Intensification: A Key Strategy to Achieve Great Animal and Environmental Beef Cattle Production Sustainability in Brachiaria Grasslands
Abstract
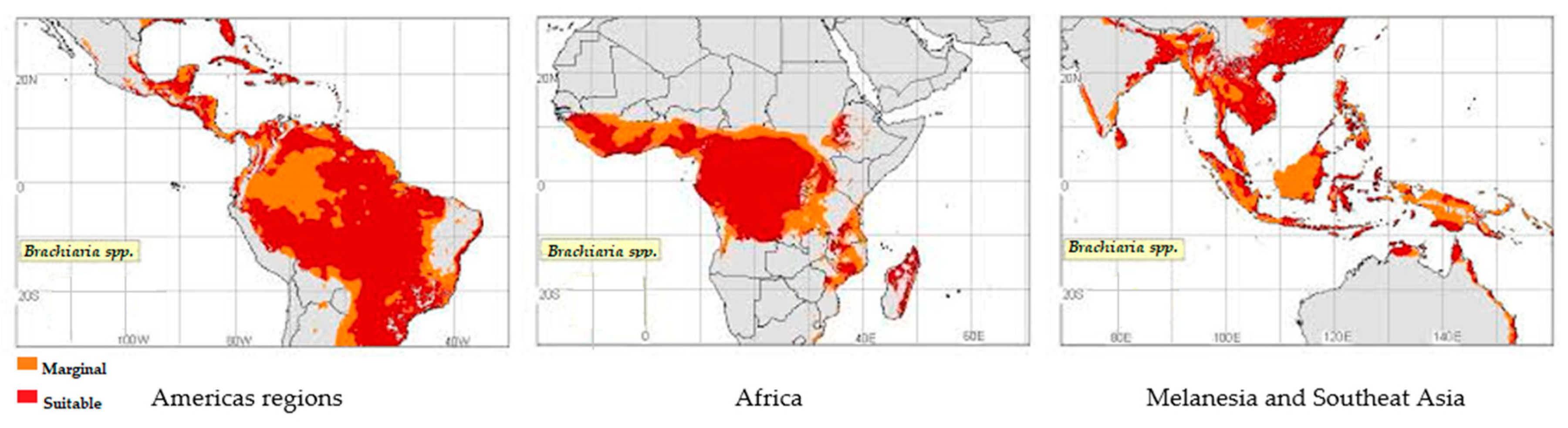
:1. Introduction
2. Concepts in Sustainable Intensification
2.1. Role of Forage in Intensification Systems
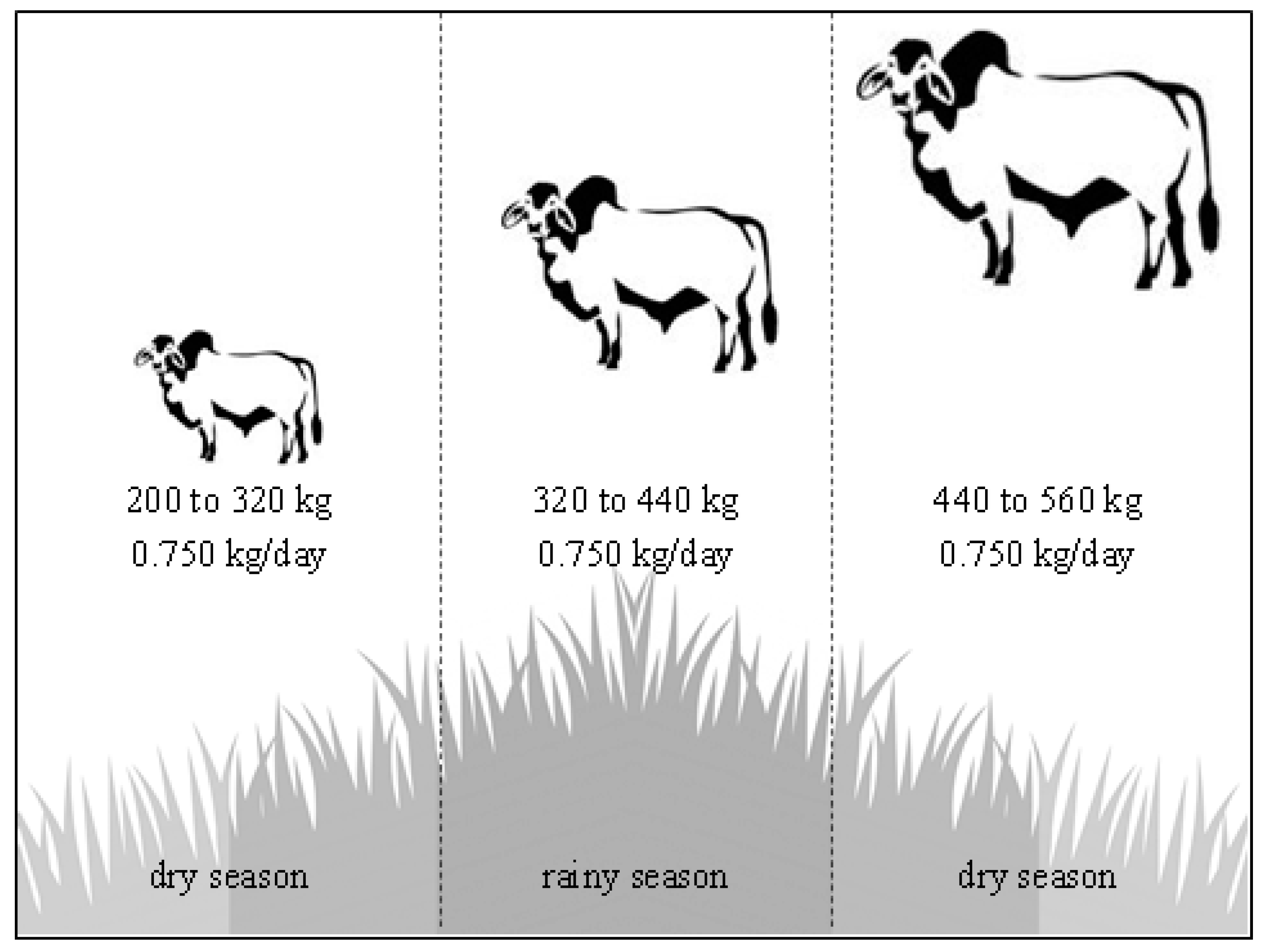
2.2. Role of Animals in Intensification Systems
2.3. Role of Supplementation in Intensification Systems
- (a)
- What is the predicted performance (average daily weight gain)?
- (b)
- What is the nutritional requirement for the predicted performance?
- (c)
- What is the estimated nutrient intake from the forage?
- (d)
- What is the deficiency (what is lacking) in relation to the requirements?
2.4. Role of Fertilizers in Intensification Systems
3. Environmental Aspects of Pasture Intensification
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Boval, M.; Dixon, R.M. The importance of grasslands for animal production and other functions: A review on management and methodological progress in the tropics. Animal 2012, 6, 748–762. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wezel, A.; Soboksa, G.; McClelland, S.; Delespesse, F.; Boissau, A. The blurred boundaries of ecological, sustainable, and agroecological intensification: A review. Agron. Sustain. Dev. 2015, 35, 1283–1295. [Google Scholar] [CrossRef]
- Boval, M.; Angeon, V.; Rudel, T.K. Tropical grasslands: A pivotal place for a more multi-functional agriculture. Ambio 2017, 46, 48–56. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barbero, R.P.; Malheiros, E.B.; Araújo, T.; Nave, R.L.G.; Mulliniks, J.; Berchielli, T.T.; Ruggieri, A.; Reis, R. Combining Marandu grass grazing height and supplementation level to optimize growth and productivity of yearling bulls. Anim. Feed. Sci. Technol. 2015, 209, 110–118. [Google Scholar] [CrossRef] [Green Version]
- Delevatti, L.M.; Cardoso, A.D.S.; Barbero, R.P.; Leite, R.G.; Romanzini, E.P.; Ruggieri, A.; Reis, R.A. Effect of nitrogen application rate on yield, forage quality, and animal performance in a tropical pasture. Sci. Rep. 2019, 9, 7596. [Google Scholar] [CrossRef]
- Koscheck, J.F.W.; Romanzini, E.P.; Barbero, R.P.; Delevatti, L.M.; Ferrari, A.C.; Mulliniks, J.T.; Mousquer, C.J.; Berchielli, T.T.; Reis, R.A. How do animal performance and methane emissions vary with forage management intensification and supplementation? Anim. Prod. Sci. 2020, 60, 1201–1209. [Google Scholar] [CrossRef]
- Peyraud, J.L. The role of grassland-based production system for sustainable protein production. In Proceedings of the 54a Reunião Anual da Sociedade Brasileira de Zootecnia, Foz do Iguaçu, Brazil, 24–28 July 2017. [Google Scholar]
- Gil, J.D.B.; Siebold, M.; Berger, T. Adoption and development of integrated crop–livestock–forestry systems in Mato Grosso, Brazil. Agric. Ecosyst. Environ. 2015, 199, 394–406. [Google Scholar] [CrossRef]
- Pedrosa, L.M.; Hoshide, A.K.; de Abreu, D.C.; Molossi, L.; Couto, E.G. Financial transition and costs of sustainable agricultural intensification practices on a beef cattle and crop farm in Brazil’s Amazon. Renew. Agric. Food Syst. 2019. [Google Scholar] [CrossRef]
- Ferrari, A.C.; Romanzini, E.P.; Leite, R.G.; Siniscalchi, D.; da Costa, T.R.F.; Lage, J.F.; Reis, R.A. Efficiency of supplementation strategies for Nellore cattle rearing in tropical grass pasture. In Proceedings of the International Tropical Agriculture Conference—TropAg, Brisbane, Australia, 12–13 November 2017. [Google Scholar]
- Barbero, R.P.; Malheiros, E.B.; Nave, R.L.; Mulliniks, J.T.; Delevatti, L.M.; Koscheck, J.F.; Romanzini, E.P.; Ferrari, A.C.; Renesto, D.M.; Berchielli, T.T.; et al. Influence of post-weaning management system during the finishing phase on grasslands or feedlot on aiming to improvement of the beef cattle production. Agric. Syst. 2017, 153, 23–31. [Google Scholar] [CrossRef]
- De Moraes, A.; Carvalho, P.C.F.; Brasil, S.; Lustosa, C.; Lang, C.R.; Deiss, L. Research on Integrated Crop–Livestock Systems in Brazil. A pesquisa em Sistemas Integrados de Produção Agropecuária no Brasil. Rev. Cienc. Agron. 2014, 45, 1024–1031. [Google Scholar] [CrossRef]
- Cardoso, A.D.S.; Brito, L.F.; Janusckiewicz, E.R.; Morgado, E.D.S.; Barbero, R.P.; Koscheck, J.F.W.; Reis, R.; Ruggieri, A. Impact of Grazing Intensity and Seasons on Greenhouse Gas Emissions in Tropical Grassland. Ecosystems 2017, 20, 845–859. [Google Scholar] [CrossRef]
- Casagrande, D.; Ruggieri, A.; Moretti, M.H.; Berchielli, T.T.; Vieira, B.R.; Roth, A.P.D.T.P.; Reis, R. Sward canopy structure and performance of beef heifers under supplementation in Brachiaria brizantha cv. Marandu pastures maintained with three grazing intensities in a continuous stocking system. Rev. Bras. Zootec. 2011, 40, 2074–2082. [Google Scholar] [CrossRef] [Green Version]
- De Oliveira, A.P.; Casagrande, D.; Bertipaglia, L.M.A.; Barbero, R.P.; Berchielli, T.T.; Ruggieri, A.; Reis, R. Supplementation for beef cattle on Marandu grass pastures with different herbage allowances. Anim. Prod. Sci. 2016, 56, 123–129. [Google Scholar] [CrossRef] [Green Version]
- Pizarro, E.A.; Hare, M.D.; Mutimura, M.; Bai, C.; Changjun, B. Brachiaria hybrids: Potential, forage use and seed yield. Trop. Grassl. Forrajes Trop. 2013, 1, 31–35. [Google Scholar] [CrossRef]
- Cook, B.G.; Pengelly, B.C.; Brown, S.D.; Donnelly, J.L.; Eagles, D.A.; Franco, M.A.; Hanson, J.; Mullen, B.F.; Partridge, I.J.; Peters, M.; et al. Tropical Forages: An Interactive Selection Tool; [CD-ROM], CSIRO, DPI and F(Qld), CIAT and ILRI: Brisbane, Australia, 2005. [Google Scholar]
- Sollenberger, L.; Coleman, S.W.; Vendramini, J.M.B. A interação planta-herbívoros em pastagens. In Forragicultura Ciência, Tecnologia E Gestão Dos Recursos Forrageiros, 1st ed.; Reis, R.A., Bernardes, T.F., Siqueira, G.R., Eds.; Gráfica Multipress: Jaboticabal, Brazil, 2014; Volume 1, pp. 69–80. [Google Scholar]
- Cândido, M.J.D.; Gomide, C.A.M.; Alexandrino, E.; Gomide, J.A.; Pereira, W.E. Morfofisiologia do dossel de Panicum maximum cv. Mombaça sob lotação intermitente com três períodos de descanso. Rev. Bras. Zootec. 2005, 34, 406–415. [Google Scholar] [CrossRef] [Green Version]
- Hodgson, J. Grazing Management: Science into Practice, 1st ed.; Longman Group UK Ltd.: Harlow, UK, 1990; p. 203. [Google Scholar]
- Santana, S.S.; Brito, L.F.; Azenha, M.V.; Oliveira, A.A.; Malheiros, E.B.; Ruggieri, A.; Reis, R. Canopy characteristics and tillering dynamics of Marandu palisade grass pastures in the rainy-dry transition season. Grass Forage Sci. 2016, 72, 261–270. [Google Scholar] [CrossRef]
- Lemaire, G.; Jeuffroy, M.-H.; Gastal, F. Diagnosis tool for plant and crop N status in vegetative stage: Theory and practices for crop N management. Eur. J. Agron. 2008, 28, 614–624. [Google Scholar] [CrossRef]
- Fagundes, J.L.; Moreira, A.L.; Freitas, A.W.D.P.; Zonta, A.; Henrichs, R.; Rocha, F.C.; Backes, A.A.; Vieira, J.S. Capacidade de suporte de pastagens de capim-tifton 85 adubado com nitrogênio manejadas em lotação contínua com ovinos. Rev. Bras. Zootec. 2011, 40, 2651–2657. [Google Scholar] [CrossRef] [Green Version]
- Silva, M.F.; Porto, E.M.V.; Alves, D.D.; Vitor, C.M.T.; Aspiazú, I. Morphogenetic characteristics of three Brachiaria brizantha cultivars submitted to nitrogen fertilization. An. Acad. Bras. Ciênc. 2013, 85, 371–377. [Google Scholar] [CrossRef] [Green Version]
- Dubeux, J.C.B., Jr.; Sollenberger, L.E.; Muir, J.P.; Tedeschi, L.O.; dos Santos, M.V.; da Cunha, M.V.; Mello, A.C.L.; Di Lorenzo, N. Sustainable intensification of livestock production on pastures. Arch. Lat. Am. Prod. Anim. 2017, 25, 3–4. [Google Scholar]
- Wedin, D.A. C4 grasses: Resource use, ecology, and global change. In Warm-Season (C4) Grasses, 1st ed.; Moser, L.E., Burson, B.L., Sollenberger, L.E., Eds.; ASA, CSSA, SSSA: Madison, WI, USA, 2004; Volume 1, pp. 15–50. [Google Scholar]
- Carvalho, L.; Pereira, L.E.T.; Hungria, M.; De Camargo, P.B.; Da Silva, S.C. Nodulation and biological nitrogen fixation (BNF) in forage peanut (Arachis pintoi) cv. Belmonte subjected to grazing regimes. Agric. Ecosyst. Environ. 2019, 278, 96–106. [Google Scholar] [CrossRef]
- Gomes, F.K.; Oliveira, M.D.B.L.; Homem, B.G.C.; Boddey, R.M.; Bernardes, T.F.; Gionbelli, M.P.; Lara, M.A.S.; Casagrande, D. Effects of grazing management in brachiaria grass-forage peanut pastures on canopy structure and forage intake 1. J. Anim. Sci. 2018, 96, 3837–3849. [Google Scholar] [CrossRef] [PubMed]
- Pereira, J.C.; Gomes, F.K.; Oliveira, M.D.; Lara, M.A.S.; Bernardes, T.F.; Casagrande, D. Defoliation management affects morphogenetic and structural characteristics of mixed pastures of brachiaria grass and forage peanut. Afr. J. Range Forage Sci. 2017, 34, 1–7. [Google Scholar] [CrossRef]
- Ferraz, J.B.S.; Felício, P.E. Production systems—An example from Brazil. Meat. Sci. 2010, 84, 238–243. [Google Scholar] [CrossRef]
- Da Silva, S.C.; Gimenes, F.M.D.A.; Sarmento, D.O.L.; Sbrissia, A.F.; Oliveira, D.E.; Hernadez-Garay, A.; Pires, A.V.; Da Silva, S.C. Grazing behaviour, herbage intake and animal performance of beef cattle heifers on marandu palisade grass subjected to intensities of continuous stocking management. J. Agric. Sci. 2012, 151, 727–739. [Google Scholar] [CrossRef] [Green Version]
- Garnett, T.; Appleby, M.C.; Balmford, A.; Bateman, I.J.; Benton, T.G.; Bloomer, P.; Burlingame, B.; Dawkins, M.; Dolan, L.; Fraser, D.; et al. Sustainable Intensification in Agriculture: Premises and Policies. Science 2013, 341, 33–34. [Google Scholar] [CrossRef]
- Chaucheyras-Durand, F.; Walker, N.; Bach, A. Effects of active dry yeasts on the rumen microbial ecosystem: Past, present and future. Anim. Feed. Sci. Technol. 2008, 145, 5–26. [Google Scholar] [CrossRef]
- Cieślak, A.; Zmora, P.; Pers-Kamczyc, E.; Szumacher-Strabel, M. Effects of tannins source (Vaccinium vitis idaea L.) on rumen microbial fermentation in vivo. Anim. Feed. Sci. Technol. 2012, 176, 102–106. [Google Scholar] [CrossRef]
- Reis, R.A.; Siqueira, G.R.; Vieira, B.R.; Moretti, M.H. Manejo alimentar na terminação em pasto. In Simpósio Sobre Nutrição De Bovinos, 1st ed.; Bittar, C.M.M., Santos, F.A.P., Moura, J.C., Faria, V.P., Eds.; FEALQ: Piracicaba, Brazil, 2011; pp. 341–381. [Google Scholar]
- Vieira, B.R.; Azenha, M.V.; Casagrande, D.; Costa, D.F.A.; Ruggieri, A.; Berchielli, T.T.; Reis, R. Ingestive behavior of supplemented Nellore heifers grazing palisadegrass pastures managed with different sward heights. Anim. Sci. J. 2016, 88, 696–704. [Google Scholar] [CrossRef]
- Hodgson, J.; Clark, D.A.; Mitchell, R.J. Foraging behavior in grazing animals and its impact on plant communities. In Forage Quality, Evaluation, and Utilization, 1st ed.; Fahey, G.C., Jr., Ed.; ASA, CSSA, SSSA Books: Madison, WI, USA, 1994; Volume 1, pp. 796–827. [Google Scholar]
- Reis, R.A.; Da Silva, S.C. Consumo de forragens. In Nutrição de Ruminantes; Berchielli, T.T., Pires, A.V., Oliveira, S.G., Eds.; FUNEP: Jaboticabal, Brazil, 2006; pp. 79–109. [Google Scholar]
- Carvalho, P.C.D.F. Harry Stobbs Memorial Lecture: Can grazing behavior support innovations in grassland management? Trop. Grassl. Forrajes Trop. 2013, 1, 137–155. [Google Scholar] [CrossRef]
- Dubeux, J.C.B., Jr.; Lira, M.A.; Santos, M.V.F.; Cunha, M.V. Fluxo de nutrientes em ecossistemas de pastagens: Impactos no ambiente e na produtividade. In As Pastagens E O Meio Ambiente, 1st ed.; Pedreira, C.G.S., De Moura, J.C., Da Silva, S.C., Faria, V.P., Eds.; FEALQ: Piracicaba, Brazil, 2006; Volume 1, pp. 439–506. [Google Scholar]
- Poppi, D.; Hughes, T.P.; L’huillier, P.J. Intake of pasture by grazing ruminants. In Livestock Feeding on Pasture. Hamilton: New Zealand Society of Animal Production, 1st ed.; Nicol, A.M., Ed.; Occasional Publication: Hamilton, New Zealand, 1987; pp. 55–64. [Google Scholar]
- Sollenberger, L.E.; VanZant, E.S. Interrelationships among Forage Nutritive Value and Quantity and Individual Animal Performance. Crop. Sci. 2011, 51, 420–432. [Google Scholar] [CrossRef]
- Reis, R.; Valente, A.L.; Dos Santos, S.M.; De Souza, F.H.; Berchielli, T.T.; Ruggieri, A.; Santana, S.S.; Serra, J.M. Performance of young Nelore bulls grazing marandu grass pasture at different heights. Trop. Grassl. Forrajes Trop. 2013, 1, 114–115. [Google Scholar] [CrossRef] [Green Version]
- Mott, G.O. Grazing pressure and the measurement of pasture production. In Proceedings of the 8th International Grasslands Congress, Reading, UK, 11–21 July 1960; Skidmore, C.L., Boyle, P.J., Raymond, L.W., Eds.; Alden Press: Oxford, UK, 1960; pp. 606–611. [Google Scholar]
- Callaway, T.R.; Edrington, T.S.; Rychlik, J.L.; Genovese, K.J.; Poole, T.L.; Jung, Y.S.; Bischoff, K.M.; Anderson, R.C.; Nisbet, D.J. Ionophores: Their use as ruminant growth promotants and impact on food safety. Curr. Issues Intest. Microbiol. 2003, 4, 43–51. [Google Scholar] [PubMed]
- Goodrich, R.D.; Garrett, J.E.; Gast, D.R.; Kirick, M.A.; Larson, D.A.; Meiske, J.C. Influence of Monensin on the Performance of Cattle. J. Anim. Sci. 1984, 58, 1484–1498. [Google Scholar] [CrossRef]
- Siqueira, G.R.; Moretti, M.H.; Fernandes, R.M.; Roth, M.T.P.; Resende, F.D.; Santos Junior, A.I. Associação pasto-confinamento na produção intensiva de carne bovina. In Proceedings of the II Simbov—II Simpósio Matogrossense de Bovinocultura de Corte, Cuiabá, Brazil, 15–17 August 2013. [Google Scholar]
- Maciel, I.; Saturnino, H.; Barbosa, F.; Malacco, V.; Júnior, J.A.; Filho, G.M.; Costa, P. Virginiamycin and sodium monensin supplementation for beef cattle on pasture. Arq. Bras. Med. Vet. Zootec. 2019, 71, 1999–2008. [Google Scholar] [CrossRef] [Green Version]
- Butaye, P.; Devriese, L.A.; Haesebrouck, F. Antimicrobial Growth Promoters Used in Animal Feed: Effects of Less Well Known Antibiotics on Gram-Positive Bacteria. Clin. Microbiol. Rev. 2003, 16, 175–188. [Google Scholar] [CrossRef] [Green Version]
- Benchaar, C.; Calsamiglia, S.; Chaves, A.V.; Fraser, G.; Colombatto, D.; McAllister, T.A.; Beauchemin, K. A review of plant-derived essential oils in ruminant nutrition and production. Anim. Feed. Sci. Technol. 2008, 145, 209–228. [Google Scholar] [CrossRef]
- Helander, I.M.; Alakomi, H.-L.; Latva-Kala, K.; Mattila-Sandholm, T.; Pol, I.; Smid, E.J.; Gorris, L.G.M.; Von Wright, A. Characterization of the Action of Selected Essential Oil Components on Gram-Negative Bacteria. J. Agric. Food Chem. 1998, 46, 3590–3595. [Google Scholar] [CrossRef]
- Cobellis, G.; Trabalza-Marinucci, M.; Yu, Z. Critical evaluation of essential oils as rumen modifiers in ruminant nutrition: A review. Sci. Total. Environ. 2016, 545, 556–568. [Google Scholar] [CrossRef]
- Busquet, M.; Calsamiglia, S.; Ferret, A.; Kamel, C. Plant Extracts Affect In Vitro Rumen Microbial Fermentation. J. Dairy Sci. 2006, 89, 761–771. [Google Scholar] [CrossRef]
- Blanch, M.; Carro, M.D.; Ranilla, M.J.; Viso, A.; Vázquez-Añón, M.; Bach, A. Influence of a blend of cinnamaldehyde and garlic oil on rumen fermentation, feeding behavior and performance of lactating dairy cows. Anim. Feed Sci. Technol. 2016, 219, 313–323. [Google Scholar] [CrossRef] [Green Version]
- Correa, D.C.C.; Cardoso, A.S.; Souza, N.C.S.; Toniello, A.D.; Reis, R.A.; Ruggieri, A.C. Effect of nitrogen source and level on ammonia volatilization in marandu grass pasturelands. In Proceedings of the 2nd International Conference on Forages, Lavras, Brazil, 28–30 May 2018; Ávila, C.L.S., Casagrande, D.R., Lara, M.A.S., Bernardes, T.F., Eds.; p. 225. [Google Scholar]
- Lamy, E.; Rawel, H.; Schweigert, F.; Capela e Silva, F.; Ferreira, A.M.; Costa, A.R.; Antunes, C.M.; De Almeida, A.M.; Coelho, A.V.; Sales-Baptista, E. The Effect of Tannins on Mediterranean Ruminant Ingestive Behavior: The Role of the Oral Cavity. Molecules 2011, 16, 2766–2784. [Google Scholar] [CrossRef] [Green Version]
- Cobellis, G.; Trabalza-Marinucci, M.; Marcotullio, M.C.; Yu, Z. Evaluation of diferente essencial oils in modulating methane and ammonia production, rumen fermentation, and rumen bacteria in vitro. Anim. Feed Sci. Technol. 2016, 215, 25–36. [Google Scholar] [CrossRef] [Green Version]
- Aboagye, I.A.; Oba, M.; Castillo, A.R.; Koenig, K.M.; Iwaasa, A.D.; Beauchemin, K.A. Effects of hydrolyzable tannin with or without condensed tannin on methane emissions, nitrogen use, and performance of beef cattle fed a high-forage diet 1. J. Anim. Sci. 2018, 96, 5276–5286. [Google Scholar] [CrossRef] [PubMed]
- Ebert, P.J.; Bailey, E.A.; Shreck, A.L.; Jennings, J.S.; Cole, N.A. Effect of condensed tannin extract supplementation on growth performance, nitrogen balance, gas emissions, and energetic losses of beef steers. J. Anim. Sci. 2017, 95, 1345–1355. [Google Scholar] [CrossRef] [PubMed]
- Dijkstra, J.; Oenema, O.; Van Groenigen, J.W.; Spek, J.W.; Van Vuuren, A.M.; Bannink, A. Diet effects on urine composition of cattle and N2O emissions. Animal 2013, 7, 292–302. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pereira, T.P.; Modesto, E.C.; Nepomuceno, D.D.D.; De Oliveira, O.F.; De Freitas, R.S.X.; Muir, J.P.; Junior, J.C.B.D.; Almeida, J.C.D.C. Characterization and biological activity of condensed tannins from tropical forage legumes. Pesqui. Agropec. Bras. 2018, 53, 1070–1077. [Google Scholar] [CrossRef] [Green Version]
- Gomide, J.A.; Noller, C.H.; Mott, G.O.; Conrad, J.H.; Hill, D.L. Effect of Plant Age and Nitrogen Fertilization on the Chemical Composition and In Vitro Cellulose Digestibility of Tropical Grasses 1. Agron. J. 1969, 61, 116–120. [Google Scholar] [CrossRef]
- Ruggieri, A.C.; Favoretto, V.; Malheiros, E.B. Características de crescimento e produção de matéria seca da Brachiaria brizantha (Hochst) stapf cv. Marandu em função de níveis de nitrogênio em regime de corte. Boletim de Indústria Animal 1994, 2, 149–155. [Google Scholar]
- Lemaire, G.; Gastal, F. Quantifying Crop Responses to Nitrogen Deficiency and Avenues to Improve Nitrogen Use Efficiency. In Crop Physiology: Applications for Genetic Improvement and Agronomy, 1st ed.; Sadras, V., Calderini, D.F., Eds.; Academic Press: San Diego, CA, USA, 2009; Volume 1, pp. 171–211. [Google Scholar]
- Peyraud, J.; Astigarraga, L. Review of the effect of nitrogen fertilization on the chemical composition, intake, digestion and nutritive value of fresh herbage: Consequences on animal nutrition and N balance. Anim. Feed. Sci. Technol. 1998, 72, 235–259. [Google Scholar] [CrossRef]
- Lessa, A.C.R.; Madari, B.E.; Paredes, D.S.; Boddey, R.M.; Urquiaga, S.; Jantalia, C.P.; Alves, B.J.R. Bovine urine and dung deposited on Brazilian savannah pastures contribute differently to direct and indirect soil nitrous oxide emissions. Agric. Ecosyst. Environ. 2014, 190, 104–111. [Google Scholar] [CrossRef]
- Balsalobre, M.A.A.; Nussio, L.G.; Júnior, G.B.M. Controle de perdas na produção de silagens de gramíneas tropicais. In A Produção Animal na Visão dos Brasileiros, Proceedings of the Reunião Anual da Sociedade Brasileira de Zootecnia, Piracicaba, Brazil, 2001; Mattos, W.R.S., Faria, V.P., Da Silva, S.C., Nussio, L.G., Moura, J.C., Eds.; FEALQ: Piracicaba, Brazil, 2001; pp. 890–911. [Google Scholar]
- Reid, D.; Strachan, N.H. The effects of a wide range of nitrogen rates on some chemical constituents of the herbage from perennial ryegrass swards with and without white clover. J. Agric. Sci. 1974, 83, 393–401. [Google Scholar] [CrossRef]
- Godde, C.M.; De Boer, I.J.; Zu Ermgassen, E.K.; Herrero, M.; Van Middelaar, C.E.; Muller, A.; Röös, E.; Schader, C.; Smith, P.; Van Zanten, H.H.E.; et al. Soil carbon sequestration in grazing systems: Managing expectations. Clim. Chang. 2020. [Google Scholar] [CrossRef]
- Lupatini, G.G.; Medeiros, S.F.; Yamamoto, W.K.; Ronchesel, J.R. Efeito de doses de adubação nitrogenada e fosfatada na recuperação da produção de Brachiaria decumbens. In Proceedings of the Simpósio de Ciências da Unesp, Dracena, Brazil, 6–8 October 2010; p. 3. [Google Scholar]
- Vicente-Chandler, J.; Pearson, R.W.; Abruña, F.; Silva, S. Potassium Fertilization of Intensively Manage Grasses under Humid Tropical Conditions 1. Agron. J. 1962, 54, 450–453. [Google Scholar] [CrossRef]
- Bernardi, A.C.D.C.; Rassini, J.B. Produção de matéria seca pelo capim-Tanzânia em função de doses e relações de nitrogênio e potássio. In Proceedings of the FertBio 2008: Desafios para o uso do solo com eficiência e qualidade ambiental, Anais, Londrina, Brazil, 15–19 September 2008. [Google Scholar]
- Dallantonia, E.; Lage, J.; Torrecilhas, J.; Leite, R.; Ferrari, A.; Rossi, L.; Fernandes, M.; Reis, R. PSXI-7 Sustainable intensification of the beef cattle production system in Brazil: Animal performance in growing phase in pasture. J. Anim. Sci. 2018, 96, 227. [Google Scholar] [CrossRef]
- Steinfeld, H.; Gerber, P.; Wassenaar, T.D.; Castel, V.; Rosales, M.; Rosales, M.; de Haan, C. Livestock’s Long Shadow: Environmental Issues and Options, 1st ed.; Food and Agriculture Organization of the United Nations (FAO): Rome, Italia, 2006; p. 493. [Google Scholar]
- Brito, L.F.; Azenha, M.V.; Janusckiewicz, E.R.; Cardoso, A.S.; Morgado, E.S.; Malheiros, E.B.; La Scala, N.; Reis, R.; Ruggieri, A. Seasonal Fluctuation of Soil Carbon Dioxide Emission in Differently Managed Pastures. Agron. J. 2015, 107, 957–962. [Google Scholar] [CrossRef] [Green Version]
- Novaes, R.M.L.; Pazianotto, R.A.A.; Brandão, M.; Alves, B.J.R.; May, A.; Folegatti-Matsuura, M.I.S. Estimating 20-year land-use change and derived CO2 emissions associated with crops, pasture and forestry in Brazil and each of its 27 states. Glob. Chang. Boil. 2017, 23, 3716–3728. [Google Scholar] [CrossRef]
- Braz, S.P.; Urquiaga, S.; Alves, B.J.R.; Jantalia, C.P.; Guimarães, A.P.; Dos Santos, C.A.; Dos Santos, S.C.; Pinheiro, E.; Boddey, R.M. Soil Carbon Stocks under Productive and Degraded Brachiaria Pastures in the Brazilian Cerrado. Soil Sci. Soc. Am. J. 2013, 77, 914–928. [Google Scholar] [CrossRef]
- Maia, S.M.; Ogle, S.M.; Cerri, C.E.P.; Cerri, C.E.P. Effect of grassland management on soil carbon sequestration in Rondônia and Mato Grosso states, Brazil. Geoderma 2009, 149, 84–91. [Google Scholar] [CrossRef]
- De Sant-Anna, S.A.C.; Jantalia, C.P.; Sá, J.M.; Vilela, L.; Marchão, R.L.; Alves, B.J.R.; Urquiaga, S.; Boddey, R.M. Changes in soil organic carbon during 22 years of pastures, cropping or integrated crop/livestock systems in the Brazilian Cerrado. Nutr. Cycl. Agroecosyst. 2017, 108, 101–120. [Google Scholar] [CrossRef] [Green Version]
- Johnson, K.A.; Johnson, D.E. Methane emissions from cattle. J. Anim. Sci. 1995, 73, 2483–2492. [Google Scholar] [CrossRef] [PubMed]
- Jarvis, S.; Lovell, R.; Panayides, R. Patterns of methane emission from excreta of grazing animals. Soil Boil. Biochem. 1995, 27, 1581–1588. [Google Scholar] [CrossRef]
- Mazzetto, A.; Barneze, A.; Feigl, B.; Van Groenigen, J.W.; Oenema, O.; Cerri, C. Temperature and moisture affect methane and nitrous oxide emission from bovine manure patches in tropical conditions. Soil Boil. Biochem. 2014, 76, 242–248. [Google Scholar] [CrossRef]
- Cardoso, A.D.S.; Quintana, B.G.; Janusckiewicz, E.; Brito, L.F.; Morgado, E.D.S.; Reis, R.A.; Ruggieri, A. How do methane rates vary with soil moisture and compaction, N compound and rate, and dung addition in a tropical soil? Int. J. Biometeorol. 2019, 63, 1533–1540. [Google Scholar] [CrossRef]
- Cardoso, A.S.; Alves BJ, R.; Urquiaga, S.; Boddey, R.M. Effect of volume of urine and mass of feces on N2O and CH4 emissions of dairy cow excreta in a tropical pasture. Anim. Prod. Sci. 2018, 58, 1079–1086. [Google Scholar] [CrossRef]
- Sordi, A.; Dieckow, J.; Bayer, C.; Alburquerque, M.A.; Piva, J.T.; Zanatta, J.A.; Tomazi, M.; Da Rosa, C.M.; De Moraes, A. Nitrous oxide emission factors for urine and dung patches in a subtropical Brazilian pastureland. Agric. Ecosyst. Environ. 2014, 190, 94–103. [Google Scholar] [CrossRef]
- Cardoso, A.S.; Oliveira, S.C.; Janusckiewicz, E.R.; Brito, L.F.; Morgado, E.S.; Reis, R.A.; Ruggieri, A.C. Effect of season on ammonia, nitrous oxide and methane emission factors for beef cattle excreta and urea fertilizer applied to tropical pasture. Soil Till. Res. 2019, 194, 104341. [Google Scholar] [CrossRef]
- Saggar, S.; Tate, K.R.; Giltrap, D.L.; Singh, J. Soil-atmosphere exchange of nitrous oxide and methane in New Zealand terrestrial ecosystems and their mitigation options: A review. Plant Soil 2008, 309, 25–42. [Google Scholar] [CrossRef]
- Intergovernmental Panel on Climate Change (IPCC). Guidelines for National Greenhouse Gas Inventories. Greenhouse Gas Inventory Reference Manual, 4. Intergovernmental Panel on Climate Change. Available online: http://www.ipccnggip.iges.or.jp/public/2006gl/vol4.html (accessed on 19 July 2019).
- Laubach, J.; Barthel, M.; Fraser, A.; Hunt, J.E.; Griffith, D.W.T. Combining two complementary micrometeorological methods to measure CH4 and N2O fluxes over pasture. Biogeosciences 2016, 13, 1309–1327. [Google Scholar] [CrossRef] [Green Version]
- Mori, A. Greenhouse Gas Sink-Source Functions of Grassland Ecosystems. Jpn. Agric. Res. Q. JARQ 2016, 50, 187–190. [Google Scholar] [CrossRef] [Green Version]
- Savian, J.V.; Schons, R.M.T.; Marchi, D.E.; De Freitas, T.S.; Neto, G.F.D.S.; Mezzalira, J.C.; Berndt, A.; Bayer, C.; Carvalho, P.C.D.F. Rotatinuous stocking: A grazing management innovation that has high potential to mitigate methane emissions by sheep. J. Clean. Prod. 2018, 186, 602–608. [Google Scholar] [CrossRef]
- Neto, A.J.; Messana, J.D.; Ribeiro, A.F.; Vito, E.S.; Rossi, L.G.; Berchielli, T.T. Effect of starch-based supplementation level combined with oil on intake, performance, and methane emissions of growing Nellore bulls on pasture 1. J. Anim. Sci. 2015, 93, 2275–2284. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krol, D.; Carolan, R.; Minet, E.; McGeough, K.; Watson, C.; Forrestal, P.; Lanigan, G.; Richards, K. Improving and disaggregating N2O emission factors for ruminant excreta on temperate pasture soils. Sci. Total. Environ. 2016, 568, 327–338. [Google Scholar] [CrossRef] [Green Version]
- Cardoso, A.S.; Junqueira, J.B.; Reis, R.A.; Ruggieri, A.C. How do greenhouse gases vary with biofertilizer type, soil temperature, and moisture, in a tropical grassland? Pedosphere 2020, 30, 1–11. [Google Scholar]
- Coutinho, E.L.M.; Silva, A.R.; Monteiro, F.A.; Rodrigues, L.R.A. Adubação potássica em forrageiras. In Simpósio Sobre Manejo da Pastagem, 1st ed.; Pedreira, C.G.S., Moura, J.C., Faria, V.P., Eds.; FEALQ: Piracicaba, Brazil, 2004; Volume 1, pp. 219–277. [Google Scholar]
- Cardoso, A.S.; Berndt, A.; Leytem, A.; Alves, B.J.; Carvalho, I.D.N.D.; Soares, L.H.D.B.; Urquiaga, S.; Boddey, R.M. Impact of the intensification of beef production in Brazil on greenhouse gas emissions and land use. Agric. Syst. 2016, 143, 86–96. [Google Scholar] [CrossRef] [Green Version]
- Filho, W.D.S.; Nunes, P.A.D.A.; Barro, R.S.; Kunrath, T.R.; De Almeida, G.M.; Genro, T.C.M.; Bayer, C.; Carvalho, P.C.D.F. Mitigation of enteric methane emissions through pasture management in integrated crop-livestock systems: Trade-offs between animal performance and environmental impacts. J. Clean. Prod. 2019, 213, 968–975. [Google Scholar] [CrossRef]
- Dos Santos, C.A.; Rezende, C.D.P.; Pinheiro, É.F.M.; Pereira, J.M.; Alves, B.J.; Urquiaga, S.; Boddey, R.M. Changes in soil carbon stocks after land-use change from native vegetation to pastures in the Atlantic forest region of Brazil. Geoderma 2019, 337, 394–401. [Google Scholar] [CrossRef]
- Tarré, R.; Macedo, R.; Cantarutti, R.; Rezende, C.D.P.; Pereira, J.; Ferreira, E.; Alves, B.; Urquiaga, S.; Boddey, R. The effect of the presence of a forage legume on nitrogen and carbon levels in soils under Brachiaria pastures in the Atlantic forest region of the South of Bahia, Brazil. Plant Soil 2001, 234, 15–26. [Google Scholar] [CrossRef]
- Carvalho, J.L.N.; Raucci, G.S.; Cerri, C.E.P.; Bernoux, M.; Feigl, B.J.; Wruck, F.J.; Cerri, C.E.P. Impact of pasture, agriculture and crop-livestock sy’tems on soil C stocks in Brazil. Soil Tillage Res. 2010, 110, 175–186. [Google Scholar] [CrossRef]
| Sex | Suppl. Level 1 | Animal Breed 2 | ADG 3 | Author |
|---|---|---|---|---|
| Female | - | PB | 0.731 | [34] |
| Female | MM 4 | CB | 0.490 | [27] |
| Female | 0.3 | CB | 0.665 | [27] |
| Male | - | PB | 0.600 | [4] |
| Male | 0.3 | PB | 1.150 | [4] |
| Male | 0.1 | PB | 0.844 | [35] |
| Male | 0.3 | PB | 0.940 | [35] |
| Male | MM 4 | PB | 0.854 | [36] |
| Male | 0.3E 5 | PB | 0.920 | [36] |
| Male | 0.3EP 5 | PB | 0.959 | [36] |
| Male | 0.3DDG 5 | PB | 0.930 | [36] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cardoso, A.d.S.; Barbero, R.P.; Romanzini, E.P.; Teobaldo, R.W.; Ongaratto, F.; Fernandes, M.H.M.d.R.; Ruggieri, A.C.; Reis, R.A. Intensification: A Key Strategy to Achieve Great Animal and Environmental Beef Cattle Production Sustainability in Brachiaria Grasslands. Sustainability 2020, 12, 6656. https://doi.org/10.3390/su12166656
Cardoso AdS, Barbero RP, Romanzini EP, Teobaldo RW, Ongaratto F, Fernandes MHMdR, Ruggieri AC, Reis RA. Intensification: A Key Strategy to Achieve Great Animal and Environmental Beef Cattle Production Sustainability in Brachiaria Grasslands. Sustainability. 2020; 12(16):6656. https://doi.org/10.3390/su12166656
Chicago/Turabian StyleCardoso, Abmael da Silva, Rondineli Pavezzi Barbero, Eliéder Prates Romanzini, Ronyatta Weich Teobaldo, Fernando Ongaratto, Marcia Helena Machado da Rocha Fernandes, Ana Cláudia Ruggieri, and Ricardo Andrade Reis. 2020. "Intensification: A Key Strategy to Achieve Great Animal and Environmental Beef Cattle Production Sustainability in Brachiaria Grasslands" Sustainability 12, no. 16: 6656. https://doi.org/10.3390/su12166656
APA StyleCardoso, A. d. S., Barbero, R. P., Romanzini, E. P., Teobaldo, R. W., Ongaratto, F., Fernandes, M. H. M. d. R., Ruggieri, A. C., & Reis, R. A. (2020). Intensification: A Key Strategy to Achieve Great Animal and Environmental Beef Cattle Production Sustainability in Brachiaria Grasslands. Sustainability, 12(16), 6656. https://doi.org/10.3390/su12166656








