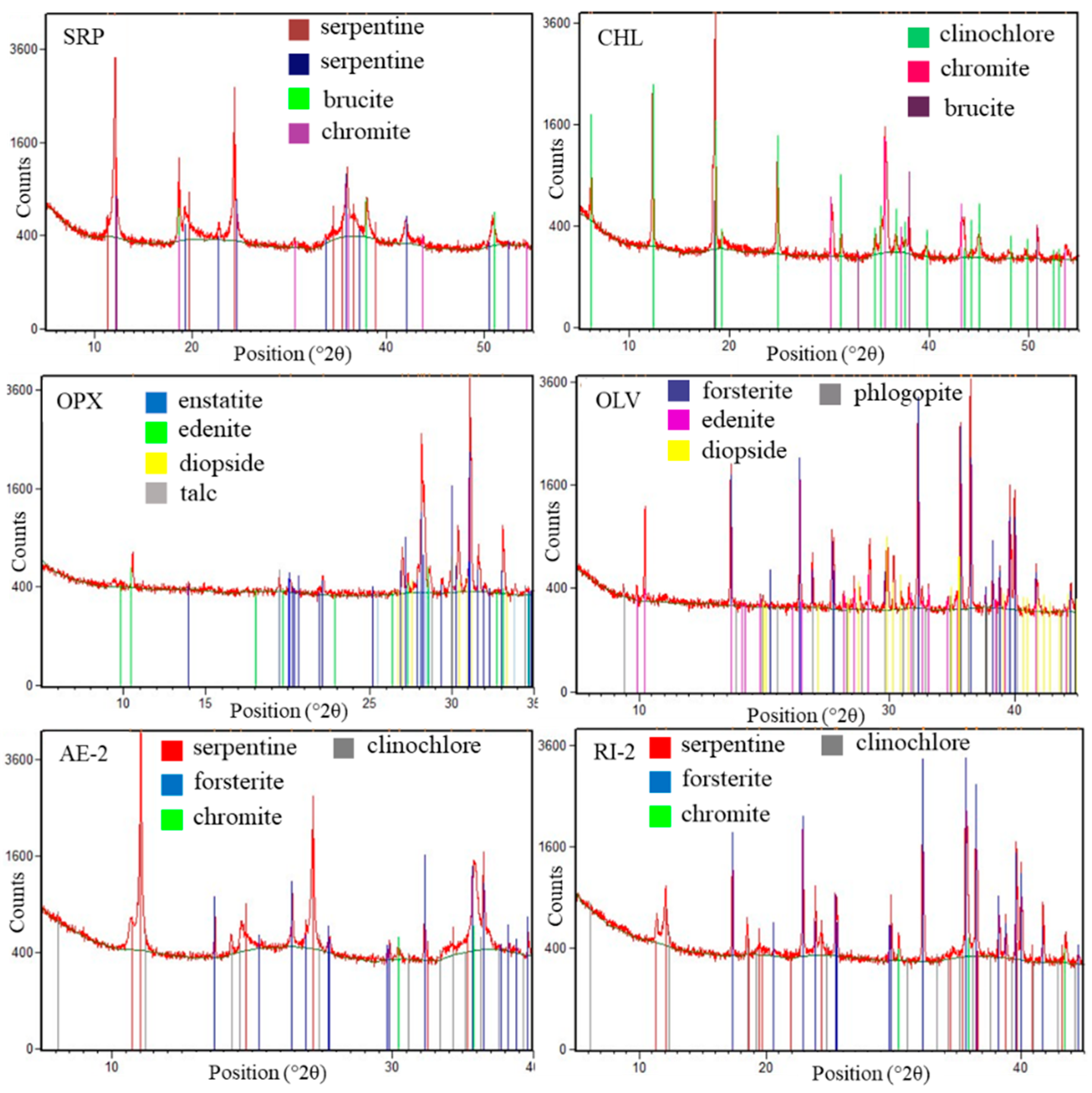
2.2. XRD Powder Diffraction
2.3. X-ray Fluorescence
2.4. Acid Demand Test
- 50 g of foundry sand
- 50 mL HCl (0.1M)
- 50 mL NaOH (0.1M)
- Purified H2O
- Mix 50 g of chromite sand with 50 mL of HCl, stir for 5 min and let the solution rest for 1 h.
- Filter the solution adding H2O until the solution reaches 250 mL.
- Measure the pH value of the solution.
- Add NaOH until the solution reaches pH 3, 4, and 5, taking note of the volume of NaOH consumed after each step.
3. Results
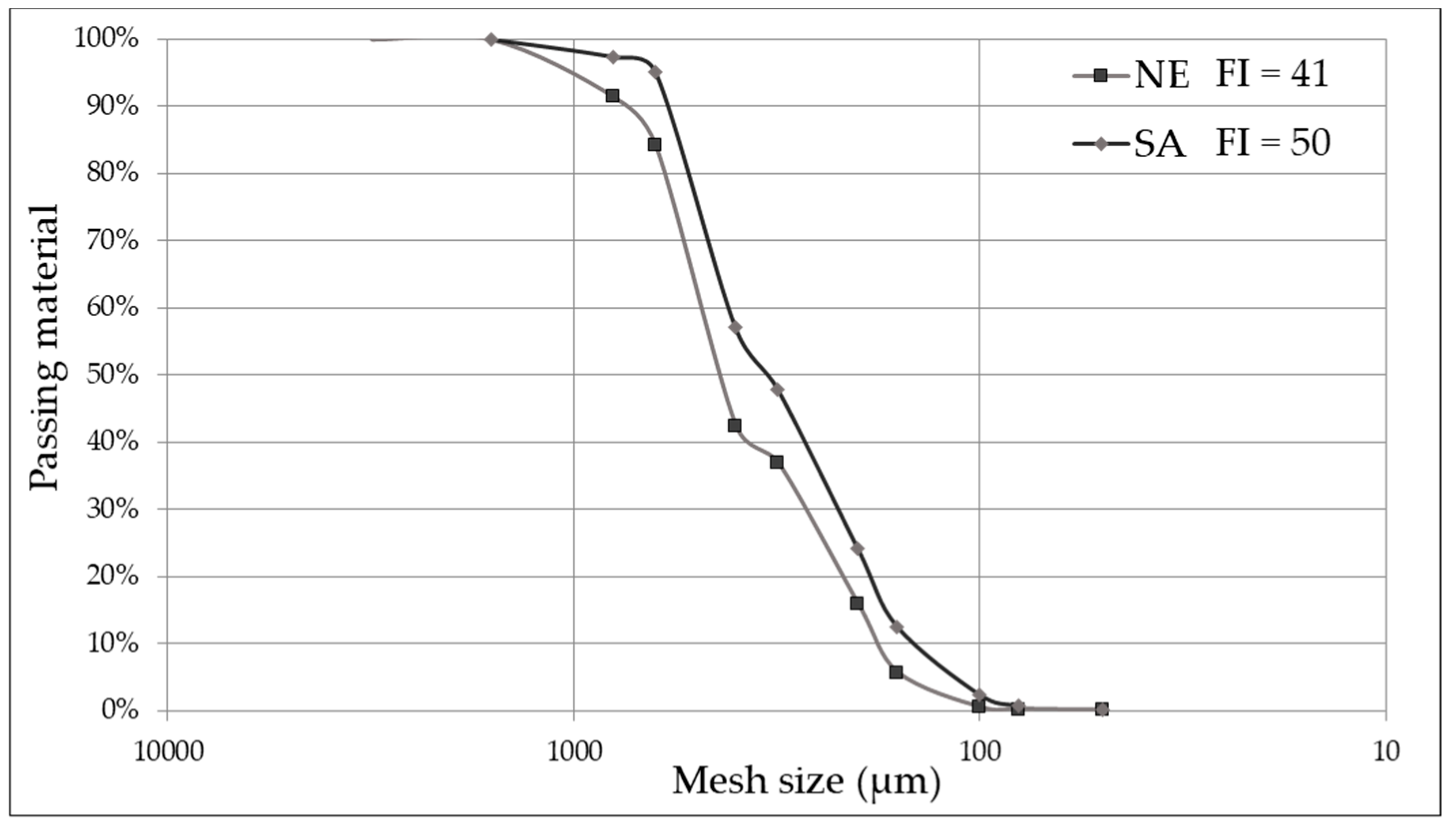
3.1. Fineness Index
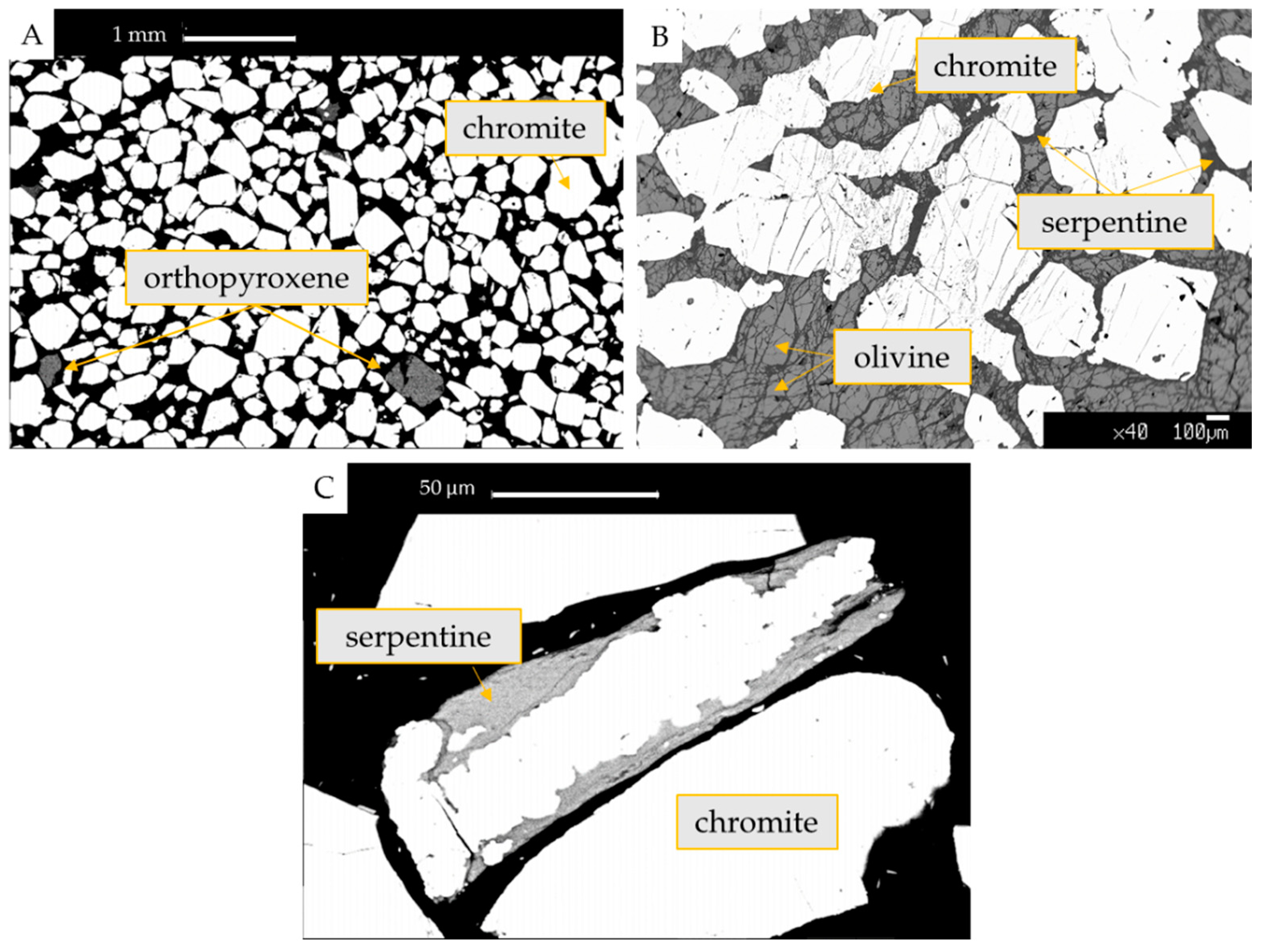
3.2. Mineralogy of Silicates
3.3. XRF
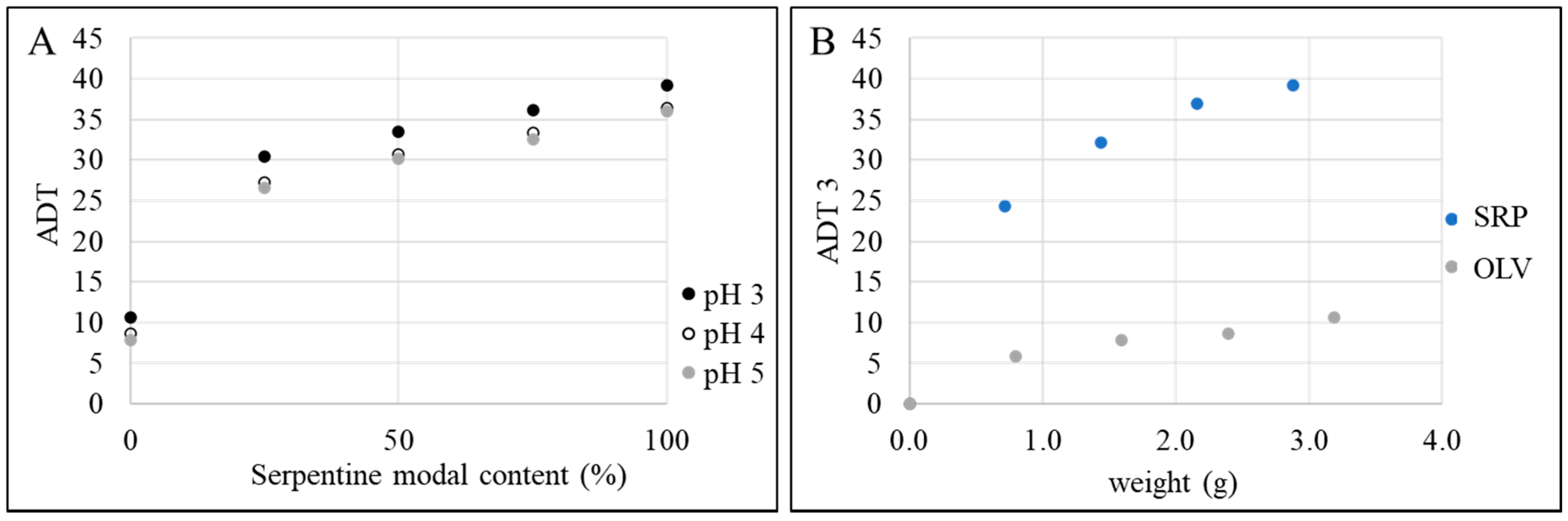
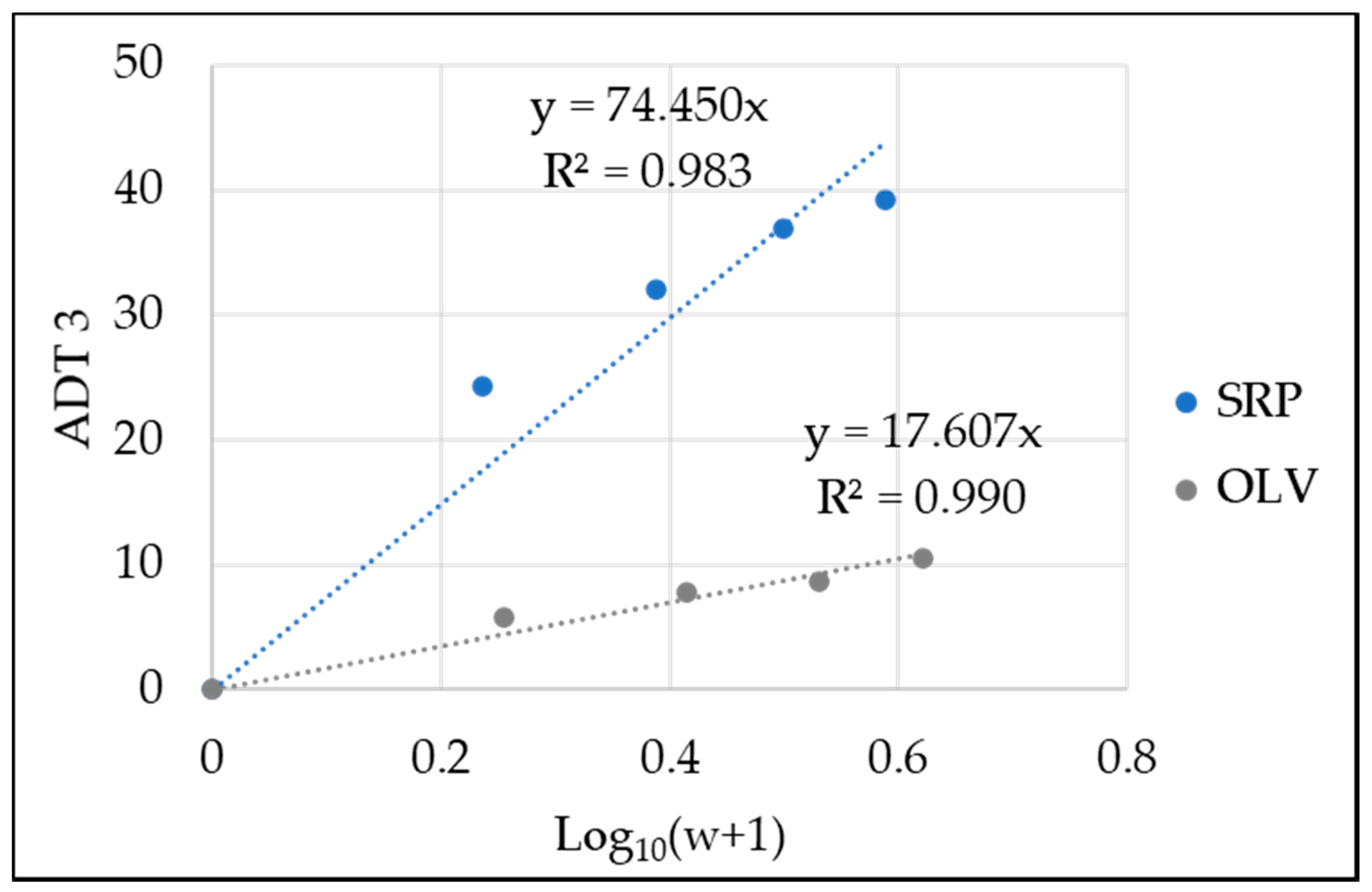
3.4. Acid Demand
4. Discussion
- (i)
- Olivine and serpentine sand mixes at different proportions (25%, 50%, and 75% of serpentine) maintaining a fixed 2.5% of SiO2, in order to simulate the behavior of partially serpentinized sands.
- (ii)
- Pure olivine and pure serpentine sands at SiO2 contents lower than 2.5% (1.875%, 1.25%, and 0.625%).
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Carey, P. Sand/binders/sand preparation and coremaking. Foundry Manag. Technol. 2002, 130, 39–52. [Google Scholar]
- Surekha, B.; Hanumantha Rao, D.; Krishna Mohana Rao, G.; Pandu, R.V.; Parappagoudar, M.B. Application of response surface methodology for modeling the properties of chromite-based resin bonded sand cores. Int. J. Mech. 2013, 7, 443–458. [Google Scholar]
- Annual Report 2014: Report on Critical Raw Materials for the EU; European Commission: Brussels, Belgium, 2014.
- Bengulur, S.; Darwada, H.R.; Gurram, K.R.; Vundavilli, P.R. Experimental Studies on Properties of Chromite-based Resin Bonded Sand System. Recent Adv. Robot. Aeronaut. Mech. Eng. 2013, 230–238. [Google Scholar]
- Acid Demand 2008; Laviosa Chimica Mineraria s.p.a.: Livorno, Italy, 2008.
- Kogel, J.E.; Trivedi, N.C.; Barker, J.M.; Krukowski, S.T. Industrial Minerals & Rocks: Commodities, Markets, and Uses; SME: Southfield, MI, USA, 2006. [Google Scholar]
- Koleli, N.; Demir, A. Chromite. Environ. Mater. Waste 2016, 245–263. [Google Scholar]
- Richard, P. Overview of the global chrome market. In Proceedings of the 1st Indinox Stainless Steel Conference, Ahmedabad, India, 25–26 January 2015. [Google Scholar]
- Rassios, A. Cooperative Development for Greek Chromite Reserves: Initial Review; Institute of Geology and Mineral Exploration: Athens Greece, 2014. [Google Scholar]
- Tzamos, E.; Kapsiotis, A.; Filippidis, A.; Koroneos, A.; Grieco, G.; Rassios, A.E.; Kantiranis, N.; Papadopoulos, A.; Gamaletsos, P.N.; Godelitsas, A. Metallogeny of the Chrome Ores of the Xerolivado-Skoumtsa Mine, Vourinos Ophiolite, Greece: Implications on the genesis of IPGE-bearing high-Cr chromitites within a heterogeneously depleted mantle section. Ore Geol. Rev. 2017, 90, 226–242. [Google Scholar] [CrossRef]
- Ghorbani, M. Economic Geology of Iran: Mineral Deposits and Natural Resources; Springer: Berlin, Germany, 2013. [Google Scholar]
- Rajabzadeh, M.A.; Nazari Dehkordi, T.; Caran, Ş. Mineralogy, geochemistry and geotectonic significance of mantle peridotites with high-Cr chromitites in the Neyriz ophiolite from the outer Zagros ophiolite belts, Iran. J. African Earth Sci. 2013, 78, 1–15. [Google Scholar] [CrossRef]
- Grieco, G.; Bussolesi, M.; Tzamos, E.; Rassios, A.E.; Kapsiotis, A. Processes of primary and re-equilibration mineralization affecting chromitite ore geochemistry within the Vourinos ultramafic sequence, Vourinos ophiolite (West Macedonia, Greece). Ore Geol. Rev. 2018, 95, 537–551. [Google Scholar] [CrossRef]
- Grieco, G.; Merlini, A. Chromite alteration processes within Vourinos ophiolite. Int. J. Earth Sci. 2012, 101, 1523–1533. [Google Scholar] [CrossRef]
- Arai, S.; Yurimoto, H. Podiform chromitites of the Tari-Misaka ultramafic complex, southwestern Japan, as mantle-melt interaction products. Econ. Geol. 1994, 89, 1279–1288. [Google Scholar] [CrossRef]
- Terre e sabbie per fonderia: Campionamento e metodi di prova; Ente Nazionale di Unificazione UNI462800: Milano, Italy, 1976.
- McLaren, C.H.; De Villiers, J.P.R. The platinum-group chemistry and mineralogy of the UG-2 chromitite layer of the Bushveld Complex. Econ. Geol. 1982, 77, 1348–1366. [Google Scholar] [CrossRef]
- Mondal, S.K.; Mathez, E.A. Origin of the UG2 chromitite layer, Bushveld Complex. J. Petrol. 2007, 48, 495–510. [Google Scholar] [CrossRef]
- Leblanc, M.; Nicolas, A. Ophiolitic Chromitites. Int. Geol. Rev. 1992, 34, 653–686. [Google Scholar] [CrossRef]
- Mondal, S.K.; Griffin, W.L. Processes and Ore Deposits of Ultramafic-Mafic Magmas through Space and Time; Elsevier: Amsterdam, The Netherlands, 2017. [Google Scholar]
- Augé, T. Chromite deposits in the northern Oman ophiolite: Mineralogical constraints. Miner. Depos. 1987, 22, 1–10. [Google Scholar] [CrossRef]
- Garuti, G.; Proenza, J.A.; Zaccarini, F. Distribution and mineralogy of platinum-group elements in altered chromitites of the Campo Formoso layered intrusion (Bahia State, Brazil): Control by magmatic and hydrothermal processes. Mineral. Petrol. 2007, 89, 159–188. [Google Scholar] [CrossRef]
- Baccolo, L.P.; Grieco, G.; Bussolesi, M.; Eslami, A. Mineralogical study of rodingitized microgabbros and associated chromitite seams from the Nain ophiolite, Central Iran. In Proceedings of the EGU General Assembly, Vienna, Austria, 7–12 April 2019; Volume 21, p. 1. [Google Scholar]
- Rassios, A.; Tzamos, E.; Dilek, Y.; Bussolesi, M.; Grieco, G.; Batsi, A.; Gamaletsos, P.N. A structural approach to the genesis of chrome ores within the Vourinos ophiolite (Greece): Significance of ductile and brittle deformation processes in the formation of economic ore bodies in oceanic upper mantle peridotites. Ore Geol. Rev. 2020, 125, 103684. [Google Scholar] [CrossRef]
- Cazzaniga, M. Parametri tessiturali di qualità delle cromiti di Aetoraches e Rizo (complesso di Vourinos, Grecia); University of Milan: Milan, Italy, 2009. [Google Scholar]
- Evans, B.W. The Serpentinite Multisystem Revisited: Chrysotile Is Metastable. Int. Geol. Rev. 2004, 46, 479–506. [Google Scholar] [CrossRef]
| Sample | Locality | Sample | Major silicates |
|---|---|---|---|
| SA | South Africa | Quality Proxy sand | Orthopyroxene |
| AE | Aetoraches (GR) | Chromite sand | Olivine + Serpentine |
| NE | Neyriz (IRN) | Chromite sand | Serpentine |
| CHL | Abdasht (IRN) | Chloritized sample | Chlorite |
| SRP | Neyriz (IRN) | Serpentinized sample | Serpentine |
| OLV | Finero (IT) | Dunite sample | Olivine |
| OPX | Ivrea Verbano (IT) | Orthopyroxenite sample | Orthopyroxene |
| RI-2 | Aetoraches (GR) | Serpentinized dunite sample | Olivine + Serpentine |
| AE-2 | Rizo (GR) | Serpentinized dunite sample | Olivine + Serpentine |
| Standard Column (µm) 1 | Sieve Parameter (a) |
|---|---|
| 3150 | 3 |
| 1600 | 5 |
| 800 | 11 |
| 630 | 18 |
| 400 | 31 |
| 315 | 38 |
| 200 | 52 |
| 160 | 66 |
| 100 | 102 |
| 80 | 130 |
| 50 | 210 |
| bottom | 300 |
| Sample | Cr2O3 | SiO2 | Al2O3 | CaO | Fe2O3 | MgO | MnO | TiO2 |
|---|---|---|---|---|---|---|---|---|
| SA | 44.66 | 0.70 | 14.52 | 0.14 | 29.07 | 9.88 | 0.19 | 0.68 |
| NE | 56.27 | 2.01 | 12.08 | 0.18 | 15.68 | 13.08 | 0.20 | 0.09 |
| AE | 48.90 | 7.72 | 7.17 | 0.33 | 19.46 | 14.74 | 0.24 | 0.12 |
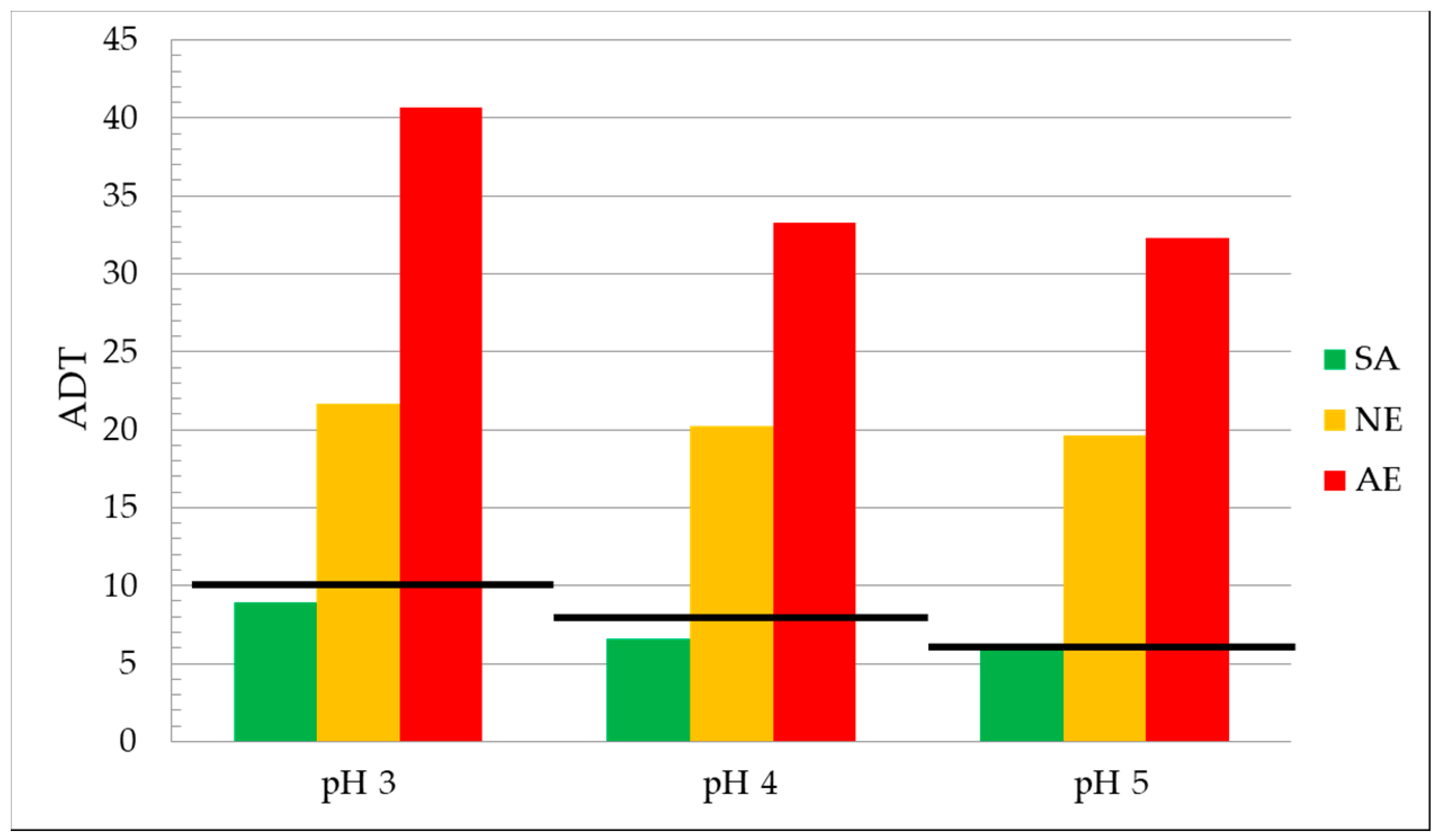
| Sample | Sand (g) | SiO2 (%) | Initial pH | ADT pH3 | ADT pH4 | ADT pH5 |
|---|---|---|---|---|---|---|
| SA | 50 | 0.70 | 1.52 | 8.9 | 6.6 | 6.0 |
| NE | 50 | 2.01 | 1.42 | 21.7 | 20.2 | 19.6 |
| AE | 50 | 7.7 | 2.62 | 40.7 | 33.3 | 32.3 |
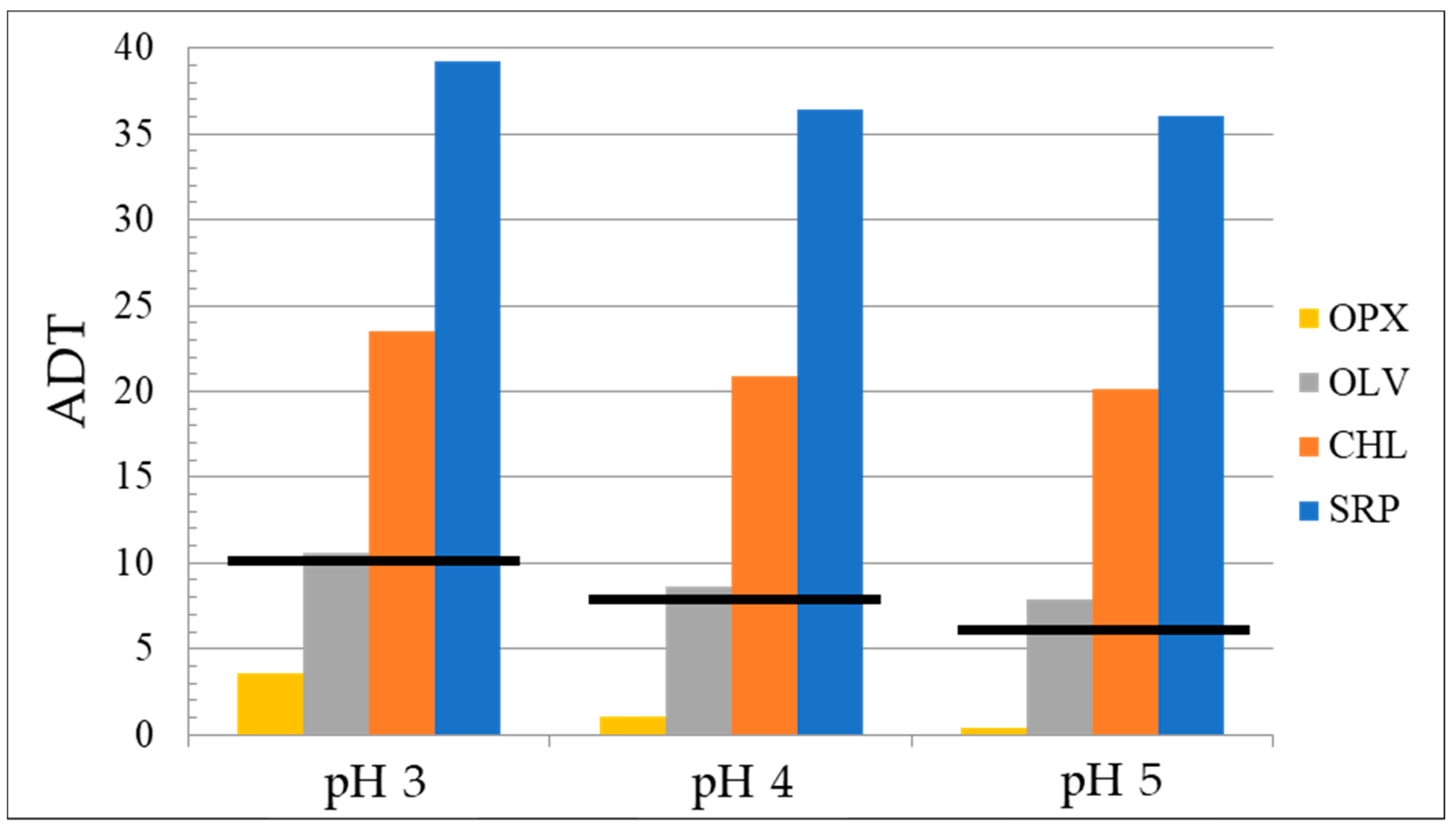
| CHL | 4.12 | 2.5 | 1.93 | 23.5 | 20.8 | 20.2 |
| OPX | 2.09 | 2.5 | 1.78 | 3.6 | 1.1 | 0.4 |
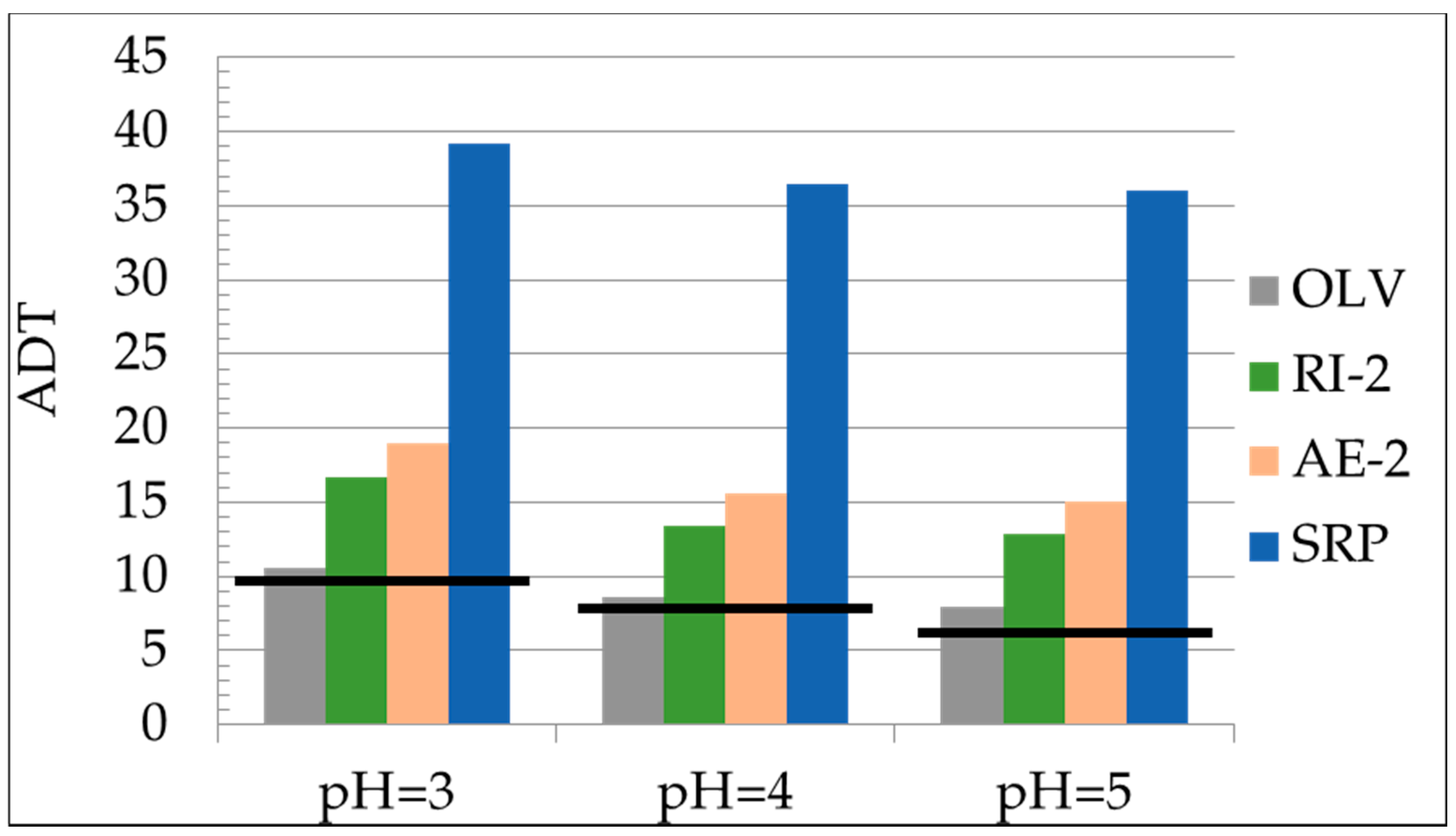
| OLV | 3.19 | 2.5 | 1.70 | 10.6 | 8.6 | 7.9 |
| SRP | 2.88 | 2.5 | 2.20 | 39.2 | 36.4 | 36.03 |
| AE-2 | 50 | 2.5 | 1.83 | 19.0 | 15.6 | 15.0 |
| RI-2 | 50 | 2.5 | 1.87 | 16.7 | 13.4 | 12.8 |
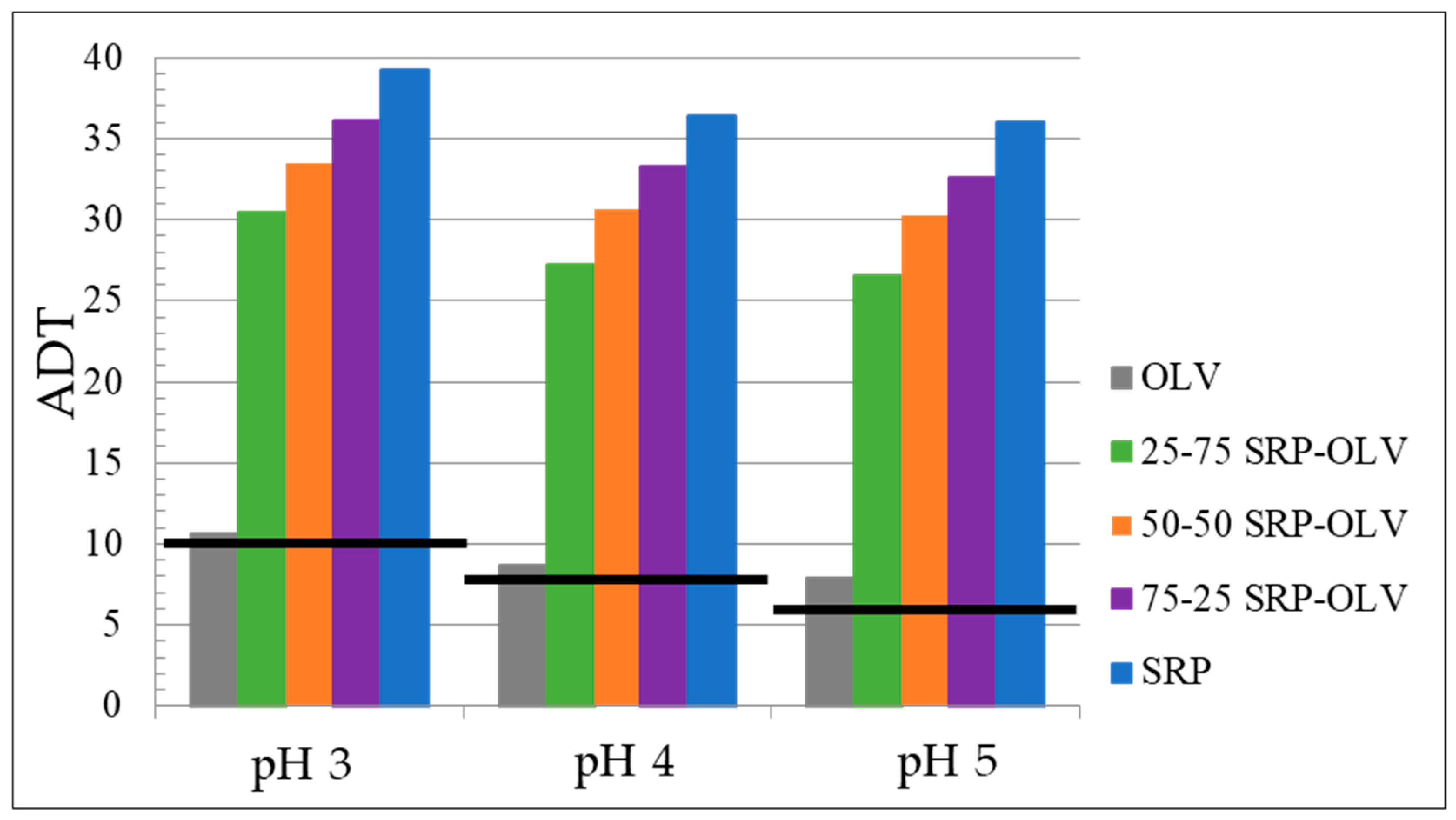
| Sample | SERP (g) | OLV (g) | SiO2% | Initial pH | ADT pH3 | ADT pH4 | ADT pH5 |
|---|---|---|---|---|---|---|---|
| 25–75 SRP-OLV | 0.76 | 2.28 | 2.50 | 2.03 | 30.5 | 27.2 | 26.6 |
| 50–50 SRP-OLV | 1.52 | 1.52 | 2.50 | 2.11 | 33.5 | 30.7 | 30.2 |
| 75–25 SRP-OLV | 2.22 | 0.74 | 2.50 | 2.15 | 36.2 | 33.3 | 32.6 |
| 25-SRP | 0.72 | 0 | 0.63 | 1.65 | 24.4 | 22.2 | 21.6 |
| 50-SRP | 1.44 | 0 | 1.25 | 1.83 | 32.1 | 29.7 | 29.0 |
| 75-SRP | 2.16 | 0 | 1.88 | 1.88 | 36.9 | 34.7 | 34.3 |
| 25-OLV | 0 | 0.80 | 0.63 | 1.45 | 5.9 | 4.3 | 4.0 |
| 50-OLV | 0 | 1.60 | 1.25 | 1.48 | 7.8 | 6.1 | 5.8 |
| 75-OLV | 0 | 2.40 | 1.88 | 1.46 | 8.7 | 6.9 | 6.4 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bussolesi, M.; Grieco, G.; Eslami, A.; Cavallo, A. Ophiolite Chromite Deposits as a New Source for the Production of Refractory Chromite Sands. Sustainability 2020, 12, 7096. https://doi.org/10.3390/su12177096
Bussolesi M, Grieco G, Eslami A, Cavallo A. Ophiolite Chromite Deposits as a New Source for the Production of Refractory Chromite Sands. Sustainability. 2020; 12(17):7096. https://doi.org/10.3390/su12177096
Chicago/Turabian StyleBussolesi, Micol, Giovanni Grieco, Alireza Eslami, and Alessandro Cavallo. 2020. "Ophiolite Chromite Deposits as a New Source for the Production of Refractory Chromite Sands" Sustainability 12, no. 17: 7096. https://doi.org/10.3390/su12177096
APA StyleBussolesi, M., Grieco, G., Eslami, A., & Cavallo, A. (2020). Ophiolite Chromite Deposits as a New Source for the Production of Refractory Chromite Sands. Sustainability, 12(17), 7096. https://doi.org/10.3390/su12177096








