Eco-Friendly Cellulose Nanofiber Extraction from Sugarcane Bagasse and Film Fabrication
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
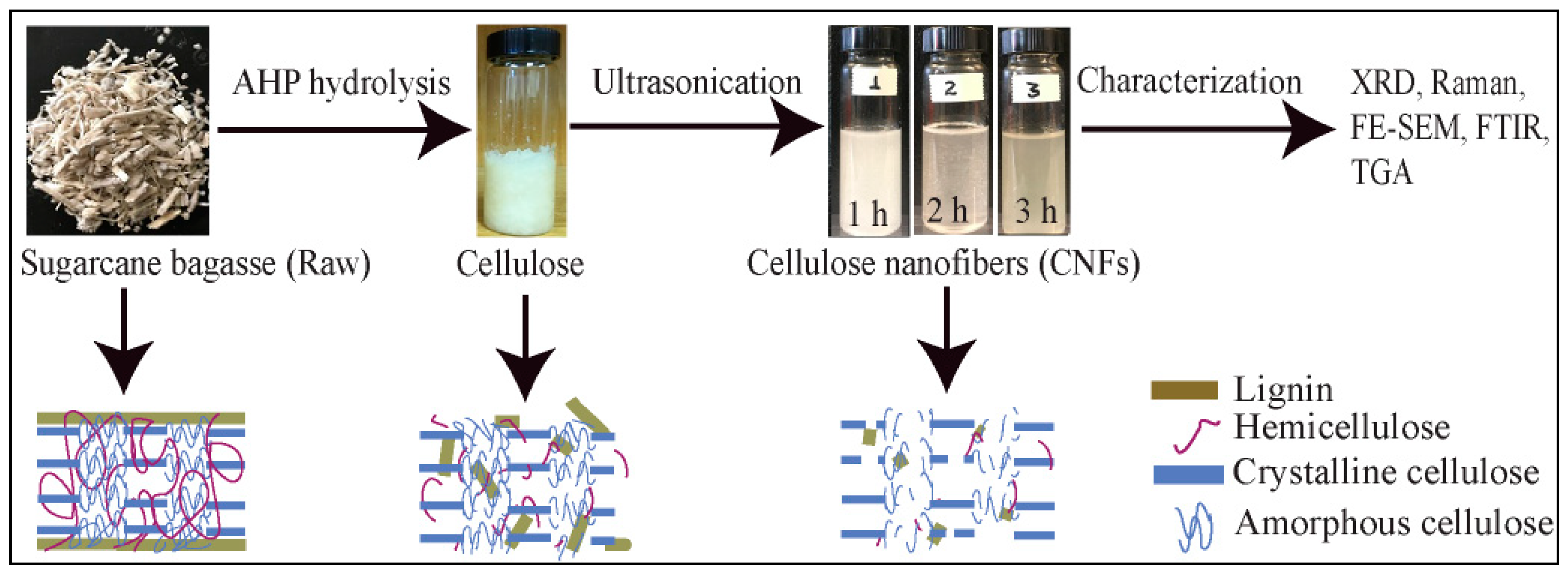
2.2. Alkaline Hydrogen Peroxide (AHP Hydrolysis)
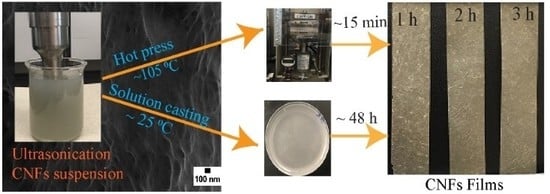
2.3. Extraction of CNF Using Ultrasonication
2.4. X-ray Diffraction (XRD)
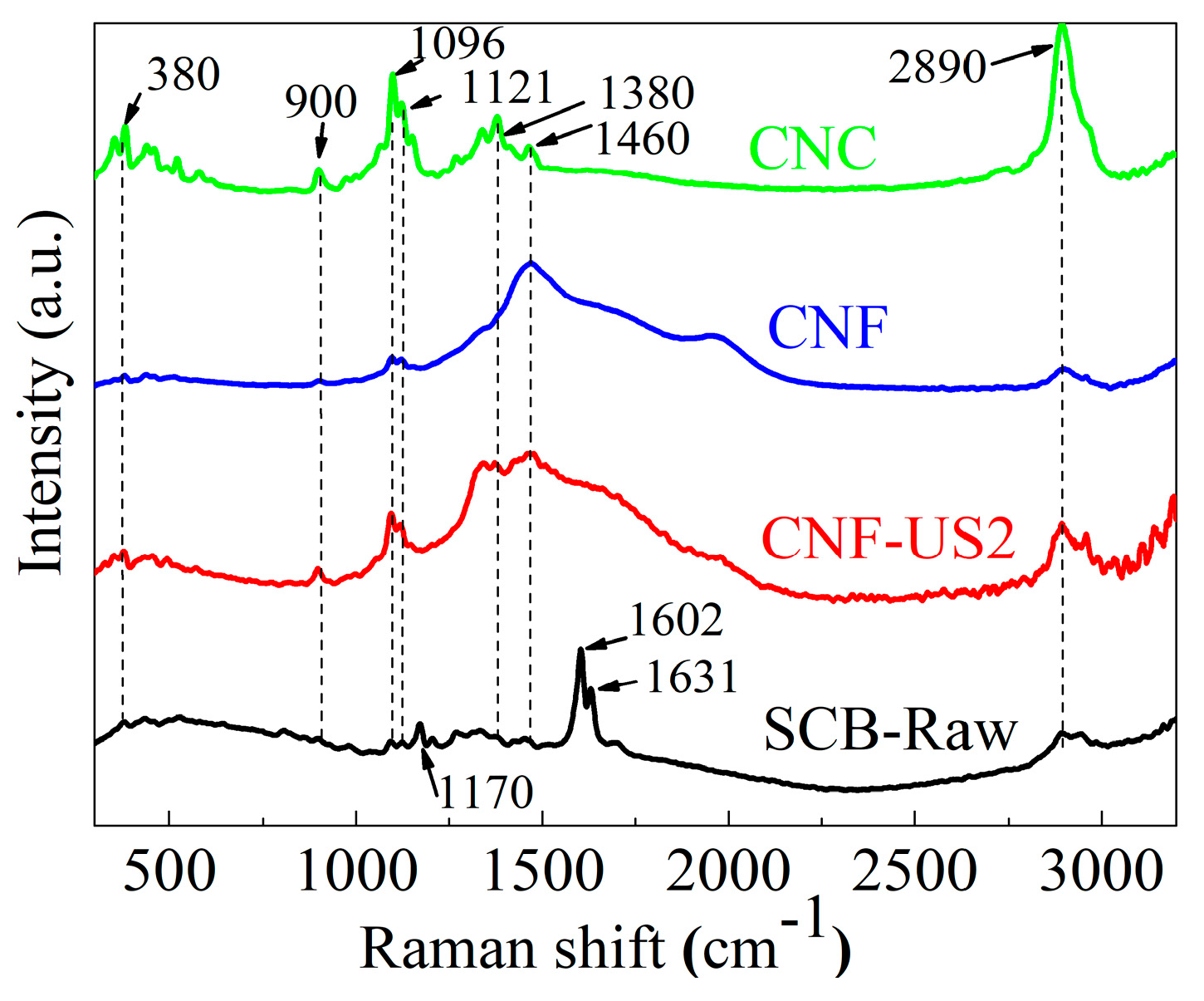
2.5. Raman Spectroscopy
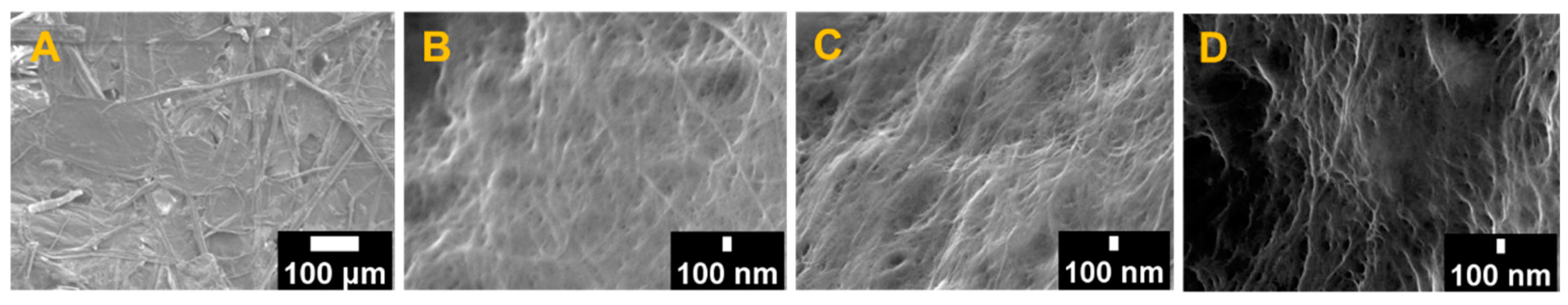
2.6. Field-Emission Scanning Electron Microscopy (FE-SEM)
2.7. Thermal Gravimetric Analysis (TGA)
2.8. Attenuated Total Reflectance Fourier Transform Infrared Spectroscopy (ATR-FTIR)
2.9. CNF Films Preparation
2.10. Mechanical Properties Testing
3. Results and Discussion
3.1. Effects of AHP Hydrolysis and Ultrasonication on Cellulose
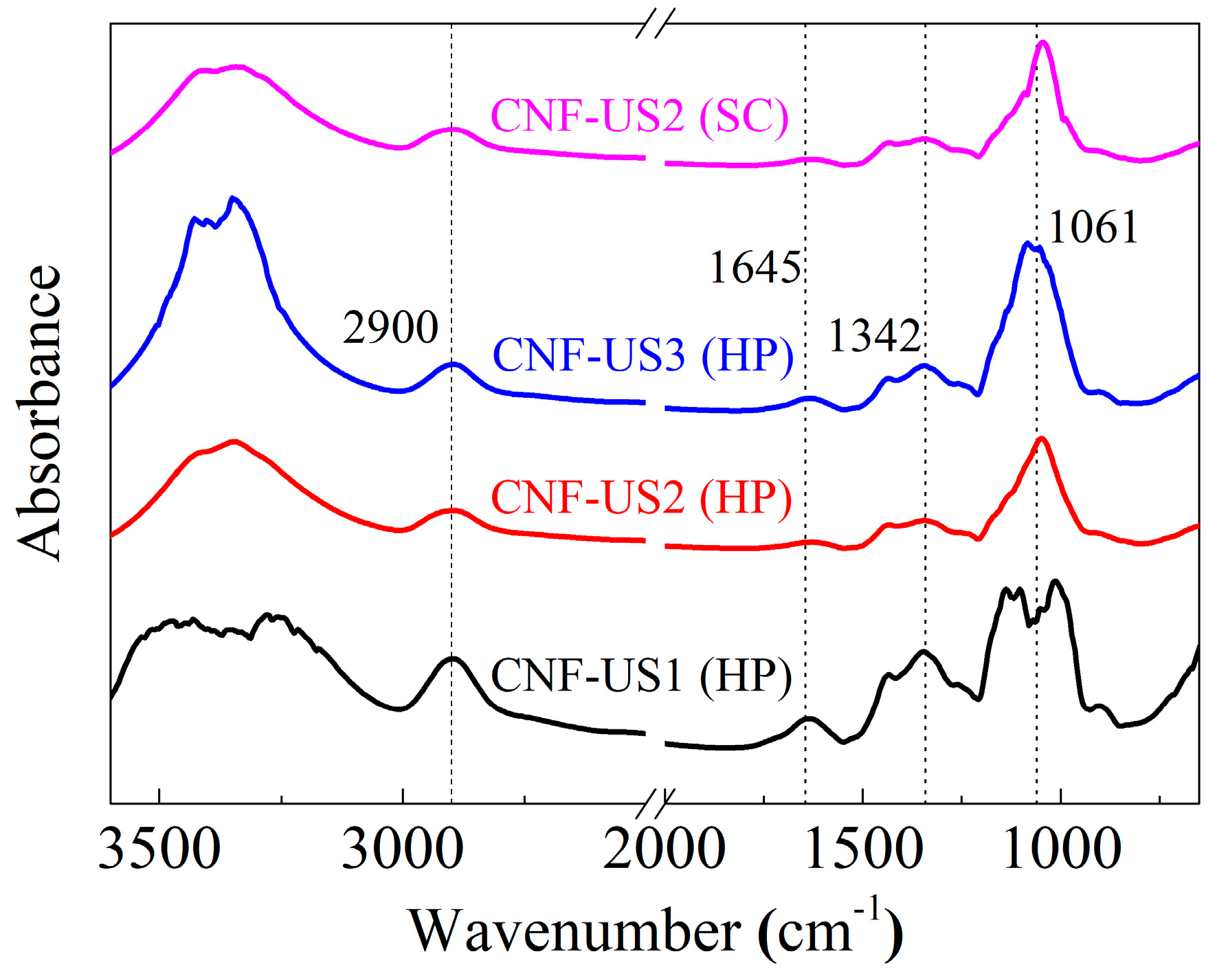
3.2. Structural Characterization of Extracted Cellulose and CNF
3.3. Morphological Characterization of the Raw SCB and CNFs
3.4. CNF Film Preparation and Mechanical Property Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Biswas, A.; Saha, B.C.; Lawton, J.W.; Shogren, R.L.; Willett, J.L. Process for obtaining cellulose acetate from agricultural by-products. Carbohydr. Polym. 2006, 64, 134–137. [Google Scholar] [CrossRef]
- Sun, J.X.; Sun, X.F.; Zhao, H.; Sun, R.C. Isolation and characterization of cellulose from sugarcane bagasse. Polym. Degrad. Stab. 2004, 84, 331–339. [Google Scholar] [CrossRef]
- Salas, C.; Nypelö, T.; Rodriguez-Abreu, C.; Carrillo, C.; Rojas, O.J. Nanocellulose properties and applications in colloids and interfaces. Curr. Opin. Colloid Interface Sci. 2014, 19, 383–396. [Google Scholar] [CrossRef]
- Xu, X.; Liu, F.; Jiang, L.; Zhu, J.Y.; Haagenson, D.; Wiesenborn, D.P. Cellulose nanocrystals vs. cellulose nanofibrils: A comparative study on their microstructures and effects as polymer reinforcing agents. ACS Appl. Mat. Interfaces 2013, 5, 2999–3009. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.; Wen, Y.; Huq, T.; Ni, Y. Cellulosic nanomaterials in food and nutraceutical applications: A review. J. Agric. Food Chem. 2018, 66, 8–19. [Google Scholar] [CrossRef] [PubMed]
- Rhim, J.W.; Kim, Y.T. Biopolymer-Based Composite Packaging Materials with Nanoparticles; Academic Press: Cambridge, MA, USA, 2013. [Google Scholar] [CrossRef]
- Yadav, S.K. Nanoscale Materials in Targeted Drug Delivery, Theragnosis and Tissue Regeneration; Springer: Berlin, Germany, 2016. [Google Scholar] [CrossRef]
- Ummartyotin, S.; Manuspiya, H. A critical review on cellulose: From fundamental to an approach on sensor technology. Renew. Sustain. Energy Rev. 2015, 41, 402–412. [Google Scholar] [CrossRef]
- Orelma, H.; Hokkanen, A.; Leppänen, I.; Kammiovirta, K.; Kapulainen, M.; Harlin, A. Optical cellulose fiber made from regenerated cellulose and cellulose acetate for water sensor applications. Cellulose 2020, 27, 1543–1553. [Google Scholar] [CrossRef]
- Khan, A.; Abas, Z.; Kim, H.S.; Kim, J. Recent progress on cellulose-based electro-active paper, its hybrid nanocomposites and applications. Sensors 2016, 16, 1172. [Google Scholar] [CrossRef]
- Voisin, H.; Bergström, L.; Liu, P.; Mathew, A. Nanocellulose-based materials for water purification. Nanomaterials 2017, 7, 57. [Google Scholar] [CrossRef]
- Feng, Y.H.; Cheng, T.Y.; Yang, W.G.; Ma, P.T.; He, H.Z.; Yin, X.C.; Yu, X.X. Characteristics and environmentally friendly extraction of cellulose nanofibrils from sugarcane bagasse. Ind. Crops Prod. 2018, 111, 285–291. [Google Scholar] [CrossRef]
- Perrone, O.M.; Colombari, F.M.; Rossi, J.S.; Moretti, M.M.S.; Bordignon, S.E.; Nunes, C.D.C.C.; Gomes, E.; Boscolo, M.; Da-Silva, R. Ozonolysis combined with ultrasound as a pretreatment of sugarcane bagasse: Effect on the enzymatic saccharification and the physical and chemical characteristics of the substrate. Bioresour. Technol. 2016, 218, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Zuluaga, R.; Putaux, J.L.; Cruz, J.; Vélez, J.; Mondragon, I.; Gañán, P. Cellulose microfibrils from banana rachis: Effect of alkaline treatments on structural and morphological features. Carbohydr. Polym. 2009, 76, 51–59. [Google Scholar] [CrossRef]
- Bang, J.H.; Suslick, K.S. Applications of ultrasound to the synthesis of nanostructured materials. Adv. Mat. 2010, 22, 1039–1059. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Singh Negi, Y.; Choudhary, V.; Bhardwaj, N.K. Characterization of cellulose nanocrystals produced by acid-hydrolysis from sugarcane bagasse as agro-waste. J. Mat. Phys. Chem. 2014, 2, 1–8. [Google Scholar] [CrossRef]
- Kim, D. Physico-chemical conversion of lignocellulose: Inhibitor effects and detoxification strategies: A mini review. Molecules 2018, 23, 309. [Google Scholar] [CrossRef] [PubMed]
- Cara, C.; Ruiz, E.; Oliva, J.M.; Sáez, F.; Castro, E. Conversion of olive tree biomass into fermentable sugars by dilute acid pretreatment and enzymatic saccharification. Bioresour. Technol. 2008, 99, 1869–1876. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Barrett, D.M.; Delwiche, M.J.; Stroeve, P. Methods for pretreatment of lignocellulosic biomass for efficient hydrolysis and biofuel production. Ind. Eng. Chem. Res. 2009, 48, 3713–3729. [Google Scholar] [CrossRef]
- Sofla, M.R.K.; Brown, R.J.; Tsuzuki, T.; Rainey, T.J. A Comparison of cellulose nanocrystals and cellulose nanofibres extracted from bagasse using acid and ball milling methods. Adv. Nat. Sci. Nanosci. Nanotechnol. 2016, 7, 035004. [Google Scholar] [CrossRef]
- Hassan, S.S.; Williams, G.A.; Jaiswal, A.K. Emerging technologies for the pretreatment of lignocellulosic biomass. Bioresour. Technol. 2018, 262, 310–318. [Google Scholar] [CrossRef]
- Mathew, A.K.; Abraham, A.; Mallapureddy, K.K.; Sukumaran, R.K. Lignocellulosic Biorefinery Wastes, or Resources? Elsevier: Amsterdam, The Netherlands, 2018. [Google Scholar] [CrossRef]
- Santucci, B.S.; Bras, J.; Belgacem, M.N.; Curvelo, A.A.D.S.; Pimenta, M.T.B. Evaluation of the effects of chemical composition and refining treatments on the properties of nanofibrillated cellulose films from sugarcane bagasse. Ind. Crops Prod. 2016, 91, 238–248. [Google Scholar] [CrossRef]
- Kim, J.S.; Lee, Y.Y.; Kim, T.H. A review on alkaline pretreatment technology for bioconversion of lignocellulosic biomass. Bioresour. Technol. 2015, 199, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Mittal, A.; Katahira, R.; Donohoe, B.S.; Black, B.A.; Pattathil, S.; Stringer, J.M.; Beckham, G.T. Alkaline peroxide delignification of corn stover. ACS Sustain. Chem. Eng. 2017, 5, 6310–6321. [Google Scholar] [CrossRef]
- Gould, J.M. Studies on the mechanism of alkaline peroxide delignification of agricultural residues. Biotechnol. Bioeng. 1985, 27, 225–231. [Google Scholar] [CrossRef] [PubMed]
- Karagöz, P.; Rocha, I.V.; Özkan, M.; Angelidaki, I. Alkaline peroxide pretreatment of rapeseed straw for enhancing bioethanol production by same vessel saccharification and co-fermentation. Bioresour. Technol. 2012, 104, 349–357. [Google Scholar] [CrossRef]
- Dutra, E.D.; Santos, F.A.; Alencar, B.R.A.; Reis, A.L.S.; de Souza, R.D.F.R.; Aquino, K.A.D.S.; Morais, M.A.; Menezes, R.S.C. Alkaline hydrogen peroxide pretreatment of lignocellulosic biomass: Status and perspectives. Biomass Convers. Biorefin. 2018, 8, 225–234. [Google Scholar] [CrossRef]
- Su, Y.; Du, R.; Guo, H.; Cao, M.; Wu, Q.; Su, R.; Qi, W.; He, Z. Fractional pretreatment of lignocellulose by alkaline hydrogen peroxide: Characterization of its major components. Food Bioprod. Process. 2015, 94, 322–330. [Google Scholar] [CrossRef]
- Shahi, N.; Joshi, G.; Min, B. Potential sustainable biomaterials derived from cover crops. BioResources 2020, 15, 5641–5652. [Google Scholar] [CrossRef]
- Csiszár, E.; Nagy, S. A comparative study on cellulose nanocrystals extracted from bleached cotton and flax and used for casting films with glycerol and sorbitol plasticisers. Carbohydr. Polym. 2017, 174, 740–749. [Google Scholar] [CrossRef]
- Abdul Khalil, H.P.S.; Davoudpour, Y.; Islam, M.N.; Mustapha, A.; Sudesh, K.; Dungani, R.; Jawaid, M. Production and modification of nanofibrillated cellulose using various mechanical processes: A review. Carbohydr. Polym. 2014, 99, 649–665. [Google Scholar] [CrossRef]
- Nakashima, K.; Ebi, Y.; Kubo, M.; Shibasaki-Kitakawa, N.; Yonemoto, T. Pretreatment combining ultrasound and sodium percarbonate under mild conditions for efficient degradation of corn stover. Ultrason. Sonochem. 2016, 29, 455–460. [Google Scholar] [CrossRef]
- Hassan, T.A.; Rangari, V.K.; Rana, R.K.; Jeelani, S. Sonochemical effect on size reduction of CaCO3 nanoparticles derived from waste eggshells. Ultrason. Sonochem. 2013, 20, 1308–1315. [Google Scholar] [CrossRef] [PubMed]
- Wanunu, M.; Sapkota, B. Porous Membranes Comprising Nanosheets and Fabrication Thereof. U.S. Patent 20190039028, 7 February 2019. [Google Scholar]
- Patist, A.; Bates, D. Ultrasonic innovations in the food industry: From the laboratory to commercial production. Innov. Food Sci. Emerg. Technol. 2008, 9, 147–154. [Google Scholar] [CrossRef]
- Bundhoo, Z.M.A.; Mohee, R. Ultrasound-assisted biological conversion of biomass and waste materials to biofuels: A review. Ultrason. Sonochem. 2018, 40, 298–313. [Google Scholar] [CrossRef] [PubMed]
- Khawas, P.; Deka, S.C. Isolation and characterization of cellulose nanofibers from bamboo using microwave liquefaction combined with chemical treatment and ultrasonication. Carbohydr. Polym. 2016, 151, 725–734. [Google Scholar] [CrossRef]
- Usmani, Z.; Sharma, M.; Gupta, P.; Karpichev, Y.; Gathergood, N.; Bhat, R.; Gupta, V.K. Ionic liquid based pretreatment of lignocellulosic biomass for enhanced bioconversion. Bioresour. Technol. 2020, 304, 123003. [Google Scholar] [CrossRef]
- Salvaggio, A.; Marino, F.; Albano, M.; Pecoraro, R.; Camiolo, G.; Tibullo, D.; Bramanti, V.; Lombardo, B.M.; Saccone, S.; Mazzei, V.; et al. Toxic effects of zinc chloride on the bone development in Danio Rerio (Hamilton, 1822). Front. Physiol. 2016, 7, 1–6. [Google Scholar] [CrossRef]
- Ciannamea, E.M.; Stefani, P.M.; Ruseckaite, R.A. Physical and mechanical properties of compression molded and solution casting soybean protein concentrate based films. Food Hydrocoll. 2014, 38, 193–204. [Google Scholar] [CrossRef]
- Panthapulakkal, S.; Sain, M. Preparation and characterization of cellulose nanofibril films from wood fibre and their thermoplastic polycarbonate composites. Int. J. Polym. Sci. 2012, 2012. [Google Scholar] [CrossRef]
- Pintiaux, T.; Viet, D.; Vandenbossche, V.; Rigal, L.; Rouilly, A. High pressure compression-molding of α-cellulose and effects of operating conditions. Materials 2013, 6, 2240–2261. [Google Scholar] [CrossRef]
- Cazón, P.; Velazquez, G.; Vázquez, M. Novel composite films from regenerated cellulose-glycerol-polyvinyl alcohol: Mechanical and barrier properties. Food Hydrocoll. 2019, 89, 481–491. [Google Scholar] [CrossRef]
- Bilanovic, D.; Starosvetsky, J.; Armon, R.H. Cross-linking xanthan and other compounds with glycerol. Food Hydrocoll. 2015, 44, 129–135. [Google Scholar] [CrossRef]
- Gogate, P.R.; Shirgaonkar, I.Z.; Sivakumar, M.; Senthilkumar, P.; Vichare, N.P.; Pandit, A.B. Cavitation reactors: Efficiency assessment using a model reaction. AIChE J. 2001, 47, 2526–2538. [Google Scholar] [CrossRef]
- Agarwal, U.P. Raman spectroscopy in the analysis of cellulose nanomaterials. In Nanocelluloses: Their Preparation, Properties, and Applications; ACS Publications: Washington, DC, USA, 2017; pp. 75–90. [Google Scholar] [CrossRef]
- Biswas, M.C.; Jeelani, S.; Rangari, V. Influence of biobased silica/carbon hybrid nanoparticles on thermal and mechanical properties of biodegradable polymer films. Compos. Commun. 2017, 4, 43–53. [Google Scholar] [CrossRef]
- Johar, N.; Ahmad, I.; Dufresne, A. Extraction, preparation and characterization of cellulose fibres and nanocrystals from rice husk. Ind. Crops Prod. 2012, 37, 93–99. [Google Scholar] [CrossRef]
- Sluiter, A.; Hames, B.; Ruiz, R.; Scarlata, C.; Sluiter, J.; Templeton, D.; Crocker, D. Determination of Structural Carbohydrates and Lignin in Biomass; LAP-002 NREL Analytical Procedure; NREL/TP-510-42618; National Renewable Energy Laboratory: Golden, CO, USA, 2004; p. 17. [Google Scholar]
- Sun, R.C.; Sun, X.F.; Ma, X.H. Effect of ultrasound on the structural and physiochemical properties of organosolv soluble hemicelluloses from wheat straw. Ultrason. Sonochem. 2002, 9, 95–101. [Google Scholar] [CrossRef]
- Ramadoss, G.; Muthukumar, K. Mechanistic study on ultrasound assisted pretreatment of sugarcane bagasse using metal salt with hydrogen peroxide for bioethanol production. Ultrason. Sonochem. 2016, 28, 207–217. [Google Scholar] [CrossRef] [PubMed]
- Bernal-Lugo, I.; Jacinto-Hernandez, C.; Gimeno, M.; Carmina Montiel, C.; Rivero-Cruz, F.; Velasco, O. Highly efficient single-step pretreatment to remove lignin and hemicellulose from softwood. BioResources 2019, 14, 3567–3577. [Google Scholar] [CrossRef]
- Segal, L.; Creely, J.J.; Martin, A.E.; Conrad, C.M. An empirical method for estimating the degree of crystallinity of native cellulose using the x-ray diffractometer. Text. Res. J. 1959, 29, 786–794. [Google Scholar] [CrossRef]
- Lupoi, J.S.; Gjersing, E.; Davis, M.F. Evaluating lignocellulosic biomass, its derivatives, and downstream Products with Raman spectroscopy. Front. Bioeng. Biotechnol. 2015, 3, 1–18. [Google Scholar] [CrossRef]
- Agarwal, U.P.; Reiner, R.R.; Ralph, S.A. Estimation of cellulose crystallinity of lignocelluloses using near-IR FT-Raman spectroscopy and comparison of the Raman and segal-WAXS methods. J. Agric. Food Chem. 2013, 61, 103–113. [Google Scholar] [CrossRef]
- Lupoi, J.S.; Singh, S.; Simmons, B.A.; Henry, R.J. Assessment of lignocellulosic biomass using analytical spectroscopy: An evolution to high-throughput techniques. Bioenergy Res. 2014, 7, 1–23. [Google Scholar] [CrossRef]
- Sacui, I.A.; Nieuwendaal, R.C.; Burnett, D.J.; Stranick, S.J.; Jorfi, M.; Weder, C.; Foster, E.J.; Olsson, R.T.; Gilman, J.W. Comparison of the properties of cellulose nanocrystals and cellulose nanofibrils isolated from bacteria, tunicate, and wood processed using acid, enzymatic, mechanical, and oxidative methods. ACS Appl. Mater. Interfaces 2014, 6, 6127–6138. [Google Scholar] [CrossRef] [PubMed]
- Alves, A.P.P.; de Oliveira, L.P.Z.; Castro, A.A.N.; Neumann, R.; de Oliveira, L.F.C.; Edwards, H.G.M.; Sant’Ana, A.C. The structure of different cellulosic fibres characterized by Raman spectroscopy. Vib. Spectrosc. 2016, 86, 324–330. [Google Scholar] [CrossRef]
- Ma, P.; Lan, J.; Feng, Y.; Liu, R.; Qu, J.; He, H. Effects of continuous steam explosion on the microstructure and properties of eucalyptus fibers. BioResources 2016, 11, 1417–1431. [Google Scholar] [CrossRef]
- Rezende, C.A.; De Lima, M.; Maziero, P.; Deazevedo, E.; Garcia, W.; Polikarpov, I. Chemical and morphological characterization of sugarcane bagasse submitted to a delignification process for enhanced enzymatic digestibility. Biotechnol. Biofuels 2011, 4. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Yan, R.; Chen, H.; Lee, D.H.; Zheng, C. Characteristics of hemicellulose, cellulose and lignin pyrolysis. Fuel 2007, 86, 1781–1788. [Google Scholar] [CrossRef]
- Yildirim, N.; Shaler, S. A study on thermal and nanomechanical performance of Cellulose Nanomaterials (CNs). Materials 2017, 10, 718. [Google Scholar] [CrossRef]
- Meng, Q.; Wang, T.J. Mechanics of strong and tough cellulose nanopaper. Appl. Mech. Rev. 2019, 71. [Google Scholar] [CrossRef]
- Ghaderi, M.; Mousavi, M.; Yousefi, H.; Labbafi, M. All-cellulose nanocomposite film made from bagasse cellulose nanofibers for food packaging application. Carbohydr. Polym. 2014, 104, 59–65. [Google Scholar] [CrossRef]
- Mosier, N.; Wyman, C.; Dale, B.; Elander, R.; Lee, Y.Y.; Holtzapple, M.; Ladisch, M. Features of promising technologies for pretreatment of lignocellulosic biomass. Bioresour. Technol. 2005, 96, 673–686. [Google Scholar] [CrossRef]
- Sun, S.; Mitchell, J.R.; MacNaughtan, W.; Foster, T.J.; Harabagiu, V.; Yihu Song, A.; Zheng, Q. Comparison of the mechanical properties of cellulose and starch films. Biomacromolecules 2010, 11, 126–132. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Baker, J.O.; Himmel, M.E.; Parilla, P.A.; Johnson, D.K. Cellulose crystallinity index: Measurement techniques and their impact on interpreting cellulase performance. Biotechnol. Biofuels 2010, 3, 10. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Halim, E.S. Chemical modification of cellulose extracted from sugarcane bagasse: Preparation of hydroxyethyl cellulose. Arab. J. Chem. 2014, 7, 362–371. [Google Scholar] [CrossRef]
- Gierlinger, N.; Keplinger, T.; Harrington, M.; Schwanninger, M. Raman imaging of lignocellulosic feedstock. Cellul. Biomass Convers. 2013. [Google Scholar] [CrossRef]

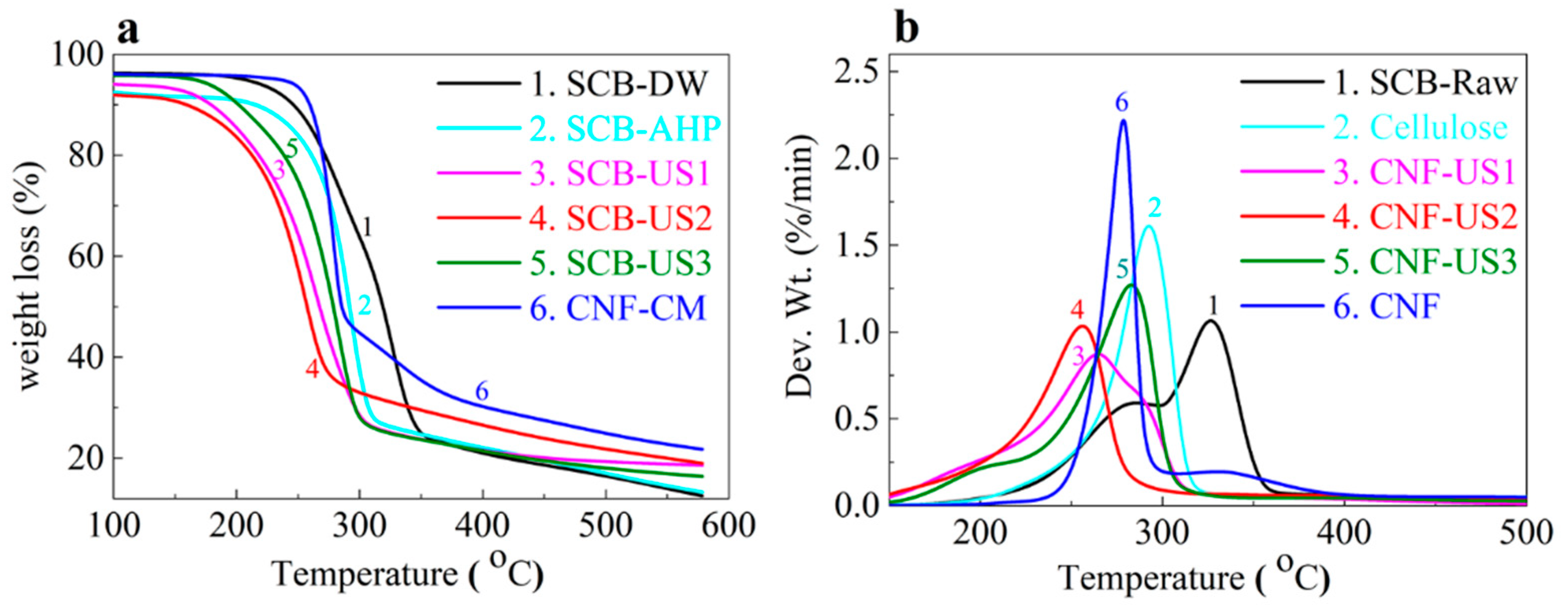

| Temp. (°C) | SCB-raw | Cellulose | CNF-US1 | CNF-US2 | CNF-US3 | CNF |
|---|---|---|---|---|---|---|
| Onset | 225 | 195 | 190 | 195 | 205 | 220 |
| Max. dec. | 325 | 290 | 255 | 265 | 280 | 280 |
| Young’s Modulus (GPa) | Tensile Strength (MPa) | Elongation (%) | |
|---|---|---|---|
| Hot Press (HP) | |||
| CNF-US1 | 1.7 ± 0.7 | 15.4 ± 3 | 1.7 ± 0.7 |
| CNF-US2 | 1.7 ± 0.5 | 25.0 ± 2 | 1.7 ± 0.5 |
| CNF-US3 | 2.3 ± 0.5 | 31.0 ± 3 | 2.0 ± 0.7 |
| Solution Casting (SC) | |||
| CNF-US1 | 0.1 ± 0.03 | 2.5 ± 0.5 | 3.3 ± 0.3 |
| CNF-US2 | 0.1 ± 0.02 | 3.5 ± 0.1 | 6.7 ± 1 |
| CNF-US3 | 0.2 ± 0.04 | 10.2 ± 2 | 6 ± 1.5 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shahi, N.; Min, B.; Sapkota, B.; Rangari, V.K. Eco-Friendly Cellulose Nanofiber Extraction from Sugarcane Bagasse and Film Fabrication. Sustainability 2020, 12, 6015. https://doi.org/10.3390/su12156015
Shahi N, Min B, Sapkota B, Rangari VK. Eco-Friendly Cellulose Nanofiber Extraction from Sugarcane Bagasse and Film Fabrication. Sustainability. 2020; 12(15):6015. https://doi.org/10.3390/su12156015
Chicago/Turabian StyleShahi, Naresh, Byungjin Min, Bedanga Sapkota, and Vijaya K. Rangari. 2020. "Eco-Friendly Cellulose Nanofiber Extraction from Sugarcane Bagasse and Film Fabrication" Sustainability 12, no. 15: 6015. https://doi.org/10.3390/su12156015
APA StyleShahi, N., Min, B., Sapkota, B., & Rangari, V. K. (2020). Eco-Friendly Cellulose Nanofiber Extraction from Sugarcane Bagasse and Film Fabrication. Sustainability, 12(15), 6015. https://doi.org/10.3390/su12156015