Steady-State Investigation of Carbon-Based Adsorbent–Adsorbate Pairs for Heat Transformation Application
Abstract
1. Introduction
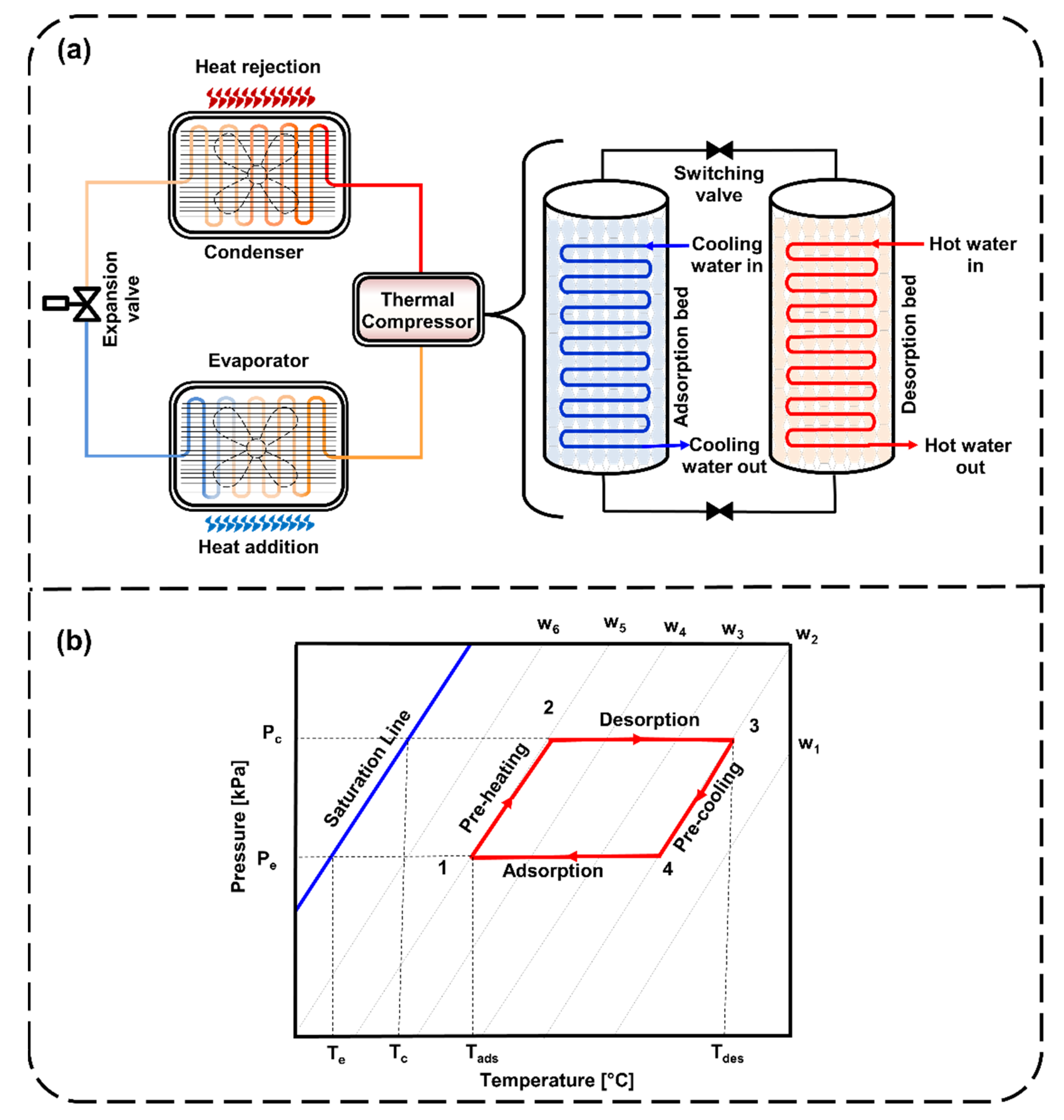
2. Materials and Methods
2.1. Adsorbent–Adsorbate Pairs
2.2. Adsorption Equilibrium Models
2.3. Steady-State Model for SCE and COP
2.4. Isosteric Heat of Adsorption
3. Results and Discussion
4. Conclusions
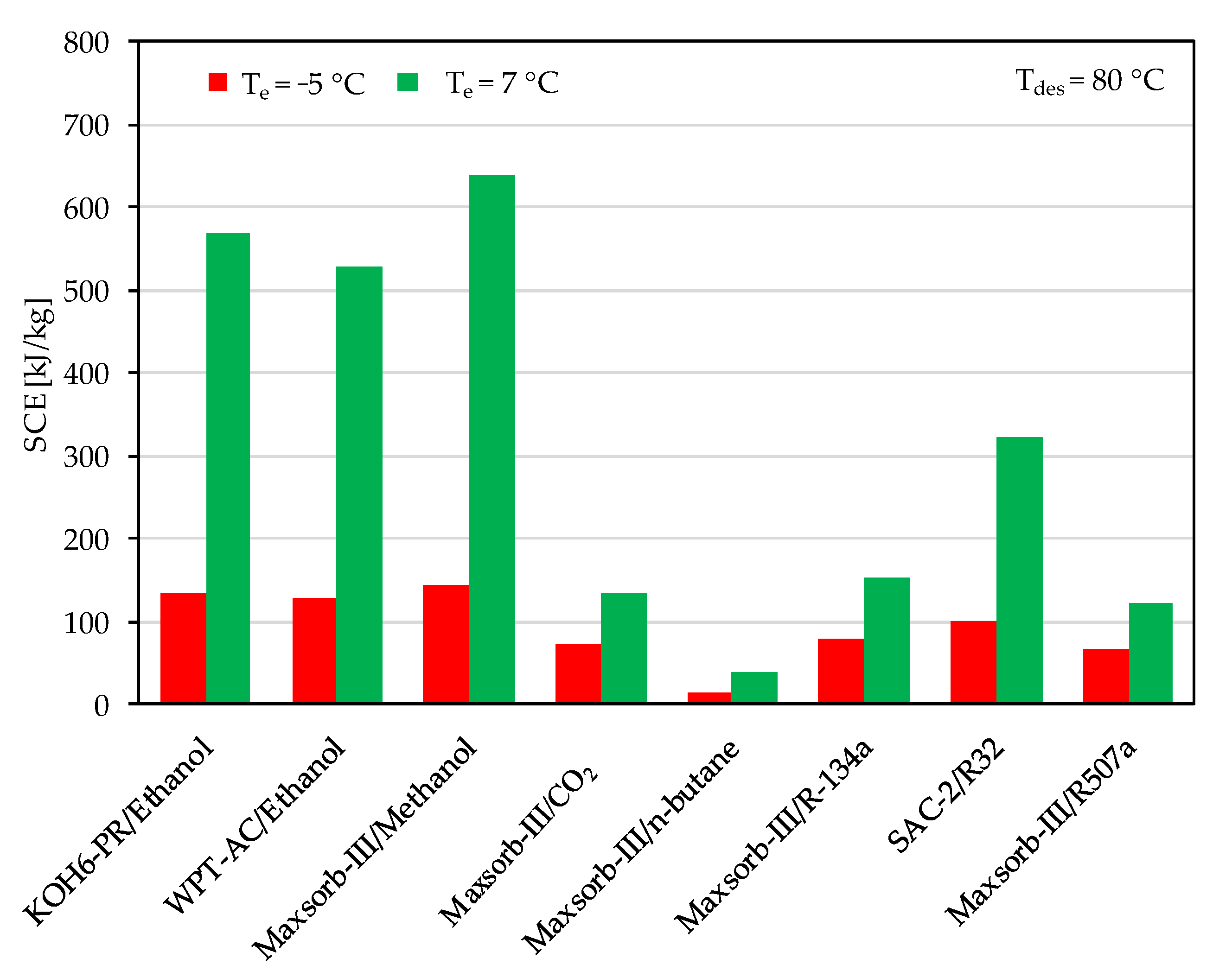
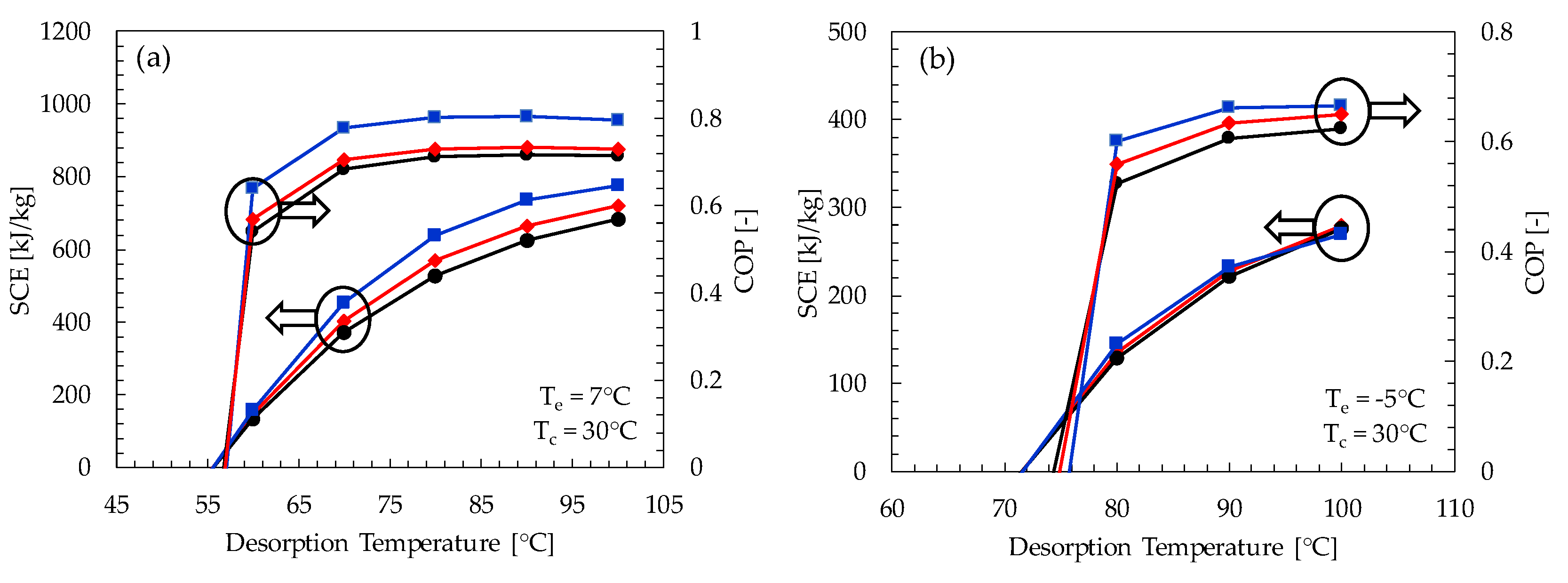
- The Maxsorb-III/methanol pair shows the highest SCE and COP for both cooling and refrigeration cycles at all ranges of desorption temperatures. At a desorption temperature of 80 °C, it has a maximum SCE and COP of 639.83 kJ/kg and 0.803, respectively, for the adsorption cooling cycle. Similarly, for the refrigeration cycle, it shows a SCE and COP of 639.83 kJ/kg and 0.803, respectively.
- The KOH6-PR/ethanol pair also has a good performance, with a SCE and COP of 144.63 kJ/kg and 0.601, respectively, under the same operating conditions.
- The performance-wise sequence of the selected pairs is as follows: Maxsorb-III/methanol > KOH6-PR/ethanol > WPT-AC/ethanol > SAC-2/R32 > Maxsorb-III/R-134a > Maxsorb-III/CO2 > Maxsorb-III/R507a > Maxsorb-III/n-butane.
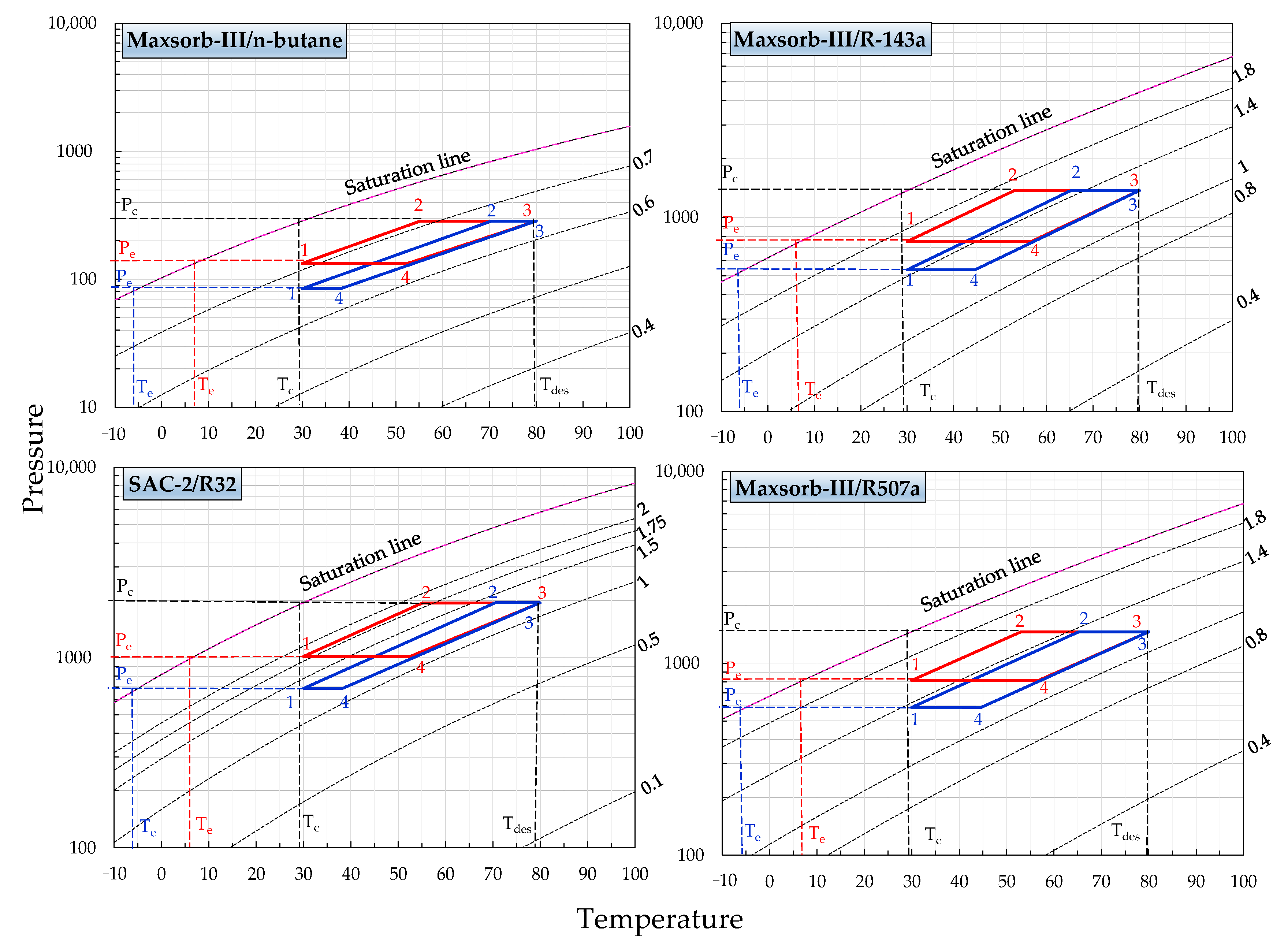
- It is found that the minimum desorption temperature required for the adsorption cooling cycle is 55 °C at the condenser and adsorption temperature of 30 °C. For the adsorption cooling cycle, the minimum desorption temperature required is about 70 °C.
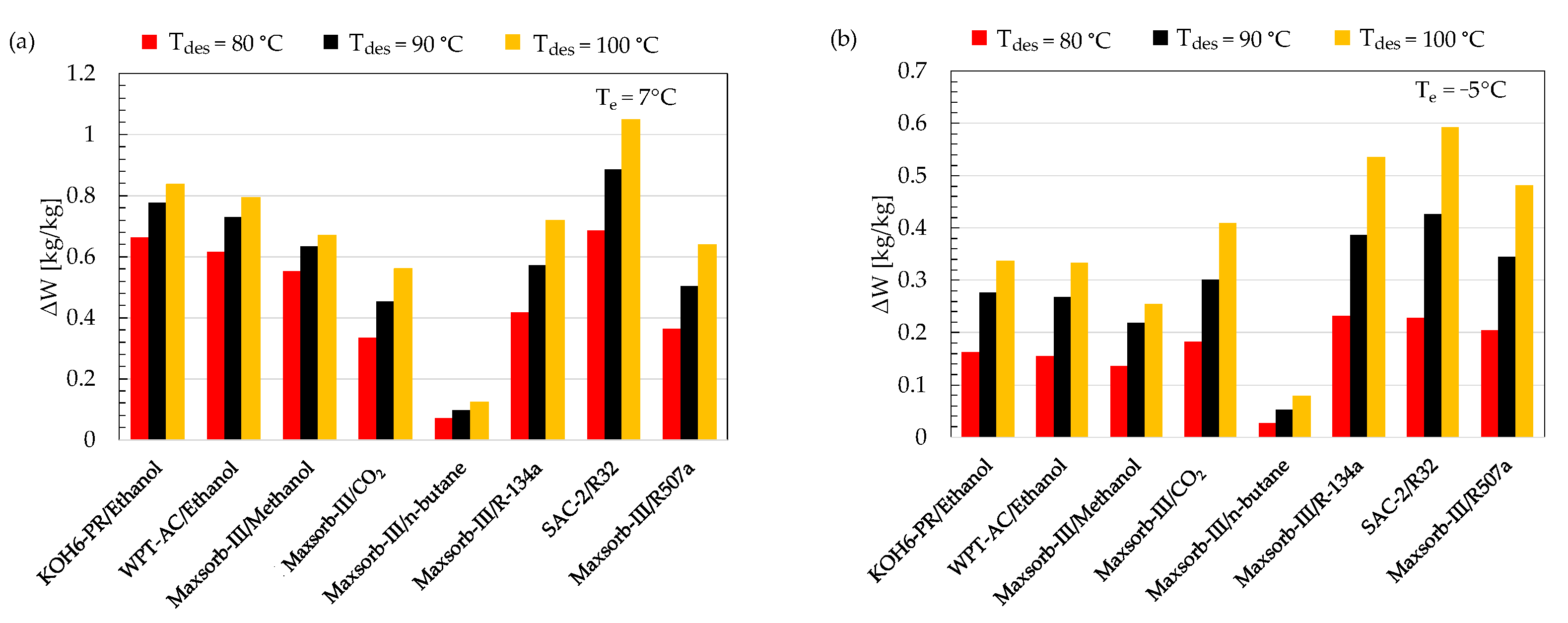
- The SCE shows a linear relation with the desorption temperature. This is due to the increase in concentration difference of adsorption pairs with an increasing desorption temperature.
- There is a significant increase in the coefficient of performance for the desorption temperature range of 60 to 80 °C. Afterwards, no considerable variation in COP is noticeable with an increasing SCE.
- Although the SAC-2/R32 pair has the highest ΔW, it shows a lower performance than Maxsorb-III/methanol, KOH6-PR/ethanol and WPT-AC/ethanol pairs due to a lower Qst value compared to these three pairs.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Nomenclature
| A | Adsorption potential (kJ/kg) |
| AC | Activated carbon |
| ACF | Activated carbon fiber |
| COP | Coefficient of performance (-) |
| Cp | Specific heat capacity (kJ/(kg.K)) |
| D-A | Dubinin–Astakhov |
| D-R | Dubinin–Radushkevich |
| E | Characteristic energy (kJ/mol) |
| k | D-A model constant for pseudo-saturated vapor pressure (-) |
| LHTe | Vaporization enthalpy at evaporator temperature (kJ/kg) |
| n | Structural heterogeneity parameter (-) |
| P | Pressure (kPa) |
| P-T-W | Pressure–temperature–concentration |
| q | Volumetric adsorption amount (cm3/g) |
| qo | Maximum volumetric adsorption capacity (cm3/g) |
| Q | Heat added (kJ/kg) |
| R | Gas constant (kJ/(kg.K)) |
| SAC | Spherical activated carbons |
| SCE | Specific cooling effect (kJ/kg) |
| T | Temperature (°C) |
| Vm | Molar volume (cm3/mol) |
| Vt | Molar volume at triple point temperature (cm3/mol) |
| W | Equilibrium adsorption uptake (kg/kg) |
| Wo | Maximum adsorption capacity (kg/kg) |
| ΔW | Concentration difference (kg/kg) |
| Thermal expansion coefficient (K−1) |
Subscripts
| ads | Adsorption |
| c | Condenser |
| cr | Critical |
| des | Desorption |
| e | Evaporator |
| max | Maximum |
| min | Minimum |
| ref | Refrigerant |
| s | Saturated |
| st | Isosteric |
| t | Triple point |
Appendix A
| Adsorbent–Refrigerant Pairs | Desorption Temperatures (°C) | |||||
|---|---|---|---|---|---|---|
| 60 | 70 | 80 | 90 | 100 | ||
| SCE | KOH6-PR/ethanol | 147.63 | 404.84 | 569.57 | 666.33 | 719.28 |
| WPT-AC/ethanol | 134.78 | 372.12 | 529.00 | 625.80 | 682.30 | |
| Maxsorb-III/methanol | 157.57 | 452.55 | 639.83 | 735.79 | 776.28 | |
| Maxsorb-III/CO2 | 34.60 | 85.48 | 135.58 | 183.35 | 227.76 | |
| Maxsorb-III/n-butane | 7.75 | 23.72 | 39.17 | 54.08 | 68.45 | |
| Maxsorb-III/R-134a | 38.13 | 95.06 | 152.52 | 209.06 | 263.59 | |
| SAC-2/R32 | 73.47 | 209.08 | 321.88 | 415.63 | 493.50 | |
| Maxsorb-III/R507a | 30.12 | 76.07 | 123.46 | 170.98 | 217.57 | |
| COP | KOH6-PR/ethanol | 0.569 | 0.705 | 0.730 | 0.734 | 0.730 |
| WPT-AC/ethanol | 0.541 | 0.684 | 0.712 | 0.717 | 0.714 | |
| Maxsorb-III/methanol | 0.639 | 0.779 | 0.803 | 0.804 | 0.797 | |
| Maxsorb-III/CO2 | 0.336 | 0.452 | 0.497 | 0.519 | 0.533 | |
| Maxsorb-III/n-butane | 0.104 | 0.204 | 0.249 | 0.275 | 0.291 | |
| Maxsorb-III/R-134a | 0.311 | 0.474 | 0.550 | 0.594 | 0.623 | |
| SAC-2/R32 | 0.341 | 0.532 | 0.592 | 0.619 | 0.632 | |
| Maxsorb-III/R507a | 0.273 | 0.436 | 0.519 | 0.570 | 0.604 | |
| Performance Index | Adsorbent–Refrigerant Pairs | Desorption Temperatures (°C) | ||
|---|---|---|---|---|
| 80 | 90 | 100 | ||
| SCE | KOH6-PR/ethanol | 134.83 | 228.13 | 279.19 |
| WPT-AC/ethanol | 128.76 | 222.10 | 276.58 | |
| Maxsorb-III/methanol | 144.63 | 232.52 | 269.60 | |
| Maxsorb-III/CO2 | 73.98 | 121.46 | 165.59 | |
| Maxsorb-III/n-butane | 13.70 | 27.58 | 40.96 | |
| Maxsorb-III/R-134a | 80.50 | 134.14 | 185.88 | |
| SAC-2/R32 | 100.81 | 189.37 | 262.94 | |
| Maxsorb-III/R507a | 65.86 | 110.80 | 154.87 | |
| COP | KOH6-PR/ethanol | 0.559 | 0.634 | 0.649 |
| WPT-AC/ethanol | 0.524 | 0.605 | 0.623 | |
| Maxsorb-III/methanol | 0.601 | 0.661 | 0.665 | |
| Maxsorb-III/CO2 | 0.399 | 0.460 | 0.491 | |
| Maxsorb-III/n-butane | 0.112 | 0.172 | 0.206 | |
| Maxsorb-III/R-134a | 0.385 | 0.477 | 0.531 | |
| SAC-2/R32 | 0.348 | 0.462 | 0.513 | |
| Maxsorb-III/R507a | 0.352 | 0.448 | 0.506 | |
References
- Nicol, J.F.; Humphreys, M.A. Adaptive thermal comfort and sustainable thermal standards for buildings. Energy Build. 2002, 34, 563–572. [Google Scholar] [CrossRef]
- Noor, S.; Ashraf, H.; Sultan, M.; Khan, Z.M. Evaporative cooling options for building air-conditioning: A comprehensive study for climatic conditions of Multan (Pakistan). Energies 2020, 13, 3061. [Google Scholar] [CrossRef]
- Omer, A.M. Energy, environment and sustainable development. Renew. Sustain. Energy Rev. 2008, 12, 2265–2300. [Google Scholar] [CrossRef]
- Choudhury, B.; Chatterjee, P.K.; Sarkar, J.P. Review paper on solar-powered air-conditioning through adsorption route. Renew. Sustain. Energy Rev. 2010, 14, 2189–2195. [Google Scholar] [CrossRef]
- Hamdy, M.; Askalany, A.A.; Harby, K.; Kora, N. An overview on adsorption cooling systems powered by waste heat from internal combustion engine. Renew. Sustain. Energy Rev. 2015, 51, 1223–1234. [Google Scholar] [CrossRef]
- El-Sharkawy, I.I.; AbdelMeguid, H.; Saha, B.B. Potential application of solar powered adsorption cooling systems in the Middle East. Appl. Energy 2014, 126, 235–245. [Google Scholar] [CrossRef]
- Hassan, H.Z.; Mohamad, A.A. A review on solar cold production through absorption technology. Renew. Sustain. Energy Rev. 2012, 16, 5331–5348. [Google Scholar] [CrossRef]
- Kalkan, N.; Young, E.A.; Celiktas, A. Solar thermal air conditioning technology reducing the footprint of solar thermal air conditioning. Renew. Sustain. Energy Rev. 2012, 16, 6352–6383. [Google Scholar] [CrossRef]
- Zhai, X.Q.; Qu, M.; Li, Y.; Wang, R.Z. A review for research and new design options of solar absorption cooling systems. Renew. Sustain. Energy Rev. 2011, 15, 4416–4423. [Google Scholar] [CrossRef]
- Sultan, M.; El-Sharkawy, I.I.; Miyazaki, T.; Saha, B.B.; Koyama, S. An overview of solid desiccant dehumidification and air conditioning systems. Renew. Sustain. Energy Rev. 2015, 46, 16–29. [Google Scholar] [CrossRef]
- Jani, D.B.; Mishra, M.; Sahoo, P.K. Solid desiccant air conditioning—A state of the art review. Renew. Sustain. Energy Rev. 2016, 60, 1451–1469. [Google Scholar] [CrossRef]
- Ge, T.S.; Dai, Y.J.; Wang, R.Z. Review on solar powered rotary desiccant wheel cooling system. Renew. Sustain. Energy Rev. 2014, 39, 476–497. [Google Scholar] [CrossRef]
- Zhai, X.Q.; Wang, R.Z. Experimental investigation and theoretical analysis of the solar adsorption cooling system in a green building. Appl. Therm. Eng. 2009, 29, 17–27. [Google Scholar] [CrossRef]
- Sultan, M.; Miyazaki, T.; Saha, B.B.; Koyama, S. Steady-state investigation of water vapor adsorption for thermally driven adsorption based greenhouse air-conditioning system. Renew. Energy 2016, 86, 785–795. [Google Scholar] [CrossRef]
- Jribi, S.; Miyazaki, T.; Saha, B.B.; Koyama, S.; Maeda, S.; Maruyama, T. CFD simulation and experimental validation of ethanol adsorption onto activated carbon packed heat exchanger. Int. J. Refrig. 2017, 74, 345–353. [Google Scholar] [CrossRef]
- Sosnowski, M. Evaluation of heat transfer performance of a multi-disc sorption bed dedicated for adsorption cooling technology. Energies 2019, 12, 4660. [Google Scholar] [CrossRef]
- Saha, B.B.; Koyama, S.; Kashiwagi, T.; Akisawa, A.; Ng, K.C.; Chua, H.T. Waste heat driven dual-mode, multi-stage, multi-bed regenerative adsorption system. Int. J. Refrig. 2003, 26, 749–757. [Google Scholar] [CrossRef]
- Miyazaki, T.; Akisawa, A.; Saha, B.B. The performance analysis of a novel dual evaporator type three-bed adsorption chiller. Int. J. Refrig. 2010, 33, 276–285. [Google Scholar] [CrossRef]
- Chorowski, M.; Pyrka, P.; Rogala, Z.; Czupryński, P. Experimental study of performance improvement of 3-Bed and 2-Evaporator adsorption chiller by control optimization. Energies 2019, 12, 3943. [Google Scholar] [CrossRef]
- Alahmer, A.; Wang, X.; Alam, K.C.A. Dynamic and economic investigation of a solar thermal-driven two-bed adsorption chiller under Perth Climatic Conditions. Energies 2020, 13, 1005. [Google Scholar] [CrossRef]
- Askalany, A.A.; Saha, B.B.; Kariya, K.; Ismail, I.M.; Salem, M.; Ali, A.H.H.; Morsy, M.G. Hybrid adsorption cooling systems—An overview. Renew. Sustain. Energy Rev. 2012, 16, 5787–5801. [Google Scholar] [CrossRef]
- Vasta, S.; Palomba, V.; La Rosa, D.; Mittelbach, W. Adsorption-compression cascade cycles: An experimental study. Energy Convers. Manag. 2018, 156, 365–375. [Google Scholar] [CrossRef]
- Lychnos, G.; Tamainot-Telto, Z. Prototype of hybrid refrigeration system using refrigerant R723. Appl. Therm. Eng. 2018, 134, 95–106. [Google Scholar] [CrossRef]
- Roumpedakis, T.C.; Vasta, S.; Sapienza, A.; Kallis, G.; Karellas, S.; Wittstadt, U.; Tanne, M.; Harborth, N.; Sonnenfeld, U. Performance results of a solar adsorption cooling and heating unit. Energies 2020, 13, 1630. [Google Scholar] [CrossRef]
- Saha, B.B.; Akisawa, A.; Kashiwagi, T. Solar/waste heat driven two-stage adsorption chiller: The prototype. Renew. Energy 2001, 23, 93–101. [Google Scholar] [CrossRef]
- Liu, Y.L.; Wang, R.Z.; Xia, Z.Z. Experimental performance of a silica gel–water adsorption chiller. Appl. Therm. Eng. 2005, 25, 359–375. [Google Scholar] [CrossRef]
- Saha, B.B.; El-Sharkawy, I.I.; Thorpe, R.; Critoph, R.E. Accurate adsorption isotherms of R134a onto activated carbons for cooling and freezing applications. Int. J. Refrig. 2012, 35, 499–505. [Google Scholar] [CrossRef]
- Habib, K.; Saha, B.B.; Rahman, K.A.; Chakraborty, A.; Koyama, S.; Ng, K.C. Experimental study on adsorption kinetics of activated carbon/R134a and activated carbon/R507A pairs. Int. J. Refrig. 2010, 33, 706–713. [Google Scholar] [CrossRef]
- El-Sharkawy, I.I.; Saha, B.B.; Koyama, S.; He, J.; Ng, K.C.; Yap, C. Experimental investigation on activated carbon-ethanol pair for solar powered adsorption cooling applications. Int. J. Refrig. 2008, 31, 1407–1413. [Google Scholar] [CrossRef]
- Qasem, N.A.A.; El-Shaarawi, M.A.I. Improving ice productivity and performance for an activated carbon/methanol solar adsorption ice-maker. Sol. Energy 2013, 98, 523–542. [Google Scholar] [CrossRef]
- Rafique, M.M. Evaluation of metal–organic frameworks as potential adsorbents for solar cooling applications. Appl. Syst. Innov. 2020, 3, 26. [Google Scholar] [CrossRef]
- Sultan, M.; El-Sharkawy, I.I.; Miyazaki, T.; Saha, B.B.; Koyama, S.; Maruyama, T.; Maeda, S.; Nakamura, T. Insights of water vapor sorption onto polymer based sorbents. Adsorption 2015, 21, 205–215. [Google Scholar] [CrossRef]
- Sultan, M.; El-Sharkawy, I.I.; Miyazaki, T.; Saha, B.B.; Koyama, S.; Maruyama, T.; Maeda, S.; Nakamura, T. Water vapor sorption kinetics of polymer based sorbents: Theory and experiments. Appl. Therm. Eng. 2016, 106, 192–202. [Google Scholar] [CrossRef]
- Zhao, H.; Jia, S.; Cheng, J.; Tang, X.; Zhang, M.; Yan, H.; Ai, W. Experimental Investigations of Composite Adsorbent 13X/CaCl2 on an Adsorption Cooling System. Appl. Sci. 2017, 7, 620. [Google Scholar] [CrossRef]
- Alghoul, M.A.; Sulaiman, M.Y.; Azmi, B.Z.; Wahab, M. Abd. Advances on multi-purpose solar adsorption systems for domestic refrigeration and water heating. Appl. Therm. Eng. 2007, 27, 813–822. [Google Scholar] [CrossRef]
- Hastürk, E.; Ernst, S.-J.; Janiak, C. Recent advances in adsorption heat transformation focusing on the development of adsorbent materials. Curr. Opin. Chem. Eng. 2019, 24, 26–36. [Google Scholar] [CrossRef]
- Edin Hamrahi, S.; Goudarzi, K.; Yaghoubi, M. Experimental study of the performance of a continues solar adsorption chiller using Nano-activated carbon/methanol as working pair. Sol. Energy 2018, 173, 920–927. [Google Scholar] [CrossRef]
- Ghazy, M.; Askalany, A.A.; Saha, B.B. Maxsorb III/HFC404A as an adsorption pair for renewable energy driven systems. Int. J. Refrig. 2020. [Google Scholar] [CrossRef]
- Manila, M.R.; Mitra, S.; Dutta, P. Studies on dynamics of two-stage air cooled water/silica gel adsorption system. Appl. Therm. Eng. 2020, 178, 115552. [Google Scholar] [CrossRef]
- Pan, Q.W.; Wang, R.Z. Study on operation strategy of a silica gel-water adsorption chiller in solar cooling application. Sol. Energy 2018, 172, 24–31. [Google Scholar] [CrossRef]
- Solovyeva, M.V.; Gordeeva, L.G.; Krieger, T.A.; Aristov, Y.I. MOF-801 as a promising material for adsorption cooling: Equilibrium and dynamics of water adsorption. Energy Convers. Manag. 2018, 174, 356–363. [Google Scholar] [CrossRef]
- Ntep, T.J.M.M.; Reinsch, H.; Hügenell, P.P.C.; Ernst, S.-J.; Hastürk, E.; Janiak, C. Designing a new aluminium muconate metal–organic framework (MIL-53-muc) as a methanol adsorbent for sub-zero temperature heat transformation applications. J. Mater. Chem. A 2019, 7, 24973–24981. [Google Scholar] [CrossRef]
- Pal, A.; Uddin, K.; Thu, K.; Saha, B.B. Activated carbon and graphene nanoplatelets based novel composite for performance enhancement of adsorption cooling cycle. Energy Convers. Manag. 2019, 180, 134–148. [Google Scholar] [CrossRef]
- Younes, M.M.; El-Sharkawy, I.I.; Kabeel, A.E.; Uddin, K.; Miyazaki, T.; Saha, B.B. Characterization of silica gel-based composites for adsorption cooling applications. Int. J. Refrig. 2020, 118, 345–353. [Google Scholar] [CrossRef]
- Shabir, F.; Sultan, M.; Miyazaki, T.; Saha, B.B.; Askalany, A.; Ali, I.; Zhou, Y.; Ahmad, R.; Shamshiri, R.R. Recent updates on the adsorption capacities of adsorbent-adsorbate pairs for heat transformation applications. Renew. Sustain. Energy Rev. 2020, 119. [Google Scholar] [CrossRef]
- El-Sharkawy, I.I.; Uddin, K.; Miyazaki, T.; Baran Saha, B.; Koyama, S.; Kil, H.S.; Yoon, S.H.; Miyawaki, J. Adsorption of ethanol onto phenol resin based adsorbents for developing next generation cooling systems. Int. J. Heat Mass Transf. 2015, 81, 171–178. [Google Scholar] [CrossRef]
- Pal, A.; Thu, K.; Mitra, S.; El-Sharkawy, I.I.; Saha, B.B.; Kil, H.S.; Yoon, S.H.; Miyawaki, J. Study on biomass derived activated carbons for adsorptive heat pump application. Int. J. Heat Mass Transf. 2017, 110, 7–19. [Google Scholar] [CrossRef]
- El-Sharkawy, I.I.; Hassan, M.; Saha, B.B.; Koyama, S.; Nasr, M.M. Study on adsorption of methanol onto carbon based adsorbents. Int. J. Refrig. 2009, 32, 1579–1586. [Google Scholar] [CrossRef]
- Jribi, S.; Miyazaki, T.; Saha, B.B.; Pal, A.; Younes, M.M.; Koyama, S.; Maalej, A. Equilibrium and kinetics of CO2 adsorption onto activated carbon. Int. J. Heat Mass Transf. 2017, 108, 1941–1946. [Google Scholar] [CrossRef]
- Saha, B.B.; Chakraborty, A.; Koyama, S.; Yoon, S.H.; Mochida, I.; Kumja, M.; Yap, C.; Ng, K.C. Isotherms and thermodynamics for the adsorption of n-butane on pitch based activated carbon. Int. J. Heat Mass Transf. 2008, 51, 1582–1589. [Google Scholar] [CrossRef]
- Loh, W.S.; Ismail, A.B.; Xi, B.; Ng, K.C.; Chun, W.G. Adsorption isotherms and isosteric enthalpy of adsorption for assorted refrigerants on activated carbons. J. Chem. Eng. Data 2012, 57, 2766–2773. [Google Scholar] [CrossRef]
- Sultan, M.; Miyazaki, T.; Saha, B.B.; Koyama, S.; Kil, H.-S.; Nakabayashi, K.; Miyawaki, J.; Yoon, S.-H. Adsorption of Difluoromethane (HFC-32) onto phenol resin based adsorbent: Theory and experiments. Int. J. Heat Mass Transf. 2018, 127, 348–356. [Google Scholar] [CrossRef]
- Dubinin, M.M. Adsorption in micropores. J. Colloid Interface Sci. 1967, 23, 487–499. [Google Scholar] [CrossRef]
- Dubinin, M.M.; Astakhov, V.A. Development of the concepts of volume filling of micropores in the adsorption of gases and vapors by microporous adsorbents. Russ. Chem Bull. 1971, 20, 8–12. [Google Scholar] [CrossRef]
- Dubinin, M.M.; Radushkevich, L.V. Evaluation of microporous materials with a new isotherm. Dokl. Akad. Nauk. SSSR 1947, 55, 331–334. [Google Scholar]
- Amankwah, K.A.G.; Schwarz, J.A. A modified approach for estimating pseudo-vapor pressures in the application of the Dubinin-Astakhov equation. Carbon 1995, 33, 1313–1319. [Google Scholar] [CrossRef]
- Uddin, K.; Islam, M.A.; Mitra, S.; Lee, J.; Thu, K.; Saha, B.B.; Koyama, S. Specific heat capacities of carbon-based adsorbents for adsorption heat pump application. Appl. Therm. Eng. 2018, 129, 117–126. [Google Scholar] [CrossRef]
- Islam, M.A.; Rocky, K.A.; Pal, A.; Thu, K.; Saha, B.B. Specific heat capacity measurement of mangrove and waste palm trunk in raw, carbonized and activated form. In Proceedings of the IMPRES 2019, Kanazawa, Japan, 20–23 October 2019. [Google Scholar]
- Askalany, A.A.; Saha, B.B. Towards an accurate estimation of the isosteric heat of adsorption—A correlation with the potential theory. J. Colloid Interface Sci. 2017, 490, 59–63. [Google Scholar] [CrossRef]



| Adsorbent–Refrigerant Pairs | Wo/qo | E (kJ/kg) | n (-) | k (-) | References |
|---|---|---|---|---|---|
| KOH-6-PR/ethanol | 1.98 (kg/kg) | 90 | 1.5 | - | [46] |
| WPT-AC/ethanol | 1.9 (kg/kg) | 91.55 | 1.43 | - | [47] |
| Maxsorb-III/methanol | 1.24 (kg/kg) | 129.28 | 2 | - | [48] |
| Maxsorb-III/CO2 | 1.5408 (cm3/g) | 119.39 | 1.326 | 4.504 | [49] |
| Maxsorb-III/n-butane | 0.8 (kg/kg) | 300 | 1.02 | - | [50] |
| Maxsorb-III/134a | 2.2 (kg/kg) | 87.25 | 1.29 | - | [51] |
| SAC-2/R32 | 3.1344 (cm3/g) | 67.24 | 1.0217 | 3.65 | [52] |
| Maxsorb-III/507a | 2.05 (kg/kg) | 76.34 | 1.34 | - | [51] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shabir, F.; Sultan, M.; Niaz, Y.; Usman, M.; Ibrahim, S.M.; Feng, Y.; Naik, B.K.; Nasir, A.; Ali, I. Steady-State Investigation of Carbon-Based Adsorbent–Adsorbate Pairs for Heat Transformation Application. Sustainability 2020, 12, 7040. https://doi.org/10.3390/su12177040
Shabir F, Sultan M, Niaz Y, Usman M, Ibrahim SM, Feng Y, Naik BK, Nasir A, Ali I. Steady-State Investigation of Carbon-Based Adsorbent–Adsorbate Pairs for Heat Transformation Application. Sustainability. 2020; 12(17):7040. https://doi.org/10.3390/su12177040
Chicago/Turabian StyleShabir, Faizan, Muhammad Sultan, Yasir Niaz, Muhammad Usman, Sobhy M. Ibrahim, Yongqiang Feng, Bukke Kiran Naik, Abdul Nasir, and Imran Ali. 2020. "Steady-State Investigation of Carbon-Based Adsorbent–Adsorbate Pairs for Heat Transformation Application" Sustainability 12, no. 17: 7040. https://doi.org/10.3390/su12177040
APA StyleShabir, F., Sultan, M., Niaz, Y., Usman, M., Ibrahim, S. M., Feng, Y., Naik, B. K., Nasir, A., & Ali, I. (2020). Steady-State Investigation of Carbon-Based Adsorbent–Adsorbate Pairs for Heat Transformation Application. Sustainability, 12(17), 7040. https://doi.org/10.3390/su12177040









