1. Introduction
Coal gangue is an inevitable product of the process of coal mining and processing and is the main pollution source in coal mines [
1]. The main source of acid coal gangue pollution and subsequent environmental disasters is the oxidation of sulfide. In the open storage process, sulfide contained in coal gangue easily forms acid mine drainage (AMD) containing high concentrations of sulfate and a diversity of harmful heavy metal ions through comprehensive actions such as air oxidation, rainwater eluviation, and microorganism activity [
2,
3]. Therefore, controlling the oxidation of sulfides in coal gangue has become an important means of mitigating the effects of acidification of coal gangue.
Studies in China and abroad have found that the oxidation process of pyrite in coal gangue is affected by the biocatalysis of oxidizing bacteria such as
Acidithiobacillus ferrooxidans (
A. ferrooxidans), significantly accelerating the conversion of Fe
2+ to Fe
3+, increasing the reaction rate by 50 to 60 times, and playing a vital role in acid pollution [
4]. In 1947, Colmer et al. discovered and isolated a strain of
A. ferrooxidans in Pittsburgh that could carry out ferrous oxidation in AMD, confirming the bio-oxidation processing of ferrous iron [
5]. Leathen et al., 1953, first proposed that acid pollution in coal gangue is controlled by the activity of microorganisms [
6]. Nosa believes that AMD is formed by the oxidation of sulfur minerals (mainly pyrite, FeS
2) in mine tailings by the combined action of chemical oxidants (O
2, Fe
3+) and
A. ferrooxidans [
7].
Antimicrobial agents can significantly affect environmental microbial activity. Researchers began to study the use of bactericides to reduce oxidation and acid production in the treatment of coal gangue in the 1980s [
8,
9]. Since 1981, Kleinmann, Schippers, and others have studied the germicidal efficacy of benzoic acid, sorbic acid, sodium alkyl benzene sulfonate, and SDS. Their results showed that the strongest bacteriostatic agent among these is SDS [
10,
11]. Peng et al. examined the effects of the surfactant Tween-80 on the sulfur metabolism of
A. ferrooxidans and confirmed that the surfactant has an effect on extracellular proteins, cell EPS composition, and expression of EPS synthesis-related genes in
A. ferrooxidans [
12]. Publishing as part of a long-term scientific cooperative project with Germany and Romania, Sand et al. concluded that isothiazoline, organic materials, and crushed limestone coatings reduce the release of metals and sulfur from coal mine wastes [
13]. Hu et al. conducted an experiment using two bactericides, namely sodium dodecyl sulfate (SDS) and sodium benzoic (SBZ), to control the acidification of coal gangue. Their results show that when the concentration of SDS is 10 mg/L or the concentration of SBZ is 30 mg/L, the oxidation of Fe
2+ can be inhibited by approximately 75% [
14].
In recent years, many scholars have focused on bactericides constituting low environmental hazards, such as food preservatives and anionic surfactants. The bactericide SDS is a food preservative with little negative impact on the environment and possesses good sterilization effects. Kathon is a nonoxidizing bactericide with high efficiency and broad spectrum, is effective over a wide pH range [
15], and it can be naturally degraded to an innocuous substance at the concentrations used, resulting in no pollution to the environment [
16]. It has been demonstrated to be effectively lethal to microorganisms in the soil [
17]. Triclosan is a broad-spectrum antibiotic with the chemical name 2,4,4′-trichloro-2′-hydroxy diphenyl ether. Triclosan can inhibit bacteria effectively and kills Gram-positive and Gram-negative bacteria, yeast, and viruses [
18]; therefore it is widely used in various household disinfectant products. As early as the 1960s, the United States began to use Triclosan to produce deodorizing soap and deodorant, and Europe began to produce toothpaste containing Triclosan [
19]. The inhibitory effect of bactericides on
A. ferrooxidans may exhibit at different points of action. To better detect the action of bactericides on
A. ferrooxidans, it is necessary to observe the protein in vivo, along with ultrastructure and lipids. Bactericide treatment may rupture of cell walls and membranes, producing a protein efflux in
A. ferrooxidans. To detect whether there is a protein release, preliminary observations were made using a protein flocculation precipitate. Scanning electron microscopy detected surface damage and dents on bacteria. The cellular lipids released were measured using time-of-flight mass spectrometry.
In this paper, three high-efficiency, environmentally friendly bactericides, SDS, Kathon, and Triclosan, were used to study their inhibitory effects on the oxidation activity of A. ferrooxidans. Moreover, optimal application concentrations and bacteriostatic mechanisms were determined. This paper discusses the possibility of using bactericides to neutralize bacterial oxidation and the generation of acidic pollution, laying a foundation for the development of fungicidal materials suitable for in situ control of acidification pollution in coal gangue dumps in China.
2. Materials and Methods
2.1. Test Strains and Drugs
The original bacterial strain used in this study came from a coal gangue dump in Yangquan, Shanxi, China. A coal gangue sample (5 g) was added to 50 mL of sterile water and oscillated at 160 rpm for 3 days at a constant temperature of 30 °C. The solution was then filtered through paper to obtain a coal gangue soak solution containing bacteria; 70 mL of iron-free 9K medium (pH = 1.80, sterilized at 121 °C, 30 min), 20 mL of coal gangue soak solution, and 10 mL of Fe2+ stock solution (filter-sterilized; containing 40 g/L Fe2+ in the form of FeSO4·7H2O) were combined. The flask was incubated in a thermostatic bath (30 °C, 160 rpm). After 7 days, the solution became reddish brown, iron precipitates formed, and the bacterial numbers reached 107–108/mL. A. ferrooxidans was sub-cultured twice in the liquid medium and after serial dilution plated on to a solid medium (0.3% sodium citrate and 0.016% CuSO4 in 9K, 2% agarose; all in w/v). Single colonies were selected for RNA extraction (FastDNA spin kit for soil, MP Biomedicals, Irvine, CA, USA) and 16S rRNA gene sequencing (ABI 3730XL, Applied Biosystems, Foster City, CA, USA). The strain was identified to be a 99.04% homologous to Acidithiobacillus ferrooxidans strain YTW (DQ062116.1) by using 16S rRNA gene sequencing and named Acidithiobacillus ferrooxidans strain AF-14 (MT774373).
The reagents used in the experiments were all analytically pure Analytical Reagent (AR). The bactericide purities of Kathon (2.5% solution, Changzhou Xinnuoke Chemical Co. LTD, Changzhou, China), SDS (powder, Biofroxx, Einhausen, Germany), and Triclosan (powder, Hefei BASF Biotechnology Co. LTD, Hefei, China) were AR, and they were sterilized by filtration through a 0.22 µm PVDF membrane.
2.2. Experimental Methods
2.2.1. Determination of Optimal Application Concentrations
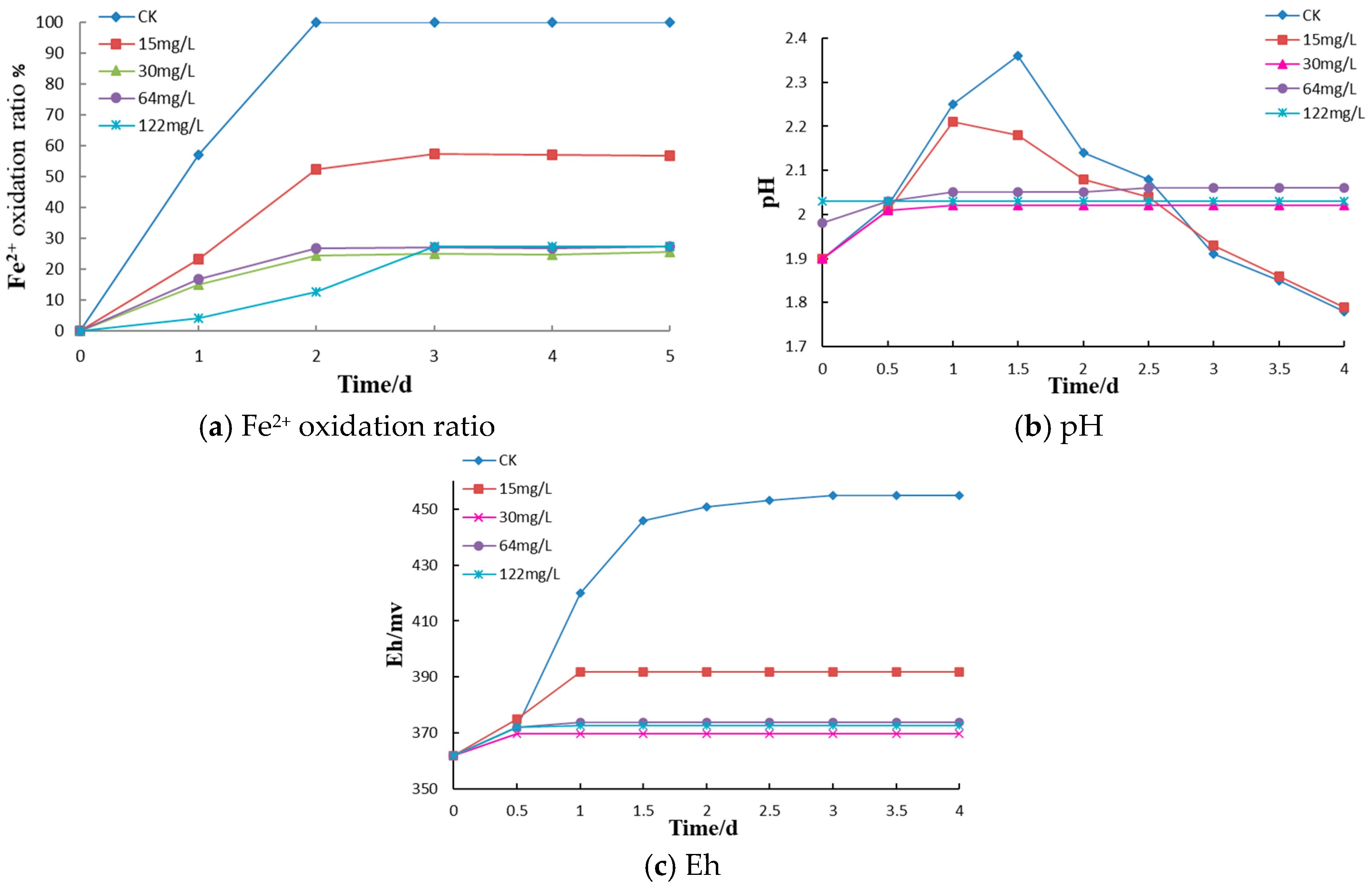
Bacterial solution (10 mL), 70 mL of iron-free 9K medium (heat sterilized as described above) and 10 mL of the Fe
2+ stock solution (also described above) were added to fifteen 250 mL sterilized conical flasks at an aseptic operation station. Kathon (10 mL) was added from sterile, 10-fold concentrated stock solutions to final concentrations 0 (control), 15, 30, 64 and 122 mg/L in the cultures. Each bactericide concentration was tested in triplicate. Similar procedure was followed with Triclosan and SDS, Triclosan with final concentrations of 0, 4, 8, 16, 28, and 36 mg/L (each tested in duplicate), SDS with final concentrations of 0, 5, 10, 30, 50, and 100 mg/L (each tested in duplicate). The control group was supplemented with 100 mL of 9K (Fe
2+) medium, sealed, and cultured at a constant temperature and rate of agitation (30 °C, 160 rpm). To determine the optimal application concentration of Kathon, the pH of the bacterial solution, the oxidation-reduction potential Eh, and the rate of Fe
2+ oxidation were measured at different times. The pH and Eh were measured by pH and redox electrode (coupled to pH and Eh meters, DZS-708L, INESA Scientific Instrument Co., Ltd. Shanghai, China). Ferrous iron concentrations were determined by the EDTA complexometric titration [
20].
2.2.2. Disk Inhibition Zone Experiment
The disk inhibition zone experiment is an experimental method to determine the bactericidal effect by the degree of spread of bactericides on solid culture medium; 0.1 mL of bacterial culture was evenly spread onto each of 12 plates containing the solid medium described in
Section 2.1. Triplicate plates were labeled No. 1–No. 4 and treated with 0.02 mL of Kathon (30 mg/L), Triclosan (16 mg/L), SDS (10 mg/L) and sterile water (control treatment), respectively. A 6 mm diameter hole was punched in the center of solid culture medium in each petri dish, and bactericide or sterile water was injected into the holes. After that, the petri dishes were placed in an incubator at a constant temperature of 30 °C for about 15 d, and the size of disk inhibition zone was observed.
2.2.3. Protein Flocculation Test
Five replications of the bacterial solution (cultures grown in 9K without the addition of any bactericide) were centrifuged at 10,000 rpm for 20 min, and the cells were collected. The cells were mixed well with 1 mL EDTA, transferred to a 2 mL EP tube, and centrifuged at 12,000 rpm for 10 min. The supernatant was discarded. This was repeated one more time, to remove metal ions from the medium.
The 1 mL cell suspensions in EDTA were used as positive controls (No. 1), with 980 μL sodium phosphate buffer (SPB) and 122 μL of MT buffer from a total DNA extraction kit (FastDNA spin kit for soil, MP Biomedicals) added. After mixing well, the solution was lysed in Matrix E tubes. When the solution temperature reached room temperature, it was shaken in a fastprep (bead mill oscillator) for 40 s. This was repeated six times before the cell beads were crushed.
Bactericide (1 mL) was added (No. 2: 1 mL, 30 mg/L for Kathon; No. 3: 1 mL, 16 mg/L for Triclosan; No. 4: 1 mL, 10 mg/L for SDS), mixed well and left to stand for 3 h.
As a negative control, 1 mL of full 9K medium (containing Fe2+) was added to No. 5, mixed, and let stand for 3 h.
All samples, No. 1–No. 5, were centrifuged at 12,000 rpm for 10 min, and the supernatant was transferred to new 2 mL EP tubes. After, the protein precipitation solution (PPS, 250 μL) was added, and the tubes were inverted. The role of the PPS solution was to denature the protein. Treatment with PPS produced a milky flocculent material. Photographs were taken and observations made with a stereomicroscope. The appearance of a milky precipitate indicated a release of proteins as a result of bactericide treatment.
2.2.4. Observations Using a Scanning Electron Microscope
The bacterial solution (300 mL, in
Section 2.1) to be tested was divided into 12 centrifuge tubes (25 mL). Triplicate plates were labeled No. 1–No. 4. Each group had 4 centrifuge tubes, centrifuged at 13,000 rpm for 20 min. The supernatants were discarded, and the precipitates containing bacteria were collected. The precipitates were then suspended in 1 mL of acidified H
2O (with HCl to pH 1.8), transferred to 1.5 mL centrifuge tubes, centrifuged for 10 min at 12,000 rpm. The supernatant was discarded, leaving the bacteria bearing precipitate (iron removal). No. 1 was suspended in 1 mL of Kathon, No. 2 with 1 mL of Triclosan, No. 3 with 1 mL of SDS, and No. 4 with 1 mL of sterile water. These were cultured for 3 h on a shaking table at 160 rpm with a constant temperature of 30 °C. They were centrifuged at 12,000 rpm for 10 min. The supernatants were removed and combined with 1 mL of phosphate buffer (0.047 mol/L g of Na
2HPO
4·12H
2O and 0.020 mol/L of KH
2PO
4, with the pH adjusted to 7.2 with H
2SO
4) and repeated 2 more times. The sample was fixed with 2.5% glutaraldehyde, held overnight at 4 °C, and centrifuged at 9000 rpm for 15 min, after which the supernatants were discarded and the samples were dehydrated with a gradient of 30%, 50%, 75%, 90%, and 100% ethanol. The cells were then freeze-dried at different gradient temperatures of −20 °C, −40 °C, −60 °C, and −80 °C, allowed to stand at each temperature step for 12 h, then observed using a scanning electron microscope (SEM).
2.2.5. Time-of-Flight Mass Spectrometry for the Determination of Intracellular Lipid Efflux
Bacterial solutions (10 mL) were mixed with iron-free 9K medium (70 mL, described above), Fe2+ stock solution (10 mL, described above), and sterile water (10 mL) in 250 mL sterilized conical flasks, which were sealed and cultured under constant temperature and agitation (30 °C, 160 rpm).
Four sample groups (10 mL) were centrifuged at 1000 rpm for 5 min. The supernatant was transferred to a new centrifuge tube and centrifuged at 10,000 rpm for 10 min. The supernatants were discarded, and the viscous material attached to the walls of the centrifuge tubes was washed with 1 mL of Kathon, Triclosan, SDS, or sterile water at a pH of 1.8. These were then cultured at 30 °C and 160 rpm for 3 h and subjected to time-of-flight mass spectrometry.
4. Conclusions
(1) Microbially mediated oxidation of pyrite in coal gangue dumps is the key process resulting in acidification and spontaneous combustion. The bactericides such as Triclosan, Kathon, and SDS can effectively inhibit the oxidation of iron by A. ferrooxidans, with the inhibition of Fe2+ oxidation reaching 75–83.5%. The optimal application concentrations of Kathon, Triclosan, and SDS for A. ferrooxidans inhibition were 30 mg/L, 16 mg/L, and 10 mg/L, respectively.
(2) All three bactericides effectively inhibited A. ferrooxidans and inactivated the cells to various extents, using different mechanisms. Among these, Kathon caused A. ferrooxidans to release small amounts of proteins and lipids, resulting from cracks and shrinkage to cell surfaces after 3 h of treatment. Triclosan caused the release of a small amount of lipid, due to cell surface shrinkage and degradation after 3 h. This caused the release of a large amount of plasma. Within 3 h, SDS caused a large release of proteins and lipids, degraded the cell surface structure, and distorted the cellular morphology of the bacteria.
(3) The main source of acid coal gangue dump pollution and consequent environmental disasters has been the oxidation of sulfides in coal gangue. Catalytic oxidation by microorganisms (A. ferrooxidans) has been identified as a major mechanism; therefore, the use of bactericides that can effectively kill A. ferrooxidans and inhibit oxidation can help reduce the acid water and the risk of spontaneous combustion in coal gangue by fixing sulfur and lowering temperatures. Together, these strategies can control the spread of pollution and promote vegetative restoration.