Efficacy of Enzymatically Induced Calcium Carbonate Precipitation in the Retention of Heavy Metal Ions
Abstract
1. Introduction
2. Background
3. Materials and Methods
3.1. Soil
3.2. Heavy Metal Contaminants
3.3. Enzyme Solutions
- Urea (CH4N2O)
- Calcium chloride (CaCl2·2H2O)
- Urease enzyme (Canavalia ensiformis (jack bean) Type III, powder, 15,000–50,000 units/g solid)
- Non-fat milk powder
3.4. EICP Treatments
3.4.1. UCS Tests
3.4.2. Measurement of Calcium Carbonate Precipitation
3.5. Extractants
3.6. Desorption Tests
4. Results and Discussions
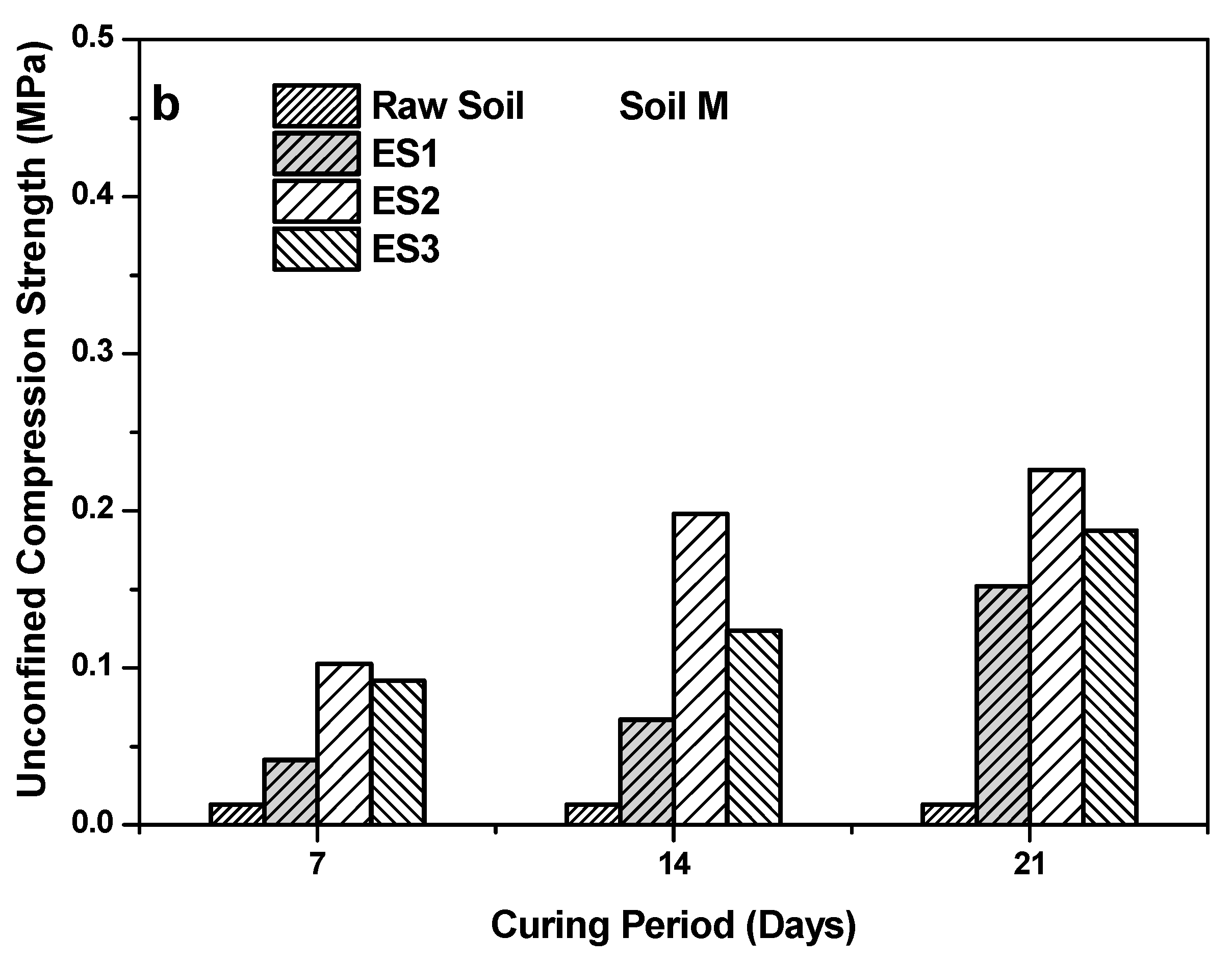
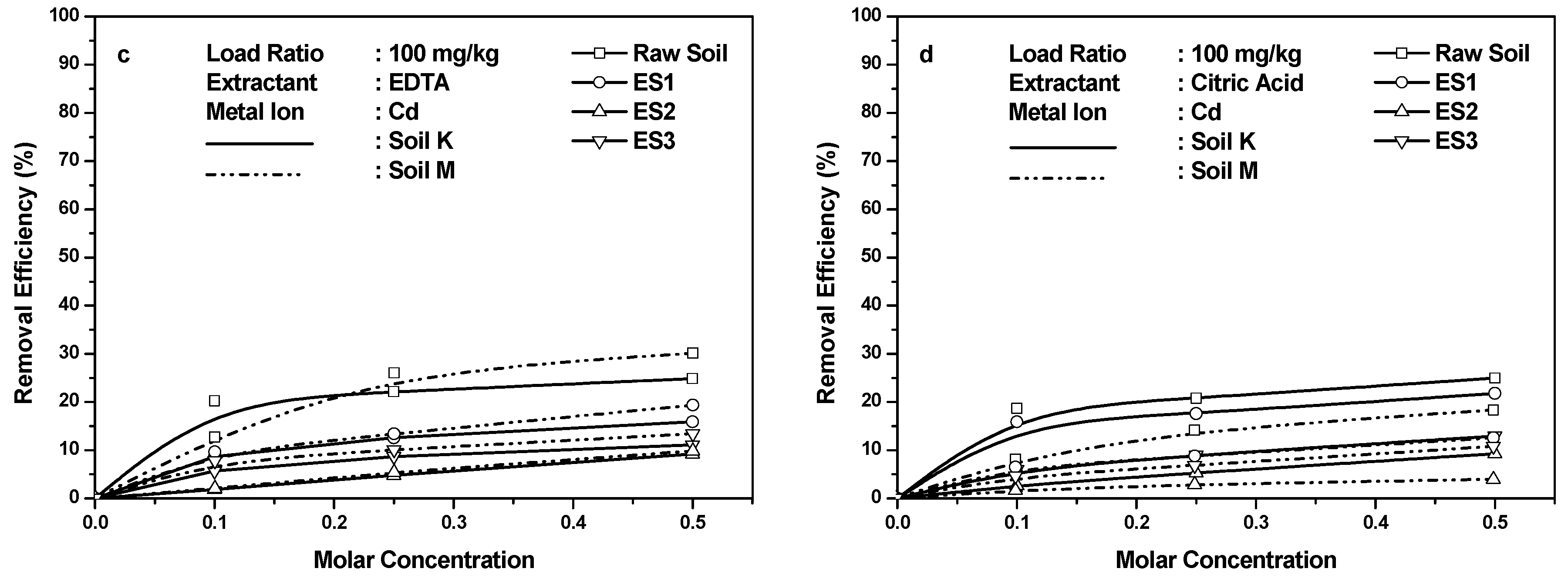
4.1. Cd Retention in Soils
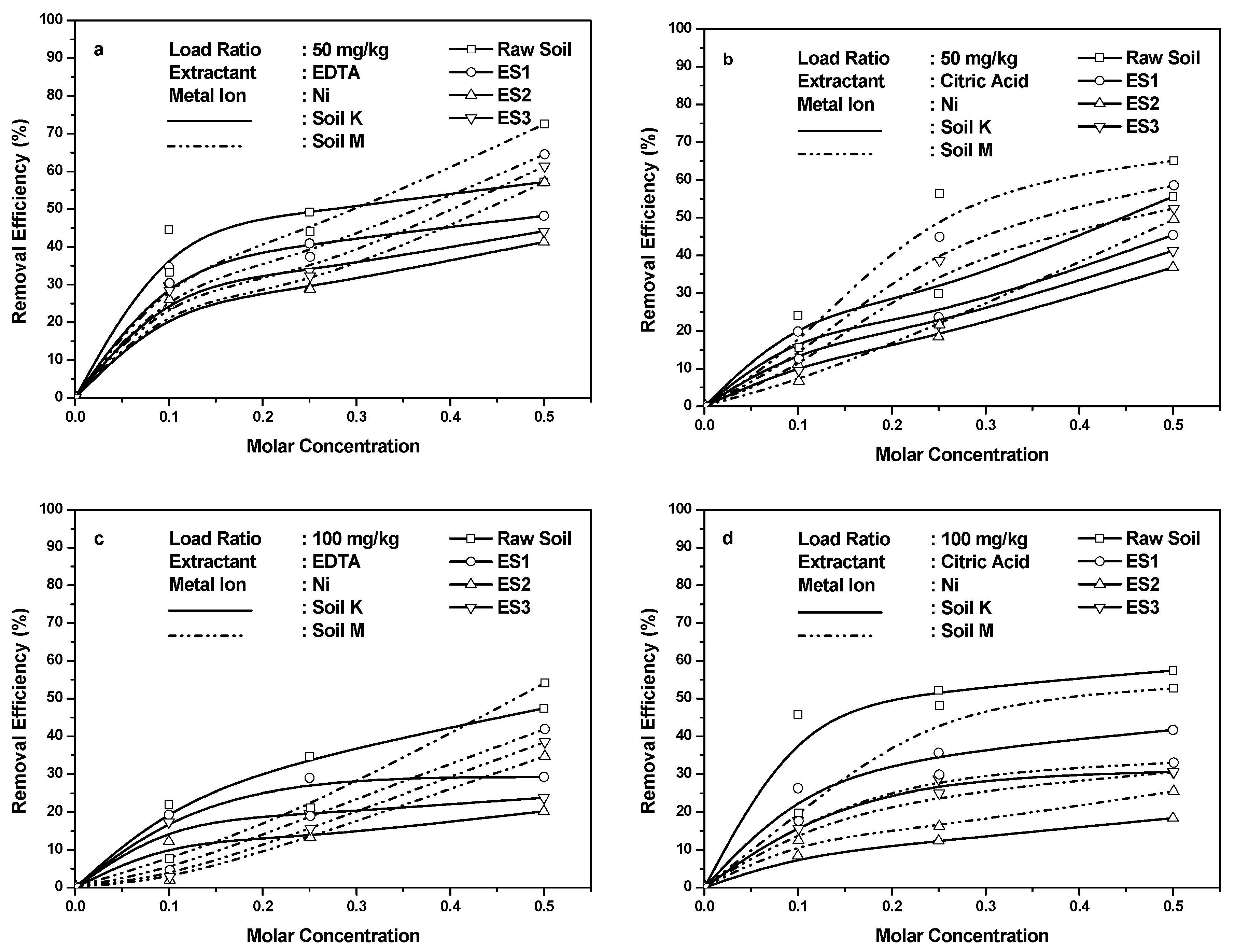
4.2. Ni Retention in Soils
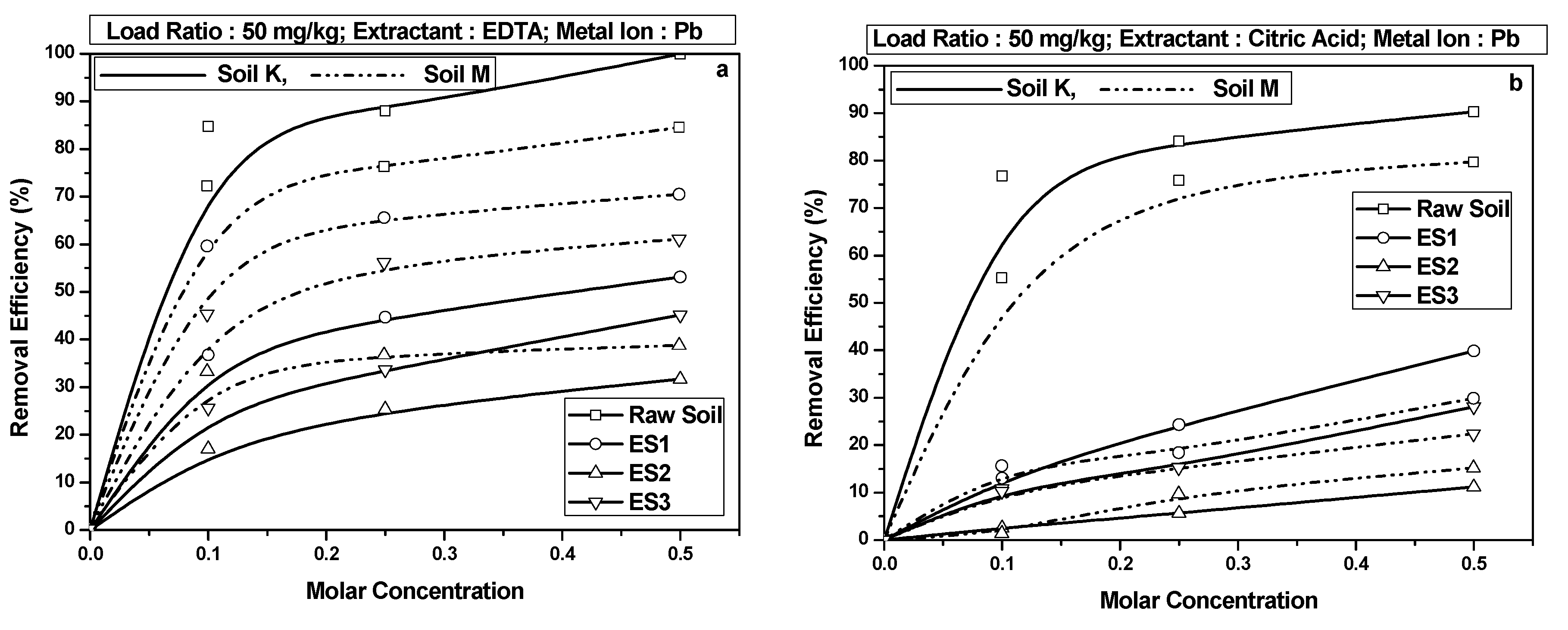
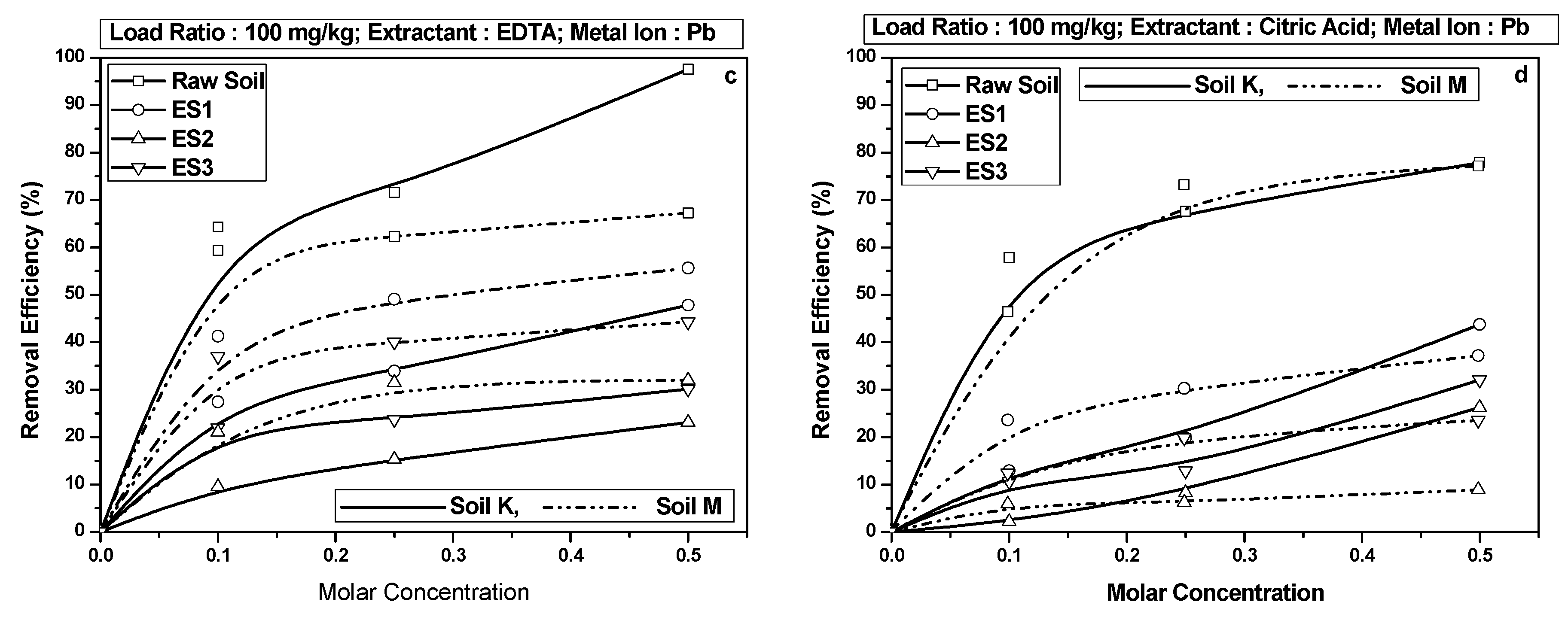
4.3. Pb Retention in Soils
5. Conclusions
- Soil grains adhered to each other due to CaCO3 precipitation initiated by the urease enzyme, and there exists a high probability that metal ions are encapsulated between the soil grains and CaCO3 precipitates. This leads to the effective retention of heavy metals in the soil matrix.
- Heavy metal retention in the soil occurred in the following order: Cd > Ni > Pb. The EICP-treated soil retained the maximum quantity of Cd among all the heavy metals. Additionally, Cd retention exceeded Ni or Pb retention even after treatment with chelants EDTA and citric acid. This was potentially due to the formation of CdCO3 in the soil matrix.
- EICP treatment using ES2 was observed to be better in terms of retaining heavy metals in the soil when compared to ES1 and ES3.
- The use of non-fat milk powder in the preparation of ES2 played a major role in boosting the UCS strength of the soil and also in retaining heavy metals in the soil due to the precipitation of CaCO3. Overall, the effects of CaCO3 precipitation due to the ESs consisted of improvements in heavy metal retention and UCS strength when compared to those of raw soils. Hence, it was concluded that the precipitates of CaCO3 hold heavy metals and improve UCS strength irrespective of whether the quantity of precipitation varies for different ESs.
- Based on these results, it is clear that EICP has sufficient ability to immobilize heavy metals in contaminated soil. This study portrayed appreciable outcomes for Cd when compared to Ni and Pb; this technique can be employed to immobilize specific contaminants by identifying its effectiveness on other heavy metals also. However, further testing in the field is necessary before drawing this conclusion.
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chalermyanont, T.; Arrykul, S.; Charoenthaisong, N. Potential use of lateritic and marine clay soils as landfill liners to retain heavy metals. Waste Manag. 2009, 29, 117–127. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, S.A.S.; Moghal, A.A.B. Soils Amended with Admixtures as Stabilizing Agent to Retain Heavy Metals. In Proceedings of the Geo-Congress, Atlanta, Georgia, 23–26 February 2014; pp. 2216–2225. [Google Scholar] [CrossRef]
- Tsang, D.C.W.; Lo, I.M.C.; Surampalli, R.Y. Chelating Agents for Land Decontamination Technologies; American Society of Civil Engineers: Reston, VA, USA, 2012; ISBN 978-0-7844-1218-3. [Google Scholar]
- Reddy, K.R.; Kumar, G.; Giri, R.K. System Effects on Bioreactor Landfill Performance Based on Coupled Hydro-Bio-Mechanical Modeling. J. Hazard. Toxic Radioact. Waste 2018, 22, 04017024. [Google Scholar] [CrossRef]
- Zhang, Q.; Lu, H.; Liu, J.; Wang, W.; Zhang, X. Hydraulic and mechanical behavior of landfill clay liner containing SSA in contact with leachate. Environ. Technol. 2018, 39, 1307–1315. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, S.K.; Ramanathan, A.L. Geochemical assessment of groundwater quality in vicinity of Bhalswa landfill, Delhi, India, using graphical and multivariate statistical methods. Environ. Geol. 2008, 53, 1509–1528. [Google Scholar] [CrossRef]
- Wuana, R.A.; Okieimen, F.E. Heavy Metals in Contaminated Soils: A Review of Sources, Chemistry, Risks and Best Available Strategies for Remediation. ISRN Ecol. 2011, 2011. [Google Scholar] [CrossRef]
- de Galvão, T.C.B.; Kaya, A.; Ören, A.H.; Yükselen, Y. Geomechanics of Landfills—Innovative Technology for Liners. Soil Sediment Contam. Int. J. 2008, 17, 411–424. [Google Scholar] [CrossRef]
- Lo, I.M.-C. Innovative Waste Containment Barriers for Subsurface Pollution Control. Pract. Period. Hazard. Toxic Radioact. Waste Manag. 2003, 7, 37–45. [Google Scholar] [CrossRef]
- Tuncan, A.; Tuncan, M.; Koyuncu, H.; Guney, Y. Use of natural zeolites as a landfill liner. Waste Manag. Res. J. Int. Solid Wastes Public Clean. Assoc. ISWA 2003, 21, 54–61. [Google Scholar] [CrossRef]
- Naeini, S.A.; Gholampoor, N.; Jahanfar, M.A. Effect of leachate’s components on undrained shear strength of clay-bentonite liners. Eur. J. Environ. Civ. Eng. 2019, 23, 395–408. [Google Scholar] [CrossRef]
- Sariosseiri, F.; Muhunthan, B. Effect of cement treatment on geotechnical properties of some Washington State soils. Eng. Geol. 2009, 104, 119–125. [Google Scholar] [CrossRef]
- Celaya, M.; Veisi, M.; Nazarian, S.; Puppala, A. Accelerated Design Process of Lime-Stabilized Clays. In Proceedings of the Geo-Frontiers, Dallas, TX, USA, 13–16 March 2011; pp. 4468–4478. [Google Scholar] [CrossRef]
- Horpibulsuk, S.; Rachan, R.; Suddeepong, A. Assessment of strength development in blended cement admixed Bangkok clay. Constr. Build. Mater. 2011, 25, 1521–1531. [Google Scholar] [CrossRef]
- Dash, S.K.; Hussain, M. Lime Stabilization of Soils: Reappraisal. J. Mater. Civ. Eng. 2012, 24, 707–714. [Google Scholar] [CrossRef]
- Fasihnikoutalab, M.H.; Asadi, A.; Kim Huat, B.; Westgate, P.; Ball, R.J.; Pourakbar, S. Laboratory-scale model of carbon dioxide deposition for soil stabilisation. J. Rock Mech. Geotech. Eng. 2016, 8, 178–186. [Google Scholar] [CrossRef]
- Sabine, C.L.; Feely, R.A.; Gruber, N.; Key, R.M.; Lee, K.; Bullister, J.L.; Wanninkhof, R.; Wong, C.S.; Wallace, D.W.R.; Tilbrook, B.; et al. The Oceanic Sink for Anthropogenic CO2. Science 2004, 305, 367–371. [Google Scholar] [CrossRef]
- Feely, R.A.; Sabine, C.L.; Lee, K.; Berelson, W.; Kleypas, J.; Fabry, V.J.; Millero, F.J. Impact of Anthropogenic CO2 on the CaCO3 System in the Oceans. Science 2004, 305, 362–366. [Google Scholar] [CrossRef]
- Yin, C.Y.; Wan Ali, W.S.; Lim, Y.P. Oil palm ash as partial replacement of cement for solidification/stabilization of nickel hydroxide sludge. J. Hazard. Mater. 2008, 150, 413–418. [Google Scholar] [CrossRef]
- Horpibulsuk, S.; Rachan, R.; Raksachon, Y. Role of Fly Ash on Strength and Microstructure Development in Blended Cement Stabilized Silty Clay. Soils Found. 2009, 49, 85–98. [Google Scholar] [CrossRef]
- Anupam, A.K.; Kumar, P.; Ransingchung, R.N.G.D. Performance evaluation of structural properties for soil stabilised using rice husk ash. Road Mater. Pavement Des. 2014, 15, 539–553. [Google Scholar] [CrossRef]
- Ali, F.H.; Adnan, A.; Choy, C.K. Geotechnical properties of a chemically stabilized soil from Malaysia with rice husk ash as an additive. Geotech. Geol. Eng. 1992, 10, 117–134. [Google Scholar] [CrossRef]
- Kampala, A.; Horpibulsuk, S. Engineering Properties of Silty Clay Stabilized with Calcium Carbide Residue. J. Mater. Civ. Eng. 2013, 25, 632–644. [Google Scholar] [CrossRef]
- Pourakbar, S.; Asadi, A.; Huat, B.B.K.; Fasihnikoutalab, M.H. Soil stabilisation with alkali-activated agro-waste. Environ. Geotech. 2015, 2, 359–370. [Google Scholar] [CrossRef]
- Almajed, A.; Tirkolaei, H.K.; Kavazanjian, E.; Hamdan, N. Enzyme Induced Biocementated Sand with High Strength at Low Carbonate Content. Sci. Rep. 2019, 9, 1135. [Google Scholar] [CrossRef] [PubMed]
- Kavazanjian, E.; Almajed, A.; Hamdan, N. Bio-Inspired Soil Improvement Using EICP Soil Columns and Soil Nails. In Proceedings of the Grouting, Honolulu, HI, USA, 9–12 July 2017; pp. 13–22. [Google Scholar] [CrossRef]
- Arab, M.; Omar, M.; Aljassmi, R.; Nasef, R.; Nassar, L.; Miro, S. EICP Cemented Sand Modified with Biopolymer. In International Congress and Exhibition“Sustainable Civil Infrastructures”; Springer: Cham, Switzerland, 2019; pp. 74–85. [Google Scholar]
- Almajed, A.; Abbas, H.; Arab, M.; Alsabhan, A.; Hamid, W.; Al-Salloum, Y. Enzyme-Induced Carbonate Precipitation (EICP)-Based methods for ecofriendly stabilization of different types of natural sands. J. Clean. Prod. 2020, 274, 122627. [Google Scholar] [CrossRef]
- Zhao, Z.; Hamdan, N.; Shen, L.; Nan, H.; Almajed, A.; Kavazanjian, E.; He, X. Biomimetic Hydrogel Composites for Soil Stabilization and Contaminant Mitigation. Environ. Sci. Technol. 2016, 50, 12401–12410. [Google Scholar] [CrossRef]
- Singh, R.K.; Datta, M.; Nema, A.K.; Pérez, I.V. Evaluating Groundwater Contamination Hazard Rating of Municipal Solid Waste Landfills in India and Europe Using a New System. J. Hazard. Toxic Radioact. Waste 2013, 17, 62–73. [Google Scholar] [CrossRef]
- Kumar, M.; Gogoi, A.; Kumari, D.; Borah, R.; Das, P.; Mazumder, P.; Tyagi, V.K. Review of Perspective, Problems, Challenges, and Future Scenario of Metal Contamination in the Urban Environment. J. Hazard. Toxic Radioact. Waste 2017, 21, 04017007. [Google Scholar] [CrossRef]
- Mohammed, S.A.S.; Sanaulla, P.F.; Moghal, A.A.B. Sustainable Use of Locally Available Red Earth and Black Cotton Soils in Retaining Cd2+ and Ni2+ from Aqueous Solutions. Int. J. Civ. Eng. 2016, 14, 491–505. [Google Scholar] [CrossRef]
- Steinnes, E.; Lierhagen, S. Geographical distribution of trace elements in natural surface soils: Atmospheric influence from natural and anthropogenic sources. Appl. Geochem. 2018, 88, 2–9. [Google Scholar] [CrossRef]
- Srinivasa Gowd, S.; Ramakrishna Reddy, M.; Govil, P.K. Assessment of heavy metal contamination in soils at Jajmau (Kanpur) and Unnao industrial areas of the Ganga Plain, Uttar Pradesh, India. J. Hazard. Mater. 2010, 174, 113–121. [Google Scholar] [CrossRef]
- Violante, A.; Pigna, M. Sorption-Desorption Processes of Metals and Metalloids in Soil Environments. Rev. Cienc. Suelo Nutr. Veg. 2008, 8, 95–101. [Google Scholar] [CrossRef][Green Version]
- dos Santos, N.M.; do Nascimento, C.W.A.; de Aguiar Accioly, A.M. Guideline Values and Metal Contamination in Soils of an Environmentally Impacted Bay. Water Air Soil Pollut. 2017, 228, 88. [Google Scholar] [CrossRef]
- DeJong, J.T.; Kavazanjian, E. Bio-mediated and Bio-inspired Geotechnics. In Geotechnical Fundamentals for Addressing New World Challenges; Lu, N., Mitchell, J.K., Eds.; Springer Series in Geomechanics and Geoengineering; Springer International Publishing: Cham, Switzerland, 2019; pp. 193–207. ISBN 978-3-030-06249-1. [Google Scholar]
- Salt, D.E.; Blaylock, M.; Kumar, N.P.B.A.; Dushenkov, V.; Ensley, B.D.; Chet, I.; Raskin, I. Phytoremediation: A Novel Strategy for the Removal of Toxic Metals from the Environment Using Plants. Bio/Technology 1995, 13, 468–474. [Google Scholar] [CrossRef] [PubMed]
- Ciumasu, I.M.; Costica, M.; Costica, N.; Neamtu, M.; Dirtu, A.C.; de Alencastro, L.F.; Buzdugan, L.; Andriesa, R.; Iconomu, L.; Stratu, A.; et al. Complex Risks from Old Urban Waste Landfills: Sustainability Perspective from Iasi, Romania. J. Hazard. Toxic Radioact. Waste 2012, 16, 158–168. [Google Scholar] [CrossRef][Green Version]
- Rojas, L.A.; Yáñez, C.; González, M.; Lobos, S.; Smalla, K.; Seeger, M. Characterization of the metabolically modified heavy metal-resistant Cupriavidus metallidurans strain MSR33 generated for mercury bioremediation. PLoS ONE 2011, 6, e17555. [Google Scholar] [CrossRef]
- Mulligan, C.N.; Yong, R.N.; Gibbs, B.F. Remediation technologies for metal-contaminated soils and groundwater: An evaluation. Eng. Geol. 2001, 60, 193–207. [Google Scholar] [CrossRef]
- Moghal, A.A.B.; Mohammed, S.A.S.; Almajed, A.; Al-Shamrani, M.A. Desorption of Heavy Metals from Lime-Stabilized Arid-Soils using Different Extractants. Int. J. Civ. Eng. 2020, 18, 449–461. [Google Scholar] [CrossRef]
- Dermont, G.; Bergeron, M.; Mercier, G.; Richer-Laflèche, M. Metal-Contaminated Soils: Remediation Practices and Treatment Technologies. Pract. Period. Hazard. Toxic Radioact. Waste Manag. 2008, 12, 188–209. [Google Scholar] [CrossRef]
- Sahu, Y.K.; Deb, M.K.; Patel, K.S.; Martín-Ramos, P.; Towett, E.K.; Tarkowska-Kukuryk, M. Bioaccumulation of Nutrients and Toxic Elements with Macrophytes. J. Hazard. Toxic Radioact. Waste 2020, 24, 05019007. [Google Scholar] [CrossRef]
- Muhammad, D.; Chen, F.; Zhao, J.; Zhang, G.; Wu, F. Comparison of EDTA- and citric acid-enhanced phytoextraction of heavy metals in artificially metal contaminated soil by Typha angustifolia. Int. J. Phytoremediat. 2009, 11, 558–574. [Google Scholar] [CrossRef]
- Mishra, S.P. Adsorption–desorption of heavy metal ions. Curr. Sci. 2014, 107, 12. [Google Scholar]
- Goldberg, S.; Criscenti, L.J.; Turner, D.R.; Davis, J.; Cantrell, K.J. Adsorption-Desorption Processes in Subsurface Reactive Transport Modeling. Vadose Zone J. 2007, 6, 407–435. [Google Scholar] [CrossRef]
- Blais, J.F.; Djedidi, Z.; Cheikh, R.B.; Tyagi, R.D.; Mercier, G. Metals Precipitation from Effluents: Review. Pract. Period. Hazard. Toxic Radioact. Waste Manag. 2008, 12, 135–149. [Google Scholar] [CrossRef]
- Moghal, A.A.B.; Reddy, K.R.; Mohammed, S.A.S.; Al-Shamrani, M.A.; Zahid, W.M. Lime-Amended Semi-arid Soils in Retaining Copper, Lead, and Zinc from Aqueous Solutions. Water Air Soil Pollut. 2016, 227, 372. [Google Scholar] [CrossRef]
- Mohammed, S.A.S.; Moghal, A.A.B. Efficacy of nano calcium silicate (NCS) treatment on tropical soils in encapsulating heavy metal ions: Leaching studies validation. Innov. Infrastruct. Solut. 2016, 1, 21. [Google Scholar] [CrossRef]
- Moghal, A.A.B.; Reddy, K.R.; Mohammed, S.A.S.; Al-Shamrani, M.A.; Zahid, W.M. Sorptive Response of Chromium (Cr+6) and Mercury (Hg+2) From Aqueous Solutions Using Chemically Modified Soils. J. Test. Eval. 2017, 45, 105–119. [Google Scholar] [CrossRef]
- Madhu, A.; Chakraborty, J.N. Developments in application of enzymes for textile processing. J. Clean. Prod. 2017, 145, 114–133. [Google Scholar] [CrossRef]
- Nathan, V.K.; Rani, M.E.; Gunaseeli, R.; Kannan, N.D. Enhanced biobleaching efficacy and heavy metal remediation through enzyme mediated lab-scale paper pulp deinking process. J. Clean. Prod. 2018, 203, 926–932. [Google Scholar] [CrossRef]
- Almajed, A.A. Enzyme Induced Carbonate Precipitation (EICP) for Soil Improvement. Ph.D. Thesis, Arizona State University, Tempe, AZ, USA, 2017. [Google Scholar]
- Almajed, A. Enzyme induced cementation of biochar-intercalated soil: Fabrication and characterization. Arab. J. Geosci. 2019, 12, 403. [Google Scholar] [CrossRef]
- Almajed, A.; Khodadadi, H.; Kavazanjian, E. Sisal Fiber Reinforcement of EICP-Treated Soil. In Proceedings of the IFCEE, Orlando, FL, USA, 5–10 March 2018; pp. 29–36. [Google Scholar] [CrossRef]
- Pasillas, J.N.; Khodadadi, H.; Martin, K.; Bandini, P.; Newtson, C.M.; Kavazanjian, E. Viscosity-Enhanced EICP Treatment of Soil. In Proceedings of the IFCEE, Orlando, FL, USA, 5–10 March 2018; pp. 145–154. [Google Scholar] [CrossRef]
- Javadi, N.; Khodadadi, H.; Hamdan, N.; Kavazanjian, E. EICP Treatment of Soil by Using Urease Enzyme Extracted from Watermelon Seeds. In Proceedings of the IFCEE, Orlando, FL, USA, 5–10 March 2018; pp. 115–124. [Google Scholar] [CrossRef]
- Oliveira, P.J.V.; Freitas, L.D.; Carmona, J.P.S.F. Effect of Soil Type on the Enzymatic Calcium Carbonate Precipitation Process Used for Soil Improvement. J. Mater. Civ. Eng. 2017, 29, 04016263. [Google Scholar] [CrossRef]
- Zhao, Q.; Li, L.; Li, C.; Li, M.; Amini, F.; Zhang, H. Factors Affecting Improvement of Engineering Properties of MICP-Treated Soil Catalyzed by Bacteria and Urease. J. Mater. Civ. Eng. 2014, 26, 04014094. [Google Scholar] [CrossRef]
- Venda Oliveira, P.J.; da Costa, M.S.; Costa, J.N.P.; Nobre, M.F. Comparison of the Ability of Two Bacteria to Improve the Behavior of Sandy Soil. J. Mater. Civ. Eng. 2015, 27, 06014025. [Google Scholar] [CrossRef]
- Hommel, J.; Akyel, A.; Frieling, Z.; Phillips, A.J.; Gerlach, R.; Cunningham, A.B.; Class, H. A Numerical Model for Enzymatically Induced Calcium Carbonate Precipitation. Appl. Sci. 2020, 10, 4538. [Google Scholar] [CrossRef]
- Chandra, A.; Ravi, K. Application of Enzyme Induced Carbonate Precipitation (EICP) to improve the shear strength of different type of soils. In Proceedings of the Indian Geotechnical Conference, Bengaluru, India, 13–15 December 2018; p. 8. [Google Scholar]
- Handley-Sidhu, S.; Sham, E.; Cuthbert, M.O.; Nougarol, S.; Mantle, M.; Johns, M.L.; Macaskie, L.E.; Renshaw, J.C. Kinetics of urease mediated calcite precipitation and permeability reduction of porous media evidenced by magnetic resonance imaging. Int. J. Environ. Sci. Technol. 2013, 10, 881–890. [Google Scholar] [CrossRef]
- Dharmakeerthi, R.S.; Thenabadu, M.W. Urease activity in soils: A review. J. Natl. Sci. Found. Sri Lanka 1996, 24, 159–195. [Google Scholar] [CrossRef]
- Blakeley, R.L.; Zerner, B. Jack bean urease: The first nickel enzyme. J. Mol. Catal. 1984, 23, 263–292. [Google Scholar] [CrossRef]
- Dejong, J.T.; Soga, K.; Kavazanjian, E.; Burns, S.; Van Paassen, L.A.; Al Qabany, A.; Aydilek, A.; Bang, S.S.; Burbank, M.; Caslake, L.F.; et al. Biogeochemical processes and geotechnical applications: Progress, opportunities and challenges. Géotechnique 2013, 63, 287–301. [Google Scholar] [CrossRef]
- Mohammed, S.A.S.; Moghal, A.A.B.; Sanaulla, P.F.; Kotresha, K.; Reddy, H.P. Cadmium Fixation Studies on Contaminated Soils Using Nano Calcium Silicate—Treatment Strategy. In Proceedings of the Geotechnical Frontiers, Orlando, FL, USA, 12–15 March 2017; pp. 434–442. [Google Scholar] [CrossRef]
- ASTM D2166/D2166M. Test Method for Unconfined Compressive Strength of Cohesive Soil; ASTM International: West Conshohocken, PA, USA, 2016. [Google Scholar]
- Khodadoust, A.P.; Reddy, K.R.; Maturi, K. Removal of Nickel and Phenanthrene from Kaolin Soil Using Different Extractants. Environ. Eng. Sci. 2004, 21, 691–704. [Google Scholar] [CrossRef]
- Gu, Y.-Y.; Yeung, A.T. Desorption of cadmium from a natural Shanghai clay using citric acid industrial wastewater. J. Hazard. Mater. 2011, 191, 144–149. [Google Scholar] [CrossRef]
- ASTM D3987. Standard Test Method for Shake Extraction of Solid Waste with Water; ASTM International: West Conshohocken, PA, USA, 2012. [Google Scholar]
- Moghal, A.A.B.; Ashfaq, M.; Al-Shamrani, M.A.; Al-Mahbashi, A. Effect of Heavy Metal Contamination on the Compressibility and Strength Characteristics of Chemically Modified Semiarid Soils. J. Hazard. Toxic Radioact. Waste 2020, 24, 04020029. [Google Scholar] [CrossRef]
- Dhami, N.K.; Reddy, M.S.; Mukherjee, A. Biomineralization of calcium carbonates and their engineered applications: A review. Front. Microbiol. 2013, 4, 314. [Google Scholar] [CrossRef]
- Krajewska, B. Urease-aided calcium carbonate mineralization for engineering applications: A review. J. Adv. Res. 2018, 13, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Almajed, A.; Khodadadi Tirkolaei, H.; Kavazanjian, E. Baseline Investigation on Enzyme-Induced Calcium Carbonate Precipitation. J. Geotech. Geoenviron. Eng. 2018, 144, 04018081. [Google Scholar] [CrossRef]
- Ran, D.; Kawasaki, S. Effective Use of Plant-Derived Urease in the Field of Geoenvironmental/Geotechnical Engineering. J. Civ. Environ. Eng. 2016, 6, 1–13. [Google Scholar] [CrossRef]
- Madrid, L.; Diaz-Barrientos, E. Influence of carbonate on the reaction of heavy metals in soils. J. Soil Sci. 1992, 43, 709–721. [Google Scholar] [CrossRef]
- Torres-Aravena, Á.E.; Duarte-Nass, C.; Azócar, L.; Mella-Herrera, R.; Rivas, M.; Jeison, D. Can Microbially Induced Calcite Precipitation (MICP) through a Ureolytic Pathway Be Successfully Applied for Removing Heavy Metals from Wastewaters? Crystals 2018, 8, 438. [Google Scholar] [CrossRef]
- Vega, F.A.; Covelo, E.F.; Andrade, M.L. A versatile parameter for comparing the capacities of soils for sorption and retention of heavy metals dumped individually or together: Results for cadmium, copper and lead in twenty soil horizons. J. Colloid Interface Sci. 2008, 327, 275–286. [Google Scholar] [CrossRef] [PubMed]
- Acosta, J.A.; Jansen, B.; Kalbitz, K.; Faz, A.; Martínez-Martínez, S. Salinity increases mobility of heavy metals in soils. Chemosphere 2011, 85, 1318–1324. [Google Scholar] [CrossRef]
- González Costa, J.J.; Reigosa, M.J.; Matías, J.M.; Covelo, E.F. Soil Cd, Cr, Cu, Ni, Pb and Zn sorption and retention models using SVM: Variable selection and competitive model. Sci. Total Environ. 2017, 593–594, 508–522. [Google Scholar] [CrossRef]
- Wang, Y.M.; Chen, T.C.; Yeh, K.J.; Shue, M.F. Stabilization of an elevated heavy metal contaminated site. J. Hazard. Mater. 2001, 88, 63–74. [Google Scholar] [CrossRef]
- Zhou, D.M.; Wang, S.Q.; Chen, H.M. Interaction of Cd and citric acid, EDTA in red soil. J. Environ. Sci. 2001, 13, 153–156. [Google Scholar]
- Manjeet, B.; Diwan, S.; Garg, V.K.; Pawan, R. Use of Agricultural Waste for the Removal of Nickel Ions from Aqueous Solutions: Equilibrium and Kinetics Studies. Int. J. Environ. Sci. Eng. 2009, 1, 108–114. [Google Scholar] [CrossRef]
- Attar, K.; Demey, H.; Bouazza, D.; Sastre, A.M. Sorption and Desorption Studies of Pb(II) and Ni(II) from Aqueous Solutions by a New Composite Based on Alginate and Magadiite Materials. Polymers 2019, 11, 340. [Google Scholar] [CrossRef] [PubMed]
- Karna, R.R.; Luxton, T.; Bronstein, K.E.; Redmon, J.H.; Scheckel, K.G. State of the science review: Potential for beneficial use of waste by-products for in situ remediation of metal-contaminated soil and sediment. Crit. Rev. Environ. Sci. Technol. 2017, 47, 65–129. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Qian, Z.; Zhou, C.; Su, W.; Hong, P.; Liu, S.; He, L.; Chen, Z.; Ji, H. Mussel-inspired synthesis of polydopamine-functionalized calcium carbonate as reusable adsorbents for heavy metal ions. RSC Adv. 2014, 4, 47848–47852. [Google Scholar] [CrossRef]
- Peters, R.W. Chelant extraction of heavy metals from contaminated soils. J. Hazard. Mater. 1999, 66, 151–210. [Google Scholar] [CrossRef]
- Cline, S.R.; Reed, B.E. Lead Removal from Soils via Bench-Scale Soil Washing Techniques. J. Environ. Eng. 1995, 121, 700–705. [Google Scholar] [CrossRef]
- Kumpiene, J.; Lagerkvist, A.; Maurice, C. Stabilization of As, Cr, Cu, Pb and Zn in soil using amendments—A review. Waste Manag. 2008, 28, 215–225. [Google Scholar] [CrossRef]
- Tian, K.; Benson, C.H.; Tinjum, J.M. Chemical Characteristics of Leachate in Low-Level Radioactive Waste Disposal Facilities. J. Hazard. Toxic Radioact. Waste 2017, 21, 04017010. [Google Scholar] [CrossRef]
- Harter, R.D. Effect of Soil pH on Adsorption of Lead, Copper, Zinc, and Nickel. Soil Sci. Soc. Am. J. 1983, 47, 47–51. [Google Scholar] [CrossRef]
- Gupta, T.B.; Lataye, D.H. Adsorption of Indigo Carmine Dye onto Acacia Nilotica (Babool) Sawdust Activated Carbon. J. Hazard. Toxic Radioact. Waste 2017, 21, 04017013. [Google Scholar] [CrossRef]
- Maturi, K.; Reddy, K.R. Extractants for the Removal of Mixed Contaminants from Soils. Soil Sediment Contam. Int. J. 2008, 17, 586–608. [Google Scholar] [CrossRef]


| Property | Soil K | Soil M |
|---|---|---|
| Color | Red | Black |
| Specific gravity | 2.6 | 2.5 |
| Liquid limit (%) | 30 | 54 |
| Plastic limit (%) | 17 | 27 |
| Plasticity index (%) | 13 | 27 |
| Classification (USCS) | CL | CH |
| pH | 5.7 | 8.3 |
| Organic content % | 0.87 | 0.77 |
| Optimum water content (%) | 16.8 | 15.2 |
| Maximum dry density (kN/m3) | 17.9 | 15.8 |
| Unconfined compressive strength (MPa) | 0.07 | 0.013 |
| Load Ratio Available with 50 mg/kg Initial Load Ratio | ||||||||
|---|---|---|---|---|---|---|---|---|
| Metal Ion | Soil K | Soil K + ES1 | Soil K + ES2 | Soil K + ES3 | Soil M | Soil M + ES1 | Soil M + ES2 | Soil M + ES3 |
| Ni | 49.37 | 48.67 | 46.19 | 47.69 | 48.37 | 47.65 | 45.61 | 46.97 |
| Cd | 48.97 | 47.68 | 44.93 | 46.37 | 48.69 | 47.86 | 45.57 | 46.59 |
| Pb | 49.01 | 48.79 | 46.82 | 48.03 | 48.04 | 47.63 | 45.39 | 46.55 |
| Load Ratio Available with 100 mg/kg Initial Load Ratio | ||||||||
| Metal Ion | Soil K | Soil K + ES1 | Soil K + ES2 | Soil K + ES3 | Soil M | Soil M + ES1 | Soil M + ES2 | Soil M + ES3 |
| Ni | 99.09 | 98.67 | 95.98 | 97.64 | 99.12 | 98.36 | 95.63 | 97.86 |
| Cd | 98.39 | 98.49 | 96.15 | 97.35 | 98.87 | 98.65 | 95.58 | 97.55 |
| Pb | 99.2 | 98.52 | 96.28 | 97.36 | 99.24 | 99.83 | 96.05 | 97.64 |
| Solution | Concentration of Component 1 | Concentration of Component 2 | Concentration of Component 3 | Concentration of Component 4 |
|---|---|---|---|---|
| Urea (CH4N2O) | Calcium Chloride (CaCl2·2H2O) | Urease Enzyme | Non-Fat Milk Powder | |
| ES1 | 1.00 M | 0.67 M | 3.00 g/L | - |
| ES2 | 1.00 M | 0.67 M | 3.00 g/L | 4.00 g/L |
| ES3 | 0.37 M | 0.25 M | 0.85 g/L | 4.00 g/L |
| Curing Period (Days) | Soil K | Soil M | ||||
|---|---|---|---|---|---|---|
| ES1 | ES2 | ES3 | ES1 | ES2 | ES3 | |
| 7 | 1.315 | 1.387 | 1.367 | 1.581 | 2.631 | 1.671 |
| 14 | 1.769 | 2.286 | 1.933 | 2.463 | 4.722 | 2.632 |
| 21 | 1.689 | 2.043 | 1.811 | 2.026 | 4.582 | 2.328 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moghal, A.A.B.; Lateef, M.A.; Mohammed, S.A.S.; Lemboye, K.; C. S. Chittoori, B.; Almajed, A. Efficacy of Enzymatically Induced Calcium Carbonate Precipitation in the Retention of Heavy Metal Ions. Sustainability 2020, 12, 7019. https://doi.org/10.3390/su12177019
Moghal AAB, Lateef MA, Mohammed SAS, Lemboye K, C. S. Chittoori B, Almajed A. Efficacy of Enzymatically Induced Calcium Carbonate Precipitation in the Retention of Heavy Metal Ions. Sustainability. 2020; 12(17):7019. https://doi.org/10.3390/su12177019
Chicago/Turabian StyleMoghal, Arif Ali Baig, Mohammed Abdul Lateef, Syed Abu Sayeed Mohammed, Kehinde Lemboye, Bhaskar C. S. Chittoori, and Abdullah Almajed. 2020. "Efficacy of Enzymatically Induced Calcium Carbonate Precipitation in the Retention of Heavy Metal Ions" Sustainability 12, no. 17: 7019. https://doi.org/10.3390/su12177019
APA StyleMoghal, A. A. B., Lateef, M. A., Mohammed, S. A. S., Lemboye, K., C. S. Chittoori, B., & Almajed, A. (2020). Efficacy of Enzymatically Induced Calcium Carbonate Precipitation in the Retention of Heavy Metal Ions. Sustainability, 12(17), 7019. https://doi.org/10.3390/su12177019









